Abstract
Background
Obstructive sleep apnea (OSA) is highly prevalent in patients with hypertrophic obstructive cardiomyopathy (HOCM). Inflammatory responses are increased in patients with OSA, meanwhile, inflammation is also associated with adverse outcomes in HOCM.
Hypothesis
To investigate the association between severity of OSA and high‐sensitivity C‐reactive protein (hs‐CRP) in patients with HOCM.
Methods
Three hundred and ninteen patients with HOCM who underwent sleep evaluations at Fuwai Hospital were retrospectively included between February 2010 and December 2018. Data from baseline clinical characteristics and polysomnography studies were collected.
Results
OSA was present in 168 (52.7%). Patients with OSA were older, more likely to be male, had a higher body mass index and more clinical comorbidities. Patients with OSA had enlarged left ventricular diameter and similar left ventricular outflow tract obstruction compared with those without. In multivariate logistic analysis, apnea‐hypopnea index (OR, 1.024; 95% CI, 1.005‐1.044; P = .014), oxygen desaturation index (OR, 1.025; 95% CI, 1.004‐1.046; P = .018) and lowest oxygen saturation (OR, 0.951; 95% CI, 0.915‐0.989; P = .011) were independently associated with high risk hs‐CRP (>3 mg/L) after adjusting for confounders. In addition, decreasing lowest oxygen saturation (β = −.159, P = .004) was also independently correlated with increasing hs‐CRP concentrations in multivariate linear analysis after adjusting for confounders.
Conclusions
Severity of OSA was independently associated with elevated hs‐CRP levels in patients with HOCM. Further studies are needed to evaluate the effects of treating OSA on hs‐CRP as well as clinical outcomes in these patients.
Keywords: high‐sensitivity C‐reactive protein, hypertrophic obstructive cardiomyopathy, inflammation, obstructive sleep apnea
1. INTRODUCTION
Obstructive sleep apnea (OSA), characterized by oxygen desaturation and sleep fragmentation due to apneas and hypopneas during sleep, has been increasingly implicated in the pathogenesis and complications of cardiovascular disease.1, 2 Elevated systemic inflammation is seen in patients with OSA because of long‐term hypoxia, sympathetic activation, and oxidative stress.3, 4 In addition, treatment of OSA significantly reduces the inflammatory responses indicating an important role of inflammation in the pathophysiological processes of OSA.5, 6, 7
Hypertrophic cardiomyopathy (HCM) is one of the most common inherited cardiac diseases, characterized by ventricular hypertrophy, myofiber disarray, and fibrosis.8, 9, 10 Inflammation also plays a critical role in the development of myocardial remodeling as well as adverse outcomes in patients with HCM.11, 12 Furthermore, hypertrophic obstructive cardiomyopathy (HOCM), a phenotype of HCM with more symptoms and higher risk of sudden death, is associated with increased inflammatory responses compared with non‐obstructive HCM.12, 13 Considering that OSA is highly prevalent in patients with HCM ranging from 32% to 71%,14, 15 we propose that OSA be associated with elevated inflammation in HCM. Of the wide array of inflammatory biomarkers that have been studied, high‐sensitivity C‐reactive protein (hs‐CRP) has received the most attention for its use in screening and risk reclassification of cardiovascular disease. 16 Therefore, the association of OSA with hs‐CRP in patients with HOCM, a more serious type of HCM, was investigated in this study.
2. MATERIALS AND METHODS
2.1. Study populations
This retrospective study included patients who were diagnosed with HOCM and underwent the first overnight diagnostic sleep examinations from in‐patient department at Fuwai Hospital between February 2010 and December 2018. The diagnosis of HOCM was made based on typical clinical, electrocardiographic, and echocardiographic features. Diagnostic criteria of HCM were consistent with the 2011 American Heart Association/American College of Cardiology and 2014 European Society of Cardiology guidelines, which mainly include unexplained septal hypertrophy with a thickness of 15 mm. We defined HOCM patients as who satisfied one of the following criteria based on echocardiography: (1) rest LVOT peak gradient ≥30 mmHg or (2) rest LVOT peak gradient <30 mmHg with provoked (valsalva maneuver, amyl nitrite or exercise) LVOT peak gradient ≥30 mmHg. Patients with both rest and provoked LVOT peak gradient <30 mmHg were defined as non‐obstructive HCM.
All patients were clinically stable who did not undergo changes in New York Heart Association (NYHA) functional class over the last 30 days and no patient was in NYHA class IV. Patients were further excluded if they had >50% central respiratory events, incomplete sleep recording data, were younger than 18 years old, or had previous septal reduction therapies (septal myectomy or alcohol septal ablation), or had history of heart transplantation surgery. No patient had systemic inflammatory disease, active infection, or trauma. All patients were not undergoing continuous positive airway pressure treatment before. Patient demographics and clinical data such as age, gender, body mass index, history of coronary artery disease, diabetes mellitus, hyperlipidemia, hypertension, and smoking were retrospectively reviewed.
All patients provided informed consent. The study was approved by the ethics committee of Fuwai Hospital. All studies were conducted in accordance with the ethical principles stated in the Declaration of Helsinki.
2.2. High‐sensitive C‐reactive protein assay
The baseline hs‐CRP values were collected from our medical records system. Briefly, fasting peripheral venous blood samples for hs‐CRP evaluation were obtained before polysomnography in the morning. After immediate centrifugation of the specimens, we stored them at 4°C prior to serum separation. The hs‐CRP was determined with an AUS5400 (Olympus, Japan) molecular analyzer at our clinical laboratory department. All samples were processed by technicians blinded to the samples. We used the recommended conventional cut‐off value for hs‐CRP of 3.0 mg/L provided by consensus conference of the American Heart Association (AHA) on the use of hs‐CRP in clinical practice. 17 The hs‐CRP concentrations were categorized into two groups as follows: high risk level (>3 mg/L) and low risk level (≤3 mg/L).
2.3. Sleep study
An overnight polysomnography was performed in all the study populations using the portable monitoring system Embletta (Medcare Flaga, Reykjavik, Iceland). This device continuously recorded finger pulse oximetry, nasal airflow by an airflow pressure transducer, thoracic and abdominal movement, body position, snoring, heart rate and ECG, and has been validated against full polysomnography. 18 The sleep was monitored automatically 30 minutes after the subjects went to bed. Apnea was defined when cessation of airflow or airflow reduction to ≤10% of the baseline value lasted for 10 seconds or more. Hypopnea was defined as a 50% or discernible decrement in airflow lasting 10 seconds with oxygen desaturation of 4%. Obstructive apneas were defined on basis of the presence of thoracic efforts. Apnea‐hypopnea index (AHI) was defined as the total number of apneas and hypopneas occurring per hour of sleep. Oxygen desaturation index (ODI) was defined as the number of oxygen level drops 4% from baseline per hour. Mean and minimal oxygen saturation (SaO2), average pulse frequency, and snoring proportion were also recorded. Diagnosis of OSA was made when the AHI in the recorded study was 5 events/hour or more, irrespectively to daytime OSA symptoms, which allowed objective evaluation of the disease severity.19, 20 Patients were classified into mild OSA (AHI: 5.0‐14.9 events/hour) and moderate to severe OSA (AHI ≥15.0 events/hour) based on widely accepted OSA severity threshold. OSA severity measures included AHI, ODI, longest apnea/hypopnea time, lowest SaO2, mean SaO2 and percent of total sleep time (TST) with SaO2 <90%.
2.4. Echocardiographic study
Echocardiography was performed using a GE Vivid 7 (GE Healthcare, Horten, Norway) with a multifrequency phased‐array transducer. Echocardiographic examinations were performed by one experienced physician. Diameters of the cardiac chambers were expressed as the maximum value of the anteroposterior diameter in cardiac cycles. The measurements of left ventricular volume, left ventricular ejection fraction, and left atrial diameter (LAD) were determined following the American Society of Echocardiography recommendations. 21 The thickness of the interventricular septum and ventricular wall was determined during diastole. Representative thickness of the interventricular septum, which was usually the thickness of the point 25 mm under the right coronary sinus nadir, was recorded to indicate overall thickness. LVOT gradient was measured in the apical views by continuous‐wave Doppler echocardiography under resting conditions and during provocative maneuvers as previously reported. 22
2.5. Statistical analysis
The results are expressed as mean ± SD, median (interquartile range), or number (percentage). Continuous variables were tested for normal distribution with the Kolmogorov‐Smirnov test. Comparison of categorical variables was performed using the χ2 or Fisher exact test, as appropriate. Differences among three groups were compared using one‐way analysis of variance or the Kruskal‐Wallis H test, as appropriate. Univariate and multivariate logistic regression analyses were used to determine the association between OSA severity measures and high risk hs‐CRP level. Significant variables in univariate analysis including age, body mass index (BMI), hypertension, fasting glucose, left ventricular end‐diastolic dimension (LVEDD), interventricular septum thickness (IVST), and supine sleep time, were included into multivariate regression analysis. Correlation between hs‐CRP concentrations and baseline characteristics were assessed through the use of the Pearson correlation coefficient for continuous variables and Spearman correlation coefficient for categorical variables. Multivariate linear regression analysis was used to identify relationship between OSA severity measures and hs‐CRP concentrations by adjusting for significant variables from correlation analysis such as age, BMI, hypertension, hyperlipidemia, LVEDD, fasting glucose, and supine sleep time. All reported probability values were 2‐tailed, and a P value of <.05 was considered statistically significant. SPSS version 24.0 (IBM Corp., Armonk, NY) and GraphPad Prism version 7.0 (GraphPad Software Inc., La Jolla, CA) were used for calculations and illustrations, respectively.
3. RESULTS
3.1. Population characteristics
A total of 319 patients with HOCM were included in the analysis (Supporting Information, Figure S1). One hundred and sixty‐eight (52.7%) were diagnosed with OSA and the median AHI value of the whole study population was markedly elevated (5.3, interquartile range [IQR] 1.5‐14.9 events/hour). Table 1 showed the demographic data, echocardiographic results, and sleep parameters of the study population grouped according to the severity of OSA. Those with more severe OSA were older, more likely to be male, had a higher BMI, be smokers, had higher NYHA cardiac function level, and had more clinical comorbidities such as hypertension, hyperlipidemia, coronary heart disease, stroke, and atrial fibrillation. The LVEDD was significantly enlarged and IVST decreased in patients with more severe OSA. There was no significant difference in LVOT gradient, LAD, and left ventricular ejection fraction among the study patients. The value of AHI, ODI, longest apnea/hypopnea time and percent of TST <90% were significantly increased, and level of lowest SaO2 and mean SaO2 were decreased with OSA severity.
TABLE 1.
Clinical characteristics of patients with HOCM grouped according to OSA severity
| Variables | None OSA (n = 151) | Mild OSA (n = 89) | Moderate to severe OSA (n = 79) | P‐value |
|---|---|---|---|---|
| Male | 95 (62.9) | 54 (60.7) | 65 (82.3) | .004 |
| Age (y) | 44.8 ± 14.0 | 55.3 ± 10.5 | 51.8 ± 14.0 | <.001 |
| BMI (kg/m2) | 24.6 ± 3.2 | 26.5 ± 3.1 | 27.5 ± 3.6 | <.001 |
| Cigarette use | 53 (35.1) | 37 (41.6) | 44 (55.7) | .011 |
| Hypertension | 31 (20.5) | 43 (48.3) | 51 (64.6) | <.001 |
| Hyperlipidemia | 20 (13.2) | 29 (32.6) | 35 (44.3) | <.001 |
| Diabetes | 7 (4.6) | 7 (7.9) | 8 (10.1) | .270 |
| Coronary heart disease | 12 (7.9) | 9 (10.1) | 16 (20.3) | .019 |
| Stroke | 0 (0.0) | 6 (6.7) | 3 (3.8) | .008 |
| NYHA class II‐III | 105 (69.5) | 74 (83.1) | 63 (79.7) | .038 |
| Familiar history of HCM | 20 (10.1) | 8 (9.0) | 7 (8.9) | .468 |
| Familiar history of SCD | 5 (3.3) | 3 (3.4) | 5 (6.3) | .505 |
| Syncope | 18 (11.9) | 18 (20.2) | 13 (16.5) | .216 |
| Atrial fibrillation | 12 (7.9) | 17 (19.1) | 16 (20.3) | .011 |
| Ventricular tachycardia | 19 (12.6) | 11 (12.4) | 10 (12.7) | .998 |
| High hs‐CRP level (%) | 17 (11.3) | 15 (16.9) | 21 (26.6) | .012 |
| Hs‐CRP (mg/L) | 0.7 (0.3–1.3) | 1.0 (0.5–2.1) | 1.4 (0.6–3.1) | <.001 |
| Fasting blood sugar (mmol/L) | 4.6 ± 0.8 | 5.1 ± 1.2 | 5.1 ± 1.3 | <.001 |
| Total cholesterol (mmol/L) | 4.4 ± 0.9 | 4.5 ± 1.0 | 4.4 ± 1.0 | .466 |
| Creatinine (mmol/L) | 81.6 ± 15.1 | 84.3 ± 16.5 | 84.5 ± 16.9 | .296 |
| Echocardiographic data | ||||
| LVOTG at rest (mm Hg) | 62.0 (41.5‐91.0) | 61.0 (42.0‐87.0) | 58.0 (34.0‐81.5) | .671 |
| LAD (mm) | 43.0 ± 6.1 | 43.8 ± 6.3 | 43.7 ± 6.8 | .508 |
| LVEDD (mm) | 41.9 ± 4.3 | 43.8 ± 4.6 | 45.6 ± 4.8 | <.001 |
| IVST (mm) | 19.9 ± 4.8 | 18.5 ± 5.1 | 18.2 ± 4.0 | .011 |
| LVEF (%) | 68.7 ± 6.0 | 67.6 ± 5.5 | 68.8 ± 5.6 | .283 |
| PSG parameters | ||||
| AHI (events/h) | 1.4 (0.6‐2.8) | 8.3 (6.4‐11.4) | 25.8 (19.9‐37.6) | <.001 |
| ODI (events/h) | 2.3 (1.0‐4.4) | 8.4 (5.9‐11.3) | 23.5 (18.4‐36.3) | <.001 |
| Longest apnea/hypopnea time (s) | 33.5 (24.0‐51.8) | 62.5 (46.2‐79.5) | 79.3 (61.2‐97.6) | <.001 |
| Lowest SaO2 (%) | 89.0 (86.0‐90.0) | 85.0 (82.0‐88.0) | 79.0 (74.0‐83.0) | <.001 |
| Mean SaO2 (%) | 94.4 (94.0‐95.0) | 93.3 (92.9‐94.3) | 93.0 (91.8‐94.0) | <.001 |
| TST with SaO2 < 90% (%) | 0.1 (0.0‐1.6) | 1.8 (0.2‐8.3) | 8.6 (2.9‐15.5) | <.001 |
| Snoring time ratio (%) | 2.8 (0.4‐6.7) | 6.0 (1.5‐11.8) | 12.9 (4.7‐19.2) | <.001 |
| HR during sleep | 71.6 ± 9.3 | 69.9 ± 10.7 | 72.2 ± 10.0 | .281 |
| Supine time (min) | 208.0 (189.5‐305.0) | 208.0 (155.0‐259.0) | 208.0 (128.0‐252.5) | .039 |
| Total recording time (min) | 506.0 (467.0‐560.0) | 491.0 (448.0‐533.0) | 457.0 (410.5‐522.0) | .069 |
Note: Values are presented as mean ± SD, as median (interquartile range), or as n (%).
Abbreviations: AHI, apnea hypopnea index; BMI, body mass index; HCM, hypertrophic cardiomyopathy; hs‐CRP, high‐sensitivity C‐reactive protein; HR, heart rate; IVST, Interventricular septum thickness; LVOTG, left ventricular outflow tract gradient; LAD, left atrial diameter; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; PSG, polysomnography; SCD, sudden cardiac death; SaO2, oxygen saturation; TST, total sleep time.
3.2. Association of OSA severity measures with high risk hs‐CRP level
We then investigated the association between severity of OSA and high risk hs‐CRP level defined as a concentration >3 mg/L. Prevalence of patients with high risk hs‐CRP level (>3 mg/L) was significantly increased with OSA severity (P = .012, Table 1). Plasma hs‐CRP concentrations were also significantly higher in patients with moderate to severe OSA (median 1.4, IQR 0.6‐3.1 mg/L) than patients with no OSA (median 0.7, IQR 0.3‐1.3 mg/L) or with mild OSA (median 1.0, IQR 0.5‐2.1, P < .001, Table 1). Compared with patients in low risk hs‐CRP group, proportion of more severe OSA types as well as AHI values were significantly higher in high risk hs‐CRP group (P = .012 and P = .005, respectively, Figure 1). In multivariate analysis, after adjusting for age, BMI, hypertension, fasting glucose, LVEDD, IVST, and supine sleep time, AHI (odds ratio [OR], 1.024; 95% confidence interval [CI], 1.005‐1.044; P = .014), ODI (OR, 1.025; 95% CI, 1.004‐1.046; P = .018), and lowest SaO2 (OR, 0.951; 95% CI, 0.915‐0.989; P = .011) were independently associated with high risk hs‐CRP level, respectively (Table 2).
FIGURE 1.
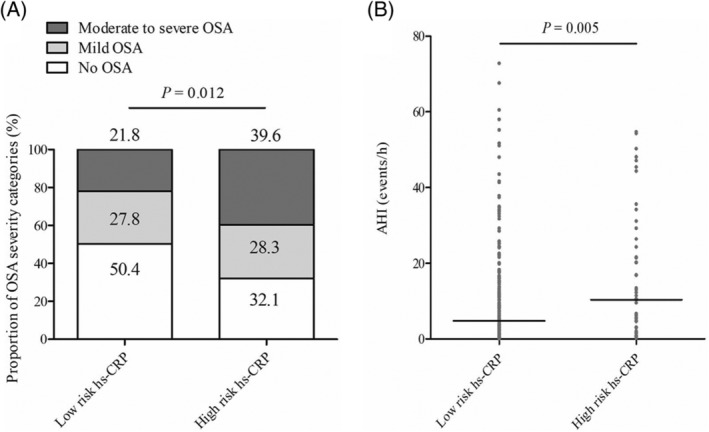
A, Prevalence of OSA in patients with low hs‐CRP level and high hs‐CRP level. B, Apnea‐hypopnea index in patients with low hs‐CRP level and high hs‐CRP level. Hs‐CRP, high‐sensitivity C‐reactive protein; OSA, obstructive sleep apnea
TABLE 2.
Univariate and multivariate logistic regression analyses to identify the association between different OSA severity measures and patients with high hs‐CRP level
| Variables | OR | 95%CI | P‐value |
|---|---|---|---|
| Univariate | |||
| Male | 1.422 | 0.744‐2.612 | .257 |
| Age (y) | 1.028 | 1.004‐1.053 | .023 |
| BMI (kg/m2) | 1.111 | 1.022‐1.208 | .014 |
| Cigarette use | 1.543 | 0.854‐2.788 | .151 |
| Hypertension | 2.348 | 1.291‐4.271 | .005 |
| Hyperlipidemia | 1.728 | 0.923‐3.236 | .087 |
| Diabetes | 2.546 | 0.984‐6.588 | .054 |
| Coronary heart disease | 2.059 | 0.930‐4.558 | .075 |
| Stroke | 1.451 | 0.293‐7.186 | .648 |
| NYHA class II‐III | 0.688 | 0.358‐1.321 | .261 |
| Familiar history of HCM | 0.619 | 0.209‐1.833 | .386 |
| Familiar history of SCD | 0.909 | 0.196‐4.225 | .903 |
| Syncope | 0.976 | 0.429‐2.220 | .953 |
| Atrial fibrillation | 1.100 | 0.481‐2.519 | .821 |
| Ventricular tachycardia | 1.074 | 0.448‐2.577 | .872 |
| Fasting blood sugar (mmol/L) | 1.353 | 1.068‐1.714 | .012 |
| Total cholesterol (mmol/L) | 1.073 | 0.793‐1.452 | .646 |
| Creatinine (mmol/L) | 1.006 | 0.988‐1.024 | .528 |
| LVOTG at rest (mm Hg) | 0.996 | 0.987‐1.005 | .390 |
| LAD (mm) | 0.990 | 0.944‐1.037 | .664 |
| LVEDD (mm) | 1.102 | 1.033‐1.177 | .003 |
| IVST (mm) | 0.932 | 0.870‐0.999 | .048 |
| LVEF (%) | 0.951 | 0.904‐1.000 | .050 |
| AHI (events/h) | 1.028 | 1.009‐1.047 | .004 |
| ODI (events/h) | 1.029 | 1.009‐1.049 | .005 |
| Longest apnea/hypopnea time (s) | 1.010 | 1.000‐1.019 | .048 |
| Lowest SaO2 (%) | 0.951 | 0.917‐0.986 | .007 |
| Mean SaO2 (%) | 0.949 | 0.806‐1.117 | .530 |
| TST with SaO2 < 90% (%) | 1.017 | 0.994‐1.040 | .145 |
| Snoring time ratio (%) | 1.021 | 0.997‐1.046 | .094 |
| HR during sleep (bpm) | 1.025 | 0.982‐1.070 | .254 |
| Supine time (min) | 0.995 | 0.992‐0.998 | .002 |
| Total recording time (min) | 0.997 | 0.994‐1.000 | .071 |
| Multivariate | |||
| AHI (events/h) | 1.024 | 1.005–1.044 | .014 |
| ODI (events/h) | 1.025 | 1.004–1.046 | .018 |
| Longest apnea/hypopnea time (s) | — | — | .202 |
| Lowest SaO2 (%) | 0.951 | 0.915–0.989 | .011 |
| Mean SaO2 (%) | — | — | .909 |
| TST with SaO2 < 90% (%) | — | — | .291 |
Note: Data are presented as odds ratio (95% confidence interval). Significant variables from univariate analysis including age, BMI, hypertension, fasting blood sugar, LVEDD, IVST, and supine time, were adjusted in multivariate analysis for different OSA severity measures, respectively.
Abbreviations: AHI, apnea hypopnea index; BMI, body mass index; CI, confidence interval; Hs‐CRP, high‐sensitivity C‐reactive protein; HCM, hypertrophic cardiomyopathy; HR, heart rate; IVST, interventricular septum thickness; LVOTG, left ventricular outflow tract gradient; LAD, left atrial diameter; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; OR, odds ratio; SCD, sudden cardiac death; SaO2, oxygen saturation; TST, total sleep time.
3.3. Linear regression analysis between OSA severity measures and hs‐CRP concentrations
Correlation between clinical characteristics and hs‐CRP concentrations were shown in Table S1. The value of hs‐CRP showed significant correlations with age (r = 0.134, P = .017), BMI (r = 0.170, P = .002), hypertension (r = 0.279, P < .001), hyperlipidemia (r = 0.203, P < .001), left ventricular end‐diastolic dimension (r = 0.144, P = .010), fasting glucose (r = 0.139, P = .013), and supine sleep time (r = −0.178, P = .001). Apnea‐hypopnea index, ODI, and longest apnea/hypopnea time were positively associated with increasing concentration of hs‐CRP, while lowest SaO2 showed inverse associations with hs‐CRP (Figure 2). In the multivariate linear analysis, decreasing lowest SaO2 (β = −.159, P = .004) was independently associated with increasing hs‐CRP concentrations after adjusting for significant variables in correlation analysis such as age, BMI, hypertension, hyperlipidemia, LVEDD, fasting glucose, and supine sleep time (Table 3).
FIGURE 2.
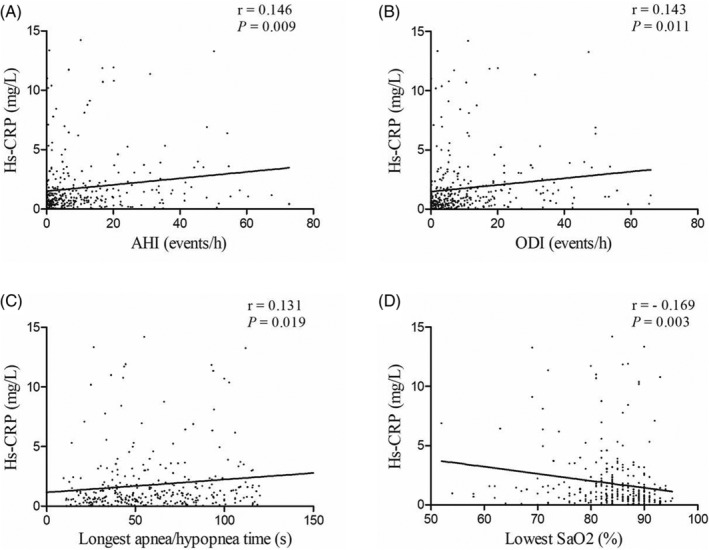
Correlation between serum levels of hs‐CRP and different severity measures of OSA. A, Correlation between hs‐CRP and AHI. B, Correlation between hs‐CRP and ODI. C, Correlation between hs‐CRP and Longest apnea/hyponea time. D, Correlation between hs‐CRP and lowest SaO2. AHI, apnea‐hypopnea index; Hs‐CRP, high‐sensitivity C‐reactive protein; OSA, obstructive sleep apnea; ODI, oxygen desaturation index; SaO2, oxygen saturation
TABLE 3.
Multivariate linear regression analysis between different OSA severity measures and hs‐CRP value adjusting for significant variables from correlation analysis
| Variables | β regression coefficients | P‐value |
|---|---|---|
| AHI (events/h) | .084 | .152 |
| ODI | .076 | .202 |
| Longest apnea/hypopnea time (s) | .078 | .181 |
| Lowest SaO2 (%) | −.159 | .004 |
| Mean SaO2 (%) | .001 | .984 |
| TST with SaO2 < 90% (%) | .069 | .220 |
Note: Significant variables from correlation analysis were adjusted in multivariate linear regression including age, BMI, hypertension, hyperlipidemia, LVEDD, fasting blood sugar, and supine time.
Abbreviations: AHI, apnea hypopnea index; BMI, body mass index; Hs‐CRP, high‐sensitivity C‐reactive protein; LVEDD, left ventricular end‐diastolic dimension; OSA, obstructive sleep apnea; ODI, oxygen desaturation index; SaO2, oxygen saturation; TST, total sleep time.
4. DISCUSSION
This study showed that OSA was highly prevalent (52.7%) in this large HOCM population. The concentrations of hs‐CRP increased with severity of OSA. OSA severity measures such as AHI, ODI, and lowest SaO2, were independently associated with high risk hs‐CRP level (>3 mg/L) after adjusting for age, BMI, hypertension, fasting glucose, LVEDD, IVST, and supine sleep time. In addition, decreasing lowest SaO2 independently correlated with increasing hs‐CRP concentrations in multivariate linear analysis.
OSA is characterized by recurrent episodes of either partial or complete upper airway obstruction during sleep, leading to episodes of interruption of respiration and intermittent hypoxia. 23 OSA is widely accepted as a risk factor for cardiovascular diseases such as hypertension, atrial fibrillation, ventricular arrhythmias, stroke, sudden cardiac death, and all‐cause mortality.1, 2 Until recently, studies showed that OSA was highly prevalent, ranging from 32% to 71%, in patients with HCM which was the most common genetic heart disease, occurring in one in 500 (0.2%) people.14, 24, 25, 26 Similarly, more than half of patients (52.7%) were diagnosed to have OSA in our study demonstrating that OSA is much common in patients with HOCM. The reason why OSA is highly prevalent in patients with HOCM is still unknown. The values of mean BMI in patients with mild OSA and moderate to severe OSA were 26.5 and 27.5 kg/m2, respectively, which indicated that obesity did not play a predominant role in propensity to OSA. It has been reported that overnight rostral fluid shift to the neck could contribute to upper airway obstruction. 27 It also has been demonstrated that even in non‐obese healthy subjects, the shift of fluid into the nuchal structures may contribute to increase neck circumference and upper airway resistance. 28 Rostral fluid shift is notable in patients with HOCM because the obstruction in left ventricular outflow tract increases left atrial and pulmonary pressure, resulting in increased blood volume and pressure in venous system, 29 which aggravate this process. 14 Therefore, overnight fluid shift could, at least in theory, play a role in the genesis of OSA among patients with HOCM. OSA was also reported to be associated with heart remodeling, atrial fibrillation, and ventricular tachycardia in HCM.15, 25 In this study, patients with HOCM and OSA were older, had a higher BMI and more clinical comorbidities which is consistent with previous studies.
Systemic inflammatory response is activated in patients with OSA because of intermittent hypoxia and reoxygenation, contributing to the cumulative burden of oxidative stress, generation of reactive oxygen species, and triggering of inflammatory cytokines. 30 C‐reactive protein, a ubiquitous protein that synthesized in the liver, is a robust biomarker of underlying systemic inflammation and is mainly regulated by inflammatory cytokines, particularly interleukin 6 (IL‐6). 31 Both increased plasma IL‐6 and CRP concentrations have been noted during hypoxic conditions. 32 CRP measured by a highly sensitive assay, namely hs‐CRP, has considerable chemical stability, requires no special precautions for sampling, and has a relatively long half‐life. 33 Thus, hs‐CRP has emerged as a leading biomarker of inflammation for clinical application. A plenty of studies had found increased levels of hs‐CRP in patients with OSA.3, 4 Additionally, hs‐CRP has also been regarded as a traditional risk factor in cardiovascular diseases. 16 Previous studies showed that hs‐CRP level >3 mg/L was independently associated with a 60% excess risk in incident cardiovascular diseases as compared with hs‐CRP level <1 mg/L. 34 Therefore, patients with hs‐CRP level >3 mg/L was considered with high risk. In this study, prevalence of patients with high risk hs‐CRP was increased with the severity of OSA. OSA severity measures such as AHI, ODI, and lowest SaO2 were independently associated with high risk hs‐CRP level. To our knowledge, this is the first study to demonstrate the association of OSA with elevated hs‐CRP levels in patients with HOCM.
It is remarkable that OSA, obstruction in upper airway, and HOCM, obstruction in LVOT, share common harmful pathways to the cardiovascular system. 14 Firstly, overstimulation of the sympathetic nervous system and subsequently elevated catecholamine levels were seen both in OSA and HOCM. 35 Secondly, large negative intrathoracic pressures generated because of increased inspiratory efforts in OSA could result in increasing left ventricular filling pressures, decreasing cardiac output, and worsening LVOT obstruction. 36 Finally, both OSA and HOCM were associated with heart remodeling, arrythmias, and sudden cardiac death.37, 38 In our study, patients with OSA had worse NYHA cardiac function class, higher prevalence of atrial fibrillation, and enlarged left ventricles indicating a high grade of cardiac remodeling. Previous studies showed that plasma value of hs‐CRP was higher in patients with HCM compared with general population. 11 Histological studies also demonstrated the infiltration of chronic inflammatory cells in the myocardium as well as the association of low‐grade inflammatory responses with myocardial fibrosis in HCM.11, 39 As hs‐CRP levels further increased with severity of OSA, we were led to speculate that elevated hs‐CRP might be a possible mechanism responsible for OSA‐related cardiovascular complications in HOCM. Future clinical trials are required to determine whether treatment of OSA could resolve inflammatory responses and improve prognosis in patients with HOCM.
This study has several limitations. Firstly, this study is a cross‐sectional study. Although our results suggested an independent association between OSA severity and hs‐CRP, the retrospective nature of this study limited our ability to determine a causal relationship. Secondly, this study was only designed to examine the hs‐CRP levels but not to other inflammatory biomarkers such as IL‐6 and tumor necrosis factor α, which have also been identified as stronger predictors of clinical events in patients with cardiovascular diseases. Thirdly, this is a single‐center study and our center is a tertiary national cardiovascular disease hospital, patients admitted to our hospital might be more symptomatic. Fourth, the values of hs‐CRP were measured at one‐time and we did not have serial measurements of hs‐CRP in this study. Finally, it is not possible to ensure that all confounding variables were fully adjusted in multivariate analysis. These facts limit the generalizability of our findings.
In conclusion, OSA is highly prevalent in patients with HOCM and hs‐CRP level increases with the severity of OSA. OSA severity measures such as AHI, ODI and lowest SaO2, were independently associated with high hs‐CRP level, a risk factor for cardiac death in this population. These results suggest that OSA should be screened for patients with HOCM and further studies are needed to evaluate the effects of treating OSA on hs‐CRP level as well as clinical outcomes for these patients over the long term.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
Supporting information
Figure S1 Study flow diagram. HOCM, hypertrophic obstructive cardiomyopathy; PSG, polysomnography.
Table S1 Correlation analysis between hs‐CRP and clinical variables.
Wang J, Xu H, Guo C, et al. Association between severity of obstructive sleep apnea and high‐sensitivity C‐reactive protein in patients with hypertrophic obstructive cardiomyopathy. Clin Cardiol. 2020;43:803–811. 10.1002/clc.23385
Juan Wang and Haobo Xu contributed equally to this work.
Contributor Information
Jiansong Yuan, Email: fwyuanjs@163.com.
Shubin Qiao, Email: dr_qiao@163.com.
REFERENCES
- 1. Lopez‐Jimenez F, Sert Kuniyoshi FH, Gami A, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. Chest. 2008;133:793‐804. [DOI] [PubMed] [Google Scholar]
- 2. Butt M, Dwivedi G, Khair O, Lip GYH. Obstructive sleep apnea and cardiovascular disease. Int J Cardiol. 2010;139:7‐16. [DOI] [PubMed] [Google Scholar]
- 3. Li K, Wei P, Qin Y, et al. Is C‐reactive protein a marker of obstructive sleep apnea? A meta‐analysis. Medicine (Baltimore). 2017;96:e6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guven SF, Turkkani MH, Ciftci B, et al. The relationship between high‐sensitivity C‐reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath. 2012;16:217‐221. [DOI] [PubMed] [Google Scholar]
- 5. Kheirandish‐Gozal L, Capdevila OS, Tauman R, Gozal D. Plasma C‐reactive protein in nonobese children with obstructive sleep apnea before and after adenotonsillectomy. J Clin Sleep Med. 2006;2:301‐304. [PMC free article] [PubMed] [Google Scholar]
- 6. Ning Y, Zhang TS, Wen WW, et al. Effects of continuous positive airway pressure on cardiovascular biomarkers in patients with obstructive sleep apnea: a meta‐analysis of randomized controlled trials. Sleep Breath. 2019;23:77‐86. [DOI] [PubMed] [Google Scholar]
- 7. Guo Y, Pan L, Ren D, Xie X. Impact of continuous positive airway pressure on C‐reactive protein in patients with obstructive sleep apnea: a meta‐analysis. Sleep Breath. 2013;17:495‐503. [DOI] [PubMed] [Google Scholar]
- 8. O'Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867‐874. [DOI] [PubMed] [Google Scholar]
- 9. Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242‐255. [DOI] [PubMed] [Google Scholar]
- 10. Gersh BJ, Maron BJ, Bonow RO, et al. ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212‐e260. [DOI] [PubMed] [Google Scholar]
- 11. Kuusisto J, Karja V, Sipola P, et al. Low‐grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart. 2012;98:1007‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu L, Zou Y, Wang Y, et al. Prognostic significance of plasma high‐sensitivity C‐reactive protein in patients with hypertrophic cardiomyopathy. J Am Heart Assoc. 2017;e004529:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dimitrow PP, Undas A, Bober M, Tracz W, Dubiel JS. Obstructive hypertrophic cardiomyopathy is associated with enhanced thrombin generation and platelet activation. Heart. 2008;94:e21. [DOI] [PubMed] [Google Scholar]
- 14. Nerbass FB, Pedrosa RP, Danzi‐Soares NJ, Drager LF, Arteaga‐Fernández E, Lorenzi‐Filho G. Obstructive sleep apnea and hypertrophic cardiomyopathy: a common and potential harmful combination. Sleep Med Rev. 2013;17:201‐206. [DOI] [PubMed] [Google Scholar]
- 15. Wang S, Cui H, Song C, et al. Obstructive sleep apnea is associated with nonsustained ventricular tachycardia in patients with hypertrophic obstructive cardiomyopathy. Heart Rhythm. 2019;16:694‐701. [DOI] [PubMed] [Google Scholar]
- 16. Yousuf O, Mohanty BD, Martin SS, et al. High‐sensitivity C‐reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013;62:397‐408. [DOI] [PubMed] [Google Scholar]
- 17. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499‐511. [DOI] [PubMed] [Google Scholar]
- 18. Santos‐Silva R, Sartori DE, Truksinas V, et al. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32:629‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sleep‐related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667‐689. [PubMed] [Google Scholar]
- 20. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:479‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440‐1463. [DOI] [PubMed] [Google Scholar]
- 22. Marwick TH, Nakatani S, Haluska B, Thomas JD, Lever HM. Provocation of latent left ventricular outflow tract gradients with amyl nitrite and exercise in hypertrophic cardiomyopathy. Am J Cardiol. 1995;75:805‐809. [DOI] [PubMed] [Google Scholar]
- 23. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature‐based analysis. Lancet Respir Med. 2019;7:687‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Konecny T, Somers VK. Sleep‐disordered breathing in hypertrophic cardiomyopathy: challenges and opportunities. Chest. 2014;146:228‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pedrosa RP, Drager LF, Genta PR, et al. Obstructive sleep apnea is common and independently associated with atrial fibrillation in patients with hypertrophic cardiomyopathy. Chest. 2010;137:1078‐1084. [DOI] [PubMed] [Google Scholar]
- 26. Konecny T, Brady PA, Orban M, et al. Interactions between sleep disordered breathing and atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2010;105:1597‐1602. [DOI] [PubMed] [Google Scholar]
- 27. Perger E, Jutant EM, Redolfi S. Targeting volume overload and overnight rostral fluid shift: a new perspective to treat sleep apnea. Sleep Med Rev. 2018;42:160‐170. [DOI] [PubMed] [Google Scholar]
- 28. Chiu KL, Ryan CM, Shiota S, et al. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006;174:1378‐1383. [DOI] [PubMed] [Google Scholar]
- 29. Covella M, Rowin EJ, Hill NS, et al. Mechanism of progressive heart failure and significance of pulmonary hypertension in obstructive hypertrophic cardiomyopathy. Circ Heart Fail. 2017;10:e003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467‐1484. [DOI] [PubMed] [Google Scholar]
- 31. Castell JV, Gomez‐Lechon MJ, David M, et al. Acute‐phase response of human hepatocytes: regulation of acute‐phase protein synthesis by interleukin‐6. Hepatology. 1990;12:1179‐1186. [DOI] [PubMed] [Google Scholar]
- 32. Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C‐reactive protein and interleukin‐6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129‐1134. [DOI] [PubMed] [Google Scholar]
- 33. Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high‐sensitivity C‐reactive protein assay. Clin Chem. 1999;45:2136‐2141. [PubMed] [Google Scholar]
- 34. Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C‐reactive protein as a risk factor for coronary heart disease: a systematic review and meta‐analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:483‐495. [DOI] [PubMed] [Google Scholar]
- 35. Limbruno U, Strata G, Zucchi R, et al. Altered autonomic cardiac control in hypertrophic cardiomyopathy. Role of outflow tract obstruction and myocardial hypertrophy. Eur Heart J. 1998;19:146‐153. [DOI] [PubMed] [Google Scholar]
- 36. Sengupta PP, Sorajja D, Eleid MF, et al. Hypertrophic obstructive cardiomyopathy and sleep‐disordered breathing: an unfavorable combination. Nat Clin Pract Cardiovasc Med. 2009;6:14‐15. [DOI] [PubMed] [Google Scholar]
- 37. Rossner S, Lagerstrand L, Persson HE, et al. The sleep apnoea syndrome in obesity: risk of sudden death. J Intern Med. 1991;230:135‐141. [DOI] [PubMed] [Google Scholar]
- 38. Elliott PM, Gimeno JR, Tome MT, et al. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J. 2006;27:1933‐1941. [DOI] [PubMed] [Google Scholar]
- 39. Allen RD, Edwards WD, Tazelaar HD, Danielson GK. Surgical pathology of subaortic septal myectomy not associated with hypertrophic cardiomyopathy: a study of 98 cases (1996‐2000). Cardiovasc Pathol. 2003;12:207‐215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Study flow diagram. HOCM, hypertrophic obstructive cardiomyopathy; PSG, polysomnography.
Table S1 Correlation analysis between hs‐CRP and clinical variables.


