Summary
Background:
Weight loss interventions can have positive ‘ripple’ effects on untreated partners in the home, but ripple effects on infants are unknown.
Objective:
To examine whether a 12-month internet-based weight loss intervention for postpartum mothers had a positive ripple effect on participants’ infants.
Methods:
A 12-month cluster randomized, assessor-blind, clinical trial enrolling 371 postpartum women at 12 Women, Infants, Children clinics in CA. Clinics were randomized to standard Women, Infants, Children or an internet-based weight loss intervention for mothers.
Results:
A total of 333 of the 371 (89.8%) mothers assented for infant participation. Infants were 5.3 ± 3.2 months; 75.9% were Hispanic and 64% were breastfeeding. Infant retention was 272/333 (82.7%) at 6 months post enrollment and 251/333 (75.3%) at 12 months post enrollment. In intent-to-treat analysis, a significant interaction between group and time was observed (p = 0.008) with the offspring of intervention mothers exhibiting lower zBMI change from study entry through 6 months (0.23 [CI, 0.03, 0.44] vs. 0.65 [0.50, 0.79] zBMI change, respectively; p = 0.001) but was not significant through 12 months (p = 0.16). Regardless of group, maternal reports at the final assessment indicated that infants (aged =17.2 ± 3.4 months) consumed sweetened beverages (0.93 ± 1.5/week), juice (2.0 ± 1.4/day), ‘junk food’ (7.8 ± 5.4/week) and fast food (2/month), and 46.7% of the infants had a TV in their bedroom.
Conclusions:
An internet-based weight loss program for low-income, postpartum mothers had a positive ‘ripple’ effect on the zBMI of infants in the home during the first 6 months of treatment.
Keywords: Infant zBMI, postpartum intervention, ripple effect
Introduction
Randomized clinical trials have shown that behavioural weight loss treatments in adults with overweight/obesity tend to promote a ~2-kg greater weight loss in untreated partners compared with control partners (1,2). This 2-kg ‘ripple’ effect on the weight of untreated partners in the home represents a positive, indirect effect of lifestyle interventions (1–4). However, few studies have examined whether the ripple effects extend to others in the home.
Infants in the home, while clearly different from untreated partners, might receive similarly positive ripple effects on growth trajectories if the lifestyle interventions targeted the weight of maternal caregivers. Maternal weight is a strong predictor of infant weight (5,6). Mothers influence about 87% of household meals (7) and have primary responsibility for feeding throughout infancy and childhood (8). Mothers and infants spend 40 to 50 h per week together (9), and, as suggested by classical models of social-cognitive development (10) and social control theories (11), time in shared activities might provide the opportunity for mothers to transmit behaviours and norms to their infants. Social contagion theory further supports that behaviours spread from mother to infant via imitation (12,13), adoption of social norms (14) and emotional contagion (15).
Since overly rapid weight gain during the first 2 years of life predicts later child obesity and cardio-metabolic risk (16,17), a positive ripple effect of maternal lifestyle interventions on infants’ weight and related behaviours would be important to document to inform future early life, obesity prevention efforts. Although weight loss interventions in postpartum women have been limited by high attrition and mixed efficacy (8–12), distally delivered interventions including the internet (18), mail (19) and phone (20) have promoted clinically significant weight loss in diverse postpartum mothers.
Fit Moms/Mamas Activas was a cluster-randomized trial that found that a primarily internet-based weight control program produced significantly greater 12-month weight loss in low-income mothers with obesity in the Women, Infants, Children (WIC) Supplemental Nutrition Programs compared with Standard WIC (18). The intervention targeted a reduction in calories, fast food and sugar sweetened beverage intake, an increase in fruit and vegetable intake, and increase in physical activity and reduction in screen time. The intervention also included recommendations to change the home food and exercise environment to prompt healthy behaviours. Interestingly, these same behaviours have been targeted in effective obesity prevention interventions in infants (21). Thus, the purpose of this study was to examine whether the Fit Moms/Mamas Activas intervention delivered to postpartum mothers had beneficial effects on infant anthropometrics, including BMI z-scores (zBMI) and skinfolds measures, and infant dietary patterns and TV viewing.
Methods
Design
Fit Moms/Mamás Activas was a cluster randomized clinical trial (18,22,23). The protocol (22) and primary outcomes (18) have been published previously.
Participants
Procedures were approved by the Cal Poly IRB, and all participants provided written informed consent. Recruitment occurred between July, 2011 and May, 2015 across 12 WIC clinics in Santa Barbara (n = 6), San Luis Obispo (n = 4) and Ventura (n = 2) counties. Women enrolled in the parent trial were between 6 weeks and 12 months postpartum, 18–40 years of age, and English-speaking or Spanish-speaking, and were currently with overweight/obesity or exceeding pre-pregnancy weight by ≥4.5 kg.(22) Women had the option to participate in this Child Measures substudy. Eligibility criteria for the Child Measures substudy included enrollment in the parent trial and informed assent for infant participation. Women who did not enrol their infant in the substudy at entry were invited again to participate at the 6 and 12-month assessments to maximize participant representation from the parent study (but analyses that included only women who enrolled at study entry resulted in the same findings). Reasons for nonparticipation in the Child Measures substudy were not formally tracked.
Interventions
The study statistician randomized (18,24) the 12 clinics to the two conditions, blocking on county (i.e., San Luis Obispo [four clinics]; Santa Barbara [six clinics]; Ventura [two clinics]), and using R (3.3.1) statistical package. Standard WIC received all aspects of standard WIC care (25) plus a brief orientation to the study and newsletters every 2 months with information about maternal weight control, exercise, nutrition and wellness; one article in a newsletter encouraged physical activity in families, but no other content was child related.
The internet-based weight control program group received all elements of standard WIC plus a 12-month weight loss program adapted from prior programs (10,12,13). The Fit Moms/Mamas Activas website, text messages and group sessions were available in English and Spanish. New content was posted each week, but the site was designed to permit flexibility in the pace of presentation of new information. Each week upon participant login, the participant completed a check-in that inquired about weight loss progress and barriers to reaching goals and received pre-programmed, tailored feedback based on progress and individual barriers. Behavioural lessons were posted weekly on the website for the intervention duration. Each new behavioural lesson was summarized in a brief, introductory video and could also be viewed through on-screen text with visuals that could be printed for reference. Topics of lessons and instructional videos included: selecting appropriate calorie goals, increasing fibre, grocery shopping, label reading, restaurant eating, beginning exercise, aerobic fitness, self-monitoring, stimulus control, problem solving, social assertion, goal setting, body image, cognitive strategies, overcoming barriers, relapse prevention training and strategies of successful weight losers. A weekly video blog of other WIC moms’ experience with weight loss and overcoming common barriers was also available in English and Spanish. Also from the study website, participants could access a secure, study-specific message board designed to provide patients with opportunities for group support, problem solving and feedback from study interventionists and peers. The website also provided an internet diary (in English and Spanish) to look up foods and record intake. The diary was integrated with a comprehensive physical activity web diary. Mothers could earn and redeem ‘diaper points’ for weight loss, positive weight behaviours, attending group and logging into the website.
The program was considered ‘primarily’ internet-based because participants also received four text messages at random days and times each week to reinforce use of the online weight loss program and provide additional encouragement and feedback to participants. Moreover, monthly face-to-face group sessions were offered at WIC to further reinforce messages of the online program and problem-solve any technical or other issues. We previously reported that similar to other postpartum studies, attendance at monthly group meetings (range, 0–12 meetings) was low with a mean 4.4 (SD, 2.7) visits overall, representing 37% of expected visit attendance. Login frequency was more strongly related to weight loss and averaged 6.0 (SD, 9.3) logins per month (18).
At the end of each weekly behavioural online lesson, a ‘Family Corner’ section encouraged mothers to be a role model and support healthy eating, activity and growth in their infant and other children and family members. No other direct infant intervention was provided.
Outcome assessments
Assessments were conducted by blinded, bilingual assessors at baseline (i.e., at the time of study enrollment) and at 6 and 12 months post enrollment. Questionnaires were selected based on their prior validity and use in English-speaking and Spanish-speaking, postpartum populations; measures that were not available in Spanish were translated and back translated by certified translators (22). Participants were provided with a US$25 honorarium for the baseline and 6-month assessments and US$50 for the 12- month visit. No additional compensation was provided for participation in the Child Measures substudy.
The primary outcome of this substudy was infant zBMI at baseline and after 6 and 12 months. Infant weight and length were objectively measured in duplicate by trained research assistants; BMI for age z scores were calculated using WHO Child Growth Standards (28–31). To estimate body fat, triceps and subscapular skinfolds were measured in duplicate, and z scores for age were calculated based on WHO reference equations (23–34). Skinfold measurement to estimate body fatness by trained research assistant has shown acceptable coefficients of reliability (75–93%) (35).
Infant demographics, including sex, age, ethnicity, and birth weight, birth length, premature birth and weeks premature, and breastfeeding, were based on maternal self-report (36). The Children’s Dietary Questionnaire (37), was used to measure dietary patterns. Mothers indicated whether their infant consumed any of a list of 18 fruits and 26 vegetables over the past 7 d. The questionnaire also assessed the frequency of infant intake of other foods and drinks over the past 7 d (from 0,1, 2,3,4 or 5+ times), including ‘non-core’ foods (e.g., peanut butter, sugar-sweetened cereals, sweet cakes, muffins, doughnut, potato chips, candies, chocolate, ice cream, pastry, cookies, French fries, hot dogs), sweetened beverages, fast food (from McDonalds, KFC, Pizza, Chinese or other) and intake of breastmilk, formula and fruit juice over past 24 h. Subscales of the Children’s Dietary Questionnaire have demonstrated satisfactory test-retest reliability (intraclass correlation coefficient 0.51 to 0.90) and an ability to detect change following intervention (37). The fruit and vegetable and noncore foods subscales have shown good internal consistency (alpha 0.76 and 0.62, respectively) (37) and criterion validity (37).
To measure ‘activity promoting’ toys in home environment, a checklist of 30 ‘active’ play items in the home was administered based on the Affordances in the Home Environment for Motor Development – Infant Scale; this measure also assessed whether a TV was present in the infant’s room (37,38). The Affordances in the Home Environment for Motor Development – Infant Scale has been shown to be valid compared with direct observation (r = 0.24) and a reliable (interrater reliability of 1 and intrarater reliability of 0.94) instrument for measuring the quality and quantity of motor development opportunities provided in the home environment for infants aged 3–18 months (37,38).
Statistical methods
Missing data were assumed to be missing at random. The approach included a likelihood-based, linear mixed effect model that included the randomized clinics (and excluded 1 clinic that withdrew) and infants in these clinics as random effects. This model allowed for Infant participants to have partial missing response data and still be included in the model without imputation. T-tests or chi-square tests were used to compare infants who did vs. did not participate in the study and those with completed vs. incomplete records on several variables, including group assignment, maternal BMI, ethnicity, and breastfeeding, and infant age. To test the primary hypothesis, a generalized linear mixed effect model was used (intervention group as a fixed effect and clinic and infant as random effects). A group × time interaction term (fixed effect) tested whether the change in zBMI over time differed significantly between groups. The model included participant-level covariates (i.e., baseline zBMI, breastfeeding status, infant sex, age, ethnicity). If the group × time interaction was statistically significant (p < 0.05), the equality of mean changes in the two groups at each intermediate time point was tested. Similar linear mixed effect models with the same participant covariates were conducted to examine changes over time in dietary factors, TV viewing and the number of ‘active toys’ in the home. Unadjusted p values were reported (19,20). SPSS and JMP (12.2.0) statistical packages were used for all analyses.
Results
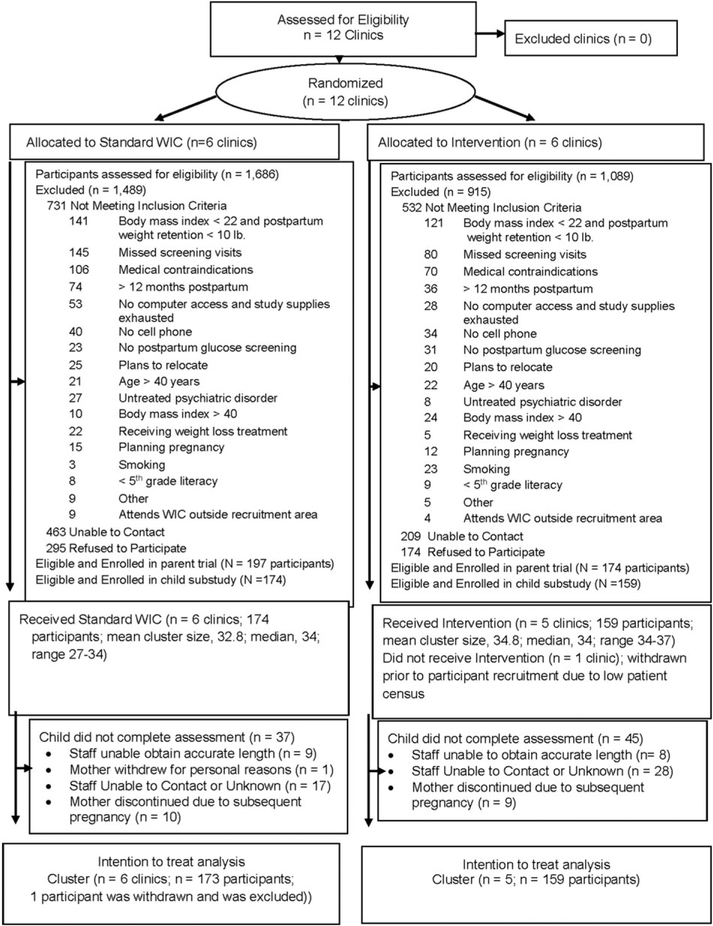
In this cluster randomized trial, the 12 WIC clinics that were approached for participation agreed to participate and were randomized at study onset (Fig. 1). Prior to initiation of participant recruitment but after randomization, one intervention clinic withdrew due to declines in staff, space and participant census. Across the 11 clinics, six received standard WIC and five received the intervention, and there were 197 and 174 participants, respectively, in each group, totalling 371 participants in the parent study. Of these, 289 (N =149 Standard Care and N =140 Intervention) consented to the Child Measures substudy at baseline and 44 (N = 25 Standard Care and N = 19 Intervention) consented at 6 months, totalling 333 (89.8%) participants in the Child Measures substudy (N = 174 standard WIC and N =159 intervention). As in the parent study, (18) mothers who participated in this substudy (N = 333) had an average (SD) BMI of 31.9 (5.3) and age (SD) of 28 (5.4) years and were 7.6 (7.1) kg above prepregnancy weight; 81.7% (Ns = 272/333) were Hispanic; 45.4% of participants spoke only or mostly Spanish; 32.4% spoke only or mostly English; and 22.2% were bilingual English/Spanish speakers. In analyses that replicated the parent study’s analyses but only included mothers whose infants participated in this substudy, (18) the intervention produced greater 12-month weight loss compared with standard WIC (3.2 kg vs. 0.9 kg, respectively; p < 0.001; 2.3-kg difference (95% CI:1.0, 3.5).
Figure 1.
Flow diagram of enrollment, randomization, follow-up and analysis. In this clinic randomized trial, 12 clinics were enrolled and randomized. After randomization but before patient recruitment, 1 Intervention clinic withdrew participation.
The infants of mothers who did vs. did not participate in this substudy did not significantly differ based on maternal group assignment, zBMI at study entry, ethnicity, breastfeeding and infant age. Infants of mothers who participated in this substudy had an average age of 5.3 ± 3.2 months; 75.9% were Hispanic and 64% were breastfeeding (Table 1). The two study groups were similar for all baseline characteristics, with the exception of infant zBMI-for-age at study entry (Table 1); this variable was adjusted for in subsequent analyses. Retention in this substudy was 272/333 (82.7%) at 6 months after enrollment and 251/333 (75.3%) at 12 months after enrollment; reasons for lost to follow-up are shown in Fig. 1. The group assignment and demographic characteristics (infant zBMI at study entry, ethnicity, breastfeeding status, sex and infant age) did not significantly differ between infants who attended vs. did not attend the 12-month visit.
Table 1.
Baseline characteristics of 333 Fit Moms/Mamas Activas infants by standard WIC (N = 174) or intervention (N = 159) groups1
| Characteristic | Overall N= 333 | Standard care n = 174 | Intervention n = 159 | p Value |
|---|---|---|---|---|
| Age, months, mean (SD) | 5.3 (3.2) | 5.0 (3.2) | 5.6 (3.0) | 0.07 |
| N = 289 | n = 172 | n = 159 | ||
| Hispanic/Latino, no. (%) | 249 (75.9) | 128 (73.9) | 121 (77.6) | 0.41 |
| N = 329 | n = 173 | n = 156 | ||
| Biological sex | 0.05 | |||
| Male, no. (%) | 169 (50.8) | 79 (45.4) | 90 (56.7) | |
| Female, no. (%) | 164 (49.3) | 95 (54.6) | 69 (43.4) | |
| N= 333 | n = 174 | n = 159 | ||
| Birth weight, grams, mean (SD) | 3486 (708) | 3455 (762) | 3519 (648) | 0.44 |
| N= 289 | n = 149 | n = 140 | ||
| Birth length, cm, mean (SD) | 50.4 (3.9) | 50.1 (3.5) | 50.7 (4.3) | 0.18 |
| N = 279 | n = 145 | n = 134 | ||
| Premature birth, no. (%) | 12 (4.2) | 6 (4.0) | 6 (4.3) | 0.57 |
| N= 289 | n = 149 | n = 140 | ||
| Currently breastfeeding, no. (%) | 213(64.0) | 111 (63.4) | 102 (64.2) | 0.52 |
| N = 333 | n = 174 | n = 159 | ||
| zBMI, Mean (SD) | −0.11 (1.2) | −0.35 (1.05) | 0.15(1.2) | 0.0001 |
| Low < −2 SD, no. (%) | 15 (5.2) | 11 (7.4) | 4 (2.9) | 0.021 |
| Medium −2 to 2 SD, no. (%) | 266 (92.0) | 137 (91.9) | 129 (92.1) | |
| High >2 SD, no. (%) | 8 (2.8) | 1 (0.7) | 7 (5.0) | |
| N = 289 | n = 149 | n = 140 | ||
| Length/age z score (SD) | 0.33 (1.2) | 0.38 (1.2) | 0.32 (1.3) | 0.71 |
| Low < −2 SD, no. (%) | 8 (2.7) | 5 (3.4) | 3 (2.1) | 0.44 |
| Medium −2 to 2 SD, no. (%) | 266 (90.5) | 138 (90.8) | 128 (90.1) | |
| High >2 SD, no. (%) | 20 (6.8) | 9 (5.9) | 11 (7.7) | |
| N = 294 | n = 149 | n = 142 | ||
| Weight for age (SD) | 0.09 (1.1) | −0.09 (1.1) | 0.30 (1.1) | 0.003 |
| Low < −2 SD, no. (%) | 7 (2.4) | 6 (4.0%) | 1 (0.7) | 0.08 |
| Medium −2 to 2 SD, no. (%) | 276 (94.2) | 140 (94%) | 132 (94.3%) | |
| High >2 SD, No. (%) | 10 (3.4) | 3 (2.0) | 7 (5.0) | |
| N = 293 | n = 149 | n = 140 | ||
| Subscapular skinfolds for age z score (SD) | .39 (1.7) | 0.37 (1.0) | 0.44 (1.3) | 0.70 |
| Low < −2 SD, no. (%) | 6 (3.0) | 1 (0.9) | 5 (5.4) | 0.09 |
| Medium −2 to 2 SD, no. (%) | 177 (89.4) | 99 (93.4%) | 78 (84.8) | |
| High >2 SD, no. (%) | 15 (7.6) | 6 (5.9) | 9 (9.8) | |
| N = 198 | n = 106 | n = 92 | ||
| Triceps skinfold for age z score (SD) | 0.51 (1.3) | 0.43(1.1) | 0.58 (1.4) | .40 |
| Low < −2 SD, no. (%) | 6 (3.0) | 1 (1.0) | 5 (4.6) | 0.21 |
| Medium −2 to 2 SD, no. (%) | 177 (89.4) | 93 (90.3) | 90 (83.3) | |
| High >2 SD, no. (%) | 9 (7.6) | 9 (8.7) | 13 (12.0) | |
| N = 192 | n = 107 | n = 110 |
Abbreviations: No, number; SD, standard deviation; g, grams.
Sample sizes varied across measures and are displayed in the table. Some participants declined to answer the marital status question (n = 2 Standard WIC, n = 2 Intervention), employment (n = 1 Standard WIC), income (n = 2 Standard WIC), education (n = 2 Standard WIC) and preconception weight (n = 3 Standard WIC). Seven participants reported having twins during their most recent pregnancy, and in such cases one infant was randomly selected for inclusion in this study.
Primary outcome
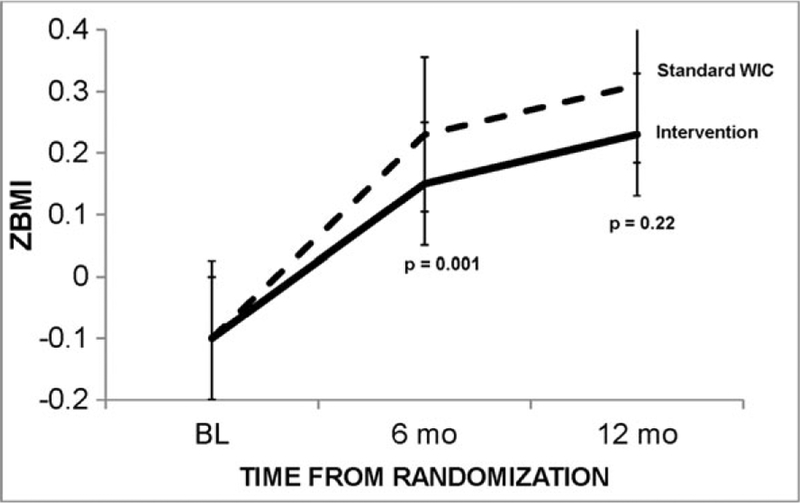
Changes in anthropometrics and skinfolds variables are summarized in Table 2. A significant interaction between group and time was observed (p = 0.008) with the offspring of intervention mothers exhibiting significantly less zBMI change over time than those of standard WIC (Fig. 2). Infants of intervention moms had zBMI scores that increased significantly less than those of standard WIC moms from study entry through 6 months post enrollment (0.23 [CI, 0.03,0.44] vs. 0.65 [0.50, 0.79] zBMI gain, respectively; p = 0.001), but were not statistically different from Standard WIC by 12 months post enrollment (p = 0.16; Table 2). Incidences of obesity (i.e., zBMI >2 SD) were low in intervention and standard WIC groups at study entry (ns = 7 and 1, respectively), 6 months post enrollment (ns = 10 and 10, respectively) and 12 months post enrollment (ns = 9 and 5, respectively). Partial correlations examining changes in infant zBMI and maternal weight (adjusting for infant age, sex, ethnicity and breastfeeding) trended toward significance from baseline to 6 months post enrollment (R = 0.13; p = 0.06) but not from baseline to 12 months post enrollment (p = 0.35).
Table 2.
Changes in infant anthropometrics over 12 months by group
| Model p values1 | ||||||
|---|---|---|---|---|---|---|
| Standard care n = 174 Mean (95% Cl) |
Intervention n = 159 Mean (95% Cl) |
Between group differences (95% Cl)2 |
Grp | Time | Grp × time | |
| BMI for age, z score | 0.58 | <0.0001 | <0.008 | |||
| Baseline zBMI | −0.16 (−0.33, 0.005) | 0.00 (−0.16, 0.17) | ||||
| Change from baseline to 6 months3 | 0.65 (0.50, 0.79) | 0.23 (0.03, 0.44) | 0.40(0.16,0.63); p = 0.001 | |||
| Change from baseline to 12 months3 | 0.81 (0.66, 0.96) | 0.62 (0.39, 0.85) | 0.19 (−.08,.46); p = 0.16 | |||
| Triceps skinfold for age, z score | 0.07 | 0.02 | 0.76 | |||
| Baseline TS, cm | ||||||
| Change from baseline to 6 months3 | 0.50 (0.27, 0.64) | 0.56 (0.38, 0.75) | ||||
| Change from baseline to 12 months3 | 0.02 (−0.27, 0.23) | −0.13 (−0.43, 0.18) | 0.15 (−.54,.23); p =.44 | |||
| 0.27 (0.02,0.51) | 0.30 (−.002, 0.61) | −0.04 (−.42,.35); p =.85 | ||||
| Subcapular skinfold for age, z score | 0.02 | 0.02 | 0.27 | |||
| Baseline SS, cm | 0.35 (0.19,0.52) | 0.40 (0.22, 0.58) | ||||
| Change from baseline to 6 months3 | −0.33 (−0.53, −0.12) | −0.16 (−0.37, 0.06) | −0.17 (−0.47, 0.13); p = 0.27 | |||
| Change from baseline to 12 months3 | −0.38 (−0.65, −0.12) | −0.07 (−0.40, 0.25) | −.30 (−0.71, 0.11); p = 0.15 | |||
Abbreviations: BL, baseline; kg, kilogram; Grp, group; zBMI, z score for body mass index; TS, triceps skinfold; SS, subscapular skinfold. z-scores computed based on World Health Organization distributions.
Model p values based on linear mixed-effects models assuming compound symmetry and including time, group and group × time as fixed effects, pre-specified covariates at fixed effects (i.e., study entry breastfeeding, infant sex, age, ethnicity and baseline anthropometric value), and clinic and subject as random effects. Mixed models were intent-to-treat and included all 333 participants (Standard Care N = 174 and Intervention N = 159). For zBMI, data were collected on infants of 149/174 (85%) Standard Care and 140/159 (88%) Intervention participants at baseline, 152/174 (87%) Standard Care and 120/159 (75%) Intervention at 6 months and 133/174 (76%) Standard Care and 114/159 (72%) Intervention at 12 months. For triceps skinfold, data were collected on the infants of 107/174 (61%) Standard Care and 110/159 (69%) Intervention participants at baseline, 151/174 (87%) Standard Care and 118/159 (74%) Intervention at 6 months and 128/174 (74%) Standard Care and 107/159 (67%) Intervention at 12 months. For subscapular skinfolds, data were collected on infants of 106/174 (61%) Standard Care and 92/159 (58%) Intervention participants at baseline, 151/174 (87%) Standard Care and 118/159 (74%) Intervention at 6 months and 129/174 (74%) Standard Care and 105/159 (66%) Intervention at 12 months.
Model estimated group differences (i.e., group × time fixed-effect parameter estimates) in outcome variables from baseline to 6 months or baseline to 12 months.
Least-squares mean (95% CI), which is the model-estimated response within each treatment group from baseline to 6 or baseline to 12 months.
Figure 2.
Adjusted weights over time by treatment group. Error bars indicate 95% confidence intervals.
The intervention had no statistically significant group × time effects on triceps or subscapular skinfold measures for age, which were measured in a subset (~57%; see Table 2 footnote) of participants. Triceps skinfold measures generally increased in both groups over time while subscapular skinfolds measures decreased (Table 2).
Examining dietary data, a significant interaction between group and time was observed (p = 0.02) for number of types of vegetables consumed, with intervention infants’ vegetable scores increasing to a lesser extent than Standard WIC (10 [9, 11.1] vs. 12 [11, 12.9] types of vegetables per week, respectively; p = 0.007). No other significant group × time effects were observed for dietary variables (Table 3). The effect of treatment group assignment on zBMI remained significant (p = 0.007) after including vegetable score into the model.
Table 3.
Changes in infant dietary intake over 12 months by group
| Model p values1 | ||||||
|---|---|---|---|---|---|---|
| Intervention n = 159 Mean (95% Cl)b |
Standard Care n = 174 Mean (95% Cl)2 |
Between group differences (95% Cl)3 |
Grp | Time | Grp × time | |
| Fruit intake, no. types overpast 7 d4 | 0.79 | 0.0001 | 0.198 | |||
| Baseline | 0.48 (0.40, 0.56) | 0.41 (0.33, 0.49) | ||||
| Change from baseline to 6 months | 0.87 (0.77, 0.99) | 0.97 (0.85, 1.1) | 0.09 (−0.06, 0.24); p = 0.25 | |||
| Change from baseline to 12 months | 1.1 (0.94, 1.2) | 1.2 (1.1, 1.4) | 0.15 (−0.03, 0.33); p = 0.10 | |||
| Vegetable intake, no. types overpast 7 d4 | 1. | 0.12 | 0.001 | 0.02 | ||
| Baseline | 3.0(2.3, 3.7) | 2.6(1.9,3.2) | ||||
| Change from baseline to 6 months | 7.4 (6.5, 8.2) | 8.4 (7.6, 9.2) | 1.1 (−0.06, 2.2); p = 0.06 | |||
| Change from baseline to 12 months | 10.0 (9.0, 11.1) | 1. (11.0, 12.9) | 2.0 (0.53, 3.4); p = 0.007 | |||
| Meat/fish, no. times overpast 7 d5 | 0.62 | 0.0001 | 0.16 | |||
| Baseline | 0.55 (0.295, 0.804) | 0.44 (0.20, 0.68) | ||||
| Change from baseline to 6 months | 1.5 (1.3, 1.6) | 1.5 (1.3, 1.6) | 0.007 (−0.22, 0.23); p = 0.21 | |||
| Change from baseline to 12 months | 2.9 (2.6, 3.2) | 3.4 (3.1, 3.7) | 0.45 (0.01, 0.89); p = 0.04 | |||
| ‘Non-core’ food, no. times overpast 7 d5 | 0.09 | 0.0001 | 0.24 | |||
| Baseline | 0.72 (0.15, 1.3) | 0.75 (0.21, 1.3) | ||||
| Change from baseline to 6 months | 2.9 (2.3, 3.6) | 3.4 (2.8, 4.0) | 0.41 (−0.48, 1.3); p = 0.37 | |||
| Change from baseline to 12 months | 6.6 (5.8, 7.4) | 7.6 (6.8, 8.4) | 0.98 (−0.15, 2.1); p = 0.09 | |||
| Fast food, no. times overpast 7 d5 | 0.64 | 0.0001 | 0.11 | |||
| Baseline | 0.11 (0.02,0.20) | 0.02 (−0.06, 0.11) | ||||
| Change from baseline to 6 months | 0.17 (−0.26, −0.9) | 0.32 (−0.40, −0.23) | 0.14(0.02, 0.26); p = 0.03 | |||
| Change from baseline to 12 months | 0.54 (0.40, 0.67) | 0.72 (0.59, 0.84) | 0.17 (−0.01, 0.36); p = 0.06 | |||
| Sweetened beverages, no. times overpast 7 d5 | 0.85 | 0.0001 | 0.29 | |||
| Baseline | 0.11 (−0.03.0.24) | 0.11 (−0.02,0.23) | ||||
| Change from baseline to 6 months | 0.14 (−0.04, 0.31) | 0.24 (0.13,0.36) | 0.10 (−0.10, 0.30); p = 0.21 | |||
| Change from baseline to 12 months | 0.82 (0.60, 1.1) | 0.69 (0.52, 0.86) | −0.14 (−0.42, 0.15); p = 0.31 | |||
| Breastmilk, no. times over past 24 h6 | 0.93 | 0.000 | 0.92 | |||
| Baseline | 3.1 (2.9, 3.4) | 3.1 (2.9, 3.3) | ||||
| Change from baseline to 6 months | −1.3 (1.0, 1.6) | −1.3 (−1.6, −1.0) | 0.002 (−0.41, 0.41); p = 0.99 | |||
| Change from baseline to 12 months | −2.2 (−2.5, −1.9) | −2.1 (−2.5, −1.8) | 0.09 (−0.37, 0.54); p = 0.71 | |||
| Formula, no. times overpast 24 h6 | 0.31 | 0.0001 | 0.15 | |||
| Baseline | 2.5(2.2,2.7) | 2.3(2.1,2.6) | ||||
| Change from baseline to 6 months | −0.79(0.44, 1.3) | −0.31 (−0.63,0.01) | 0.47 (0.004, 0.94); p = 0.05 | |||
| Change from baseline to 12 months | −2.1 (−2.4, −1.7) | −1.9 (−2.3,−1.6) | 0.16 (−0.36, 0.67); p = 0.56 | |||
| Juice, no. times overpast 24 h6 | 0.05 | 0.0001 | 0.13 | |||
| Baseline | 0.32 (0.16,0.48) | 0.29 (0.14,0.45) | ||||
| Change from baseline to 6 months | 0.74 (0.57,0.91) | 1.1 (0.88, 1.3) | 0.34 (0.07, 0.60); p = 0.01 | |||
| Change from baseline to 12 months | 1.6 (1.3, 1.8) | 1.8 (1.5,2.0) | 0.20 (−0.12, 0.52); p = 0.22 | |||
Abbreviation: Grp, group.
Model p values based on linear mixed-effects models assuming compound symmetry and including time, group and group × time as fixed effects, pre-specified covariates at fixed effects (i.e., study entry breastfeeding, infant sex, age, ethnicity and baseline value), and clinic and subject as random effects. Mixed models were intent-to-treat and included all 333 participants (Standard Care N = 174 and Intervention N = 159).
Least-squares mean (95% CI), which is the model-estimated response within each treatment group from baseline to 6 or baseline to 12 months.
Model estimated group differences (i.e., group × time fixed-effect parameter estimates) in outcome variables from baseline to 6 months or baseline to 12 months.
Mothers indicated whether their infant consumed any of a list of 18 fruits and 26 vegetables over the past 7 days; frequency of intake was not assessed by this measure.37
Mothers reported the frequency of infant intake of food and drinks over the past 7 days (from 0, 1, 2, 3, 4 or 5 + times), including ‘non-core’ foods (e.g., peanut butter, sugar-sweetened cereals, sweet cakes, muffins, doughnut, potato chips, candies, chocolate, ice cream, pastry, cookies, French fries, hot dogs), sweetened beverages and fast food (from McDonalds, KFC, Pizza, Chinese or other).37
Mothers reported infant intake of breastmilk, formula and fruit juice over past 24 h.37
Changes in TV viewing were also examined, and no significant (p = 0.80) group × time interaction effect was observed. Infants in both groups watched 1.5 (CI, 0.84, 2.1) hours of TV per week at study entry, and this increased (p = 0.0001) to 4.9 (CI, 4.2, 5.5) hours per week over 12 months. No significant group × time interaction was observed for TVs in the bedroom; 46.7% of participants reported having a television in their infant’s bedroom overall and across time. Changes in the number of ‘activity promoting’ toys in the home did not significantly differ by group (p = 0.80). Both groups reported an average (CI) number of 3.1 (2.9, 3.2) ‘activity promoting’ toys in the home at study entry, which increased (p = 0.0001) to 3.6 (3.4, 3.7) active toys in the home by 12 months.
Discussion
This study is the first to document that a behavioural weight loss treatment delivered primarily online to postpartum mothers in WIC had a positive impact on the measured zBMI of the untreated infants during the first 6 months of the program. This pattern held true regardless of the infants’ age, ethnicity, sex or breastfeeding status. Prior interventions in WIC have directly targeted child obesity and not found significant effects on child zBMI (41–44). Findings from this study suggest that a potential novel approach to child obesity prevention efforts might be to promote maternal postpartum weight loss with brief messages encouraging healthy habits and growth in children. Since overly rapid weight gain at any time during the first year of life predicts later child obesity, future research is needed to determine whether the positive ripple effect observed in the current study is protective of longer-term child health.
For mothers, the intervention promoted clinically significant weight loss and maintenance during the entire 12-month program. However, in infants, the positive ‘ripple’ effect on growth was pronounced during the initial 6 months of maternal treatment and then was not significantly different from standard WIC at 12 months post enrollment. The reasons the intervention had a positive effect on infant growth during the initial but not subsequent 6 months remain unknown. The first 6 months of the program was when mothers were actively losing weight; active weight loss could exert a more powerful ripple effect than weight loss maintenance. It is also possible that more intensive maternal interventions would have had a greater and more enduring ‘ripple’ effect on infant zBMI trajectories. It is also possible developmental factors influenced the waning ripple effect. The infants were on average 5 months of age at study initiation. The subsequent 6 month window (i.e., aging from 5 to 11 months) is a period of development in which infants typically remain reliant on mothers, who may still be breastfeeding and carrying the child during daily activities (45). Maternal/infant contagious processes are likely most pronounced during this window when infants still lack ‘self–other differentiation.’ (10,12) After 12 months of age, most infants start walking independently and are increasingly self-feeding and drinking from a cup, and are developing understanding of the other person as an independent intentional agent (i.e., self-other differentiation) (10,12). Initiation of self-feeding skills has been linked with greater intake (45). These factors could have reduced maternal influence and intervention-related ripple effects. However, interindividual and intraindividual developmental differences exist among infants of the same age, and developmental milestones and time mothers and children spend together were not measured in this study. Future research is needed to disentangle unique developmental pathways as moderators of intervention ripple effects in infancy.
Intervention mothers self-reported smaller increase than standard WIC mothers in the number of types of vegetables that they fed to their infants over time. This finding raises some concern because of the high nutritional value of vegetables. This finding is also surprising because the intervention encouraged women to increase their vegetable intake. It is possible that the smaller increases in vegetables might reflect a general approach among intervention mothers (vs. those in Standard WIC) to feed their infants less. Trends (in Table 3) suggested that mothers in the intervention also fed their infants less meat/fish, ‘junk food’, juice and fast food, and these latter three were intervention targets in the maternal program. Future maternal intervention studies aiming to maximize ripple effects on offspring should consider including messages underscoring the nutritional value of vegetables in children. Also, future research with more detailed assessment of infant food and beverage intake and activity is needed to determine the mechanisms through which behavioural weight loss interventions affect untreated infants.
Regardless of group and by the end of the program, infants already had several risk factors for future obesity, including maternal reports of frequent consumption of sweetened beverages (0.93/week), juice (2.0/day), ‘junk food’ (7.8/week) and fast food (2/month), and 46.7% of the infants had a TV in their bedroom. Prior intervention studies have had mixed efficacy in reducing these behaviours in infants. A home-based obesity prevention intervention that was initiated when children were 1 month old reduced child zBMI (0.29 reduction) and showed positive effects on children’s vegetable consumption and TV viewing at age 2 years (21). Responsive parenting interventions targeting infant sleep and maternal feeding practices have also shown success in reducing rapid weight gain during the first 6 months of life and preventing overweight status at age 1 year (46). However, few effective early life obesity prevention interventions have been documented in WIC or in primarily Latina mothers. Woo Baidal et al. (44), found that an obesity prevention intervention in children ages 2 through 4 years in WIC improved some obesity risk factors, including sugar-sweetened beverage consumption and sleep, but resulted in only modest improvements in zBMI and only in non-Asian children. Other research has suggested that promotion of breastfeeding might be the most optimal method to prevent child obesity in WIC (47). Given the widespread reach of WIC, which enrolled 8 million women and children in 2015, future research in WIC is needed to examine ripple effects of maternal interventions in diverse WIC populations and identify additional effective interventions (48–50) that might reduce obesity-risk factors in infants and children.
The study’s strengths include its cluster randomized design and objective measurement of infant zBMI by trained research assistants and mixed model analysis that allowed for inclusion of 333 participants. The study included measures of infant food and beverage intake and TV viewing. Limitations include use of self-report measures and the lack of measurement of other early life risk factors for obesity, including maternal feeding behaviours (e.g., responsive feeding), timing of introduction of solids. The study’s primary outcome was based on the WHO growth standards, which reflected growth patterns among children in several countries, including the USA, who were predominantly breastfed, which might not accurately represent this study’s population. While zBMI measurement is practical and inexpensive for clinical settings, error may result from a child who is uncooperative or who moves during the assessment. Skinfolds were only obtained in about 57% of the sample; thus, these results should be interpreted with caution. Also, there is controversy in the best methods to measure body composition in infants because of low correlation between skinfolds and body fat estimated by more accurate methods including hydrometry and MRI (51). Finally, it remains unknown whether maternal weight loss or the ‘Family Corner’ information provided at the end of each lesson could have led to a direct effect observed on infant zBMI.
In conclusion, this study found that a primarily internet-based intervention for postpartum mothers had a positive ‘ripple’ effect on the zBMI of infants in the home during the first 6 months of the program. The magnitude of the intervention’s effect on zBMI in the infants was smaller than that observed in their actively treated mothers, but these benefits were obtained without the assistance of a more structured and costly child behavioural program. Systematic reviews suggest that a multipronged approach will likely be needed to effectively prevent childhood obesity (52). Findings from the current study suggest that early life, obesity prevention interventions should consider including maternal behavioural weight loss as part of its armament.
Acknowledgements
S.P. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. S.P. and D.T. conceived and carried out the study. S.P. conducted the analysis and interpretation of data. S.P., T.H., A.B. and E.H. were responsible for data collection and management. S.P. drafted the manuscript. S.P., D.T., A.S., A.B., T.H., K.M.C., A.M. and A.V provided critical revision of the manuscript for important intellectual content. S.P. and D.T. obtained funding. A.B., K.E.H., T.H., K.M.C. and A.B. provided administrative, technical and material support. S.P. and D.T. provided study supervision. We thank Naomi Stotland, MD, of University of California at San Francisco and Barbara M. O’Brien, MD, formerly of Women and Infants Hospital in Rhode Island, for serving without pay as the data safety officers on this project. We thank Patricia Gradziel, PhD, RD at California State WIC, Linda McClure, RD at San Luis Obispo County WIC, Caro Stinson, RD, at Santa Barbara County WIC and Kathleen Rowe, MS, RD at Ventura County WIC, and the WIC staff who supported the study without additional pay and without whom this study would be impossible. We thank our paid research team members at Cal Poly, including Maria Legato, B.S., Teresa Sanchez, B.S. and Nick Katsantones, and, at the University of North Carolina at Chapel Hill, Molly Diamond, MPH. Wethankthe WIC moms who participated in the study receiving minimal compensation.
This research was supported by National Institutes of Health grant DK087889; the NIH was not involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication. Dr Tate reports non-financial support from Weight Watchers International, outside the submitted work. Dr Phelan reports grants from Weight Watchers International, also outside the submitted work.
Footnotes
Conflict of interest statement
Phelan reports receiving a grant from Weight Watchers International. Tate reports being a member of the scientific advisory board of Weight Watchers International. The authors have no other conflict of interest to report.
Reference
- 1.Gorin AA, Wing RR, Fava JL, et al. Weight loss treatment influences untreated spouses and the home environment: evidence of a ripple effect. Int J Obes (Lond) November 2008; 32: 1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagobian TA, Phelan S, Gorin AA, Phipps MG, Abrams B, Wing RR. Effects of maternal lifestyle intervention during pregnancy on untreated partner weight: Results from fit for delivery study. Obesity (Silver Spring) January 2016; 24: 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shattuck AL, White E, Kristal AR. How women’s adopted low-fat diets affect their husbands. Am J Public Health September 1992; 82: 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golan R, Schwarzfuchs D, Stampfer MJ, Shai I. Halo effect of a weight-loss trial on spouses: the DIRECT-Spouse study. Public Health Nutr April 2010; 13: 544–549. [DOI] [PubMed] [Google Scholar]
- 5.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol November 2008; 112:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brynhildsen J, Sydsjo A, Ekholm-Selling K, Josefsson A. The importance of maternal BMI on infant’s birth weight in four BMI groups for the period 1978–2001. Acta Obstet Gynecol Scand 2009; 88: 391–396. [DOI] [PubMed] [Google Scholar]
- 7.Agricultural Research Service Community Nutrition Research Group. Results from USDA’s 1994–96 Diet and Health Knowledge Survey: Table Set 19: U.S. Department of Agriculture;2000. [Google Scholar]
- 8.Savage JS, Fisher JO, Birch LL. Parental influence on eating behavior: conception to adolescence. J Law Med Ethics. Spring 2007; 35: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JP, Ellwood M. Feeding patterns and emotional care in breastfed infants. Soc Indic Res April 2011; 101: 227–231. [Google Scholar]
- 10.Paulus M The emergence of prosocial behavior: Why do infants and toddlers help, comfort, and share? Child Dev Perspect June 2014; 8: 77–81. [Google Scholar]
- 11.Eccles JS, Wigfield A. Motivational beliefs, values, and goals. Annu Rev Psychol 2002; 53: 109–132. [DOI] [PubMed] [Google Scholar]
- 12.Barresi J, Moore C. Intentional relations and social understanding. Behav Brain Sci March 1996; 19: 107. [Google Scholar]
- 13.Kenward B Gredeback G Infants help a non-human agent. Plos One September 18 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christakis NA, Fowler JH. Social contagion theory: examining dynamic social networks and human behavior. Stat Med February 20 2013; 32: 556–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kartner J, Keller H, Yovsi RD. Mother-infant interaction during the first 3 months: The emergence of culture-specific contingency patterns. Child Dev Mar-Apr 2010; 81: 540–554. [DOI] [PubMed] [Google Scholar]
- 16.Zheng M, Lamb KE, Grimes C, et al. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obes Rev October 2017:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ October 22 2005; 331: 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phelan S, Hagobian T, Brannen A, et al. Effect of an Internet-Based Program on Weight Loss for Low-Income Postpartum Women: A Randomized Clinical Trial. JAMA June 20 2017; 317: 2381 –2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leermakers EA, Anglin K, Wing RR. Reducing postpartum weight retention through a correspondence intervention. Int J Obes (Lond) 1998; 22: 1103–1109. [DOI] [PubMed] [Google Scholar]
- 20.Huseinovic E, Bertz F, Leu Agelii M, Hellebo Johansson E, Winkvist A, Brekke HK. Effectiveness of a weight loss intervention in postpartum women: results from a randomized controlled trial in primary health care. Am J Clin Nutr August 2016; 104: 362–370. [DOI] [PubMed] [Google Scholar]
- 21.Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Effectiveness of home based early intervention on children’s BMI at age 2: randomised controlled trial. BMJ. June 26 2012;344:e3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phelan S, Brannen A, Erickson K, et al. ‘Fit Moms/Mamas Activas’ internet-based weight control program with group support to reduce postpartum weight retention in low-income women: study protocol for a randomized controlled trial. Trials February 25 2015; 16: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin CL, Tate DF, Schaffner A, et al. Acculturation influences postpartum eating, activity, and weight retention in low-income Hispanic women. J Womens Health (Larchmt) December 2017; 26: 1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esserman D, Allore HG, Travison TG. The Method of Randomization for Cluster-Randomized Trials: Challenges of Including Patients with Multiple Chronic Conditions. Int J Stat Med Res January 8 2016; 5: 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States. Dept. of Agriculture. WIC The special supplemental nutrition program for women, infants, and children. Food and Nutrition Service: Washington, DC, 2008. [Google Scholar]
- 26.Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med August 14–28 2006; 166: 1620–1625. [DOI] [PubMed] [Google Scholar]
- 27.Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA April 9 2003; 289: 1833–1836. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. World Health Organization: Geneva, 2006. [Google Scholar]
- 29.Rifas-Shiman SL, Gillman MW, Oken E, Kleinman K, Taveras EM. Similarity of the CDC and WHO weight-for-length growth charts in predicting risk of obesity at age 5 years. Obesity (Silver Spring). June 2012; 20: 1261–1265. [DOI] [PubMed] [Google Scholar]
- 30.Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. September 2009; 124:S23–S34. [DOI] [PubMed] [Google Scholar]
- 31.Gutin B, Johnson MH, Humphries MC, et al. Relationship of visceral adiposity to cardiovascular disease risk factors in black and white teens. Obesity (Silver Spring). April 2007; 15: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization., World Health Organization. Nutrition for Health and Development. WHO child growth standards: head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: methods and development. World Health Organization: Geneva, 2007. [Google Scholar]
- 33.Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol. October 1995; 173:1176–1181. [DOI] [PubMed] [Google Scholar]
- 34.Slaughter MH, Lohman TG, Boileau RA, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. October 1988; 60: 709–723. [PubMed] [Google Scholar]
- 35.WHO Multicentre Growth Reference Study Group. Reliability of anthropometric measurements in the WHO Multicentre Growth Reference Study. Acta Paediatr Suppl. April 2006; 450: 38–46. [DOI] [PubMed] [Google Scholar]
- 36.Ohlin A, Rossner S. Factors related to body weight changes during and after pregnancy: the Stockholm Pregnancy and Weight Development Study. Obes Res. May 1996; 4: 271–276. [DOI] [PubMed] [Google Scholar]
- 37.Spurrier NJ, Magarey AA, Golley R, Curnow F, Sawyer MG. Relationships between the home environment and physical activity and dietary patterns of preschool children: a cross-sectional study. Int J Behav Nutr Phys Act. 2008; 5: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cacola P, Gabbard C, Santos DC, Batistela AC. Development of the Affordances in the Home Environment for Motor Development-Infant scale. Pediatr Int. December 2011; 53: 820–825. [DOI] [PubMed] [Google Scholar]
- 39.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol June 17 2002; 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. April 18 1998;316(7139):1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davison KK, Edmunds LS, Wyker BA, Young LM, Sarfoh VS, Sekhobo JP. Feasibility of increasing childhood outdoor play and decreasing television viewing through a family-based intervention in WIC, New York State, 2007-2008. Prev Chronic Dis. May 2011; 8: A54. [PMC free article] [PubMed] [Google Scholar]
- 42.Crawford PB, Gosliner W, Strode P, et al. Walking the talk: Fit WIC wellness programs improve self-efficacy in pediatric obesity prevention counseling. Am J Public Health. September 2004; 94: 1480–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sekhobo JP, Egglefield K, Edmunds LS, Shackman G. Evidence of the adoption and implementation of a statewide childhood obesity prevention initiative in the New York State WIC Program: the NY Fit WIC process evaluation. Health Educ Res. April 2012; 27: 281–291. [DOI] [PubMed] [Google Scholar]
- 44.Woo Baidal JA, Nelson CC, Perkins M, et al. Childhood obesity prevention in the women, infants, and children program: Outcomes of the MA-CORD study. Obesity (Silver Spring). July 2017; 25: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carruth BR, Ziegler PJ, Gordon A, Hendricks K. Developmental milestones and self-feeding behaviors in infants and toddlers. J Am Diet Assoc. January 2004; 104: s51–s56. [DOI] [PubMed] [Google Scholar]
- 46.Savage JS, Birch LL, Marini M, Anzman-Frasca S, Paul IM. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year: A randomized clinical trial. JAMA pediatrics August 1 2016; 170: 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nianogo RA, Wang MC, Wang A, et al. Projecting the impact of hypothetical early life interventions on adiposity in children living in low-income households. Pediatric obesity. October 2017; 12: 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khanal S, Welsby D, Lloyd B, Innes-Hughes C, Lukeis S, Rissel C Effectiveness of a once per week delivery of a family-based childhood obesity intervention: a cluster randomised controlled trial. Pediatric obesity. December 2016; 11: 475–483. [DOI] [PubMed] [Google Scholar]
- 49.Norman G, Huang J, Davila EP, et al. Outcomes of a 1-year randomized controlled trial to evaluate a behavioral ‘stepped-down’ weight loss intervention for adolescent patients with obesity. Pediatric obesity. February 2016; 11: 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rhodes ET, Vernacchio L, Mitchell AA, et al. A telephone intervention to achieve differentiation in dietary intake: a randomized trial in paediatric primary care. Pediatric obesity. December 2017; 12: 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toro-Ramos T, Paley C, Pi-Sunyer FX, Gallagher D. Body composition during fetal development and infancy through the age of 5 years. Eur J Clin Nutr. December 2015; 69: 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Cai L, Wu Y, et al. What childhood obesity prevention programmes work? A systematic review and metaanalysis. Obes Rev. July 2015; 16: 547–565. [DOI] [PMC free article] [PubMed] [Google Scholar]