Abstract
Repeated stimuli elicit attenuated responses in visual cortex relative to novel stimuli. This adaptation can be considered as a form of rapid learning and a signature of perceptual memory. Adaptation occurs not only when a stimulus is repeated immediately, but also when there is a lag in terms of time and other intervening stimuli before the repetition. But how does the visual system keep track of which stimuli are repeated, especially after long delays and many intervening stimuli? We hypothesized that the hippocampus and medial temporal lobe (MTL) support long-lag adaptation, given that this memory system can learn from single experiences, maintain information over delays, and send feedback to visual cortex. We tested this hypothesis with fMRI in an amnesic patient, LSJ, who has encephalitic damage to the MTL resulting in extensive bilateral lesions including complete hippocampal loss. We measured adaptation at varying time lags between repetitions in functionally localized visual areas that were intact in LSJ. We observed that these areas track information over a few minutes even when the hippocampus and extended parts of the MTL are unavailable. LSJ and controls were identical when attention was directed away from the repeating stimuli: adaptation occurred for lags up to three minutes, but not six minutes. However, when attention was directed toward stimuli, controls now showed an adaptation effect at six minutes but LSJ did not. These findings suggest that visual cortex can support one-shot perceptual memories lasting for several minutes but that the hippocampus and surrounding MTL structures are necessary for adaptation in visual cortex after longer delays when stimuli are task-relevant.
Keywords: repetition suppression, amnesia, medial temporal lobe, lateral occipital cortex, parahippocampal place area, fMRI
Introduction
Repeated visual stimuli elicit weaker responses in visual cortex than novel stimuli. This adaptation phenomenon (also called repetition suppression or repetition attenuation) can be considered a form of rapid learning and a signature of perceptual memory for previously viewed stimuli. Adaptation has been observed in many visual regions including the lateral occipital cortex (LOC) for repeated presentations of objects (e.g., Li et al., 1993; Grill-Spector et al., 1999; Grill-Spector & Malach, 2001; Anderson et al., 2008; Hatfield et al., 2016; Kim et al., 2009; Konen & Kastner, 2008) and in the parahippocampal place area (PPA) for repeated presentations of scenes (Epstein et al., 1999; Epstein et al., 2008; Kim et al., 2015). Adaptation occurs not only when a stimulus is repeated immediately (with no intervening stimuli), but also after a time lag of several minutes or longer during which intervening stimuli are presented (e.g., van Turennout et al., 2000; van Turennout et al., 2003; Henson et al., 2000; Henson et al., 2004; Weiner et al., 2010; Brozinsky et al., 2005). Although immediate adaptation may reflect a refractory period caused by temporary physiological changes in the local visual area being stimulated (e.g., synaptic depression; see Grill-Spector et al., 2006), the neural mechanisms underlying long-lag adaptation remain unknown.
For repetitions outside an immediate refractory period, adaptation requires more durable memories for previous stimuli. The memories could be stored in the adapting area itself (i.e., a long-term form of the local changes underlying immediate adaptation), or in other brain regions that provide feedback to the area. The first possibility is called into question by the observation that adaptation can occur for stimuli that have only been seen once before, because cortical learning processes may not support one-shot, long-term memory formation. Indeed, cortex is thought to learn long-term memories only gradually after many exposures and opportunities for consolidation (e.g., Norman & O’Reilly, 2003). Moreover, adaptation can occur after numerous, often highly similar, intervening stimuli, and these stimuli would interfere with sensory representations of the prior stimuli.
For these reasons, here we evaluate the second possibility — that long-lag adaptation in visual cortex is supported by memories stored elsewhere. We focus on the role of the medial temporal lobe (MTL), which includes the hippocampus, entorhinal cortex (ERC) that provides hippocampal input and output, and perirhinal cortex (PRC) and parahippocampal cortex (PHC) that connect ERC to broader cortical networks. We propose that the MTL memory system is a good candidate for supporting adaptation because: (a) it is positioned at the top of the ventral visual stream, with anatomical connections from and to many visual areas (Felleman & Van Essen, 1991); (b) it can learn rapidly from even a single experience (e.g., Norman & O’Reilly, 2003); (c) it can reinstate memories in visual cortex (e.g., Turk-Browne et al., 2010; Staresina et al., 2012; Bosch et al., 2014); (d) it can keep multiple similar stimuli distinct because of sparse coding and pattern separation (e.g., O’Reilly & Rudy, 2001); (e) it distinguishes between repeated and novel stimuli of different types (e.g., Brozinsky et al., 2005; Rolls et al., 1993); and (f) it has been linked to cortical adaptation for the repetition of associations (Vannini et al., 2013; Kremers et al., 2014).
We asked whether the hippocampus and surrounding MTL are necessary for long-lag adaptation, and in particular what role they play in cortical adaptation for individual stimuli. To establish necessity, we examined patient LSJ (Gregory et al., 2014; Gregory et al., 2016; Schapiro et al., 2014; Valtonen et al., 2014) who has extensive bilateral MTL lesions including complete hippocampal loss (Figure 1A). We hypothesized that if the lag between repetitions extends beyond the timescale at which local physiological changes can produce (immediate) adaptation, LSJ will differ from healthy control participants and fail to show adaptation in visual cortex due to the lack of hippocampal and MTL feedback. Alternatively, if LSJ shows similar adaptation effects as controls, it is possible that visual cortex can keep track of visual information over certain delays independent of these structures. Because it is unknown over which timescales the hippocampus and surrounding MTL are required, we varied the repetition lag across several experiments: immediate (in a block design), 30 seconds, 3 minutes, and 6 minutes (all in event-related designs).
Figure 1. LSJ and localizer stimuli.
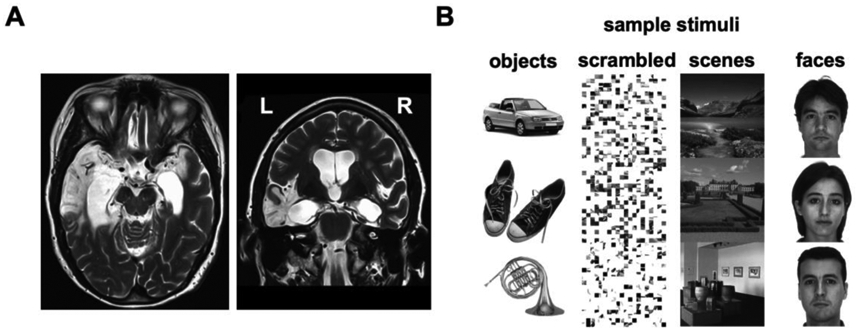
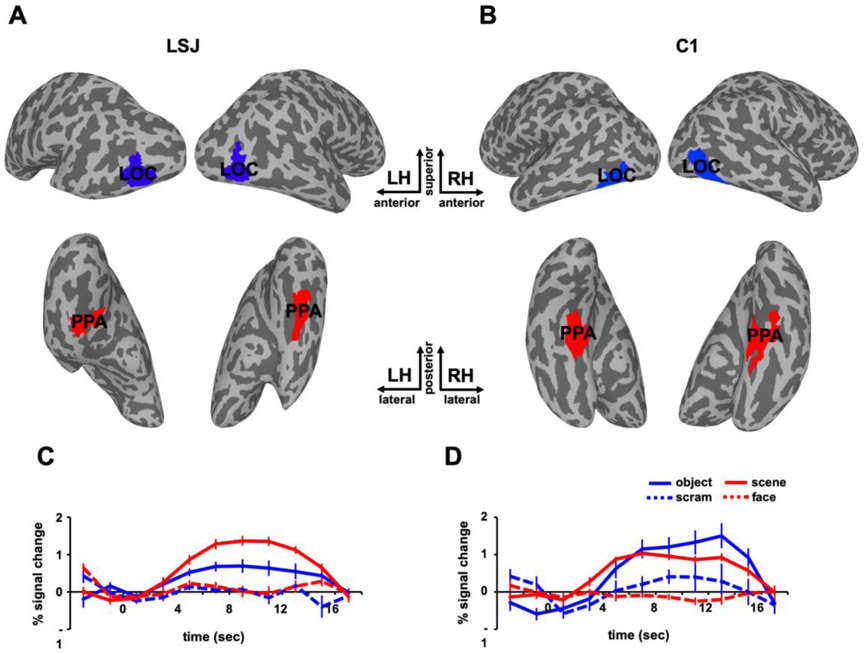
(A) T2-weighted MRI scan of LSJ’s brain reveals lesions in the bilateral medial temporal lobes (in white), extending laterally to the anterior temporal lobe especially in the left hemisphere. More than 98% of her hippocampus was destroyed bilaterally (27). See Experimental Procedures for further details on the case history. (B) Sample stimuli from the functional localizers used to define object- and scene-selective ROIs. LOC was defined by the contrast of objects vs. scrambled. PPA was defined by the contrast of scenes vs. faces.
Another factor that might affect hippocampal and MTL involvement is whether the repeated stimuli are attended and task-relevant. Goal-directed attention modulates the hippocampus and MTL (Chun & Turk-Browne, 2007; Aly & Turk-Browne, 2016b), which in turn determines what is learned (Uncapher & Rugg, 2009; Carr et al., 2013; Aly & Turk-Browne, 2016a). Moreover, attention influences adaptation in visual cortex by increasing selectivity or specificity of the neural population representing the attended stimuli (Murray & Wojciulik, 2003; Moore et al., 2013; Eger et al., 2004; Yi & Chun, 2005; see also Bar et al., 2006; Peelen & Kastner, 2014). Thus, for the longest lag (6 minutes), which we expected to be most likely to require the MTL, we ran two experiments, one with attended and one with non-attended stimuli.
Methods
Case History
LSJ is a 68 year-old (62 at the time of the first and 63 at the time of last scan session), right-handed, college-educated woman. She is a highly successful artist and amateur musician. She contracted herpes encephalitis in 2007. High-resolution anatomical MRI revealed that more than 98% of her hippocampus was destroyed bilaterally (Schapiro et al., 2014). She also has extensive damage to MTL cortex, with approximately half of the volume remaining in her right ERC, PRC, and PHC, and little to no remaining tissue in left ERC, PRC, and PHC. There is additional damage in the left lateral and anterior temporal lobe and potentially in other regions that would normally receive projections from the MTL (Figure 1A). Her medical history prior to this event was unremarkable. LSJ suffers from anterograde and retrograde amnesia and her score on the Wechsler Memory Scale’s General Memory is < 0.1%. Her basic sensory and language abilities appear to be intact. A thorough examination of LSJ’s memory functions is detailed in Gregory et al.'s study (Gregory et al., 2014; for additional reports concerning LSJ’s memory and learning abilities, see Gregory et al., 2016; Schapiro et al., 2014; Valtonen et al., 2014).
Participants
A total of 18 age-matched control subjects participated in the experiments (all right-handed, two males, mean age = 62.8 (range 56–69), no history of neurological disorder). For each experiment, there were 8 control participants. The same 8 controls participated in Experiments 1-3 and three of these controls also participated in Experiments 4 and 5. The other 10 participants, who were tested in Experiments 4 and 5, were distinct individuals. Because of the extensive nature of the experiments, we were unfortunately unable to recruit the same control participants for all experiments. But for any given lag-duration the same 8 controls participated in both the object and scene experiments. In addition to the adaptation experiments, all participants completed functional localizer and retinotopy scans, as described below. LSJ and control participants were scanned in multiple sessions at Princeton University. All participants had normal or corrected-to-normal vision and gave informed consent to a protocol approved by Princeton University’s Institutional Review Board.
fMRI Acquisition and Preprocessing
Participants were scanned in multiple scan sessions with a Siemens Skyra 3T scanner. During each session, high-resolution T1-weighted anatomical scans using the MPRAGE sequence were obtained with the following parameters: TR = 2.3s, TE = 1.97ms, flip angle = 9°, matrix = 256 x 256, resolution = 1mm isotropic, slices = 176. These anatomical scans were used to align the functional data across sessions. The fMRI scans were acquired with a T2*-weighted echo planar imaging sequence: retinotopy scans, TR = 2.5s, TE = 30ms, flip angle = 75°, matrix = 64 x 64, resolution = 3mm isotropic, slices = 39; functional localizers and adaptation experiments, TR = 2s, TE = 30ms, flip angle = 72°, matrix = 64 x 64, resolution = 3mm isotropic, slices = 36.
The functional scans were preprocessed in AFNI (http://afni.nimh.nih.gov/afni), including de-spiking, slice time correction, motion correction, and de-trending. Although LSJ had significantly more head motion during some of the experiments than compared to controls, the amount of head motion that LSJ exhibited did not differ reliably across different experiments. See Figure S4 for LSJ’s motion parameters for all experiments and for comparison of LSJ and controls’ motion parameters. Thus, any differences between LSJ and controls cannot be merely attributed to differences in head motion during scanning.
Data were smoothed with a 4mm full-width half-maximum Gaussian kernel and normalized to percent signal change by dividing the time series by its mean intensity. All functional scans were co-registered to each session’s anatomical scan. FreeSurfer (http://surfer.nmr.mgh.harvard.edu) and SUMA (http://afni.nimh.nih.gov/afni/suma) were used to make inflated and flat cortical surface reconstructions. After preprocessing, the retinotopy and localizer runs were projected onto the inflated brains and voxels that fell within the gray matter boundary were used to define regions-of-interest (ROIs).
ROI Localization and Retinotopic Mapping
Functional localizers were used to define LOC, PPA, transverse occipital sulcus, (TOS), retrosplenial cortex (RSC) and fusiform face area (FFA) in each participant. In alternating blocks of trials, LSJ and age-matched controls passively viewed series of either objects vs. scrambled objects, or scenes vs. faces. LOC was defined based on the contrast of greater blood oxygen level-dependent (BOLD) responses to objects than scrambled objects. PPA, TOS and RSC, were defined based on the contrast of greater BOLD responses to scenes than faces. As control regions, retinotopic early visual areas (V1-V4) and the face-selective FFA were defined for each participant. Retinotopic areas were defined using a standard topographic mapping procedure from a separate scanning session (Kim et al., 2015; Bandettini et al., 1993), and FFA was defined based on the contrast faces vs. scenes.
The ROIs were defined using a thresholded t-map of p < .05, Bonferroni corrected. See Figure 1B for sample stimuli. Each localizer run (2.6 min) began and ended with 8 seconds of fixation. There were eight 12-s blocks in each run, each separated by 6 s of fixation. Each block consisted of 12 different images, randomly selected without replacement from a total of 40 images per category. Stimuli subtended 11° and were presented for 500 ms with an inter-stimulus interval (ISI) of 500 ms. Each participant was scanned in two object localizer runs and two scene/face localizer runs.
Retinotopic areas V1, V2, V3, V4 were defined in each participant across four runs. Each run started with a 10 s fixation period followed by 5 cycles of a wedge that rotated around a central fixation (32 s for a full rotation). The wedge spanned 1-13.5° in eccentricity with an arc length of 45° and was filled with 1000 dots (0.1°, 65 cd/m2) moving in random direction at a rate of 7°/s. The wedge rotated either clockwise or counter-clockwise in alternating runs. To delineate visual areas a Fourier analysis was used where the amplitude and phase of the harmonic at the stimulus frequency was determined. The statistical threshold used to delineate ROIs was p < 0.001, uncorrected, derived from the F ratio of the Fourier transform. Similar phase encoding parameters and procedures were used previously (Kim et al., 2015; Bandettini et al., 1993; Silver & Kastner, 2009) and the details of the statistical analyses were reported previously (Schneider et al., 2004; Arcaro et al., 2009).
Adaptation Experiments
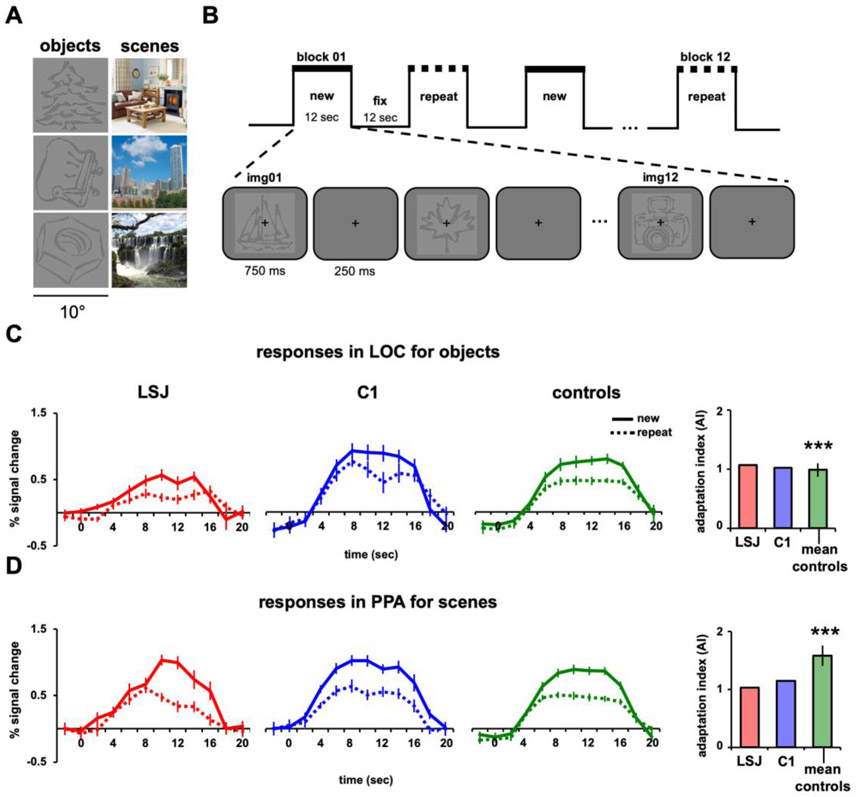
A large set of line drawing objects and colored scene photographs were used for the adaptation experiments. Different sets of object and scene stimuli were used for each experiment and stimuli used for the functional localizers and adaptation experiments did not overlap. Examples are shown in Figure 3A.
Figure 3. Block design and immediate repetition.
(A) Example line-drawing objects and scenes used as stimuli. (B) Blocks alternated between “new” (12 different stimuli) and “repeat” (1 stimulus repeated 12 times) conditions, with order counterbalanced across runs. Objects and scenes were shown in separate runs. BOLD time courses and AIs for LSJ (red), a representative control C1 (blue), and the average of all controls (green) for (C) objects in LOC and (D) scenes in PPA. The asterisks above the control average denote significant adaptation effects from zero using a one-sample t-test (*** p < 0.001). Error bars in the line graphs for LSJ and C1 denote standard errors across blocks of trials. The error bars for average controls (for this and subsequent figures) denote standard errors across subjects. Using the Crawford and Howell’s t-test for case-control comparisons (38), LSJ’s AIs did not reliably differ from control AIs for both LOC and PPA.
Immediate Aadaptation (Experiment 1)
Every participant was scanned in six object and six scene runs. Each run started with 8 s of fixation followed by six blocks each lasting 12 s followed by 12 s of fixation, for a total of 2.5 mins (Figures 3B). The new and repeat blocks were presented in an alternating fashion. During the new block, 12 distinct objects or scenes were presented. In the repeat block, one object or scene was repeated 12 times. Each image subtended 10° and was presented centrally for 750 ms with an inter-trial-interval of 1s. Squarewave functions matching the timecourse of the design were convolved and regressors for each timepoints for each block were used in a multiple regression model. Additional nuisance regressors included motion parameters, linear drifts within runs, and shifts between runs. The resulting beta values of this regression model were used for the timecourse analyses. To quantify adaptation effects, an adaptation index (AI) was computed for each ROI and participant using similar methods previously published (Kim et al., 2015; Pinsk et al., 2009):
where PeakNew and PeakRepeat are defined as the average response over the timepoints at the peak of the hemodynamic response (8-16 s after block onset) for new and repeat blocks, respectively, and σ2New and σ2RePeat are the variance of these peak responses across new and repeat blocks, respectively. AIs greater than 0 indicate greater responses for new vs. repeat blocks.
Long-Lag Adaptation (Experiments 2-5)
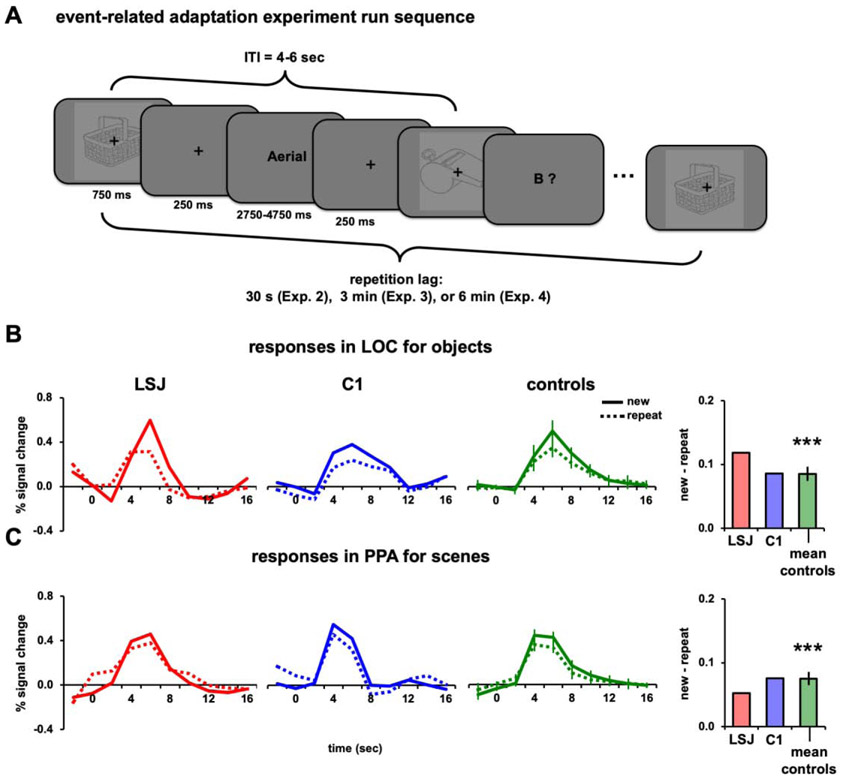
The long-lag adaptation experiments were conducted using a rapid event-related design. Each run started with 8 s of fixation period followed by 36 trials, and ended with 6 s of fixation. During each trial, a stimulus was presented centrally with a fixation cross for 750 ms followed by 250 ms of fixation (Figure 4A). The ITI was 4 s for two-thirds of the trials and 6 s for the remaining one-third of trials, randomized across runs for each subject. During the fixation period of each trial of Experiments 2-4, either a word or a letter prompt (e.g., “A?”) for the alphabet game (described below) was presented for 3 s. There were eight runs for each for the object and scene conditions within every experiment. Repetitions occurred at different lags across experiments: Experiment 2, 30 s (range 2-10 intervening stimuli); Experiment 3, 3 mins (range 36-54 intervening stimuli); Experiment 4 and 5, 6 mins (range 56-70 intervening stimuli). There were 144 new trials and 144 repeat trials for each experiment. Across different experiments, different sets of objects and scenes were used because some of the subjects participated in more than one experiment. BOLD responses were estimated within ROIs using a general linear model with stimulus events and motion parameters from the preprocessing. Stimulus events were modeled with a series of finite impulse response functions, one regressor for each of ten 2-s timepoints. The beta values were converted to percent signal change and averaged over the time period of the peak hemodynamic response (4-8 s after stimulus onset). The difference between the average peak response for the new minus repeat conditions was used to compute AIs for the event-related designs. Since all trials for each condition were used to model the hemodynamic response functions for the new and repeat conditions, these AI computations differed from the AI quantification for the block design experiment, which took into consideration the peak responses for each condition as proportion to the variance across blocks.
Figure 4. Event-related design and 30-s lag repetition.
(A) Example sequence for the lagged adaptation experiments with rapid-event related design. Each stimulus was repeated just once, after 30 s (Experiment 2), 3 mins (Experiment 3), or 6 mins (Experiment 4) on average. Participants performed a demanding alphabet task interleaved with the stimuli, in which the computer generated a word starting with one letter of the alphabet and then the participant generated a word covertly starting with the next letter. BOLD timecourses and AIs for repetitions after a 30-s lag of (B) objects in LOC and (C) scenes in PPA. For calculating AIs, peak responses were defined as the average BOLD response 4-8 s post-stimulus.
Behavioral Tasks
FMRI runs were designed to be shorter than usual — i.e., no longer than 3 mins, estimated as LSJ’s attention span based on observations and verbal reports from LSJ’s family. LSJ and control participants engaged in a perceptual preference task during the experiments with block designs (i.e., functional localizer and Experiment 1), which encouraged participants to focus on the visual stimuli being presented. After each block of trials, participants were prompted with: “Press a button if you liked what you saw.” To ensure that LSJ did not forget the task during stimulus presentation, task instructions were also shown on the screen during the fixation period before each block: “Now you are going to see some [objects/textures/scenes/faces]. Pay attention!”.
Although this task is subjective in nature, results suggest that LSJ and controls mostly liked the stimuli being presented and responded after most of the blocks. For the localizer runs, controls reported to like the stimuli on 78.1% of the object blocks and 92.2% of the scene blocks. LSJ’s behavioral performance was similar to that of controls (87.5% for both object and scene blocks) and did not differ significantly (t17 = 0.39, p = 0.70 and t17 = −0.29, p = 0.77, respectively). Similarly, for Experiment 1, LSJ (83.3% for objects and 94.4% for scenes) and controls (88.9% for objects and 91.7% for scenes) responded that they liked most of the blocks presented and LSJ’s preference scores did not differ reliably from controls (t7 = −1.31, p = 0.23 and t7 = 0.28, p = 0.76, respectively).
While preparing for the study the authors met with LSJ, her family members, and other researchers to assess what kinds of tasks LSJ would be able and motivated to perform, given her limited memory span. On many occasions, we observed that LSJ likes to play an alphabet game where she and another player (e.g., her sister) take turns going through the alphabet and generating a unique word for each letter. With the help of the alphabet game, which provides sequential structure, we observed that LSJ can maintain a fluid conversation for several minutes. Given these observations, we designed a virtual alphabet game between the computer and LSJ that was administered during fMRI. This task encouraged LSJ and control participants to fixate the centrally presented letters and stay engaged during retinotopy and most event-related designs (Experiments 2-4). LSJ and the computer went through the alphabet letter-by-letter, taking turns generating words that begin with the prompted letter. For example, the computer might start by generating a word that starts with an “A” (e.g., “Admire”) and this word is shown at fixation. Then, “B?” would be displayed at fixation and participants needed to generate a word that starts with the letter B.
The words generated on the computer’s turns consisted of mostly non-object words (e.g., verbs and abstract nouns) to avoid interfering with the object and scene stimuli as much as possible and they were not repeated across runs or experiments. When object names were used, we made sure they did not overlap with the object photographs. Letters and words were displayed on the screen every 4 s. When generating words, participants were instructed to only think of the word and not to say the word out loud, to reduce the possibility of head motion and artifacts related to producing speech. This task allowed us to examine long-lag adaptation when objects/scenes were task-irrelevant.
In Experiment 5, participants performed a go/no-go categorization task on the objects/scenes, to evaluate the role of task relevance. For objects, participants were instructed to press a button if the presented object was a natural object (e.g., plants, animals) and to withhold a response if the object was artificial. For scenes, participants were instructed to press a button if the presented scene was an indoor scene (e.g., kitchen, office) and to withhold a response if the scene was outdoor. Equal numbers of natural and artificial objects and indoor and outdoor scenes were used. All trials were included in the fMRI analysis because of high accuracy rates in LSJ and the controls.
Single Case Study Statistics
The statistical comparison of LSJ to the control group was done with a modified independent samples two-tailed t-test (Crawford & Howell, 1998), which accounts for the limited size of the control group and tests the null hypothesis that the single case comes from a population of controls. This method has been widely used in neuropsychological case studies (Kim et al., 2015; Behrmann et al., 2006; Konen et al., 2011) and has advantages over other single case statistics such as the modified ANOVA or z-score inferences (Crawford et al., 2004). As a visual benchmark, we compared LSJ’s results to a representative control participant C1 for each experiment. We confirmed that C1 was representative by comparing their data (e.g., size of ROIs, amplitude of adaptation index) to the rest of the control group for each experiment. None of the tests resulted in a significant difference (all ps > 0.05).
Results
Visual Selectivity
To examine adaptation effects in visual cortex, we used functional localizers to define ROIs for object-selective LOC and scene-selective PPA in each participant. Figure 2 depicts the locations and BOLD time courses of LOC and PPA for LSJ and a representative control participant C1 for visualization purposes. The statistical comparison of LSJ to the control group for this and subsequent analyses was done with a modified independent samples two-tailed t-test (Crawford & Howell, 1998), which accounts for the limited size of the control group and tests the null hypothesis that the single case (LSJ) comes from a population of controls (see Experimental Procedures). Because ROIs were functionally localized in every subject, the comparisons of ROIs between LSJ and controls below are collapsed across experiments. The sizes of LOC and PPA did not differ reliably between LSJ and controls, (t17 = −1.32, p = 0.22 and t17 = −1.36, p = 0.20, respectively). The sizes of other visual ROIs also did not differ between LSJ and controls (V1: t17 = −0.27, p = 0.79; V2: t17 = 1.31, p = 0.22; V3: t17 = 0.77, p = 0.50; V4: t17 = 0.67, p = 0.52; TOS: t17 = −0.03, p = 0.98; RSC: t17 = −1.64, p = 0.13; FFA: t17 = −0.97, p = 0.35). Despite extensive MTL damage, LSJ showed intact object selectivity in LOC and scene selectivity in PPA and none of the functionally localized ROIs differed in size to that of controls. These results suggest that MTL is not necessary for the basic function and organization of category-selective and retinotopic visual cortex.
Figure 2. Localizer results.
Object-selective LOC (blue) and scene-selective PPA (red) are superimposed on the inflated brains of LSJ (A) and a representative control participant C1 (B). The BOLD time courses in the localizer from (C) LSJ’s and (D) C1’s LOC and PPA are shown for the sake of visualization. The error bars in this figure reflect standard errors across different blocks of trials.
Immediate Adaptation
In Experiment 1, we used a block design to examine adaptation for immediate stimulus repetitions (Figures 3B). Previous studies in healthy adults (e.g., Grill-Spector et al., 1999; Konen & Kastner, 2008; Epstein et al., 1999) have shown robust adaptation in LOC and PPA when comparing blocks that contain, respectively, one object or scene presented repeatedly (“repeat”) with blocks of the same total number of stimuli but with each stimulus being novel (“new”). We hypothesized that such short-lag adaptation is mediated by the visual system and thus does not depend on the hippocampus and MTL. Consequently, we expected that both LSJ and controls would show adaptation effects in this paradigm. This first experiment was an important step to establish that the adaptation paradigm was a feasible way to test LSJ in an fMRI study, given the difficulties of working with severely amnesic patients in such an environment.
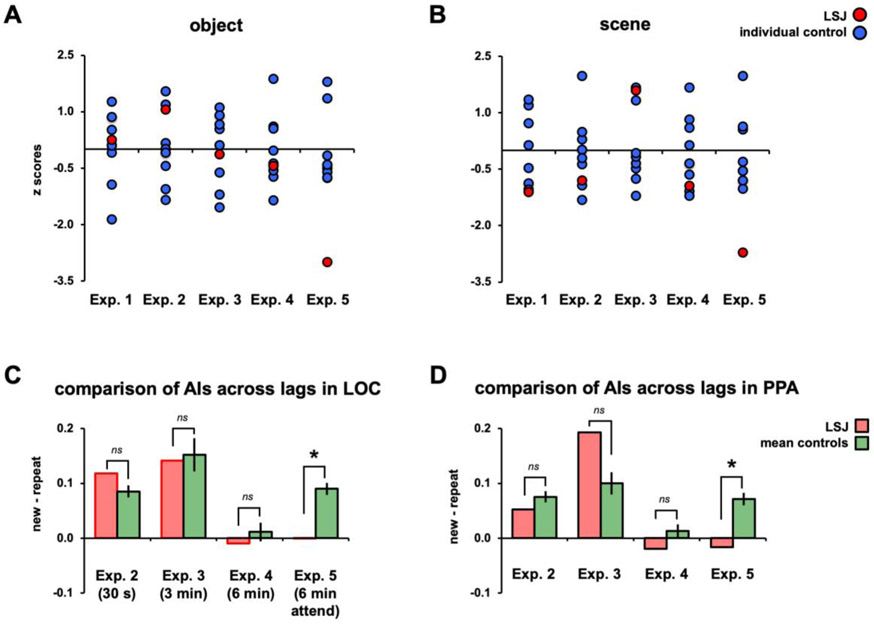
Figures 3C and 3D show the BOLD time courses in LOC for objects and PPA for scenes, respectively, from LSJ, C1, and averaged controls (n = 8). As expected, control participants showed greater BOLD responses to the new vs. repeat blocks. To quantify these effects, we computed, for each ROI in each participant, an adaptation index (AI): the difference in peak responses to the new and repeat conditions as a proportion of the variance across blocks from the combined conditions (see Experimental Procedures). AI values greater than zero denote greater BOLD responses for the new than repeat conditions. Controls had AIs reliably greater than zero for objects in LOC (t7 = 8.58, p < 0.0001) and scenes in PPA (t7 = 8.90, p < 0.0001). LSJ also showed positive AIs for both objects in LOC and scenes in PPA. Furthermore, LSJ’s AI for LOC (t7 = 0.21, p = 0.84) and PPA (t7 = −1.04, p = 0.33) did not differ reliably from controls (see Figures 8A-8B to compare LSJ’s AI values against individual controls). See Figure S1 and Tables S1-S2 for adaptation effects in other ROIs (for this and subsequent Exps.). To examine whether or not LSJ’s adaptation effects are expected by chance, we performed a permutation test to compute the null distribution of AIs by randomizing the condition labels and computing AIs across 1000 iterations (see Figure S2). This analysis showed that LSJ’s AIs for LOC and PPA (for this and subsequent Exps. where LSJ showed adaptation effects) was not due to chance and well below the 5% tail of the permutation distribution.
Figure 8. Summary across experiments.
Normalized (z-scores against mean and SD from controls) AIs from Experiments 1-5 are plotted for LSJ (in red) and individual controls (in blue) for objects (A) and scenes (B). For Experiments 1-4, LSJ’s AIs are within the distribution of controls’ AIs. In Experiment 5, for both object and scene, LSJ’s AI values are about 3 SDs below the mean of controls. Mean control AIs from Experiments 2-5 are re-plotted (in green) against LSJ’s AIs (in red) for objects in LOC (C) and scenes in PPA (D).
Neither controls nor LSJ showed hemispheric differences in AIs for this and subsequent experiments (see Figure S3 and Tables S3-S4). Consistent with our hypothesis, these results suggest that the hippocampus and MTL are not necessary for immediate adaptation effects in ventral visual cortex. It also demonstrated the feasibility to test the patient in this type of study.
Long-Lag Adaptation: Task-Irrelevant Stimuli
In Experiment 1, at the boundary condition of immediate repetitions (i.e., zero lag), it appeared that local physiological changes within visual cortex were sufficient for adaptation, or at least that such adaptation could occur independently of the hippocampus and MTL. However, these physiological changes may dissipate some period of time after stimulus presentation, which might correspond to the lag at which longer-term memories are required and adaptation would become MTL dependent. As we did not know a priori at which timescale this might happen, we manipulated lag parametrically across the remaining experiments.
30-s Lag
Experiment 2 used a rapid event-related design in which each stimulus was repeated once at a lag of approximately 30 s or 6 intervening stimuli (Figure 4A). We again examined BOLD responses to objects in LOC and scenes in PPA (in separate runs). While passively viewing the stimuli, participants engaged in an “alphabet game” at the center of the screen that required them to think of words that began with a cued letter (see Experimental Procedures). This task was chosen because it is one of LSJ’s favorite activities and it was intended to reduce attention to the object and scene stimuli, as they were task-irrelevant and presented in the background of the letters.
Figures 4B and 4C show the BOLD time courses and AIs for LSJ, C1, and the control group, averaged for object presentations in LOC and scene presentations in PPA. The AI quantification for this experiment (and all subsequent event-related experiments) was the difference between the average peak responses (time points 4-8 s post stimulus onset) for the new and repeat conditions (see Experimental Procedures). The controls’ AIs for objects in LOC were reliably positive (t7 = 7.76, p < 0.001), indicating adaptation approximately 30 s after first exposure to a stimulus. LSJ also showed a positive AI, and it did not differ from controls (t7 = 1.00, p = 0.35; see Figure 8A to compare LSJ’s AI values against individual controls). Likewise, the controls’ AIs for scenes in PPA were reliably positive (t7 = 7.34, p < 0.001; see Figure 8B to compare LSJ’s AI values against individual controls). LSJ also showed a positive AI, which again did not differ from controls (t7 = −0.74, p = 0.48).
Similar to immediate adaptation, both controls and LSJ showed reliable adaptation in LOC and PPA, suggesting that the hippocampus and MTL are not necessary to bridge across repetitions spaced by 30 s and that cortex keeps track of visual input over this period of time. It is notable that these effects occurred despite the fact that the stimuli were not task-relevant and attention was drawn to a different task, suggesting a highly automated tracking of stimuli by the visual system across this time interval.
3-min Lag
Having observed reliable adaptation in both LSJ and controls at a 30-s lag, in Experiment 3, we increased the lag to approximately 3 mins or 42 intervening stimuli. Anecdotally, LSJ seems to have a shorter time window of memory than this (e.g., she repeats questions, and forgets having done tasks, after 1-2 mins), and so this lag seemed like a reasonable timescale for possibly observing engagement of the hippocampus and MTL. Everything else was the same as in Experiment 2, except that the longer lag meant that the initial and repeated presentations of each stimulus occurred in different runs. The stimuli were shown in the background while LSJ and controls engaged in the alphabet game at the center of the screen.
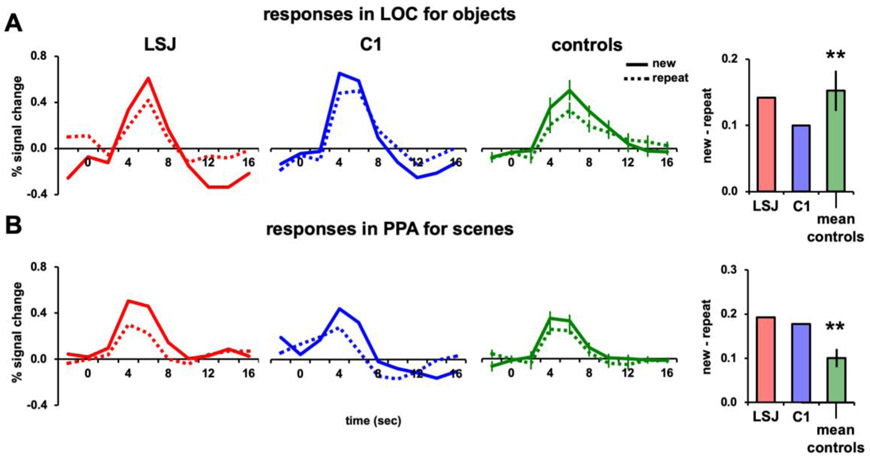
Figures 5A and 5B show the BOLD time courses and AIs for LSJ, C1, and the control average for objects in LOC and scenes in PPA. The control AIs for objects in LOC were again reliably positive (t7 = 5.04, p < 0.002) and not different from LSJ’s AI (t7 = −0.12, p = 0.91; see Figure 8A to compare LSJ’s AI values against individual controls). Likewise, the control AIs for scenes in PPA were again reliably positive (t7 = 4.89, p < 0.002) and not different from LSJ’s AI (t7 = 1.51, p = 0.18; see Figure 8B to compare LSJ’s AI values against individual controls). Thus, the pattern of results for a 3-min lag was very similar to that for the 30-s lag.
Figure 5. 3-min lag repetition.
BOLD timecourses and AIs for repetitions after a 3-min lag of (A) objects in LOC and (B) scenes in PPA (** p < 0.01).
6-min Lag
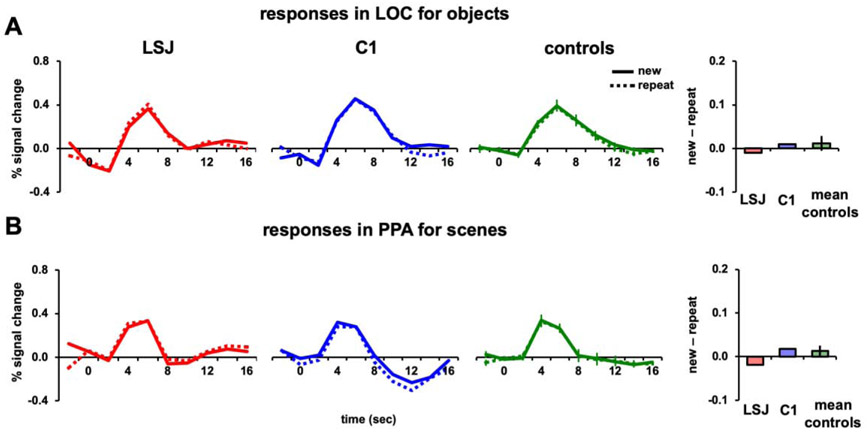
Given that increasing the lag from 30 s to 3 mins did not impact the results, in Experiment 4 we doubled the lag to approximately 6 mins or 68 intervening stimuli.
Figures 6A and 6B show the BOLD time courses and AIs for LSJ, C1, and the control average for objects in LOC and scenes in PPA, respectively. In contrast to the prior experiments, the increased lag eliminated the long-lag adaptation effect observed previously. The control AIs did not reliably differ from 0 for either objects in LOC (t7 = 0.67, p = 0.52) or scenes in PPA (t7 = 1.06, p = 0.33). Using an independent samples t-test, the AIs for the 6-min lag were reliably weaker than for the 30-s lag in both LOC and PPA (t14 = 3.66, p = 0.003 and t14 = 3.91, p = 0.002, respectively) and compared to the 3-min lag (t14 = 4.06, p = 0.001 and t14 = 3.66, p = 0.003, respectively). LSJ was not different from controls for either objects in LOC (t7 = −0.41, p = 0.69) or scenes in PPA (t7 = −0.88, p = 0.41; see Figures 8A-8B to compare LSJ’s AI values against individual controls).
Figure 6. 6-min lag repetition.
BOLD timecourses and AIs for repetitions after a 6-min lag of (A) objects in LOC and (B) scenes in PPA.
Although LSJ no longer showed adaptation, the lack of adaptation in controls prevents us from concluding based on this experiment that the hippocampus and MTL are necessary. Instead, it appears that the automatic tracking of stimulus information performed by the visual system occurs on the order of a few minutes, but reaches a limit within a 3-6 minute time frame.
Long-Lag Adaptation: Task-Relevant Stimuli
Why did we not observe adaptation in controls after a 6-min lag, despite the fact that previous studies (van Turennout et al., 2000; van Turennout et al., 2003; Meister et al., 2005) have found such effects at even longer lags? One difference is that these prior studies employed behavioral tasks that made the stimuli task- relevant, requiring participants to attend to them. For instance, participants might be instructed to name objects (e.g., Meister et al., 2005; van Turennout et al., 2000; van Turennout et al., 2003) or perform a categorization task such as deciding whether an object is bigger or smaller than a shoebox (Henson et al., 2004; Koutstaal et al., 2001; Dobbins et al., 2004). In contrast, Experiments 2 - 4 used a task that was orthogonal to the repeating stimuli; the parallel task rendered the visual stimuli task-irrelevant and drew attention away from them. Consistent with the importance of stimuli being task-relevant, selective attention has been shown to modulate long-lag adaptation (Moore et al., 2013; Yi & Chun, 2005; Turk-Browne et al., 2006; Vuilleumier et al., 2006).
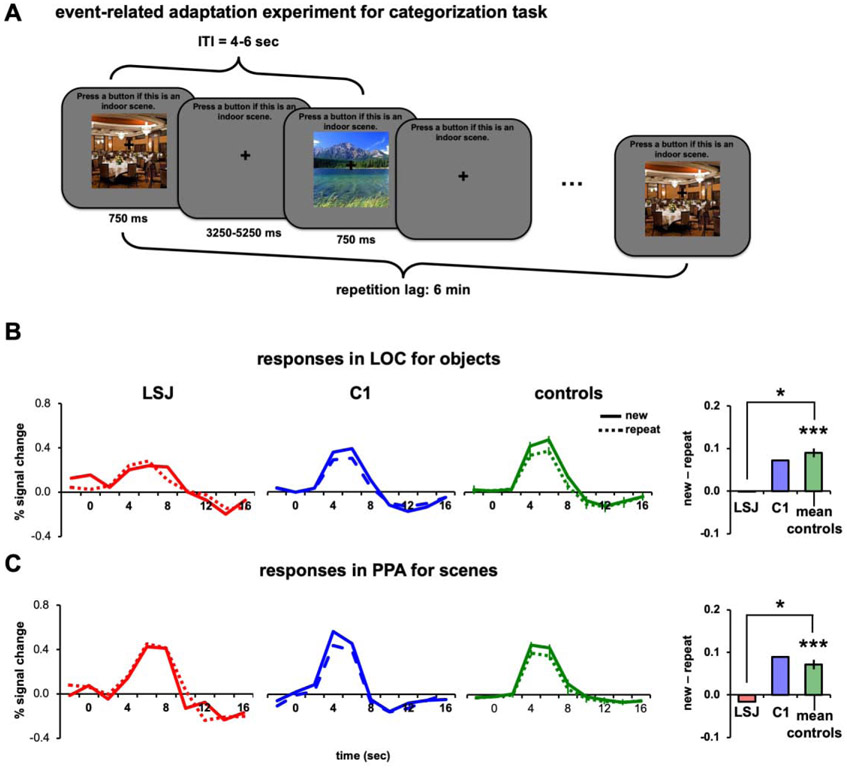
To examine the role of task relevance in long-lag adaptation as well as possible interactions with hippocampus and surrounding MTL, in Experiment 5 we asked participants to perform a go/no-go categorization on the stimuli (Figure 7A). All other experimental parameters were identical to Experiment 4 (e.g., lag was 6 minutes). For objects, participants were instructed to press a button if the presented object was a natural object; and for scenes, if the presented scene was an indoor scene. To help LSJ remember the task, a task prompt (“Press a button if this is a natural object” for objects and “Press a button if this is an indoor scene” for scenes) was shown on top of the screen throughout the experimental runs for all subjects.
Figure 7. Task-relevant design and 6-min lag repetition.
(A) Example trial sequence using an event-related design with a categorization task. For scenes (shown here), participants were instructed to press a button if the presented scene was an indoor scene. For objects, participants were instructed to press a button if the presented object was a natural object. The task prompt was shown on the top of the screen throughout the experiment to minimize the memory demands of the task on LSJ. BOLD time courses and AIs for LSJ, C1, and the average of all controls for (B) objects in LOC and (C) scenes in PPA. (* p < 0.05).
In contrast to Experiment 4 controls showed reliable adaptation effects in LOC for objects (t7 = 8.38, p < 0.0001; Figure 7B) and PPA for scenes (t7 = 6.26, p < 0.001; Figure 7C). Strikingly, LSJ did not show any adaptation, with AIs near 0 and lower than all controls for objects in LOC (t7 = −2.83, p < 0.03) and scenes in PPA (t7 = −2.56, p < 0.04; see Figure 8A-8B to compare LSJ’s AI values against individual controls). These findings suggest that the hippocampus and MTL are necessary for tracking stimuli over a time period of 6 minutes after initial presentation when the repeating stimuli are task-relevant. The comparison of AIs across experiments for controls and LSJ are summarized in Figures 8C and 8D.
Behavioral accuracy in the tasks was high for both LSJ (88.6% for objects and 89.7% for scenes) and controls (mean = 94.6% for objects and 94.2% for scenes), and LSJ’s accuracies did not differ reliably from those of controls (t7 = −1.19, p = 0.27 for objects and t7 = −1.06, p = 0.33 for scenes). Response times (RTs) did differ between LSJ and controls for objects (789 vs. mean of 593 ms, respectively; t7 = 2.96, p = 0.02), but not for scenes (687vs. mean of 610 ms, respectively; t7 = 1.20, p = 0.27).
Making the stimuli task relevant also let us examine response priming in behavior by comparing RTs for the first vs. second exposure to each stimulus. Controls were reliably faster for the second than the first exposure of objects (mean difference = 43.8 ms, t7 = 3.90, p < 0.006) and marginally faster for scenes (mean difference = 10.9 ms, t7 = 2.03, p < 0.08). LSJ showed a numerical benefit for second vs. first exposures (8.1 ms for objects and 10.9 ms for scenes), and these differences were not reliably different from controls (t7 = −1.06, p = 0.32 and t7 = −0.32, p = .76, respectively). Controls did not exhibit greater accuracy on the categorization tasks for the second vs. first exposure of objects (mean difference = −0.44%, t7 = 0.91, p = .39) or scenes (mean difference = 0.21%, t7 = −0.60, p = 0.56). LSJ showed similar accuracy results (difference of 0.41% for objects, t7 = 0.58, p = 0.57 and 0.22% for scenes, t7 < 0.01, p > 0.99).
Together, these results suggest that LSJ was able to perform the behavioral tasks. The only reliable difference from controls was the overall RT in the object task, and thus any adaptation effects that hold for both objects and scenes cannot be fully explained by differences in task performance or difficulty. The behavioral priming for controls and LSJ is consistent with prior studies showing preserved long-lasting behavioral priming in hippocampal amnesia (Warrington & Weiskrantz, 1974; Gabrieli et al., 1990; Cave & Squire, 1992). Moreover, the presence of this behavioral priming effect does not entail that adaptation occurred in visual cortex, as behavioral response priming has been shown to be supported by frontal and other structures also preserved in LSJ (Dobbins et al., 2004; Maccotta & Buckner, 2004; McMahon & Olson, 2007).
To further examine the involvement of the hippocampus in driving the observed effects, we analyzed the functional connectivity between hippocampus and LOC and PPA in control subjects using data from Experiment 4 and Experiment 5 where the lag between the repeated stimuli were held constant but the experiments differed in terms of task relevance. Hippocampus was anatomically defined in each control participant using Freesurfer’s (http://surfer.nmr.mgh.harvard.edu) subcortical segmentation tool (Fischl et al., 2002). We reasoned that if making the stimuli task-relevant engaged the hippocampus in a way that produces adaptation in LOC and PPA for objects and scenes, respectively, we would expect enhanced connectivity for Experiment 5 vs. 4. We adopted an approach to functional connectivity based on the correlations of responses in the hippocampus and LOC/PPA, akin to a beta series analysis but with the response quantified from the preprocessed BOLD activity rather than a GLM. Specifically, we examined the functional correlations of peak responses (timepoints 4-8 seconds post stimulus onset) for repeat trials in control participants between the hippocampus and LOC or PPA, as a function of both experiment and stimulus class.
When objects were task-relevant (Experiment 5), we observed a significant correlation between the hippocampus and LOC (r = 0.73, p = 0.04), but not between the hippocampus and PPA (r = 0.50, p = 0.20). When objects were passively viewed (Experiment 4), hippocampus and LOC responses did not show a reliable correlation (r = 0.30, p = 0.47). Similarly, when scenes were task-relevant (Experiment 5), we observed a significant correlation between the hippocampus and PPA (r = 0.82, p = 0.01), but not between the hippocampus and LOC (r = 0.46, p = 0.25). When scenes were passively viewed (Experiment 4), hippocampus and PPA responses did not show a reliable correlation (r = 0.54, p = 0.16).
Note that the positive correlation between the hippocampus and LOC when objects were task-relevant was specific to repeat trials (new trials: r = 0.50, p = 0.21). In contrast, the positive correlation between the hippocampus and PPA when scenes were task-relevant was found for both repeat and new trials (r = 0.79, p = 0.02). The higher overall correlations for scenes and PPA and their lack of modulation by repetition may suggest that the hippocampus plays a persistent role in scene perception (Lee et al., 2012). In contrast, the hippocampus may be more selectively recruited for object memory than object perception, exhibiting reliable interactions with LOC only for repetitions (see Figure S5 for additional analyses on visually evoked responses in the hippocampus for controls).
Discussion
We conducted a case study of LSJ, a patient with extensive bilateral MTL lesions including complete hippocampal loss. She completed an extraordinary amount of fMRI testing for a single patient, across a series of five fMRI experiments spanning repetition lags from immediate to several minutes, and with two sessions per experiment to replicate the findings across object and scene stimulus classes. LSJ is a unique patient given that she is cognitively high-functioning despite her extensive MTL lesions and severe memory impairment. Importantly, LSJ was comfortable and reported enjoying participating in experiments, and thus we were able to acquire substantial high-quality data. Even though single-case studies like ours necessarily have limitations, including the inability to draw population-level inferences and difficulty generalizing to other MTL patients who show different cognitive profiles, such studies can nevertheless provide rare insights into brain function after perturbation. Our findings should be interpreted with these caveats in mind.
The reported experiments provide a comprehensive account of the role of the hippocampus and MTL in neural adaptation (summarized in Figure 8). Using localizers, we first defined ROIs for object-selective LOC and scene-selective PPA, as well as other category-selective (TOS, RSC, FFA) and early visual areas (V1-V4). None of these ROIs differed in size from controls, suggesting that the organization and selectivity of ventral visual cortex does not depend upon the MTL. Also similar to controls, LSJ showed adaptation for repetitions of task-relevant object and scene stimuli in LOC and PPA, respectively, whether they occurred immediately, after 30 s, or after 3 mins. LSJ did not show adaptation for repetitions of task-irrelevant stimuli after 6 mins, but neither did the controls. Importantly, when the object and scene stimuli became task-relevant, repetitions led to adaptation in LOC and PPA, respectively, but only in controls and not LSJ.
These results suggest that visual cortex, independent of a contribution from the hippocampus and MTL, is able to sustain perceptual memory of task-irrelevant stimuli viewed once for somewhere between 3 and 6 minutes. Beyond that timeframe, however, the MTL is necessary for adaptation and the stimuli must be task-relevant during the initial and/or repeated exposures. Below we consider potential mechanisms both for preserved adaptation at the shorter lags despite MTL damage and for the disrupted adaptation at the longest lag as a result of MTL damage.
Preserved Short-Lag Adaptation in Amnesia
We show that without the hippocampus and much of MTL cortex, LOC and PPA can track task-irrelevant visual information for at least three (but less than six) minutes. This is consistent with the suggestion that LOC and PPA have “temporal receptive windows” lasting several minutes (Hasson et al., 2015). This refers to the time window over which inputs can be integrated, in our case the impact of the first exposure to a stimulus on later processing of its repetition. Such windows are typically mapped with a stimulus that has higher-order structure (i.e., a movie with a continuous plot) and the receptive window is defined as the timescale over which scrambling the stimulus changes the response. For example, a visual area that faithfully represents the current sensory stimulus would be insensitive to scrambling the movie across windows of a few contiguous seconds. In contrast, a brain region that represents entire scenes lasting a few minutes would be disrupted by scrambling over seconds, but not scrambling across windows longer than a few minutes. The stimuli in our experiments appeared in a random order, however, with no overarching structure in the sequence or repetition of their identity. This poses a problem for an account based on temporal receptive windows, as it is akin to scrambling over seconds that would destroy long-timescale responses.
An alternative account could be that working memory supports adaptation up to a few minutes by maintaining a stimulus over this delay and shielding it from the many intervening stimuli. However, this seems unlikely because it would require that all of the hundreds of stimuli encountered are maintained, both far exceeding the known capacity of working memory and occurring in the background while subjects performed an unrelated behavioral task. This alphabet-naming task was the primary focus and diverted attention away from the stimuli, discouraging their active maintenance in working memory during the experiment.
Even more striking than LOC and PPA is that LSJ seemed to show adaptation up to three minutes in early visual areas V1-V4 (Figure S1). Whether stimulus information is maintained this long in such areas is unclear, especially because they are typically believed to have a relatively short temporal window over which sensory information is integrated (Hasson et al., 2015). One possibility is that higher-level areas like LOC and PPA track the information and send feedback to early visual areas. Another possibility is that brain regions outside of visual cortex and other than the hippocampus and MTL are involved in providing feedback. For example, the intact frontal cortex of LSJ may have automatically maintained recent stimuli up to this lag.
Disrupted Long-Lag Adaptation in Amnesia
A key result of our study is that the MTL appears to be necessary for long-lag adaptation beyond three minutes when stimuli are task-relevant. Because of the extent of MTL damage in LSJ, the precise locus of this deficit cannot be identified. Additional patients would need to be tested in our paradigm, varying in the location and extent of lesions. This could include patients with more selective damage to either the hippocampus or MTL, as well as cortical structures. With such patients, it would be possible to adjudicate between a few potential explanations consistent with the results from LSJ.
One such explanation is that the deficit results from the memory functions of the MTL and specifically the hippocampus. By this memory account, the hippocampus encodes the initial presentation of each task-relevant stimulus as a result of its unique abilities for one-shot learning and to form distinct representations of similar inputs via pattern separation (Norman & O’Reilly, 2003). Such hippocampal memory traces are all that remains after six minutes. When a repeated stimulus is subsequently perceived, the memory of the initial presentation is retrieved and reinstated in visual cortex (e.g., Bosch et al., 2014; Gordon et al., 2014; Danker et al., 2017). This reinstatement could have the effect of facilitating sensory processing in the visual cortex, leading to an overall reduction in visual activity (Li et al., 1993; Anderson et al., 2008; Kremers et al., 2014). By this account, such facilitation does not occur in LSJ because she lacks the requisite hippocampal memories. Patients with focal hippocampal lesions would be expected to show the same pattern of long-lag adaptation in LOC/PPA as LSJ. This proposed relationship between hippocampal long-term memory and long-lag adaptation is consistent with prior studies that have linked adaptation to behavioral measures of long-term memory (e.g., Turke-Browne et al., 2006; Manelis et al., 2011). It is also consistent with the fact that LSJ’s deficit was revealed only when stimuli were task-relevant, given the close connection between attention and memory in the hippocampus (Aly & Turk-Browne, 2017), including the role of selective attention in enhancing encoding (Uncapher & Rugg, 2009; Carr et al., 2013; Aly & Turk-Browne, 2016a).
Another potential explanation similarly focuses on the hippocampus but emphasizes its role in prediction rather than memory per se. The premise for this account is that adaptation in visual cortex reflects a reduced response for stimuli that are expected (Summerfield & de Lange, 2014). Because the environment is stable over time, we are more likely to encounter a recently seen stimulus than a new stimulus. Accordingly, repeated stimuli should be more expected than novel stimuli. In predictive coding models, this potentiates or “explains away” sensory representations of expected stimuli, preventing them from producing a net increase in neural activity. As a result, the visual system preferentially represents unexpected stimuli (i.e., prediction errors), responding more strongly to novel than repeated stimuli, in the service of learning to generate better predictions over time. The hippocampus has recently emerged as a candidate source for generating visual expectations (Hindy et al., 2016), in which case both LSJ and patients with more selective hippocampal damage would not be able to form such expectations, resulting in less adaptation in visual cortex.
Both the memory and prediction accounts as formulated above identify the hippocampus as the critical piece of LSJ’s broader MTL damage necessary for long-lag adaptation. We found partial empirical support for this in a follow-up analysis of evoked fMRI activity in the intact hippocampus of the control participants. Specifically, they showed reliable hippocampal responses at the longest lag when scenes were task-relevant but not when they were task-irrelevant (Figure S5), paralleling the conditions under which long-lag adaptation was observed for task-relevant scenes in PPA. We describe this evidence as partial because the same pattern was not found for objects, and hippocampal responses were also observed for scenes at one of the shorter lags.
This dissociation in hippocampal responses based on stimulus category suggests a third potential explanation. Rather than treating the hippocampus as a domain-general memory system or prediction generator, a different class of theories suggests that MTL subregions are stimulus-selective, representing different types of information (Fahy et al., 1993; Xiang & Brown, 1998; Davachi, 2006; Saksida & Bussey, 2010). In our case, the hippocampus would be involved in encoding scenes and the PRC in encoding objects (Lee et al., 2012; Turk-Browne, 2019). Accordingly, LSJ may show a deficit in long-lag adaptation for task-relevant scenes in PPA because of her hippocampal lesions, whereas her deficit in long-lag adaptation for task-relevant objects in LOC would result from her PRC lesions. In other words, what looks like a common pattern of results for LSJ across stimulus types actually reflects two separate underlying mechanisms that are both damaged as a result of the large extent of her MTL lesions. This representational account leads to different hypotheses than the memory and prediction accounts above for patients with more selective MTL damage. Focal hippocampal lesions should disrupt long-lag adaptation in visual cortex for scenes but not objects, and focal PRC lesions should disrupt long-lag adaptation in visual cortex for objects but not scenes.
Regardless of whether future studies determine that long-lag adaptation depends upon common or dissociable MTL mechanisms, such studies would only establish that the MTL plays a necessary role. This should not be confused with arguing that the MTL is sufficient for long-lag adaptation in visual cortex. Indeed, the hippocampus, PRC, and PHC are all closely integrated into broader cortical networks (Raganath & Ritchey, 2012). MTL damage can cause dysfunction in these networks, which might in turn disrupt the function of connected regions (Hayes et al., 2012; Rudebeck et al., 2013; Henson et al., 2016). It could be that such connected regions are additionally necessary for long-lag adaptation in visual cortex. This could be examined in future studies relating MTL functional connectivity to long-lag adaptation in healthy controls.
Despite future work being needed to better resolve the mechanism of LSJ’s deficit in long-lag adaptation, we do not believe that it can be attributed to more basic confounds that can arise in patient studies, such as task difficulty. Most importantly, accuracy did not differ between LSJ and controls in either the object or scene tasks. Even though LSJ was slower than controls in the object task, she was not reliably slower in the scene task, and both stimulus categories produced the same pattern of differential adaptation effects. Moreover, the responses of LOC and PPA to new stimuli were comparable between LSJ and controls, suggesting intact perceptual and attentional processing of the stimuli. For these reasons, we interpret the observed deficit in long-lag adaptation as attributable to the cognitive functions specifically impaired by MTL damage, rather than to peripheral differences in task performance or engagement.
Indeed, the use of a task in LSJ at the longest lag allowed us to characterize both neural adaptation and behavioral priming. Interestingly, LSJ exhibited some evidence for faster RTs to repetitions of objects and scenes without adaptation in LOC or PPA, respectively. This is consistent with previous findings of preserved behavioral priming in hippocampal patients (Warrington & Weiskrantz, 1974; Gabrieli et al., 1990; Cave & Squire, 1992). Despite severe impairment in recognizing previously presented stimuli, these patients can exhibit long-lasting priming similar to healthy controls (Cave & Squire, 1992). Behavioral priming is often accompanied by adaptation in ventral visual cortex (e.g., Henson et al., 2004; Dobbins et al., 2004; Maccotta & Buckner, 2004; McMahon & Olson, 2007; Wig et al., 2005), but can be dissociated (Xu et al., 2007) and is thought to depend partly on frontal cortex (Dobbins et al., 2004; Maccotta & Buckner, 2004; McMahon & Olson, 2007; Wig et al., 2005).
Conclusions
In summary, the present study offers insights into the role of the MTL in the functions of visual cortex. Although an intact hippocampus and MTL do not appear to be required for adaptation from seconds to a few minutes, they may be required for longer delays under conditions of enhanced attentional processing. This clarifies the situations under which memory systems contribute to visual processing, helping account for the position of the MTL at the apex of the ventral visual stream.
Supplementary Material
Acknowledgements
We thank LSJ’s sister for her extensive help and feedback, and dedicate this article to the memory of their mother, who also provided considerable support. This research was funded by NIH EY02316601 (JGK); Johns Hopkins Brain Science Institute (BL & MM); NIH R01 EY021755 (NTB); NSF BCS 1025149 (SK); NIH 2RO1 MH64043 (SK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aly M, Turk-Browne NB (2016a) Attention promotes episodic encoding by stabilizing hippocampal representations. Proc Natl Acad Sci USA 113:E420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aly M, Turk-Browne NB (2016b) Attention stabilizes representations in the human hippocampus. Cereb Cortex 26:783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aly M, & Turk-Browne NB (2017). How hippocampal memory shapes, and is shaped by, attention. Chapter in Hannula DE & Duff MC (Eds.), The Hippocampus from Cells to Systems (pp. 369–403). Springer. [Google Scholar]
- 4.Anderson B, Mruczek REB, Kawasaki K, Sheinberg D (2008). Effects of familiarity on neural activity in monkey inferior temporal lobe. Cereb Cortex 18:2540–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arcaro MJ, McMains SA, Singer BD, Kastner S (2009) Retinotopic organization of human ventral visual cortex. J Neurosci 29:10638–10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS (1993) Processing strategies for time-course data sets in functional MRI of the human brain. Magn Resort Med 30:161–173. [DOI] [PubMed] [Google Scholar]
- 7.Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, Hamalainen MS, Marinkovic K, Schacter DL, Rosen BR, Halgren E (2006) Top-down facilitation of visual recognition. Proc Natl Acad Sci USA 103:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behrmann M, Peterson MA, Moscovitch M, Suzuki S (2006) Independent representation of parts and the relations between them: Evidence from integrative agnoisa. J Exp Psychol 32:1169–1184. [DOI] [PubMed] [Google Scholar]
- 9.Bosch SE, Jehee JFM, Fernandez G, Doeller CF (2014) Reinstatement of associative memories in early visual cortex is signaled by the hippocampus. J Neurosci 34:7493–7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brozinsky CJ, Yonelinas AP, Kroll NEA, Ranganath C (2005) Lag-sensitive repetition suppression effects in the anterior parahippocampal gyrus. Hippocampus 15:557–561. [DOI] [PubMed] [Google Scholar]
- 11.Carr VA, Engel SA, Knowlton BJ (2013) Top-down modulation of hippocampal encoding activity as measured by high-resolution functional MRI. Neuropsychologia 51:1829–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cave BC, Squire LR (1992) Intact and long-lasting repetition priming in amnesia. J Exp Psychol 18:509–520. [DOI] [PubMed] [Google Scholar]
- 13.Chun MM, Turk-Browne NB (2007) Interactions between attention and memory. Curr Opirt Neurobiol 17:177–184. [DOI] [PubMed] [Google Scholar]
- 14.Crawford JR, Garthwaite PH, Howell DC, Gray CD (2004) A single case with a control sample: Modified t-tests versus Mycroft et al.’s (2002) modified ANOVA. Cog Neuropsych 21:750–755. [DOI] [PubMed] [Google Scholar]
- 15.Crawford JR, Howell DC (1998) Regression equations in clinical neuropsychology: An evaluation of statistical methods for comparing predicted and obtained scores. J Clin Exp Neuropsychol 20:755–762. [DOI] [PubMed] [Google Scholar]
- 16.Danker JF, Tompary A, Davachi L (2017) Trial-by-trial hippocampal encoding activation predicts the fidelity of cortical reinstatement during subsequent retrieval. Cereb Cortex 27:3515–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davachi L (2006). Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology, 16(6), 693–700. [DOI] [PubMed] [Google Scholar]
- 18.Dobbins IG, Schnyer DM, Verfaellie M, Schacter D (2004) Cortical activity reductions during repetition priming can restult from rapid response learning. Nature 428:316–319. [DOI] [PubMed] [Google Scholar]
- 19.Eger E, Henson RNA, Driver J, Dolan RJ (2004) BOLD repetition decreases in object-responsive ventral visual areas depend on spatial attention. J Neurophysiol 92:1241–1247. [DOI] [PubMed] [Google Scholar]
- 20.Epstein R, Harris A, Stanley D, Kanwisher N (1999) The parahippocampal place area: recognition, navigation, or encoding? Neuron 23:115–125. [DOI] [PubMed] [Google Scholar]
- 21.Epstein RA, Parker WE, Feiler AM (2008) Two kinds of fMRI repetition suppression? Evidence for dissociable neural mechanisms. J Neurophys 99:2877–2886. [DOI] [PubMed] [Google Scholar]
- 22.Fahy FL, Riches IP, Brown MW (1993) Neuronal activity related to visual recognition memory: Long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp Brain Res 96:457–472. [DOI] [PubMed] [Google Scholar]
- 23.Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1:1–47. [DOI] [PubMed] [Google Scholar]
- 24.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- 25.Gabrieli JDE, Milberg W, Keane MM, Corkin S (1990) Intact priming of patterns despite impaired memory. Neuropsychologia 28:417–427. [DOI] [PubMed] [Google Scholar]
- 26.Gordon AM, Rissman J, Kiani R, Wagner AD (2014) Cortical reinstatement mediates the relationship between content-specific encoding activity and subsequent recollection decisions. Cereb Cortex 24:3350–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R (1999) Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron 24:187–203. [DOI] [PubMed] [Google Scholar]
- 28.Gregory E, McCloskey M, Landau B (2014) Profound loss of general knowledge in retrograde amnesia: evidence from an amnesic artist. Front Hum Neurosci 8:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregory E, McCloskey M, Ovans Z, Landau B (2016) Declarative memory and skill-related knowledge: Evidence from a case of amnesia and implications for theories of memory. Cog Neuropsychol 33:220–240. [DOI] [PubMed] [Google Scholar]
- 30.Grill-Spector K, Henson R, Martin A (2006) Repetition and the brain: Neural models of stimulus-specific effects. Trends Cog Sci 10:14–23. [DOI] [PubMed] [Google Scholar]
- 31.Grill-Spector K, Malach R (2001) FMR-adaptation: A tool for studying the functional properties of human cortical neurons. Acta Psychol 107:293–321. [DOI] [PubMed] [Google Scholar]
- 32.Hasson U, Chen J, Honey CJ (2015) Hierarchical process memory: Memory as an integral compoent of information processing. Trends Cogn Sci 19:304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatfield M, McCloskey M, Park S (2016) Neural representation of object orientation: A dissociation between MVPA and repetition suppression. Neuroimage 139:136–148. [DOI] [PubMed] [Google Scholar]
- 34.Hayes SM, Salat DH, & Verfaellie M (2012). Default network connectivity in medial temporal lobe amnesia. Journal of Neuroscience, 32(42), 14622–14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henson RN, Greve A, Cooper E, Gregori M, Simons JS, Geerligs L, … & Browne G (2016). The effects of hippocampal lesions on MRI measures of structural and functional connectivity. Hippocampus, 26(11), 1447–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henson RN, Ross ARE, Vuilleumeir P, Rugg MD (2004) The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. Neuroimage 21:1674–1689. [DOI] [PubMed] [Google Scholar]
- 37.Henson R, Shallice T, Dolan R (2000) Neuroimaging evidence for dissociable forms of repetition priming. Science 287:1269–1272. [DOI] [PubMed] [Google Scholar]
- 38.Hindy NC, Ng FY, Turk-Browne NB (2016) Linking pattern completion in the hippocampus to predictive coding in visual cortex. Nat Neurosci 19:665–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JG, Aminoff EM, Kastner S, Behrmann M (2015) A neural basis for developmental topographic disorientation. J Neurosci 35:12954–12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JG, Biederman I, Lescroart MD, Hayworth KJ (2009) Adaptation to objects in the lateral occipital complex (LOC): Shape or semantics? Vision Res 49:2297–2305. [DOI] [PubMed] [Google Scholar]
- 41.Konen CS, Behrmann M, Nishimura M, Kastner S (2011) The functional neuroanatomy of object agnosia: A case study. Neuron 71:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konen CS, Kastner S (2008) Two hierarchically organized neural systems for object information in human visual cortex. Nat Neurosci 11:224–231. [DOI] [PubMed] [Google Scholar]
- 43.Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL (2001) Perceptual specificity in visual object priming: Functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia 39:184–199. [DOI] [PubMed] [Google Scholar]
- 44.Kremers NAW, Deuker L, Kranz TA, Oehrn C, Fell J, Axmacher N (2014) Hippocampal control of repetition effects for associative stimuli. Hippocampus 24:892–902. [DOI] [PubMed] [Google Scholar]
- 45.Lee ACH, Yeung L-K, & Barense MD (2012). The hippocampus and visual perception. Frontiers in Human Neuroscience, 6, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L, Miller EK, Desimone R (1993) The representation of stimulus familiarity in anterior inferior temporal cortex. J Neurophys 69:1918–1929. [DOI] [PubMed] [Google Scholar]
- 47.Maccotta L, Buckner RL (2004) Evidence for neural effects of repetition that directly correlate with behavioral priming. J Cog Neurosci 16:1625–1632. [DOI] [PubMed] [Google Scholar]
- 48.Manelis A, Wheeler ME, Paynter CA, Storey L, Reder LM (2011) Opposing patterns of neural priming in same-exemplar vs. different-exemplar repetition predict subsequent memory. NeuroImage 55:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McMahon DBT, Olson CR (2007) Repetition suppression in monkey inferotemporal cortex: Relation to behavioral priming. J Neurophysiol 97:3532–3543. [DOI] [PubMed] [Google Scholar]
- 50.Meister IG, Weidemann J, Foltys H, Brand H, Willmes K, Krings T, Thron A, Topper R, Boroojerdi B (2005) The neural correlate of very-long-term picture priming. Eu. J Neurosci 21:1101–1106. [DOI] [PubMed] [Google Scholar]
- 51.Moore KS, Yi D-J, Chun M (2013) The effect of attention on repetition suppression and multivoxel pattern similarity. J Cog Neurosci 25:1305–1314. [DOI] [PubMed] [Google Scholar]
- 52.Murray SO, Wojciulik E (2003) Attention increases neural selectivity in the human lateral occipital complex. Nat Neurosci 7:70–74. [DOI] [PubMed] [Google Scholar]
- 53.Norman KA, O’Reilly RC (2003) Modeling hippocampal and neocortical contributions to recognition memory: A contemporary-learning-systems approach. Psych Rev 110:611–646. [DOI] [PubMed] [Google Scholar]
- 54.O’Reilly RC, Rudy JW (2001) Conjunctive representation in learning and memory: Principles of cortical and hippocampal function. Psych Rev 108:311–345. [DOI] [PubMed] [Google Scholar]
- 55.Peelen MV, Kastner S (2014). Attention in the real world: Towards understanding its neural basis. TICS 18, 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinsk MA, Arcaro M, Weiner KS, Kalkus JF, Inati SJ, Gross CG, Kastner S (2009) Neural representations of faces and body parts in macaque and human cortex: A comparative fMRI study. J Neurophysiol 101:2581–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ranganath C, & Ritchey M (2012). Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience, 73(10), 713–726. [DOI] [PubMed] [Google Scholar]
- 58.Rolls ET, Cahusac PMB, Feigenbaum JD, Miyashita Y (1993) Responses of single neurons in the hippocampus of the macaque related to recognition memory. Exp Brain Res 93:299–306. [DOI] [PubMed] [Google Scholar]
- 59.Rudebeck SR, Filippini N, & Lee AC (2013). Can complex visual discrimination deficits in amnesia be attributed to the medial temporal lobe? An investigation into the effects of medial temporal lobe damage on brain connectivity. Hippocampus, 23(1), 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saksida LM, & Bussey TJ (2010). The representational-hierarchical view of amnesia: Translation from animal to human. Neuropsychologia, 48(8), 2370–2384. [DOI] [PubMed] [Google Scholar]
- 61.Schapiro AC, Gregory E, Landau B, McCloskey M, Turk-Browne NB (2014) The necessity of the medial temporal lobe for statistical learning. J Cogn Neurosci 26:1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider KA, Richter MC, Kastner S (2004) Retinotopic organization and functional subdivisions of the human lateral geniculate nucleus: A high-resolution functional magnetic resonance imaging study. J Neurosci 24:8975–8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silver M, Kastner S (2009). Topographic maps in human frontal and parietal cortex. TICS 13, 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Staresina BP, Henson RNA, Kriegeskorte N, Alink A (2012) Episodic reinstatement in the medial temporal lobe. J Neurosci 32:18150–18156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Summerfield C, de Lange FP (2014) Expectation in perceptual decision making: Neural and computational mechanisms. Nat Rev Neurosci 15:745–756. [DOI] [PubMed] [Google Scholar]
- 66.Turk-Browne NB (2019). The hippocampus as a visual area organized by space and time: A spatiotemporal similarity hypothesis. Vision Research, 165, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turk-Browne NB, Scholl BJ, Johnson MK, Chun MM (2010) Implicit perceptual anticipation triggered by statistical learning. J Neurosci 30:11177–11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turk-Browne NB, Yi D-J, Chun MM (2006) Linking implicit and explicit memory: Common encoding factors and shared representations. Neuron 49:917–927. [DOI] [PubMed] [Google Scholar]
- 69.Uncapher MR, Rugg MD (2009) Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. J Neurosci 29:8270–8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valtonen J, Gregory E, Landau B, McCloskey M (2014) New learning of music after bilateral medial temporal lobe damage: Evidence from an amnesic patient. Front Hum Neurosci 8:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Turennout M, Bielamowicz L, Martin A (2003) Modulation of neural activity during object namging: Effects of time and practice. Cereb Cortex 13:381–391. [DOI] [PubMed] [Google Scholar]
- 72.van Turennout M, Ellmore T, Martin A (2000) Long-lasting cortical plasticity in the object naming system. Nature 3:1329–1334. [DOI] [PubMed] [Google Scholar]
- 73.Vannini P, Hedden T, Sullivan C, Sperling RA (2013) Differential functional response in the posteromedial cortices and hippocampus to stimulus repetition during successful encoding. Hum Brain Mapp 34:1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vuilleumier P, Schwartz S, Duhoux S, Dolan RJ, Driver J (2006) Selective attention modulates neural substrates of repetition priming and “implicit” visual memory: Suppressions and enhancements revealed by fMRI. J Cogn Neurosci 17:1245–1260. [DOI] [PubMed] [Google Scholar]
- 75.Warrington EK, Weiskrantz L (1974) The effect of prior learning on subsequent retention in amnesic patients. Neuropsychologia 12:419–428. [DOI] [PubMed] [Google Scholar]
- 76.Weiner KS, Sayres R, Winberg J, Grill-Spector K (2010) FMRI-adaptation and category selectivity in human ventral temporal cortex: Regional differences across time scales. J Neurophys 103:3349–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wig GS, Grafton ST, Demos KE, Kelley WM (2005) Reductions in neural activity underlie behavioral components of repetition priming. Nat Neurosci 8:1228–1135. [DOI] [PubMed] [Google Scholar]
- 78.Xiang J-X, Brown MW (1998) Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacol 37:657–676. [DOI] [PubMed] [Google Scholar]
- 79.Xu Y, Turk-Browne NB, Chun MM (2007) Dissociating task performance from fMRI repetition attenuation in ventral visual cortex. J Neurosci 27:5981–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yi D-J, Chun MM (2005) Attentional modulation of learning-related repetition attenuation effects in human parahippocampal cortex. J Neurosci 25:3593–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.