Abstract
Study Objectives
Estimate the genetic and environmental influences on the relationship between onset of regular cannabis use and young adult insomnia.
Methods
In a population-based twin cohort of 1882 twins (56% female, mean age = 22.99, SD = 2.97) we explored the genetic/environmental etiology of the relationship between onset of regular cannabis use and insomnia-related outcomes via multivariate twin models.
Results
Controlling for sex, current depression symptoms, and prior diagnosis of an anxiety or depression disorder, adult twins who reported early onset for regular cannabis use (age 17 or younger) were more likely to have insomnia (β = 0.07, p = 0.024) and insomnia with short sleep on weekdays (β = 0.08, p = 0.003) as young adults. We found significant genetic contributions for the onset of regular cannabis use (a2 = 76%, p < 0.001), insomnia (a2 = 44%, p < 0.001), and insomnia with short sleep on weekdays (a2 = 37%, p < 0.001). We found significant genetic correlations between onset of regular use and both insomnia (rA = 0.20, p = 0.047) and insomnia with short sleep on weekdays (rA = 0.25, p = 0.008) but no significant environmental associations between these traits.
Conclusions
We found common genetic liabilities for early onset of regular cannabis use and insomnia, implying pleiotropic influences of genes on both traits.
Keywords: insomnia, short sleep duration, cannabis onset, development, twin studies
Statement of Significance.
This study strengthens the small collection of research that shows an association of early cannabis use and sleep deficits in young adulthood, extending the effects to clinical sleep outcomes. Furthermore, it provides novel insight into shared genetics between early regular cannabis use and adult insomnia, implying a pleotropic genetic influence on both traits. Insomnia with short sleep is considered the most severe phenotype of insomnia and understanding developmental risk factors may prove to be useful for prevention efforts.
Introduction
Insomnia is one of the most common sleep disorders [1] with symptoms and diagnosis prevalence rates as high as 30% and 5%–10%, respectively, in the general population [2]. It is a burdensome diagnosis associated with increased health care costs [3], poor quality of life [4], and occupational impairments [5, 6]. The development, consistency, and severity of insomnia are often attributed to psychological factors: stress and cognitive-emotional arousal are frequently premorbid with diagnoses [7, 8], and insomnia has high comorbidity with psychiatric disorders like depression and anxiety [9, 10]. Cannabis use is cross-sectionally associated with increased rates of insomnia and insomnia symptoms [11–16]. Given evidence that early cannabis use can predict later sleep problems [17, 18], research is needed to understand the influence of early cannabis use on specific adult clinical sleep outcomes such as insomnia.
In addition to insomnia, cannabis use has been linked with poor subjective sleep quality [11, 15, 19–23], reduced time in the rapid eye movement phase of sleep [24, 25], eveningness (a preference for later sleep–wake timing) [26], later bed times [27], prolonged latency to sleep onset [28], and shorter sleep duration [27–33]. Both premorbid insomnia [12] and more generalized sleep problems [17, 34–38] significantly predict later cannabis use, but only a small number of studies provide evidence for early cannabis use predicting later sleep components such as tiredness, trouble sleeping [17], and sleep duration [18, 35], and no studies have focused on specific sleep disorders. With longitudinal evidence in both directions, it is possible that sleep problems could influence cannabis use, cannabis use could influence sleep problems, or an underlying shared liability such as common genetics could be responsible for their association.
Recent evidence suggests that genetics may play a role in the etiology of the relationship between early cannabis use and adult sleep problems [18], and that there could be a common genetic liability for cannabis use and sleep problems that explain their relationship. Several lines of evidence are consistent with this common genetic model: the presence of genes believed to be involved with circadian rhythm/sleep in genome-wide associations studies of lifetime cannabis use [39–41], clock gene genetic variants that are associated with cannabis addiction [42], and the possible role of the endocannabinoid system in the circadian rhythm/sleep–wake cycle [43–46]. Further research is needed on the possible shared genetics between cannabis use and clinical sleep outcomes such as insomnia.
Insomnia with short sleep duration is a subclassification of insomnia described as insomnia with an average sleep time of less than 6 h per night and elevated morbidity [47]. It is frequently conceptualized as the most biologically severe phenotype of the disorder [48] and is associated with distinctive consequences including biomarkers that are focused on physiologic hyperarousal [49–51], cardiometabolic morbidities and health related problems [52–55], and increased neurocognitive impairments [56, 57]. Furthermore, individuals with insomnia with short sleep may not respond as well to cognitive behavioral therapy for insomnia and might be at greater risk for chronic sleep disturbance [58], demonstrating the challenges of treating insomnia with short sleep as well as the need to identify developmental factors that could heighten vulnerability for this diagnosis. No prior study has looked at either the predictive phenotypic or potential genetic relationship between early cannabis use and insomnia disorder and insomnia with short sleep.
Twin studies can be used to estimate the genetic and environmental correlations between traits [59]. We used a young adult twin sample to dissect the relationship between the onset of regular cannabis use and both insomnia and insomnia with short sleep on weekdays. First, we tested if onset of regular cannabis use was associated with insomnia and insomnia with short sleep on weekdays, controlling for known correlates including sex [60], current depression symptoms [61], prior diagnoses of a depression or anxiety disorder [9, 10], and shift-work [62]. Next, we used univariate twin analyses to estimate the genetic and environmental etiological components of each trait individually. We then tested a sequence of nested bivariate twin models that decomposed the genetic and environmental overlap between onset of regular cannabis and both insomnia and insomnia with short sleep on weekdays.
Methods
Participants
Participants were 1882 individual twins (472 monozygotic [MZ] pairs, 304 dizygotic [DZ] same-sex pairs, 165 opposite-sex pairs, 56% female) from the Colorado community twin sample and longitudinal twin study [63] who completed both an online sleep survey and a substance use questionnaire. The average age was 22.99 years (range = 18–33, SD = 2.97) at the time of the online sleep survey, which was administered on average 2.31 years (SD = 1.75) before the substance use assessment (mean age = 25.29, range = 21–33, SD = 2.71). We excluded 39 participants due to reporting “small children keep awake” or being “pregnant” as reasons for sleep issues. Seven individuals were missing an insomnia classification because not enough information was provided to determine their diagnoses. Twenty-three subjects had missing responses on the sleep duration questionnaire. We assigned subjects NA for sleep duration if they responded as receiving more than 12 h (17 subjects) or 0 h of average weekday sleep (one subject). One subject was excluded due to an implausible response of age of first regular cannabis use being 1. All research protocols were reviewed and approved by the University of Colorado’s Investigational Review Board.
Procedure
Twins completed an online survey about sleep problems that took between 30 and 45 min (it also included the depression and anxiety questions used in our analysis). At a later time, they completed the online substance use questionnaire as part of a large battery of assessments that took between 2 and 3 h for the third wave of a longitudinal three-wave study.
Measures
Short sleep duration on weekdays.
Weekday self-reported sleep duration was assessed via a question asking, “During the past month, thinking about your average WEEKDAY, how long did you ACTUALLY sleep, EACH night (or your longest sleep period if you work a night shift or rotating shift)?” Participants entered responses in open text boxes for both hours and minutes. We averaged responses that were in the form of ranges (e.g. 7–9 h would become 8 h) (n = 41). We summed hours and minutes to create a total average weekday sleep measure (M = 7.07, SD = 1.31, Median = 7). We coded short sleep duration if the summed response was less than 6 h and compared outcomes to those with sleep duration responses that summed 6 or more. Weekday sleep was utilized because it captures the most normative insomnia sleep disturbances, free of the flexibility and variation of weekend schedules as well as the potential confounds from compensatory sleep of weekend sleep [54].
Insomnia.
We based insomnia diagnosis on DSM-IV-TR criteria [64]. The questionnaire asked how often in the past month participants experienced difficulty falling asleep, difficulty staying asleep, and had nonrefreshing sleep (never, sometimes, usually, or always). Participants who did not report “never” for any of these items were asked follow-up questions, including how long they had the sleep problem (years and months), and to what extent it interfered with their “daily functioning (daytime fatigue, ability to function at work/daily chores, concentration, memory, mood, etc.),” with answers “not at all interfering” = 0, “a little” =1, “somewhat” = 2, “much” = 3, and “very much interfering” = 4. Participants met criteria for insomnia if they responded to at least one of the three problems (falling asleep, staying asleep, or non-refreshing sleep) as “usually” or “always” for the duration of at least a month, and with it interfering with daily functioning at least “somewhat.” Insomnia (n = 391) was coded as a binary variable.
Insomnia with short sleep duration on weekdays.
A four-category ordinal variable was created to characterize insomnia with short sleep on weekdays for our twin models. We coded subjects who met criteria for both insomnia and short sleep duration on weekdays of less than 6 h as a 3 (n = 120), subjects with insomnia and with sleep duration 6 h or more as a 2 (n = 265), subjects who did not have insomnia but had short sleep duration on weekdays of less than 6 h as a 1 (n = 118), and subjects who did not have insomnia or sleep duration 6 h or more as a 0 (n = 1342). This ordinal categorization allowed us to score nearly all participants and resulted in similar group sizes for each category of insomnia.
Covariates of sleep.
Our phenotypic analyses controlled for known correlates of sleep: sex [60], current depression symptoms [61], prior diagnoses of a depression or anxiety disorder [9, 10], and shift-work [62]. Depression symptoms were assessed at the same time as the sleep questions with the Center for Epidemiological Studies-Depression (CES-D) scale [65]. We used a log-transformed sum of 19 items; the sleep disturbance question from the CES-D composite score was removed due to its direct overlap with our outcome measure. Prior anxiety and depressive disorder diagnoses were assessed at the time of the sleep survey via questions asking, “Have you ever been diagnosed with an anxiety disorder?” and “Have you ever been diagnosed with depression?” We coded anxiety and depression disorder as binary variables. Of those who endorsed having insomnia with short sleep on weekdays, 29% endorsed having a shift-work job such as a regular evening shift (n = 6), regular night shift (n = 8), or rotating shift (n = 21). As a form of quality control, we coded those who had a shift-work job as a 1 (n = 526) and those who did not as a 0 (n = 1348) and conducted a series of models with our other covariates that both included and excluded the shift-work variable as a control.
Onset of regular cannabis use.
Age of onset of regular cannabis use (M = 17.53 years, SD = 2.99, Median = 17) was assessed in a self-report addendum to the Composite International Diagnostic Interview Substance Abuse Module (CIDI-SAM) [66]. Participants who endorsed any lifetime cannabis use were asked “How old were you when you began using marijuana on a regular basis, that is at least once per month?” We coded responses as an ordinal variable: 2 = age of first regular use at age 17 or earlier as (early onset group) (n = 300); 1 = age of first regular use after age 17 (late onset group) (n = 246); 0 = never used cannabis regularly (n = 1336). This categorization was selected based on the mean and median age of 17 and because it divided the distribution of age of onset into approximately equal groups of early- and late-onset of regular cannabis use. Utilizing a three-category ordinal variable, rather than a binary categorization, improves the performance of multivariate genetic and environmental modeling [67].
Statistical analyses
Phenotypic models.
R version 3.5.1 [68] was used for all descriptive statistics. Mplus version 8.1 [69] was utilized for our phenotypic analysis, structural equation modeling, and comparison tests. To correct for the nonindependence of the twin pairs in the phenotypic analyses, we used the TYPE = COMPLEX command to cluster data by family. This method uses a weighted likelihood function to obtain scaled χ 2 and standard errors corrected for nonindependence using a sandwich estimator. This technique effectively corrects for the nonindependence of twin data [70]. Probit regression with a means and variances adjusted weighted least squares (WLSMV) estimator was used to examine predictors of insomnia and insomnia with short sleep on weekdays.
Univariate twin models.
We used biometrical twin ACE modeling [59] in Mplus to estimate the genetic and environmental contributions to the onset of regular cannabis use, insomnia, and insomnia with short sleep on weekdays. These models assume three latent factors are responsible for the variance of an individual trait: additive genetic effects (A), shared environmental (C), and nonshared or unique environmental factors (E). MZ twins correlate perfectly for A because they share all their all their additive genetic influences (alleles identical by descent). DZ twins correlate 0.5 for A because they share on average half of their alleles identical by descent. C is fixed to correlate at 1 for MZ and DZ twin pairs, by definition for the environmental influences they share. E is fixed to a zero correlation, by definition, for MZ and DZ twin pairs. Classical twin modeling leverages the differences between MZ and DZ pairs to estimate the contribution of each latent variable (A, C, or E) to a trait. Twins were randomly assigned to twin1 and twin2, but males from opposite sex twin pairs were assigned to twin1.
Because the data were ordinal, we assumed an underlying normal distribution and estimated threshold models using the WLSMV estimator [71]. We assessed fit with the omnibus χ 2 statistic, supplemented with root-mean-square error of approximation (RMSEA) and confirmatory fit index (CFI). RMSEA < 0.06 and CFI > 0.95 indicate good model fit [72]. We used χ 2 difference tests to check each individual trait for sex differences in the distributions of ordinal variable and the magnitude of A, C, and E estimates in males and females (scalar sex differences). We used χ 2 difference tests to determine best fitting models by checking if individual parameters (A, C, or E) could be dropped with no significant decrement in fit.
Bivariate twin models.
We used Cholesky decompositions to decompose the environmental/genetic variances and covariances between the onset of regular cannabis use and both insomnia and insomnia with short sleep on weekdays. These analyses allow us to estimate both the unique and overlapping contributions of genetic/environmental pathways between traits. Once the genetic overlap is estimated using the Cholesky decomposition, a simple conversion of the Cholesky path coefficients can provide the proportion of genetic variation shared between two traits, i.e. the genetic correlation:.
Results
Phenotypic analysis
Table 1 displays descriptive statistics and the frequencies of depressive symptoms, prior anxiety diagnoses, prior depression diagnoses, no insomnia and sleep 6 h or more on weekdays, no insomnia with short sleep on weekdays (<6 h), insomnia, and insomnia with short-sleep duration on weekdays for the full sample and by sex. Table 2 displays the descriptive statistics and frequencies for each group of regular cannabis onset (early, late, none).
Table 1.
Descriptive statistics and frequencies for all variables at the time of the sleep questionnaire (with the exception of age of onset of regular cannabis use which was taken from the substance use assessment)
| Mean (SD) sample characteristics | |||
|---|---|---|---|
| Full sample (n = 1882) | Female (n = 1061) | Male (n = 821) | |
| Age (years) | 22.99 (2.97) | 22.79 (2.87) | 23.24 (3.07) |
| Age of onset of regular cannabis use (years) | 17.53 (2.99) | 17.61 (3.16) | 17.48 (2.85) |
| Sleep duration (h) | 7.08 (1.31) | 7.02 (1.31) | 7.14 (1.32) |
| Current depression symptoms (CES-D) | 11.02 (8.62) | 12.05 (9.09) | 9.69 (7.77) |
| Frequencies | |||
| Full sample | Female | Male | |
| Prior anxiety diagnosis | 9.15% (n = 171) | 12.26% (n = 129) | 5.14% (n = 42) |
| Prior depression diagnosis | 15.19% (n = 286) | 19.22% (n = 202) | 10.27% (n = 84) |
| No insomnia and sleep 6 h or more on weekdays | 72.74% (n = 1342) | 69.21% (n = 717) | 77.26% (n = 625) |
| No insomnia with short sleep on weekdays (<6 h) | 6.39% (n = 118) | 6.37% (n = 66) | 6.43% (n = 52) |
| Insomnia | 20.83% (n = 391) | 24.29% (n = 257) | 16.36% (n = 134) |
| Insomnia with short sleep on weekdays (<6 h) | 6.50% (n = 120) | 7.14% (n = 74) | 5.69% (n = 46) |
CES-D = Center for Epidemiological Studies-Depression scale.
Table 2.
Descriptive statistics and frequencies for all variables for each onset of regular cannabis use group
| Mean (SD) sample characteristics | |||
|---|---|---|---|
| Onset of regular cannabis use | |||
| 17 and younger (n = 300) | After 17 (n = 246) | No onset (n = 1336) | |
| Sleep duration (h) | 6.99 (1.45) | 7.21 (1.41) | 7.07 (1.26) |
| Current depression symptoms (CES-D) | 12.07 (8.87) | 12.17 (9.49) | 10.58 (8.35) |
| Frequencies | |||
| Onset of regular cannabis use | |||
| 17 and younger | After 17 | No onset | |
| Prior anxiety diagnosis | 13.67% (n = 41) | 12.50% (n = 30) | 7.52% (n = 100) |
| Prior depression diagnosis | 21.67% (n = 65) | 21.07% (n = 51) | 12.81% (n = 170) |
| No insomnia and sleep ≥6 h on weekdays | 62.46% (n = 183) | 74.17% (n = 178) | 74.77% (n = 981) |
| No insomnia with short sleep on weekdays (<6 h) | 8.53% (n = 25) | 4.58% (n = 11) | 6.25% (n = 82) |
| Insomnia | 28.96% (n = 86) | 20.82% (n = 51) | 19.03% (n = 254) |
| Insomnia with short sleep on weekdays (<6 h) | 9.89% (n = 29) | 7.92% (n = 19) | 5.49% (n = 72) |
CES-D = Center for Epidemiological Studies-Depression scale.
Coding onset as an ordinal variable and controlling for sex, onset of regular cannabis use significantly predicted insomnia (standardized β = 0.14, p = <0.001) and insomnia with short sleep on weekdays (standardized β = 0.14, p = <0.001). Controlling for sex, depressive symptoms, and prior diagnoses of an anxiety or depression disorder, onset of regular cannabis use significantly predicted insomnia (standardized β = 0.07, p = 0.024) and insomnia with short sleep on weekdays (standardized β = 0.08, p = 0.003). Lastly, in a model including all covariates and shift-work, onset of regular cannabis use still significantly predicted insomnia (standardized β = 0.07, p = 0.025) and insomnia with short sleep on weekdays (standardized β = 0.08, p = 0.003), suggesting that shift-work did not explain the association of onset of early regular cannabis use with insomnia and insomnia with short sleep on weekdays.
Genetic analyses
Cross-twin and cross-twin cross-trait correlations.
We found higher cross-twin correlations among MZ twin pairs than DZ twin pairs for all traits (see Table 3), indicating genetic influences on onset of regular cannabis use, insomnia, and insomnia with short sleep on weekdays. Table 3 also displays cross-twin cross-trait correlations among MZ twin pairs and DZ twin pairs, suggesting common genetic influences between the onset of regular cannabis use and both insomnia and insomnia with short sleep on weekdays.
Table 3.
Cross-twin and cross-twin cross-trait correlations for all main study variables
| Zygosity | Onset of regular cannabis use | Insomnia | Insomnia with short sleep on weekdays (<6 h) | Onset and insomnia | Onset and insomnia with short sleep on weekdays (<6 h) |
|---|---|---|---|---|---|
| MZ | 0.74* (p < 0.001) | 0.46* (p < 0.001) | 0.39* (p < 0.001) | 0.11 (p = 0.102) | 0.13* (p = 0.019) |
| DZ | 0.49* (p < 0.001) | 0.16 (p = 0.086) | 0.15 (p = 0.134) | 0.07 (p = 0.227) | 0.06 (p = 0.220) |
MZ = monozygotic; DZ = dizygotic.
* p < 0.05.
Univariate analyses.
To determine the genetic/environmental contributions to the variance of each individual trait we conducted univariate twin models. Table 4 reports the model fit statistics and model comparisons for each individual trait. We found significant sex differences in thresholds for onset of regular cannabis use (χ 2diff(2) = 52.227, p < 0.001), insomnia (χ 2diff(1) = 15.216, p < 0.001), and insomnia with short sleep on weekdays (χ 2diff(3) = 23.295, p < 0.001). We did not find scalar sex differences in the variance components for any traits (all χ 2diff(3) < 0.933, p > 0.817) suggesting no significant differences in the genetic and environmental contributions for these traits. Therefore, our models allowed separate thresholds for each sex, but equated A, C, and E parameters.
Table 4.
Fit and comparison tests of univariate twin models for onset of regular cannabis use, insomnia, and insomnia with short sleep on weekdays (<6 h)
| Measure | Model | Model fit | Standardized paths | χ 2 test with full ACE model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ 2 | df | P | RMSEA | CFI | A | C | E | df | χ 2 | P | ||
| Onset of regular cannabis use | ACE | 11.332 | 22 | 0.9697 | 0.000 | 1 | 0.708 | 0.486 | 0.512 | – | – | – |
| AE | 14.557 | 23 | 0.9098 | 0.000 | 1 | 0.872 | – | 0.489 | 1 | 3.747 | 0.0529 | |
| CE | 22.304 | 23 | 0.5019 | 0.000 | 1 | – | 0.814 | 0.580 | 1 | 13.060 | <0.001 | |
| AC | 50.879 | 23 | 0.0007 | 0.080 | 0.937 | 1 | 0 | – | 1 | 47.438 | <0.001 | |
| Insomnia | ACE | 3.553 | 12 | 0.9902 | 0.000 | 1 | 0.664 | 0 | 0.748 | – | – | – |
| AE | 3.554 | 13 | 0.9951 | 0.000 | 1 | 0.664 | – | 0.748 | 1 | 0.00 | 0.9986 | |
| CE | 9.706 | 13 | 0.7178 | 0.000 | 1 | – | 0.583 | 0.813 | 1 | 6.145 | 0.0132 | |
| AC | 31.932 | 13 | 0.0025 | 0.088 | 0.497 | 1 | 0 | – | 1 | 28.351 | <0.001 | |
| Insomnia with short sleep on weekdays (<6 h) | ACE | 17.461 | 32 | 0.9827 | 0.000 | 1 | 0.610 | 0 | 0.793 | – | – | – |
| AE | 17.587 | 33 | 0.9871 | 0.000 | 1 | 0.610 | – | 0.793 | 1 | 0.00 | 1.00 | |
| CE | 22.182 | 33 | 0.9235 | 0.000 | 1 | – | 0.532 | 0.847 | 1 | 5.802 | 0.0160 | |
| AC | 109.127 | 33 | 0.00 | 0.111 | 0 | 1 | 0 | – | 1 | 115.650 | <0.001 |
Models in bold indicate the best fitting model based on χ 2 difference tests.
We then tested each etiological pathway with a χ 2 difference test to determine the most parsimonious model. Shared environmental influences were not significantly different from zero for all univariate models (all χ 2diff(1) < 3.747, p > 0.0529), but model comparisons determined A and E could not be dropped (all χ 2diff(1) > 5.825, p < 0.0160). In the most parsimonious A and E models, additive genetic and nonshared environmental factors contributed to the onset of regular cannabis use (a2 = 76% and e2 = 24%, all χ 2diff(1) > 43.691, p ≤ 0.001), insomnia (a2 = 44% and e2 = 56%, all χ 2diff(1) > 40.920, p = <0.001), and insomnia with short sleep on weekdays (a2 = 37% and e2 = 63%, all χ 2diff(1) > 40.479, p ≤ 0.001).
Bivariate twin models.
We conducted separate bivariate Cholesky decompositions for onset of regular cannabis with insomnia and onset of regular cannabis use with insomnia with short sleep on weekdays. We found no significant scalar sex differences for either of our bivariate models (all χ 2diff(9) < 4.583, p > 0.869), suggesting no genetic and/or environmental sex differences. Table 5 includes model fits for bivariate twin models without sex differences. We found that C paths could be dropped from both models without a significant decrement in fit (all χ 2diff(3) < 3.773, p > 0.287), similar to our univariate results.
Table 5.
Fit and comparison tests of bivariate twin models for onset of regular cannabis use with both insomnia and insomnia with short sleep on weekdays (<6 h)
| Measure | Model | Model fit | χ 2 test with full Cholesky ACE decomposition | ||||||
|---|---|---|---|---|---|---|---|---|---|
| χ 2 | df | P | RMSEA | CFI | df | χ 2 | P | ||
| Onset of regular cannabis use and insomnia | Full Cholesky ACE | 40.923 | 51 | 0.8426 | 0.000 | 1.000 | – | – | – |
| Dropping all A paths | 58.191 | 54 | 0.3238 | 0.020 | 0.991 | 3 | 18.375 | <0.001 | |
| Dropping all C paths | 44.599 | 54 | 0.8154 | 0.000 | 1.000 | 3 | 3.736 | 0.2914 | |
| Dropping all E paths | 142.629 | 54 | 0.0000 | 0.093 | 0.809 | 3 | 125.396 | <0.001 | |
| Onset of regular cannabis use and insomnia with short sleep on weekdays (<6 h) | Full Cholesky ACE | 54.866 | 71 | 0.9215 | 0.000 | 1.000 | – | – | – |
| Dropping all A paths | 71.443 | 74 | 0.5626 | 0.000 | 1.000 | 3 | 19.147 | <0.001 | |
| Dropping all C paths | 58.465 | 74 | 0.9071 | 0.000 | 1.000 | 3 | 3.772 | 0.2872 | |
| Dropping all E paths | 189.233 | 74 | 0.0000 | 0.091 | 0.758 | 3 | 182.649 | <0.001 |
Models in bold indicate the best fitting model via χ 2 difference tests.
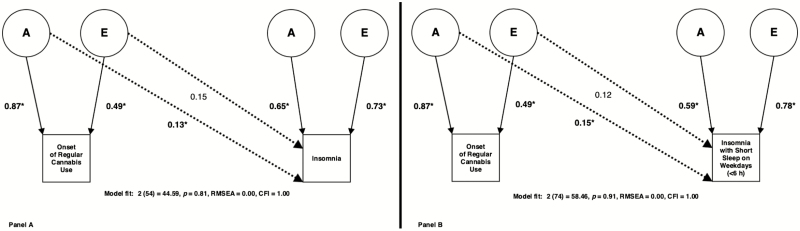
Figure 1 illustrates the best fitting A and E Cholesky decompositions and their genetic/environmental cross paths. The A and E Cholesky decomposition with the best fit between onset of regular cannabis use and insomnia indicated a significant genetic overlap (a cross-path = 0.13, rA = 0.20, χ 2diff(1) = 3.934, p = 0.047) between the traits. The best fitting A and E Cholesky decomposition between onset of regular cannabis use and insomnia with short sleep on weekdays also suggested a significant genetic overlap (a cross-path = 0.15, rA = 0.25, χ 2diff(1) = 6.942, p = 0.008). Neither of these Cholesky decompositions suggested significant environmental overlap (all χ 2diff(1) < 2.300, p > 0.129).
Figure 1.
Best fitting bivariate AE Cholesky decompositions between onset of regular cannabis use and both insomnia (Panel A) and insomnia with short sleep on weekdays (<6 h) (Panel B). Solid lines with arrows represent non-standardized additive genetic (A) and unique environmental (E) variance paths for each univariate trait. Dashed lines with arrows represent additive genetic (A) and unique environmental (E) cross paths between onset of regular cannabis use and insomnia/insomnia with short sleep on weekdays (<6 h). Parameters that are significant (p < 0.05) are indicated via asterisks (all parameters significant besides the cross E paths in both decompositions).
Discussion
We tested the hypothesis that early onset of regular cannabis use predicted higher rates of young adult insomnia. Controlling for sex, current depression symptoms, and prior anxiety and depression diagnoses, we found that early onset of regular cannabis use was associated with increased rates of young adult insomnia and insomnia with short sleep on weekdays. Using a genetically informative twin design, we found both genetic and nonshared environmental contributions to the etiology of age of regular cannabis use onset, insomnia, and insomnia with short sleep on weekdays individually. These findings of significant genetic contribution are consistent with prior research on cannabis initiation [73] and insomnia [74] but this is the first report of genetic contributions to insomnia with short sleep on weekdays. Lastly, we found evidence of significant overlapping additive genetic influences on onset of regular cannabis use and both insomnia and insomnia with short sleep on weekdays, implying common genetic liabilities, but we did not find significant environmental overlap.
Our genetic and environmental variance estimates for insomnia resemble a prior twin study that utilized the same cohort [75], but our additional results on insomnia with short sleep on weekdays are novel. We found that additive genetics contributed more to insomnia alone, and that unique environment contributed more to insomnia with short sleep on weekdays. The higher environmental contribution to insomnia with short sleep on weekdays may reflect the fact that the unique environment largely contributes to the majority of the variance in adult sleep duration (e2 = 67–80% and a2 = 20–32%) [18, 76, 77]. Thus, inclusion of the short sleep with insomnia might lead the trait to have a larger contribution from unique environment. Our findings of genetic contribution to the onset of regular cannabis use are consistent with studies of lifetime cannabis use and problem cannabis use [73], and align with a prior twin study focused on the onset of regular cannabis use and adult sleep duration that used a subsample of the current study’s sample [18]. Although our best fitting and most parsimonious model for onset of regular cannabis use consisted of A and E parameter, the presence of some C (though only marginally significant) is consistent with previous studies of substance use [78].
These findings are consistent with prior studies [17, 18] and build on the current body of research regarding the relationship between early cannabis use and adult sleep problems. We find early regular cannabis use predicts higher rates of insomnia and insomnia with short sleep on weekdays in young adulthood. Thus, our findings provide evidence in support of the theory that early regular cannabis use is associated with subsequent sleep problems, extending this effect to clinical sleep outcomes. The differing time frames of the measures strengthen the support for possible long-lasting effects of early cannabis on sleep problems in young adulthood and our study reinforces the evidence of possible pleotropic influence of genes on early cannabis use and sleep [18].
Several possible behavioral, developmental, or neurological mechanisms have previously been proposed that could explain how early regular cannabis use might influence later sleep and insomnia in young adulthood. For example, early cannabis users could develop maladaptive behaviors or long-lasting sleep problems that could progress into adulthood affecting sleep [35]. Additionally, circadian pathway disturbances resulting from substance use [79–81] and early cannabis use could lead to developmental disruptions in circadian rhythms and late sleep timing that could advance into adulthood. Lastly, early cannabis use could result in altered brain development that affects adult insomnia [18], as there is evidence of alterations of the prefrontal cortex (PFC) for both traits. Functional and structural imaging studies have shown early cannabis use is associated with alterations in the PFC and early users suffer impairments in neurocognitive performance associated with those area [82]. Correspondingly, insomnia is associated with deficits in neurocognitive performance in executive function tasks, suggesting PFC impairments [56, 57, 83] and there is evidence of insomnia being related to structural alterations in regions of the PFC [84–86], changes in functional PFC connectivity as well as in networks involving the PFC [87–92], and variations in EEG sleep patterns in the PFC [93, 94].
However, our results suggest that the relationship of onset of regular cannabis use with insomnia and insomnia with short sleep on weekdays may also be due to pleiotropic influence of genes on these traits. A recent study using similar twin models found that shared genetics may play a role in the etiology of the relationship between early cannabis use and adult sleep duration [18], and there are various additional lines of evidence consistent with this common genetic liability theory. Endocannabinoids may play a large part in this relationship, as the endocannabinoid system may be involved in the circadian sleep–wake cycle [43–46]. Endocannabinoids influence sleep, and their levels vary with time of day [45]. Additionally, several large genome-wide association studies (GWASs) of sleep-related variables, including chronotype [95], sleep duration [96, 97], and insomnia [98] have found significant genes and genetic pathways linked with cannabis use [40, 99] or cannabinoid activity [100–106].
Genes related to circadian rhythm and sleep could also play an important role in this relationship. Several GWASs of lifetime cannabis use [39–41] have found significant genes associated that are believed to be involved in circadian rhythm and sleep behaviors [107–111], and a recent study found that several clock gene polymorphisms were significant risk factors for cannabis addiction [42]. Despite our best fitting model implying that shared genetics explain the association between these traits, it is entirely plausible there is a causal link between cannabis use and sleep deficits; a previous analysis found that a causal model between early regular cannabis use and sleep duration provided the best fit (although there are limitations to consider with that design) [18]. GWAS methods such as Mendelian randomization [112] can provide further information on causal mechanisms between the genetics of these traits. Further research should consider the role of genetics in the relationship between early regular cannabis use and later sleep (specifically endocannabinoid and clock/circadian/sleep genes), with a focus on clinically important outcomes such as insomnia.
Limitations
There are several limitations worth addressing. First, our sleep duration measure was self-reported and only a small collection of insomnia with short sleep studies has used self-reported measures [54, 113]. The reliability of self-reported reports, and their consistency with more commonly used objective measures like polysomnography (PSG) and actigraphy [114] are not perfect. But there are also critiques of objective laboratory sleep assessments, as they are commonly limited to one night or a few nights of recording and could lack accuracy with respect to at-home sleep variability [115]. Criticisms of actigraphy include its inability to distinguish sleep disorders [116] and its tendency to overestimate sleep times [117–119]. A recent comprehensive review of insomnia with short sleep called for research with self-reported as well as objective measures [114], and research on self-reported sleep duration measures is clearly needed as an alternative when objective measures are too costly and time intensive. Development of accurate, valid, and easy to use wearable technology that can better assess sleep in the home environment is needed. Our definition of short sleep (<6 h) with insomnia is consistent with the existing literature, but it should be noted that more than 7 h sleep is recommended for promoting health in adults [120], thus future research should explore other cutoffs of short sleep with insomnia.
Second, the lower bound for the age range at the time of the substance use assessment was 18, which is very close to the cut-off age for early regular cannabis use and perhaps does not allow enough time to pass to develop insomnia symptomology. Third, our measures were collected via self-report procedures and our onset of regular cannabis use measure was a retrospective question which could be prone to memory discrepancies or report bias [121]. Lastly, due to time interval between the substance use interview and sleep questionnaire, we could not separately assess the contributions of current cannabis or other substance use to insomnia as we did not have frequency of use measures collected at the time of administration of the sleep questionnaire. Lack of concurrent substance use measures is a limitation of the current study, as use of other substances including alcohol [122, 123] and tobacco [124–126], as well as cannabis [15], have been linked to sleep deficits and insomnia. Future studies should consider and include appropriate substance use covariates as well as objective measures of sleep to address the mentioned concerns.
Summary
Our findings are consistent with the theory that early cannabis use is associated with increased rates of young adult insomnia and insomnia with short sleep on weekdays. These results extend the current body of research regarding the relationship of early cannabis use with adult sleep to include clinical sleep outcomes as well as provide novel insight into shared genetics between these traits. This is also the first twin study to estimate, and find, genetic influences on insomnia with short sleep on weekdays, which exhibited similar etiology to other sleep traits. Further research is needed to understand the potential developmental impact of early regular cannabis use on adult sleep, specifically on clinically important outcomes such as insomnia. Future studies should focus on genetic factors and would benefit from using both self-reported and object measurers of sleep duration.
Ethical Approval
Documented consent was obtained from all study participants and all procedures of this study followed the ethical standards of the University of Colorado Institutional Review Board.
Funding
Supported by grants P60 DA011015, R01 DA042755, T32 DA017637, and funding from the Henry Ford Health System.
Conflict of interest statement: This study was not financially supported by industry. Christopher L. Drake has received grant support from Cephalon, Zeo Inc., Merck Inc., and Takeda Inc. and participated in speaking engagements for Cephalon and Asante Communications all unrelated to this study. Kenneth P. Wright Jr. has received research support from the National Institutes of Health, Office of Naval Research, Pac-12, and SomaLogic, Inc., consulting fees from or served as a paid member of scientific advisory boards for the Sleep Disorders Research Advisory Board—National Heart, Lung and Blood Institute, CurAegis Technologies, Circadian Therapeutics, Ltd and has received speaker/educational/travel consultant honorarium fees from the American Academy of Sleep Medicine, American College of Chest Physicians, American College of Sports Medicine, American Diabetes Association, Associated Professional Sleep Societies, Kellogg Company, Obesity Medicine Association, and The European Association for the Study of Obesity. All other authors have no potential conflicts of interest to disclose.
Acknowledgments
We would like to thank all authors for their insightful comments and suggestions regarding the design, analysis, and wording of the manuscript.
References
- 1. National Institutes of Health state of the science conference statement: manifestations and management of chronic insomnia in adults June 13–15, 2005. Sleep. 2005;28(9):1049–1057. [DOI] [PubMed] [Google Scholar]
- 2. Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007:3(5 Suppl):S7. [PMC free article] [PubMed] [Google Scholar]
- 3. Wade AG. The societal costs of insomnia. Neuropsychiatr Dis Treat. 2011;7:1–18. doi:10.2147/NDT.S15123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Léger D, et al. SF-36: evaluation of quality of life in severe and mild insomniacs compared with good sleepers. Psychosom Med. 2001;63(1):49–55. doi:10.1097/00006842-200101000-00006 [DOI] [PubMed] [Google Scholar]
- 5. Kuppermann M, et al. Sleep problems and their correlates in a working population. J Gen Intern Med. 1995;10(1):25–32. [DOI] [PubMed] [Google Scholar]
- 6. Léger D, et al. Medical and socio-professional impact of insomnia. Sleep. 2002;25(6):621–625. [PubMed] [Google Scholar]
- 7. Fernández-Mendoza J, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010;72(4):397–403. [DOI] [PubMed] [Google Scholar]
- 8. Drake CL, et al. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. Sleep. 2014;37(8):1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vollrath M, et al. The Zurich study. VIII. Insomnia: association with depression, anxiety, somatic syndromes, and course of insomnia. Eur Arch Psychiatry Neurol Sci. 1989;239(2):113–124. [DOI] [PubMed] [Google Scholar]
- 10. Taylor DJ, et al. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–1464. [DOI] [PubMed] [Google Scholar]
- 11. Johnson EO, et al. Sleep problems and substance use in adolescence. Drug Alcohol Depend. 2001;64(1):1–7. doi:10.1016/S0376-8716(00)00222-2 [DOI] [PubMed] [Google Scholar]
- 12. Roane BM, et al. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 2008;31(10):1351–1356. [PMC free article] [PubMed] [Google Scholar]
- 13. Freeman D, et al. Persecutory ideation and insomnia: findings from the second British National Survey of Psychiatric Morbidity. J Psychiatr Res. 2010;44(15):1021–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alwan H, et al. Association between substance use and psychosocial characteristics among adolescents of the Seychelles. BMC Pediatr. 2011;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conroy DA, et al. Marijuana use patterns and sleep among community-based young adults. J Addict Dis. 2016;35(2):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong MM, et al. Insomnia symptoms, cannabis protective behavioral strategies, and hazardous cannabis use among U.S. college students. Exp Clin Psychopharmacol. 2019;27(4):309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong MM, et al. Childhood sleep problems, early onset of substance use and behavioral problems in adolescence. Sleep Med. 2009;10(7):787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Winiger E, et al. The relationship between early regular cannabis use & adult sleep duration: genetic variation and the implications of a predictive relationship. Drug Alcohol Depend. 2019;204:107517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klonoff H, et al. Drug patterns in the chronic marijuana user. Int J Addict. 1976;11(1):71–80. [DOI] [PubMed] [Google Scholar]
- 20. Fakier N, et al. Associations among sleep problems, learning difficulties and substance use in adolescence. J Adolesc. 2011;34(4):717–726. [DOI] [PubMed] [Google Scholar]
- 21. Ogeil RP, et al. Risky drug use and effects on sleep quality and daytime sleepiness. Hum Psychopharmacol. 2015;30(5):356–363. [DOI] [PubMed] [Google Scholar]
- 22. Maple KE, et al. Dose-dependent cannabis use, depressive symptoms, and FAAH genotype predict sleep quality in emerging adults: a pilot study. Am J Drug Alcohol Abuse. 2016;42(4):431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ogeil RP, et al. Early adolescent drinking and cannabis use predicts later sleep-quality problems. Psychol Addict Behav. 2019;33(3):266–273. [DOI] [PubMed] [Google Scholar]
- 24. Pivik RT, et al. Delta-9-tetrahydrocannabinol and synhexl: effects on human sleep patterns. Clin Pharmacol Ther. 1972;13(3):426–435. [DOI] [PubMed] [Google Scholar]
- 25. Feinberg I, et al. Effects of marijuana extract and tetrahydrocannabinol on electroencephalographic sleep patterns. Clin Pharmacol Ther. 1976;19(6):782–794. [DOI] [PubMed] [Google Scholar]
- 26. Hasler BP, et al. Eveningness and later sleep timing are associated with greater risk for alcohol and marijuana use in adolescence: initial findings from the National Consortium on Alcohol and Neurodevelopment in Adolescence Study. Alcohol Clin Exp Res. 2017;41(6):1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Troxel WM, et al. Examining racial/ethnic disparities in the association between adolescent sleep and alcohol or marijuana use. Sleep Health. 2015;1(2):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bolla KI, et al. Sleep disturbance in heavy marijuana users. Sleep. 2008;31(6):901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ebin VJ, et al. Acculturation and interrelationships between problem and health-promoting behaviors among Latino adolescents. J Adolesc Health. 2001;28(1):62–72. [DOI] [PubMed] [Google Scholar]
- 30. Mednick SC, et al. The spread of sleep loss influences drug use in adolescent social networks. PLoS One. 2010;5(3):e9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Glozier N, et al. Short sleep duration in prevalent and persistent psychological distress in young adults: the DRIVE study. Sleep. 2010;33(9):1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McKnight-Eily LR, et al. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev Med. 2011;53(4–5):271–273. [DOI] [PubMed] [Google Scholar]
- 33. Ames ME, et al. Patterns of marijuana use and physical health indicators among Canadian youth. Int J Psychol. 2018. doi:10.1002/ijop.12549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wong MM, et al. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol Clin Exp Res. 2004;28(4):578–587. [DOI] [PubMed] [Google Scholar]
- 35. Pasch KE, et al. Longitudinal bi-directional relationships between sleep and youth substance use. J Youth Adolesc. 2012;41(9):1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hasler BP, et al. Restless sleep and variable sleep timing during late childhood accelerate the onset of alcohol and other drug involvement. J Stud Alcohol Drugs. 2016;77(4):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miller MB, et al. The prospective association between sleep and initiation of substance use in young adolescents. J Adolesc Heal. 2017;60(2):154–160. doi:10.1016/j.jadohealth.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nguyen-Louie TT, et al. Effects of sleep on substance use in adolescents: a longitudinal perspective. Addict Biol. 2018;23(2):750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stringer S, et al. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl Psychiatry. 2016;6:e769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gage SH, et al. Assessing causality in associations between cannabis use and schizophrenia risk: a two-sample Mendelian randomization study. Psychol Med. 2017;47(5):971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pasman JA, et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat Neurosci. 2018;21(9):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saffroy R, et al. Several clock genes polymorphisms are meaningful risk factors in the development and severity of cannabis addiction. Chronobiol Int. 2019;36(1):122–134. [DOI] [PubMed] [Google Scholar]
- 43. Vaughn LK, et al. Endocannabinoid signalling: has it got rhythm? Br J Pharmacol. 2010;160(3):530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murillo-Rodriguez E, et al. The emerging role of the endocannabinoid system in the sleep–wake cycle modulation. Cent Nerv Syst Agents Med Chem. 2011;11(3):189–196. [DOI] [PubMed] [Google Scholar]
- 45. Prospéro-García O, et al. Endocannabinoids and sleep. Neurosci Biobehav Rev. 2016;71:671–679. [DOI] [PubMed] [Google Scholar]
- 46. Hodges EL, et al. Aging circadian rhythms and cannabinoids. Neurobiol Aging. 2019;79:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. American Academy of Sleep Medicine. The International Classification of Sleep Disorders (ICSD-3); Darien, IL: American Academy of Sleep Medicine, 2014. [Google Scholar]
- 48. Vgontzas AN, et al. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–254. doi:10.1016/j.smrv.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Floam S, et al. Sleep characteristics as predictor variables of stress systems markers in insomnia disorder. J Sleep Res. 2015;24(3):296–304. [DOI] [PubMed] [Google Scholar]
- 50. D’Aurea C, et al. Objective short sleep duration is associated with the activity of the hypothalamic-pituitary-adrenal axis in insomnia. Arq Neuropsiquiatr. 2017;73(6):516–519. doi:10.1590/0004-282x20150053 [DOI] [PubMed] [Google Scholar]
- 51. Fernandez-Mendoza J, et al. Insomnia is associated with cortical hyperarousal as early as adolescence. Sleep. 2016;39(5):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vgontzas AN, et al. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009; 32(4):491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sivertsen B, et al. Midlife insomnia and subsequent mortality: the Hordaland health study. BMC Public Health. 2014;14:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kalmbach DA, et al. DSM-5 insomnia and short sleep: comorbidity landscape and racial disparities. Sleep. 2016;39(12):2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bertisch SM, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep. 2018;41(6). doi:10.1093/sleep/zsy047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shekleton JA, et al. Neurobehavioral performance impairment in insomnia: relationships with self-reported sleep and daytime functioning. Sleep. 2014;37(1):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Khassawneh BY, et al. Neurocognitive performance in insomnia disorder: the impact of hyperarousal and short sleep duration. J Sleep Res. 2018;27(6):e12747. [DOI] [PubMed] [Google Scholar]
- 58. Bathgate CJ, et al. Insomnia patients with objective short sleep duration have a blunted response to cognitive behavioral therapy for insomnia. Sleep. 2017;40(1). doi:10.1093/sleep/zsw012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Neale MC, et al. Methodology for Genetic Studies of Twins and Families. 2013. doi:10.1007/978-94-015-8018-2 [Google Scholar]
- 60. Krishnan V, et al. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12(6):383–389. [DOI] [PubMed] [Google Scholar]
- 61. Grandner MA, et al. Criterion validity of the Pittsburgh Sleep Quality Index: investigation in a non-clinical sample. Sleep Biol Rhythms. 2006;4(2):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Drake CL, et al. Shift work, shift-work disorder, and jet lag. In: Principles and Practice of Sleep Medicine: Sixth Edition; Elsevier Science Health Science; 2016. doi:10.1016/B978-1-4160-6645-3.00071-2 [Google Scholar]
- 63. Rhea S-A, et al. Colorado twin registry: an update. Twin Res Hum Genet. 2013;16(1):351–357. doi:10.1017/thg.2012.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR); Amer Psychiatric Pub Inc. 2000. doi:10.1176/appi.books.9780890423349 [Google Scholar]
- 65. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi:10.1177/014662167700100306 [Google Scholar]
- 66. Cottler LB, et al. The reliability of the CIDI‐SAM: a comprehensive substance abuse interview. Br J Addict. 1989;84(7):801–814. doi:10.1111/j.1360-0443.1989.tb03060.x [DOI] [PubMed] [Google Scholar]
- 67. Heath AC, et al. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Res. 2002;5(2):113–124. [DOI] [PubMed] [Google Scholar]
- 68. R Foundation for Statistical Computing. R. Development Core Team: R: A language and environment for statistical computing;2015. ISBN 3-900051-07-0 http//wwwR-project.org.
- 69. Muthén LK, et al. Mplus User’s Guide. 8th ed Los Angeles, CA: Muthén Muthén; 2017. doi:10.1111/j.1600-0447.2011.01711.x [Google Scholar]
- 70. Rebollo I, et al. Phenotypic factor analysis of family data: correction of the bias due to dependency. Twin Res Hum Genet. 2006;9(3):367–376. [DOI] [PubMed] [Google Scholar]
- 71. Asparouhov T, et al. Weighted least squares estimation with missing data. Technical Appendix, 2010;1–10. [Google Scholar]
- 72. Hu LT, et al. Fit Indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol Methods. 1998;3(4):424. doi:10.1037/1082-989X.3.4.424 [Google Scholar]
- 73. Verweij KJ, et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction. 2010;105(3):417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lind MJ, et al. Genetic pathways to insomnia. Brain Sci. 2016;6(4):64. doi:10.3390/brainsci6040064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Drake CL, et al. Sleep reactivity and insomnia: genetic and environmental influences. Sleep. 2011;34(9):1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Genderson MR, et al. Genetic and environmental influences on sleep quality in middle-aged men: a twin study. J Sleep Res. 2013;22(5):519–526. doi:10.1111/jsr.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hublin C, et al. Genetic factors in evolution of sleep length—a longitudinal twin study in Finnish adults. J Sleep Res. 2013;22(5):513–518. [DOI] [PubMed] [Google Scholar]
- 78. Rhee SH, et al. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60(12):1256–1264. [DOI] [PubMed] [Google Scholar]
- 79. Shibley HL, et al. Adolescents with insomnia and substance abuse: consequences and comorbidities. J Psychiatr Pract. 2008;14(3):146–153. [DOI] [PubMed] [Google Scholar]
- 80. Hasler BP, et al. Circadian rhythms, sleep, and substance abuse. Sleep Med Rev. 2012;16(1):67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hasler BP, et al. Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol. 2015;49(4):377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jacobus J, et al. Effects of cannabis on the adolescent brain. Curr Pharm Des. 2014;20(13):2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fernandez-Mendoza J, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33(4):459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Altena E, et al. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67(2):182–185. [DOI] [PubMed] [Google Scholar]
- 85. Joo EY, et al. Brain gray matter deficits in patients with chronic primary insomnia. Sleep. 2013;36(7):999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Suh S, et al. Cortical thinning and altered cortico-cortical structural covariance of the default mode network in patients with persistent insomnia symptoms. Sleep. 2016;39(1):161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Drummond SPA, et al. Neural correlates of working memory performance in primary insomnia. Sleep. 2013;36(1):1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li Y, et al. Functional connectivity changes between parietal and prefrontal cortices in primary insomnia patients: evidence from resting-state fMRI. Eur J Med Res. 2014;19(1):32. doi:10.1186/2047-783X-19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nie X, et al. Functional connectivity of paired default mode network subregions in primary insomnia. Neuropsychiatr Dis Treat. 2015;11:3085–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li C, et al. Abnormal whole-brain functional connectivity in patients with primary insomnia. Neuropsychiatr Dis Treat. 2017;13:427. doi:10.2147/NDT.S128811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Altena E, et al. Prefrontal hypoactivation and recovery in insomnia. Sleep. 2008;31(9):1271–1276. [PMC free article] [PubMed] [Google Scholar]
- 92. Son YD, et al. fMRI brain activation in patients with insomnia disorder during a working memory task. Sleep Breath. 2018;22(2):487–493. [DOI] [PubMed] [Google Scholar]
- 93. Perrier J, et al. Specific EEG sleep pattern in the prefrontal cortex in primary insomnia. PLoS One. 2015;10(1):e0116864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Colombo MA, et al. Wake high-density electroencephalographic spatiospectral signatures of insomnia. Sleep. 2016;39(5):1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jones SE, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 2019;10(1):343. doi:10.1038/s41467-018-08259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dashti HS, et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun. 2019;10(1):1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Doherty A, et al. GWAS identifies 14 loci for device-measured physical activity and sleep duration. Nat Commun. 2018;9(1):5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jansen PR, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51(3):394. doi:10.1038/s41588-018-0333-3 [DOI] [PubMed] [Google Scholar]
- 99. Philibert RA, et al. Transcriptional profiling of subjects from the Iowa adoption studies. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(5):683–690. [DOI] [PubMed] [Google Scholar]
- 100. Rubino T, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19(8):763–772. [DOI] [PubMed] [Google Scholar]
- 101. Cardinal P, et al. Cannabinoid type 1 (CB1) receptors on Sim1-expressing neurons regulate energy expenditure in male mice. Endocrinology. 2015;156(2):411–418. [DOI] [PubMed] [Google Scholar]
- 102. Colizzi M, et al. Interaction between functional genetic variation of DRD2 and cannabis use on risk of psychosis. Schizophr Bull. 2015;41(5):1171–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Szutorisz H, et al. Cross-generational THC exposure alters the developmental sensitivity of ventral and dorsal striatal gene expression in male and female offspring. Neurotoxicol Teratol. 2016;58:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pagotto U, et al. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27(1):73–100. [DOI] [PubMed] [Google Scholar]
- 105. Flores A, et al. Cannabinoid-hypocretin cross-talk in the central nervous system: what we know so far. Front Neurosci. 2013;7:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kozela E, et al. Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J Neuroinflammation. 2016;13(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yan J, et al. Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput Biol. 2008. doi:10.1371/journal.pcbi.1000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Stadler F, et al. Lack of calbindin-D28k alters response of the murine circadian clock to light. Chronobiol Int. 2010;27(1):68–82. [DOI] [PubMed] [Google Scholar]
- 109. Tabuchi S, et al. Influence of inhibitory serotonergic inputs to orexin/hypocretin neurons on the diurnal rhythm of sleep and wakefulness. Sleep. 2013;36(9):1391–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jiang P, et al. A systems approach identifies networks and genes linking sleep and stress: implications for neuropsychiatric disorders. Cell Rep. 2015;11(5):835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yang SY, et al. Effects of genetic variants of ST8SIA2 and NCAM1 genes on seasonal mood changes and circadian preference in the general population. Chronobiol Int. 2018;35(3):405–415. doi:10.1080/07420528.2017.1410827 [DOI] [PubMed] [Google Scholar]
- 112. Smith GD, et al. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33(1):30–42. doi:10.1093/ije/dyh132 [DOI] [PubMed] [Google Scholar]
- 113. Fortier-Brochu E, et al. Cognitive impairment in individuals with insomnia: clinical significance and correlates. Sleep. 2014;37(11):1787–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fernandez-Mendoza J. The insomnia with short sleep duration phenotype: an update on it’s importance for health and prevention. Curr Opin Psychiatry. 2017;30(1):56–63. [DOI] [PubMed] [Google Scholar]
- 115. Vgontzas AN, et al. Insomnia with short sleep duration: nosological, diagnostic, and treatment implications. Sleep Med Clin. 2013;8(3):309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ancoli-Israel S, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 117. Kushida CA, et al. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi:10.1016/S1389-9457(00)00098-8 [DOI] [PubMed] [Google Scholar]
- 118. Vallières A, et al. Actigraphy in the assessment of insomnia. Sleep. 2003;26(7):902–906. [DOI] [PubMed] [Google Scholar]
- 119. Lichstein KL, et al. Actigraphy validation with insomnia. Sleep. 2006;29(2):232–239. [PubMed] [Google Scholar]
- 120. Watson NF, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hassan ES. Recall bias can be a threat to retrospective and prospective research designs. Internet J Epidemiol. 2005;3(2):339–412. [Google Scholar]
- 122. Roehrs T, et al. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5(4):287–297. [DOI] [PubMed] [Google Scholar]
- 123. Haario P, et al. Bidirectional associations between insomnia symptoms and unhealthy behaviours. J Sleep Res. 2013;22(1):89–95. [DOI] [PubMed] [Google Scholar]
- 124. Brook DW, et al. Trajectories of cigarette smoking in adulthood predict insomnia among women in late mid-life. Sleep Med. 2012;13(9):1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jaehne A, et al. How smoking affects sleep: a polysomnographical analysis. Sleep Med. 2012;13(10):1286–1292. [DOI] [PubMed] [Google Scholar]
- 126. Cohrs S, et al. Impaired sleep quality and sleep duration in smokers-results from the German multicenter study on nicotine dependence. Addict Biol. 2014;19(3):486–496. [DOI] [PubMed] [Google Scholar]