Abstract
Study Objectives
Obstructive sleep apnea (OSA) is a serious and costly public health problem. The main medical treatment, continuous positive airway pressure, is efficacious when used, but poorly tolerated in up to 50% of patients. Upper airway reconstructive surgery is available when medical treatments fail but randomized trial evidence supporting its use is limited. This protocol details a randomized controlled trial designed to assess the clinical effectiveness, safety, and cost-effectiveness of a multilevel upper airway surgical procedure for OSA.
Methods
A prospective, parallel-group, open label, randomized, controlled, multicenter clinical trial in adults with moderate or severe OSA who have failed or refused medical therapies. Six clinical sites in Australia randomly allocated participants in a 1:1 ratio to receive either an upper airway surgical procedure consisting of a modified uvulopalatopharyngoplasty and minimally invasive tongue volume reduction, or to continue with ongoing medical management, and followed them for 6 months.
Results
Primary outcomes: difference between groups in baseline-adjusted 6 month OSA severity (apnea–hypopnea index) and subjective sleepiness (Epworth Sleepiness Scale). Secondary outcomes: other OSA symptoms (e.g. snoring and objective sleepiness), other polysomnography parameters (e.g. arousal index and 4% oxygen desaturation index), quality of life, 24 hr ambulatory blood pressure, adverse events, and adherence to ongoing medical therapies (medical group).
Conclusions
The Sleep Apnea Multilevel Surgery (SAMS) trial is of global public health importance for testing the effectiveness and safety of a multilevel surgical procedure for patients with OSA who have failed medical treatment.
Clinical Trial Registration
Multilevel airway surgery in patients with moderate-severe Obstructive Sleep Apnea (OSA) who have failed medical management to assess change in OSA events and daytime sleepiness. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366019&isReview=true Australian New Zealand Clinical Trials Registry ACTRN12614000338662, prospectively registered on 31 March 2014.
Keywords: obstructive sleep apnea, surgery, UPPP, tongue reduction
Statement of Significance.
Patients with obstructive sleep apnea (OSA) frequently do not accept or adhere to medical treatments such as CPAP, or obtain a subtherapeutic response to others, e.g. oral devices. Upper airway surgery is an alternative treatment for such patients, but there is a paucity of randomized controlled trial evidence to support its use. This trial aims to fill this evidence gap. The Sleep Apnea Multilevel Surgery (SAMS) randomized controlled trial will test the effectiveness, safety, and cost effectiveness of this treatment for people with OSA who have failed medical treatment and would otherwise remain without effective treatment.
Introduction
Obstructive sleep apnea (OSA) is a common and serious medical disorder with a high public health cost [1].
OSA can be efficaciously treated medically, but poor adherence to therapy is a major challenge [2]. Although continuous positive airway pressure (CPAP) [3] and the use of a mandibular advancement splint (MAS) [4] have proven efficacy, many patients find the treatments difficult to tolerate. A 2009 review showed that as few as 17% of people with OSA may be adherent to CPAP treatment after 5 years [3]. As a consequence, many people with OSA remain untreated or under-treated.
Upper airway reconstructive surgery for OSA is reported to be beneficial in well-controlled observational studies [5–9]. However, multicenter randomized clinical trial evidence is limited and is critically needed to inform clinical practice, particularly for multilevel surgery [10–12]. This paucity of randomized trial evidence has led some to argue for “disinvestment” from OSA surgery [13–15].
The most common surgical treatment for OSA is uvulopalatopharyngoplasty (UPPP), where the aim is to open and stabilize the oropharyngeal and velopharyngeal inlets [16]. There are now a number of variations to UPPP, moving towards newer reconstructive approaches. These refinements have demonstrated improved outcomes and reduced morbidity over traditional UPPP in small trials and observational studies [10, 17–20].
The posterior tongue is another major site of upper airway collapse, and narrowing at this level is correlated with apnea–hypopnea index (AHI) in the supine sleep position [21]. Surgery to address tongue obstruction appears to improve surgical outcomes in the treatment of OSA [12, 16, 22, 23], although traditional methods of tongue resection are associated with major morbidity [24]. Isolated minimally invasive techniques using radiofrequency to reduce the tongue base improve AHI and clinical outcomes, especially in mild-moderate OSA, but the magnitude of its effect is small or moderate [12, 25–27]. An observational study combining modified UPPP and minimally invasive tongue reduction conducted by our group prior to the current trial showed encouraging results with respect to effectiveness and safety [17].
The surgical technique being evaluated in this trial was designed to minimize morbidity while addressing the two main sites of OSA obstruction: the soft palate and the posterior tongue.
Aims and Hypotheses
The aim of this randomized trial is to assess the clinical effectiveness, safety, and cost-effectiveness of a multilevel upper airway surgical procedure for OSA. We will accomplish this aim by testing three hypotheses:
For moderate or severe symptomatic OSA with failed CPAP treatment, surgery will be superior to ongoing medical management in improving the primary outcomes of OSA severity measured by change in AHI (lower limit of 95% confidence intervals (CI) for mean difference ≥ 20 events/hr) and subjective daytime sleepiness measured by change in the Epworth Sleepiness Scale (ESS; lower limit of 95% CI for mean difference ≥ 3) at 6 months compared with baseline.
The postoperative serious adverse event rate from surgery will not be different (upper limit 95% CI for mean difference ≤ 10%) from ongoing medical management at 6 months.
Surgery will be cost effective when compared with ongoing medical management: the incremental cost effectiveness ratio of surgery over ongoing medical management will be <AUD$50,000 per quality adjusted life year gained.
In addition, we aim to analyze magnetic resonance imaging (MRI) of the surgery group to define the anatomical effects of the surgery and to test the relationship between baseline anatomical findings and treatment response. If successful, this relationship may help derive a clinical prediction model that may aid preoperative assessment and selection of patients for these surgical procedures in the future.
Methods
Study design
The trial protocol was designed in accordance with the Standard Protocol Items: Recommendations for Investigative Trials (SPIRIT) declaration and checklist [28] (Supplementary Table S3). This study is a prospective, parallel-group, open label, randomized, controlled, multicenter clinical trial. Participants are from six participating Australian study sites (five academic centers providing services to public and private patients and one private hospital) located in South Australia, New South Wales, and Western Australia. Ethical approvals were obtained from the human research ethics committees (EC00188, EC00271, EC00443, and EC000266) and governance approvals from local research governance offices associated with each hospital. The study is being conducted according to the principles of the Declaration of Helsinki, following the Therapeutic Goods Administration guidelines for Good Clinical Practice. Informed consent is obtained from all study participants by sleep physicians prior to randomization to a treatment group. Surgical consent is obtained by otolaryngologists. Participants are free to withdraw consent at any time.
Sample size estimation and statistical power
All calculations assume a 2-sided type I error rate of α = 0.05. We estimated the need for a total sample of 102 participants (51 per group). Of the primary outcome variables (AHI and ESS), the ESS required a larger sample size and therefore was the basis for our sample size estimation. We set an a priori superiority margin of 3 in ESS change between the groups at 6 months [29]. Review of patients from our preliminary study with preoperative ESS > 8 and an AHI > 20 (n = 17), matching this trial eligibility criteria, showed the mean change in ESS with surgery was 7.5 with a standard deviation 5.0 [17]. From these data and assuming a similar standard deviation in the ongoing medical management group, we calculated that 44 participants were needed in each group in the present trial for a lower limit of the 95% CI = 3 for the difference in the change in ESS between groups with 80% power, assuming a normal distribution and using Student’s t-test. In the unlikely event that change was non-normally distributed, the asymptotic relative efficiency [30] of the Mann–Whitney U test compared with the Student’s t-test would be >0.864 for any distribution of change scores under the alternative, requiring 44/0.864 = 51 participants per group. Thus, we planned a total sample, N = 102.
In our preliminary study, patients with preoperative ESS > 8 and an AHI > 20 (n = 17) had a mean change in AHI pre-post-surgery of 33 events per hour with a standard deviation of 27 [17]. From these data and assuming a similar standard deviation in the ongoing medical management group, the sample of 102 participants (51 per group) provides 96% power to detect a mean difference of 20 in the change in AHI between groups. Although AHI typically is not normally distributed, change in AHI commonly is normally distributed [12].
Participants
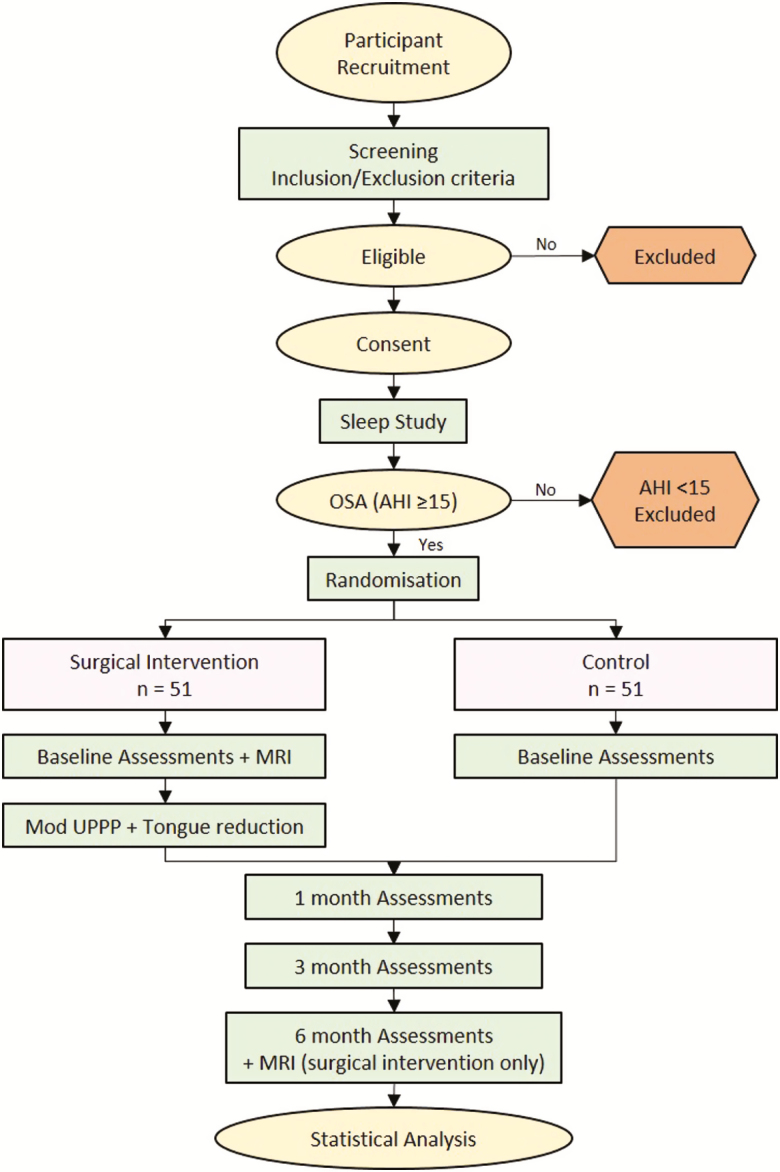
Recruitment occurred by advertisement and directly from sleep medicine and otolaryngology specialist services (August 2014–October 2017). Participants who met the eligibility criteria (Supplementary Table S1) and who consented to the study were enrolled by on-site study staff and randomly allocated to receive either the surgical intervention or ongoing medical management. Participants are followed for 6 months from baseline assessments (ongoing medical treatment group) or from surgery. They are reviewed by a sleep physician at 1, 3, and 6 months at which times outcome measures are collected. Surgical group participants are seen by an otolaryngologist as needed postoperatively and at 1, 3, and 6 months (Figure 1 and Supplementary Table S2).
Figure 1.
Study process.
Randomization
Eligible participants were randomized 1:1 to receive either the surgical intervention or ongoing medical management. To facilitate trial integrity and ensure allocation separation from study personnel, an independent central service randomized participants using computerized random allocation provided upon real-time email request by on-site investigators and staff at the time of group assignment. A minimization program (MinimPy) [31] was employed to ensure balance between variables with the potential to affect outcomes: study site, gender, age (<50 and ≥50 years), AHI (<50 and ≥50 events/hr), and BMI (<28 and ≥28 kg/m2).
Interventions
Surgical treatment of OSA
The surgical procedure was a modified UPPP plus minimally invasive tongue reduction [17], standardized at each site using a study Surgical Manual of Procedures. The modified UPPP was a reconstructive procedure to open the lateral velopharyngeal port [17] that included a bilateral tonsillectomy when palatine tonsils were present. It aimed to preserve mucosa and included resection of only the tonsils, lateral palatal space fat pad [17, 32], and part of the uvula. Minimally invasive tongue reduction was performed using a Coblation ReFlex XP wand (Smith & Nephew, London, United Kingdom) on Power setting 6 for 15 s per channel with chilled saline. The standard protocol included 7–9 tongue channels: 4 lateral (2 left, 2 right) and 3–5 midline/para-midline. Lesions were performed at 1 cm intervals with a posterior limit of 1 cm anterior to circumvallate papillae and an anterior limit of 2.5 cm from the tip of the tongue. A more detailed description of the surgical procedures is available in the Surgical Manual of Procedures (see Supplemental Material), which also includes details of anesthesia and peri-operative care.
Medical treatment of OSA
Participants assigned to ongoing medical management for their OSA received advice about weight loss, avoiding sleeping in the supine position during sleep where relevant, and other therapies such as nasal steroids [33]. Participants were given the opportunity to consider or reconsider nonsurgical treatments for the management of OSA (i.e. retrial of CPAP and MAS) during the trial but not surgical reconstruction of the upper airway.
Primary outcome measures
The two primary outcomes to compare clinical effectiveness of the surgical intervention versus ongoing medical management after 6 months follow-up are defined as the difference between groups in baseline-adjusted 6 month OSA severity (AHI) and subjective sleepiness (ESS). In participants allocated to the surgical intervention, the second evaluation occurs 6 months after the surgery is performed, to allow for the expected and unavoidable delay after randomization in scheduling the surgery. In the ongoing medical management group, the follow-up diagnostic study at 6 months was performed off CPAP or MAS. All sleep studies were scored according to the AASM 2007 alternate criteria [34].
Secondary outcome measures
The following sleep measures are determined at baseline and again at 6 months. Variables marked below with an asterisk (*) had an additional measurement taken at 3 months.
The Multiple Sleep Latency Test (MSLT), an objective laboratory measure of daytime sleepiness [35, 36]
-
Polysomnography measures including:
Sleep stages (NREM 1, 2, and 3 and REM)
Arousal Index
3% and 4% oxygen desaturation indices
Lowest oxygen saturation
% of sleep time spent with oxygen saturation <90%
Apnea index
Supine and nonsupine: sleep time percentage, AHI and lowest oxygen saturation
ESS (primary outcome at 6 months and secondary outcome at 3 months)*[37]
Snoring Severity Scale questionnaire [38], completed by the bed partner when possible*
Functional Outcomes of Sleep Questionnaire, a comprehensive measure of the impact of sleep disorders on daily functioning and quality of life [39]
Fiberoptic nasendoscopy (% and pattern of collapse at the velopharynx and tongue base) measured at baseline (all participants) and 6 months (surgical group only)
Adherence to other OSA therapies (i.e., CPAP adherence via CPAP use download or self-report, and MAS use via self-report)*
Other measures include general and otolaryngology specific quality of life and cardiovascular outcomes:
EuroQoL 5-dimension 5-level questionnaire (EQ-5D-5L), a participant-reported health-related quality of life instrument* [40]
Glasgow Benefit Inventory questionnaire, a quality of life change (transition) instrument, measured at 6 months only [41, 42]
Institute for Medical Technology Assessment Productivity Cost Questionnaire, measure of indirect costs of medical intervention from a societal perspective; measured at baseline, 1 month, and 6 months [43]
Morning seated office blood pressure, average of 3 measurements*
24 hour ambulatory blood pressure, analyzed as means, systolic, and diastolic and night versus daytime
Anthropometrics: age, height, weight, neck circumference, waist circumference, hip circumference
MRI upper airway
Participants randomized to surgery had upper airway MRI scans at baseline and again at 6 months using a MRI protocol described previously [44] to which a Dixon imaging sequence was added to optimize fat visualization in the tongue [45]. The scanning protocol was standardized across sites.
Blinding
In designing the study, it was decided not to undertake a sham surgical procedure as a control for the following reasons: (1) A sham would not provide the desired placebo control because participants in the surgical arm would feel postoperative pain and see surgical changes in their oral cavity and oropharynx, whereas those in the control arm would not; and (2) there were ethical and safety concerns about undertaking a general anesthetic for participants to undergo a sham procedure. Participants were therefore not blinded with respect to the intervention. Sleep study measures including one of two primary outcomes (change in AHI) are least likely to be subject to participant bias. To further limit potential bias, all polysomnographies (PSG) and MSLT tests are analyzed by technicians who are blinded to treatment allocation. To make surgical bookings and MRI appointments, trial coordinators had to be aware of participant treatment allocation. Participants (and bed partners) completed questionnaires with no guidance from the investigators, trial coordinators, or research assistants.
Adverse event reporting
Adverse events are reported for all study participants regardless of their group allocation or whether they are deemed serious. The mechanisms for reporting serious adverse events are based on the guidelines adopted by the International Conference on Harmonization Good Clinical Practice (ICH GCP) [46]. For this trial, adverse events will be deemed serious if they resulted in patient death; life-threatening illness or injury; permanent impairment of body structure or function; in-patient hospitalization (>24 hr) or prolongation of existing hospitalization; and medical or surgical intervention to prevent permanent impairment to body structure or body function. The study documentation recognizes minor postoperative bleeding as a relatively frequent event. In Australia, it has become common practice for any patient that presents to the hospital emergency department with a history of bleeding following a tonsillectomy procedure to be admitted for observation (several hours/overnight), no matter how minor the bleed. This hospital admittance reflects hospital policy and is not in keeping with the serious nature of an SAE notification, and therefore, it was decided a priori that a postoperative bleed hospital admittance requiring surgical intervention would be considered an SAE. See Surgical Manual of Procedures in Supplementary Material for a description of the normal expected postoperative occurrences, those considered an adverse event and the bleeding classification [47] used.
Data and safety monitoring committee
The study is being overseen by a data and safety monitoring committee comprising three independent experts: one each in the fields of sleep medicine, trial methodology, and upper airway surgery. The members have no direct involvement in the conduct of the study, and no financial or professional interest that would compromise impartial decision-making. The primary objective of the committee is to monitor the safety of the intervention and the validity and integrity of the data for the study. The committee also evaluates recruitment pace and makes recommendations to the steering committee regarding the continuation, modification, or termination of the study. The committee was formed and the study protocol reviewed prior to the onset of participant recruitment for the study. The committee meets every 6 to 12 months to evaluate the trial conduct, recruitment, participant safety, data integrity, and scientific validity of the study.
Data analysis
A detailed statistical analysis plan for the primary and secondary outcomes was prepared and approved by the trial statistician and the trial coordinating principal investigator on behalf of the steering committee. See statistical analysis plan in Supplementary Material.
Primary outcomes
Anticipating that the study participants would have severe OSA on average, we considered a mean AHI reduction of ≥20 events per hour of sleep would be clinically meaningful and needed to justify the cost and potential morbidity of surgery. By incorporating cost and potential morbidity, this “sufficiently important difference” [48] is greater than the minimal clinically important difference alone, which is defined as “the smallest difference in score in the domain of interest which patients perceive as beneficial and which would mandate, in the absence of troubling side effects and excessive cost, a change in the patient’s management” [49]. We set an a priori superiority margin of 3 in ESS change after intervention between the groups [29]. This is a slightly higher minimum difference than we have used previously [29, 50] and others have recommended [51] in determining the sample size in our randomized trials of other OSA treatments, as we believe clinicians would consider this change enough to justify the cost and potential morbidity of surgery in a patient with OSA (i.e. sufficiently important difference).
For the two primary outcome variables, AHI and ESS, the effect of treatment will be analyzed on the basis of 6 month follow-up between allocation groups using linear mixed effects models with adjustment for baseline values of the relevant outcome. As a sensitivity analysis, we will use multiple imputation using chained equations for outcomes with missing data at 6 months and repeat the analysis described above. All analyses will be conducted primarily on an intention-to-treat basis, and the trial will be reported according to CONSORT guidelines [52].
Secondary outcomes
We plan multiple secondary analyses, including testing the secondary outcome measures as listed above. This analysis will examine 6 month between group differences for OSA symptoms and symptom severity, quality of life, and demographics. These data will be analyzed as described above for primary outcome analysis. In addition, participants will be classified according to the proportion who achieve an AHI < 10 events per hour of sleep (i.e. normal/very mild). For comparison to the literature, response rates according to other definitions (e.g. AHI < 15 and AHI > 10 with AHI reduction > 50%) will be reported. Categorical outcome data will be assessed using the chi-squared test or Fisher’s exact tests. If there is substantial imbalance in dropout, crossover, or baseline variables between groups, then we will also perform per-protocol secondary analyses with adjustment for potential confounding variables.
MRI analysis
Analysis of all MRI scans will be centralized and performed according to published segmentation methods [53]. Preoperative and postoperative anatomical scans will be aligned and volumetric analysis of the airway space, tongue, and soft palate will be performed and used to define the anatomical effects of the surgery and their relationship to the treatment response [45, 54]. Tongue fat will be identified on the Dixon images and the tongue fat content calculated. Craniofacial skeletal landmarks will be identified using 3D cephalometry to create a “box.” A soft tissue volume to box volume ratio will be used as an assessment of “anatomical balance.” Baseline MRI measurements will be analyzed according to treatment response in an attempt to derive a clinical prediction model that may aid preoperative assessment and selection of patients for these surgical procedures in the future.
Health economic analysis
The economic analysis will be conducted from the perspective of the health service with a 6 month time horizon. The primary measure of outcome for this analysis will be the incremental cost per unit of improvement in functional outcomes of sleep using the FOSQ [39]. We will also calculate the incremental cost per quality adjusted life year gained as measured by the EQ-5D-5L [55]. See Supplementary Material for further details.
Additional substudies
Subsequent to reporting the main study findings, a number of preplanned analyses will be conducted. These are briefly described in Supplementary Material.
Trial status
This trial is currently ongoing and in the participant follow-up phase. Recruitment has been completed with 102 participants randomized into the trial: 51 allocated to undergo the surgical treatment and 51 allocated to the ongoing medical management group. Data analysis will be conducted in accordance with the statistical analysis plan after the last patient visit and data lock has occurred.
Discussion
There are many challenges to executing a valid randomized trial of a surgical treatment [12, 56–58]. In the present trial, we found that the ethics and governance reviews and approval turn-around times at public hospitals, private hospitals, and independent clinics were often lengthy. Reassuringly, our justification of equipoise for surgery in this group of patients was accepted and we found support from our lead ethical committee regarding the need for a high-quality RCT in this area.
There can be no doubt that recruitment was our main obstacle towards trial completion. Although CPAP failure is common, sourcing patients for such a trial can be difficult because patients rarely present back to sleep clinics. Patients presenting to surgeons do so with the expectation that they will be considered for surgery and many declined randomization or even initial assessment on this basis. We required >3 years to recruit the target 102 participants, which was twice as long as anticipated. One approach to this challenge is to advertize in the community. However, our advertisements of a surgical procedure to a population where the main medical therapy (CPAP) is difficult to embrace led to an overwhelming response from patients dissatisfied with CPAP but still using it. The determination of CPAP failures in this large group was challenging as rules for defining clinically beneficial CPAP use remain unclear. For example, 2 nights of 10 hr use per week versus 5 nights of 4 hr use per week each provides 20 hr use per week, but it is unclear which, if either, constitutes adequate treatment or failure. We decided on the definition of CPAP failure as <7 nights use in the last month (on average) or an average nightly use of <2 hr per night, both representing approximately 25% of ideal CPAP use.
Another challenge involved coordinating trial otolaryngologists to perform a standardized surgical procedure. This was achieved with a comprehensive manual (see Supplementary Material), videos, a teleconference, and encouragement to attend an upper airway cadaver dissection course led by the trial otolaryngologist chief investigators. Although over 80% of trial surgeons attended the course, there were obstacles preventing 100% recruitment. Some surgeons initially expressed concern regarding the rigidity of the surgical protocol, preferring a multilevel surgical procedure specifically tailored to individual patients. Several challenging teleconferences were necessary before consensus on the surgical protocol was finally achieved. We aimed to make the research practices as similar to clinical care as possible; however, standard clinical care varies across hospitals and clinics. Medication regimens also required standardization as usual protocols varied based on the preferences of individual otolaryngologists.
Another major issue for us was that the ICH GCP definition of an SAE does not easily translate to a surgical trial. Patients have a variable response to surgery, analgesia, and postoperative function. A low-risk return to hospital for optimization of analgesia, for example, would not be regarded by most surgeons as a major issue but might be regarded as an SAE by some observers. A lengthy debate between the investigators reached consensus that, when looking at Australian postoperative outcomes, a ward readmission exceeding 24 hr was something that should be relatively uncommon. It was considered important to be open and transparent in reporting all adverse events and preferable to err on the conservative side when reporting a surgical adverse event as serious. Thus, we agreed that a postoperative ward admission of >24 hr would be classified as an SAE.
There is clinical concern that positive results in a surgery trial could be surgeon-dependent. In the current trial, the surgical techniques and equipment are readily available to most (if not all) otolaryngologists around the world. Thus, there could be widespread applicability of such techniques if outcomes are favorable. This trial tested a standardized surgical methodology across multiple sites, including both academic (with supervised trainees) and community-based practices, which enhances generalizability of the results.
We are also aware of the need to ascertain longer-term outcomes of surgery, including both beneficial and adverse effects. To this end, we plan an ongoing observational study of participants who received surgery (including participants who were randomized to ongoing medical management of OSA and later receive surgery after RCT completion).
Surgery has a very important potential advantage over other therapies used for OSA because once operated on, patient adherence does not affect the effectiveness of the treatment. Like with MAS [59], it may be that surgery has less efficacy than CPAP in control of OSA at a single time point, but may carry effectiveness comparable to CPAP over time when low CPAP adherence affects outcome [8]. Surgery outcome will be enhanced by developing a clinical prediction model to identify those patients most likely to succeed with surgery in the future. Data from this trial will facilitate such a prediction model, which will require independent validation in future studies.
It is not the goal of this trial to test the effectiveness of surgery versus CPAP, because surgery has less certain efficacy and carries additional risk of morbidity. Instead, we aim to test whether surgery should be used to treat OSA in patients who fail CPAP therapy. Our hypothesis is that this randomized trial will demonstrate the effectiveness, safety, and cost-effectiveness of this relatively straightforward surgical procedure, thereby reducing OSA burden amongst the many people with OSA who have failed CPAP treatment and would otherwise remain untreated or under-treated.
Funding
The study has received project funding from the Australia National Health and Medical Research Council (Project Grant 1059510, CIA’s Antic and McEvoy), the Repat Foundation Prabha Seshardri Research Grant (CIA Antic), and the Flinders University Near-Miss Grant (CIA Antic). Dr. Weaver’s effort was supported by the United States National Institutes of Health (R01 HL084139, PI Weaver) and Veterans Affairs Puget Sound Health Care System, Seattle, WA; the contents do not represent the views of the United States Department of Veterans Affairs or the United States Government. Prof. McEvoy was supported by an Australian National Health and Medical Research Council Practitioner Fellowship.
Conflict of interest statement. Prof. Carney is a board member of the not-for-profit organization Asia Pacific Otolaryngology Surgical Training which receives financial support from Olympus, Medtronic, and Smith & Nephew. He is a consultant for Olympus and has received speaking honoraria from Smith & Nephew. Prof. McEvoy reports receiving research grants from Philips Respironics and Fisher & Paykel; research equipment grants from Philips Respironics, ResMed, and Airliquide; and speaker fees from ResMed. Prof. Cistulli has an appointment to an endowed academic Chair at the University of Sydney that was established from ResMed funding (a CPAP manufacturer). He has received research support from ResMed, SomnoMed, and Zephyr Sleep Technologies. He is a consultant/adviser to Zephyr Sleep Technologies, and Narval. He has a pecuniary interest in SomnoMed related to a previous role in R&D (2004). Dr. Chai-Coetzer has received equipment/product donations for research from ResMed, Philips Respironics, SomnoMed, and Biotech Pharmaceuticals. Prof. MacKay reports receiving studying funding from Nyxoah S.A. Potential professional conflicts of interest: Profs. Carney, MacKay, and Weaver perform OSA surgery, and Prof. McEvoy, Dr. Chai-Coetzer, and Prof. Cistulli are practicing sleep physicians and prescribe medical devices (CPAP, MAS).
Supplementary Material
Acknowledgments
The authors wish to acknowledge the following: Dr. Samuel Robinson (deceased) for his pioneering work in the field leading to this clinical trial. Participating sleep physicians and otolaryngologists; without their support this study would have been impossible. Trial coordinators: Ms. SueEllen Holmes, Ms. Kelsey Johnson, Ms. Alison Teare, Ms. Natasha Umrigar, Ms. Aimee Lowth, Ms. Melanie Madronio, Ms. Nina Sarkissian, and Ms. Siobhan Clare Rea. The Data and Safety Monitoring Committee: Prof. Matthew Naughton (chair), A/Prof. Nathaniel Marshall and Prof. William Coman. Prof. Julie Radcliffe for health economic analysis advice. Dr. Stephen Quinn for assistance with sample size calculation and planned analysis. Prof. Nick Antic is acknowledged posthumously as an author. Prof. Antic was the instigator and the principal investigator of this trial up until the time of his premature death in November 2016. The authors and everyone involved in the study who knew Prof. Antic, wish to acknowledge, and pay tribute to his inspiration, drive, and collaborative spirit, without which the study would not have been possible.
References
- 1. Young T, et al. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. [DOI] [PubMed] [Google Scholar]
- 2. Weaver TE, et al. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weaver TE, et al. Management of obstructive sleep apnea by continuous positive airway pressure. Oral Maxillofac Surg Clin North Am. 2009;21(4):403–412. [DOI] [PubMed] [Google Scholar]
- 4. Bamagoos AA, et al. Mandibular advancement splints. Sleep Med Clin. 2016;11(3):343–352. [DOI] [PubMed] [Google Scholar]
- 5. Lee HM, et al. Uvulopalatopharyngoplasty reduces the incidence of cardiovascular complications caused by obstructive sleep apnea: results from the national insurance service survey 2007-2014. Sleep Med. 2018;45:11–16. [DOI] [PubMed] [Google Scholar]
- 6. Weaver EM, et al. ; SLEEP Study Investigators Studying life effects & effectiveness of palatopharyngoplasty (SLEEP) study: subjective outcomes of isolated uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 2011;144(4):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weaver EM, et al. Survival of veterans with sleep apnea: continuous positive airway pressure versus surgery. Otolaryngol Head Neck Surg. 2004;130(6):659–665. [DOI] [PubMed] [Google Scholar]
- 8. Robinson S, et al. Upper airway reconstructive surgery long-term quality-of-life outcomes compared with CPAP for adult obstructive sleep apnea. Otolaryngol Head Neck Surg. 2009;141(2):257–263. [DOI] [PubMed] [Google Scholar]
- 9. Keenan SP, et al. Long-term survival of patients with obstructive sleep apnea treated by uvulopalatopharyngoplasty or nasal CPAP. Chest. 1994;105(1):155–159. [DOI] [PubMed] [Google Scholar]
- 10. Sommer UJ, et al. Tonsillectomy with uvulopalatopharyngoplasty in obstructive sleep apnea. Dtsch Arztebl Int. 2016;113(1-02):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Browaldh N, et al. SKUP3 randomised controlled trial: polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea. Thorax. 2013;68(9):846–853. [DOI] [PubMed] [Google Scholar]
- 12. Woodson BT, et al. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2003;128(6):848–861. [DOI] [PubMed] [Google Scholar]
- 13. Elshaug AG, et al. An analysis of the evidence-practice continuum: is surgery for obstructive sleep apnoea contraindicated? J Eval Clin Pract. 2007;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 14. Elshaug AG, et al. Over 150 potentially low-value health care practices: an Australian study. Med J Aust. 2012;197(10):556–560. [DOI] [PubMed] [Google Scholar]
- 15. Franklin KA, et al. Effects and side-effects of surgery for snoring and obstructive sleep apnea–a systematic review. Sleep. 2009;32(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- 16. Schellenberg JB, et al. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. Am J Respir Crit Care Med. 2000;162(2 Pt 1):740–748. [DOI] [PubMed] [Google Scholar]
- 17. MacKay SG, et al. Modified uvulopalatopharyngoplasty and coblation channeling of the tongue for obstructive sleep apnea: a multi-centre Australian trial. J Clin Sleep Med. 2013;9(2):117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caples SM, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33(10):1396–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cahali MB, et al. Lateral pharyngoplasty versus uvulopalatopharyngoplasty: a clinical, polysomnographic and computed tomography measurement comparison. Sleep. 2004;27(5):942–950. [DOI] [PubMed] [Google Scholar]
- 20. Pang KP, et al. Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2007;137(1):110–114. [DOI] [PubMed] [Google Scholar]
- 21. Kim HY, et al. The correlation between pharyngeal narrowing and the severity of sleep-disordered breathing. Otolaryngol Head Neck Surg. 2008;138(3):289–293. [DOI] [PubMed] [Google Scholar]
- 22. Friedman M, et al. Combined uvulopalatopharyngoplasty and radiofrequency tongue base reduction for treatment of obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2003;129(6):611–621. [DOI] [PubMed] [Google Scholar]
- 23. Sher AE, et al. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19(2):156–177. [DOI] [PubMed] [Google Scholar]
- 24. Woodson BT. Updated hypopharyngeal surgery for sleep apnea. Adv Otorhinolaryngol. 2017;80:81–89. [DOI] [PubMed] [Google Scholar]
- 25. Lin HC, et al. Effects of minimally invasive surgery for patients with OSA on quality of life. Ann Otol Rhinol Laryngol. 2018;127(2):118–123. [DOI] [PubMed] [Google Scholar]
- 26. Verse T, et al. Multi-level surgery for obstructive sleep apnea. Lingual tonsillectomy vs. hyoid suspension in combination with radiofrequency of the tongue base. Sleep Breath. 2015;19(4):1361–1366. [DOI] [PubMed] [Google Scholar]
- 27. Baba RY, et al. Temperature controlled radiofrequency ablation at different sites for treatment of obstructive sleep apnea syndrome: a systematic review and meta-analysis. Sleep Breath. 2015;19(3):891–910. [DOI] [PubMed] [Google Scholar]
- 28. Chan AW, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Antic NA, et al. A randomized controlled trial of nurse-led care for symptomatic moderate-severe obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179(6):501–508. [DOI] [PubMed] [Google Scholar]
- 30. Hodges JL, et al. The efficiency of some nonparametric competitors of the t-test. Ann. Math. Stat. 1956;27 (2):324–335. [Google Scholar]
- 31. Saghaei M, et al. Implementation of an open-source customizable minimization program for allocation of patients to parallel groups in clinical trials. J Biomed Sci Eng. 2011;04(11):734–739. [Google Scholar]
- 32. Woodson BT, et al. Radiofrequency ablation of the lateral palatal space for snoring. World J Otorhinolaryngol Head Neck Surg. 2017;3(2):106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Epstein LJ, et al. ; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 34. Iber C, Ancoli-Israel S, Chesson AL Jr, Quan S editors. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. In: American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 35. Littner MR, et al. ; Standards of Practice Committee of the American Academy of Sleep Medicine Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28(1):113–121. [DOI] [PubMed] [Google Scholar]
- 36. Carskadon MA, et al. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9(4):519–524. [DOI] [PubMed] [Google Scholar]
- 37. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 38. Lim PV, et al. A new method for evaluating and reporting the severity of snoring. J Laryngol Otol. 1999;113(4):336–340. [DOI] [PubMed] [Google Scholar]
- 39. Weaver TE, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843. [PubMed] [Google Scholar]
- 40. Herdman M, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robinson K, et al. Measuring patient benefit from otorhinolaryngological surgery and therapy. Ann Otol Rhinol Laryngol. 1996;105(6):415–422. [DOI] [PubMed] [Google Scholar]
- 42. Hendry J, et al. The glasgow benefit inventory: a systematic review of the use and value of an otorhinolaryngological generic patient-recorded outcome measure. Clin Otolaryngol. 2016;41(3):259–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bouwmans C, et al. Manual of the iMTA Productivity Cost Questionnaire (iPCQ). Rotterdam: iMTA, Erasmus University Rotterdam; 2013. [Google Scholar]
- 44. Lee RW, et al. Relationship between surface facial dimensions and upper airway structures in obstructive sleep apnea. Sleep. 2010;33(9):1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim AM, et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014;37(10):1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. ICH Guideline for Good Clinical Practise E6(R2), 2016. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf Accessed March 9, 2019. [Google Scholar]
- 47. Sarny S, et al. Hemorrhage following tonsil surgery: a multicenter prospective study. Laryngoscope. 2011;121(12):2553–2560. [DOI] [PubMed] [Google Scholar]
- 48. Barrett B, et al. Sufficiently important difference: expanding the framework of clinical significance. Med Decis Making. 2005;25(3):250–261. [DOI] [PubMed] [Google Scholar]
- 49. Jaeschke R, et al. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–415. [DOI] [PubMed] [Google Scholar]
- 50. Chai-Coetzer CL, et al. Primary care vs specialist sleep center management of obstructive sleep apnea and daytime sleepiness and quality of life: a randomized trial. JAMA. 2013;309(10):997–1004. [DOI] [PubMed] [Google Scholar]
- 51. Crook S, et al. Minimum important difference of the Epworth Sleepiness Scale in obstructive sleep apnoea: estimation from three randomised controlled trials. Thorax. 2018. https://thorax.bmj.com/content/early/2018/08/11/thoraxjnl-2018-211959.info Accessed March 9, 2019. [DOI] [PubMed] [Google Scholar]
- 52. Schulz KF, et al. ; CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Open Med. 2010;4(1):e60–e68. [PMC free article] [PubMed] [Google Scholar]
- 53. Chan AS, et al. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax. 2010;65(8):726–732. [DOI] [PubMed] [Google Scholar]
- 54. Humbert IA, et al. Simultaneous estimation of tongue volume and fat fraction using IDEAL-FSE. J Magn Reson Imaging. 2008;28(2):504–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ratcliffe J, et al. A randomised controlled trial of acupuncture care for persistent low back pain: cost effectiveness analysis. BMJ. 2006;333(7569):626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. MacKay SG, et al. Surgical approaches to obstructive sleep apnea. Sleep Med Clin. 2016;11(3):331–341. [DOI] [PubMed] [Google Scholar]
- 57. Powell NB, et al. Radiofrequency treatment of turbinate hypertrophy in subjects using continuous positive airway pressure: a randomized, double-blind, placebo-controlled clinical pilot trial. Laryngoscope. 2001;111(10):1783–1790. [DOI] [PubMed] [Google Scholar]
- 58. Weaver EM. Judging sleep apnea surgery. Sleep Med Rev. 2010;14(5):283–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Phillips CL, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187(8):879–887. [DOI] [PubMed] [Google Scholar]
- 60. Fairbanks DNF, et al. Snoring and obstructive sleep apnea. 3rd ed. Philadelphia, PA:Lippincott Williams & Williams; 2003. [Google Scholar]
- 61. Hsu PP, et al. Clinical predictors in obstructive sleep apnea patients with computer-assisted quantitative videoendoscopic upper airway analysis. Laryngoscope. 2004;114(5):791–799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.