Abstract
In a large, multicenter, contemporary, 8-year, cohort study, one third of allogeneic-hematopoietic cell transplant (HCT) recipients with Pseudomonas aeruginosa (PSA) infection developed a recurrent infection within 3 months. Antibiotic treatment duration of ≥14 days was the only significantly associated variable with reduced recurrence rates of PSA infections in allogeneic-HCT recipients.
Keywords: allogeneic hematopoietic cell transplant recipients, duration treatment, Pseudomonas aeruginosa, recurrent infection
In a large retrospective multicenter 8-year cohort study, Pseudomonas aeruginosa (PSA)-infections in allogeneic hematopoietic cell transplant recipients were associated with 30% recurrence rate. A minimum of 14-day antibiotic treatment was required in order to prevent recurrent PSA-infections.?
Pseudomonas aeruginosa (PSA) is the most commonly isolated pathogen in lower respiratory tract (LRT), and the third most common Gram-negative organism in bloodstream infections (BSI) during the first 3 and 6 months after allogeneic hematopoietic cell transplantation (allo-HCT), respectively [1, 2]. Furthermore, the prevalence of PSA-antimicrobial resistance has been steadily increasing [2]. In a recent European study, 38% of PSA isolates were resistant to carbapenems and 29% were multidrug resistant (MDR) [2]. These data are relevant, considering that the mortality attributable to MDR-PSA infections in patients with hematologic malignancies reaches 40% [3].
There are limited data on the management of PSA BSI and/or LRT infection (LRTI) in allo-HCT recipients. Retrospective data suggested that treatments of <14 days for PSA LRTI were associated with a higher relapse rate (6 of 19, 31.6%) compared with longer courses (4 of 40, 10%; P = .06) [4]. Although not conclusive, these data represent the only available evidence to suggest that longer treatment courses may be required in allo-HCT recipients with PSA infections. We conducted a retrospective, multicenter cohort study using the Swiss Transplant Cohort Study (STCS) to evaluate the incidence and timing of primary and recurrent PSA BSI and/or LRTI in allo-HCT recipients with focus on optimal treatment duration.
METHODS
Study Design
This was a retrospective cohort study including all adult allo-HCT recipients between January 2011 and May 2018 with available signed informed consent form participating in the STCS [5]. The study was approved by the relevant Ethical Committees.
Inclusion and Exclusion Criteria
Cases included all adult (≥18 years) allo-HCT recipients with a proven diagnosis of PSA BSI and/or LRTI and ≥6-month follow-up post-HCT. For patients who received >1 allo-HCT recipients, the latest transplant was considered. Patients were excluded if data on PSA-active treatment were not available.
Outcomes
The primary outcome was the incidence of recurrent PSA infection within the first 90-days posttreatment completion of the primary PSA infection. The following secondary outcomes were assessed: (1) time to first recurrence of PSA infection, (2) predictors of PSA infection recurrence, and (3) all-cause mortality.
Definitions
Proven PSA BSI was defined as the presence of ≥1 blood culture positive for PSA [4]. Pseudomonas aeruginosa LRTI was defined based on previously described clinical, radiological, and microbiological criteria [4]. Primary PSA infection was defined as the first documented episode of a proven PSA infection post-HCT. Recurrent infection was defined as PSA infection occurring within 90 days after completion of treatment and documented resolution of the previous episode. Patients were excluded from analyses if they died during or within 7 days after the end of treatment of their primary PSA infection. Two physicians (F.O. and D.N.) independently reviewed all cases, without any discrepancies in their assessments. Bacterial, viral, and invasive fungal infections (IFIs) were defined based on the STCS and prior consensus guidelines [5, 6].
Data Collection
The following data included in the STCS database were recorded: demographics, HCT-associated variables, and post-HCT infectious complications 1 month before and 3 months postdiagnosis of a PSA infection, including bacterial BSI and/or LRTIs, viral infections, and proven/probable IFI. Specific data on PSA infection diagnosis, disease severity, and treatment were recorded by chart review.
Statistical Analyses
A χ 2 test and Student t test were used for categorical and continuous variable comparisons, respectively. Cumulative incidence of primary PSA infection was estimated from HCT date to PSA infection among all allo-HCT recipients during the study period, whereas the cumulative incidence of recurrent PSA infection was estimated from the date of treatment completion of the primary PSA infection, censoring for death and loss to follow-up. Mortality was analyzed using the Kaplan-Meier method. A 2-sided P < .05 was considered statistically significant for all tests. Statistical analysis was performed using STATA 16.0 (StataCorp, College Station, TX).
RESULTS
Incidence of Pseudomonas aeruginosa Infections
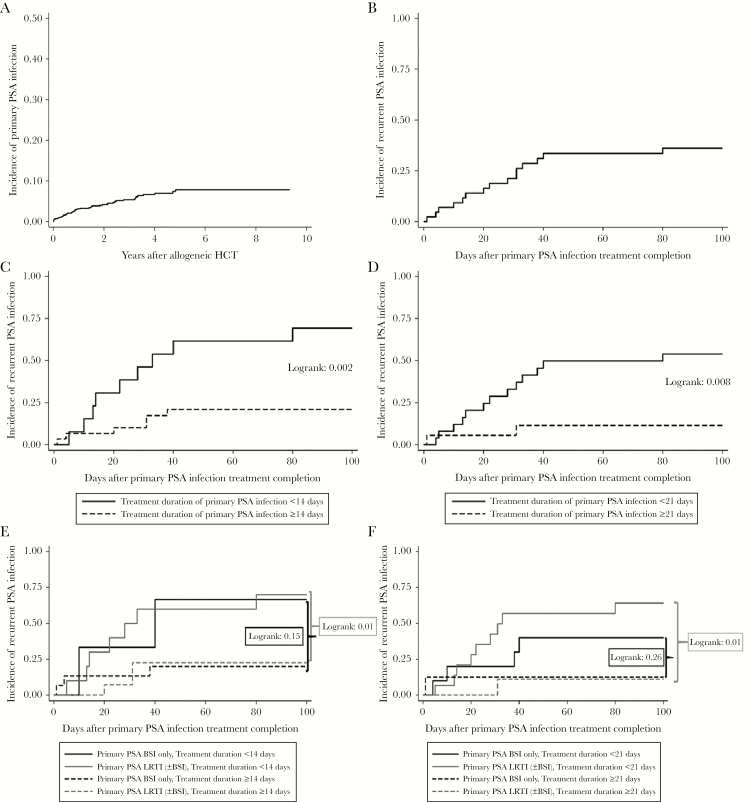
Fifty-five patients with primary PSA infections were identified among 1314 allo-HCT recipients, with a cumulative incidence of 4.2% (Figure 1a). Overall, 22 of 55 (40%), 24 of 55 (44%), and 9 of 55 (16%) patients were diagnosed with PSA BSI, LRTI, and concomitant BSI and LRTI (BSI + LRTI), respectively. The patient baseline characteristics are detailed in Supplementary Table 1. Most of available PSA isolates (30 of 48, 62.5%) were susceptible to all antibiotic classes tested. Combination antibiotic therapy with 2 agents from different classes were administered during the first 7 days of treatment in 15 of 55 (27.3%) patients. Sequential administration of different agents was observed in 36 of 55 (65.5%) patients. Median duration of antibiotic treatment for primary PSA infection was 15.5 (interquartile range [IQR], 13–29) days. There were no BSIs complicated by osteoarticular, endocarditis/endovascular, or central nervous system infections.
Figure 1.
(a) Incidence of primary Pseudomonas aeruginosa (PSA) infections in a cohort of 1314 allogeneic hematopoietic cell transplant (HCT) recipients. (b) Ninety-day incidence of recurrent PSA infections among 43 allogeneic-HCT recipients after completion of treatment of their primary PSA infection. (c) Ninety-day incidence of recurrent PSA infections based on treatment duration of primary infection for <14 vs ≥14 days. (d) Ninety-day incidence of recurrent PSA infections based on treatment duration of primary infection for <21 vs ≥21. (e) Ninety-day incidence of recurrent PSA infections based on primary PSA infection type (bloodstream infection [BSI] only vs lower respiratory tract infection [LRTI] with and without BSI) and treatment duration of primary infection for <14 vs ≥14 days. (f) Ninety-day incidence of recurrent PSA infections based on primary PSA infection type (BSI only vs LRTI with and without BSI) and treatment duration of primary infection for <21 vs ≥21 days.
A total of 12 patients who died either during the treatment of the primary PSA infection (N = 8) or within 7 days after treatment completion (N = 4) were excluded from recurrent infection analyses. Hence, for all subsequent analyses, only the remaining 43 patients were used (Supplementary Table 2). Among them, there were 14 (32.6%) patients who received monotherapy with the same agent throughout their treatment course, followed by 15 (34.9%) patients who received sequential treatment only, 12 (27.9%) patients who received both sequential and combination treatment, and 2 (4.6%) patients who received combination treatment only. Fifteen (15 of 43, 34.9%) patients developed a recurrent PSA infection (Figure 1b) at a median of 28 days (IQR, 10–33) after completion of treatment for the primary PSA infection: 9 of 15 (60%) presented a recurrence within 30 days, 5 of 15 (33.3%) between 31–60 days, and 1 of 15 (6.7%) between 61 and 90 days. Recurrent PSA infections were observed equally among patients with primary BSI (5 of 18, 27.8%), LRTI (8 of 19, 42.1%) and BSI + LRTI (2 of 6, 33.3%) (P = .65). Three (20%) patients had >1 recurrent PSA infections (Supplementary Table 3). In 13 of 15 patients, antibiograms for the primary and first recurrent PSA infection were available: 8 of 13 (61.5%) patients had identical antibiograms between the 2 episodes. In 5 of 13 (38.5%) patients, antibiograms differed: 1, 2, and 1 patients developed resistance to cephalosporins, carbapenems, and fluoroquinolones, respectively. In 1 patient, an initially fluoroquinolone-resistant isolate in the primary episode was fluoroquinolone susceptible in the recurrent PSA infection.
Risk Factors for Recurrent Pseudomonas aeruginosa Infections
The independent variables evaluated as recurrence predictors in univariable analyses are presented in Supplementary Table 4. Patients with recurrent PSA infections were more likely to have received shorter treatment courses for their primary infection (median, 13 days; IQR, 11–16) compared with those without recurrence (median, 21 days; IQR, 14–31; P = .04). Recurrent infections were observed in 6 of 30 (20%) versus 9 of 13 (69.2%) patients, who received treatment for ≥14 and <14 days, respectively (log rank = 0.002) (Figure 1c). Recurrent infections were observed in 2 of 17 (11.7%) versus 13 of 26 (50%) patients who received treatments for ≥21 and <21 days, respectively (log rank = 0.008) (Figure 1d). Longer treatment duration of the primary PSA infection (≥14 days, odds ratio [OR] = 0.11, P = .004; ≥21 days, OR = 0.11, P = .01) was the only significant factor protective against recurrent PSA infection. Considering the small number of cases and lack of other important predictors, multivariable analyses were not performed.
When the effect of treatment duration was assessed by type of primary PSA infection (BSI versus LRTI with or without BSI), recurrent infections were more likely to occur among patients with LRTI, who received treatment for <14 (7 of 10, 70%) versus ≥14 days (3 of 15, 20%) (log rank = 0.01) (Figure 1e). Likewise, recurrent infections were significantly more likely to occur among patients with PSA LRTI, who received treatment for <21 (9 of 15, 60%) versus ≥21 days (1 of 10, 10%) (log rank = 0.01) (Figure 1f).
All-Cause Mortality
All-cause mortality by day 14, 30, 60, and 90 post-PSA infection diagnosis was 10.9% (6 of 55), 12.7% (7 of 55), 18.2% (10 of 55), and 23.6% (13 of 55), respectively. All-cause mortality was higher among patients with (35 of 55, 63.6%) versus those without PSA infection (494 of 1259, 39.2%; P < .001). All-cause mortality was similar among patients with (7 of 15, 46.6%) and without (15 of 28, 53.6%) recurrent PSA infection (P = .67).
DISCUSSION
In this retrospective, multicenter, 8-year, cohort study on the incidence of recurrent PSA infections in allo-HCT recipients, we report a high rate of infection recurrence particularly among patients who received shorter than 14-day treatment courses. One in three patients with primary PSA infection had a recurrence within the first 3 months after their primary PSA infection. This is higher than the 16% recurrence rate reported by the Seattle group more than 1 decade ago [4]. The higher rates of recurrent PSA infection observed in our cohort may be, in part, due to higher risk patients and HCT practices compared with prior decades, the higher numbers of LRTI included in this cohort (60% vs 30%), and more delayed post-HCT presentation (median: 9 vs 3 months) compared with the Seattle cohort. Later diagnosis could suggest that most patients were diagnosed in an outpatient setting, with longer times to healthcare presentation and treatment initiation leading to more dismal outcomes.
Our data confirm the findings by Hakki et al [4] that treatment duration of <14 days for PSA infection may be associated with higher recurrence rates in allo-HCT recipients. Treatment for a minimum of 2 weeks decreased the risk of recurrence, with an absolute risk reduction of almost 50% and a number needed to treat of 2 patients to prevent 1 recurrent infection. This observation becomes quite pertinent, during an era when shorter treatment courses are preferred for bacterial pneumonias [7, 8]. In fact, recent data suggest that shorter (7–9 days) treatment duration may be adequate for the treatment of Gram-negative (and PSA) bacteremia [9, 10]. It is notable that only a few allo-HCT recipients were included in those studies, which did not allow researchers to make any further conclusions on treatment duration in high-risk allo-HCT recipients. Moreover, our data suggest that treatment courses as long as 3 weeks may prevent disease recurrence even further, particularly among HCT recipients with PSA LRTI (with and without bacteremia). It is likely that certain patients with PSA LRTI may have higher organism inoculum, requiring prolonged treatment duration for sufficient source control and adequate lung tissue penetration. Whether longer than 2-week treatment courses are required for patients with PSA LRTI versus BSI requires further research.
CONCLUSIONS
This study has several limitations, including its retrospective nature and limited sample size. Molecular typing was not performed; therefore, definitive evidence of infection recurrence due to the same isolate was not established. However, based on the timing of most recurrent infections within the first 2 months of the primary event and similar antibiograms in more than 60% of the cases, we believe that they represent true recurrences. In conclusion, PSA infections in allo-HCT recipients are associated with high recurrence rates, and a minimum of 14 days of targeted antibiotic treatment seems to be required to prevent recurrent infections. Further prospective studies are needed to evaluate these findings and define the optimal duration of treatment to prevent infection recurrence.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank all patients, doctors, and nurses associated with the Swiss Transplant Cohort Study (STCS).
Financial support. This study has been conducted in the framework of the Swiss Transplant Cohort Study (FUP129), supported by the Swiss National Science Foundation and the Swiss University Hospitals and transplant centers. Data quality audits are funded by the Federal Office of Public Health of Switzerland.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Lossos IS, Breuer R, Or R, et al. . Bacterial pneumonia in recipients of bone marrow transplantation. A five-year prospective study. Transplantation 1995; 60:672–8. [DOI] [PubMed] [Google Scholar]
- 2. Averbuch D, Tridello G, Hoek J, et al. . Antimicrobial resistance in Gram-negative rods causing bacteremia in hematopoietic stem cell transplant recipients: intercontinental prospective study of the Infectious Diseases Working Party of the European Bone Marrow Transplantation Group. Clin Infect Dis 2017; 65:1819–28. [DOI] [PubMed] [Google Scholar]
- 3. Trecarichi EM, Tumbarello M, Caira M, et al. . Multidrug resistant Pseudomonas aeruginosa bloodstream infection in adult patients with hematologic malignancies. Haematologica 2011; 96:e1–3; author reply e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hakki M, Limaye AP, Kim HW, et al. . Invasive Pseudomonas aeruginosa infections: high rate of recurrence and mortality after hematopoietic cell transplantation. Bone Marrow Transplant 2007; 39:687–93. [DOI] [PubMed] [Google Scholar]
- 5. Koller MT, van Delden C, Müller NJ, et al. . Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long-term follow-up cohort. Eur J Epidemiol 2013; 28:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Pauw B, Walsh TJ, Donnelly JP, et al. ; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uranga A, España PP, Bilbao A, et al. . Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med 2016; 176:1257–65. [DOI] [PubMed] [Google Scholar]
- 8. Klompas M, Li L, Menchaca JT, Gruber S. Centers for disease control and prevention epicenters program. Ultra-Short-Course antibiotics for patients with suspected ventilator-associated pneumonia but minimal and stable ventilator settings. Clin Infect Dis 2017; 64:870–6. [DOI] [PubMed] [Google Scholar]
- 9. Yahav D, Franceschini E, Koppel F, et al. ; Bacteremia Duration Study Group Seven versus 14 days of antibiotic therapy for uncomplicated Gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis 2019; 69:1091–8. [DOI] [PubMed] [Google Scholar]
- 10. Fabre V, Amoah J, Cosgrove SE, Tamma PD. Antibiotic therapy for Pseudomonas aeruginosa bloodstream infections: how long is long enough? Clin Infect Dis 2019; 69:2011–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.