Abstract
STUDY QUESTION
Are women who fill a benzodiazepine prescription before conception at increased risk of ectopic pregnancy?
SUMMARY ANSWER
Risk of ectopic pregnancy is 50% higher among women who fill a benzodiazepine prescription before conception.
WHAT IS KNOWN ALREADY
Benzodiazepine use in pregnancy increases the risk of miscarriage, adverse birth outcomes and adverse child development outcomes.
STUDY DESIGN, SIZE, DURATION
Using data from US commercial insurance claims, we performed a cohort study of 1 691 366 pregnancies between 1 November 2008 and 30 September 2015.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We identified ectopic pregnancies using diagnosis and procedure codes and used unadjusted and inverse probability of treatment (IPT)-weighted log-binomial models to calculate relative risks (RR) of ectopic pregnancy for pregnant women who did and did not fill any prescriptions for benzodiazepines in the 90 days before conception. Two sub-groups of women with specific indications for benzodiazepine use were also examined—women who had a least one diagnosis for anxiety disorder and women who had at least one diagnosis of insomnia in the year before conception.
MAIN RESULTS AND THE ROLE OF CHANCE
Of the 1 691 366 pregnancies, 1.06% filled at least two benzodiazepine prescriptions totaling at least 10 days supply in the 90 days before conception. Among women with a benzodiazepine prescription, there was an excess of 80 ectopic pregnancies per 10 000 pregnancies, and their IPT-weighted risk of ectopic pregnancies was 1.47 (95% CI 1.32 to 1.63) times greater relative to women without benzodiazepine prescriptions before conception. The IPT-weighted RR between ectopic pregnancy and benzodiazepine use was 1.34 (95% CI 1.18 to 1.53) among women with anxiety disorder diagnoses and 1.28 (95% CI 0.99 to 1.68) among women with an insomnia diagnosis.
LIMITATIONS, REASONS FOR CAUTION
We relied on outpatient prescription data to identify benzodiazepine use before conception, which could result in over- or under-estimation of actual benzodiazepine consumption. We relied on medical claim codes to identify pregnancies and conception date, which may result in misclassification of pregnancy outcomes and gestational length.
WIDER IMPLICATIONS OF THE FINDINGS
This study found that women who have a benzodiazepine prescription before conception are at an increased risk of ectopic pregnancy. This information can help women, and their healthcare providers make more fully informed decisions about benzodiazepine use in their reproductive years.
STUDY FUNDING/COMPETING INTEREST(S)
Funding for this project was provided by a Banting Postdoctoral Fellowship and a Stanford Maternal and Child Health Research Institute Postdoctoral Award. Data access for this project was provided by the Stanford Center for Population Health Sciences Data Core. The PHS Data Core is supported by a National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085) and internal Stanford funding. The authors have no competing interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: ectopic pregnancy, benzodiazepines, cohort studies, pharmacoepidemiology, anxiety disorders
Introduction
Ectopic pregnancy occurs in 1 to 2% of the approximately 6.3 million recognized pregnancies in the USA every year, resulting in up to 126 000 ectopic pregnancies annually (Hoover et al., 2010; Curtin et al., 2013). Ectopic pregnancy is responsible for 6–13% of all pregnancy-related deaths, and hemorrhage from ectopic pregnancy is the leading cause of pregnancy-related death in the first trimester (Chang et al., 2003; Creanga et al., 2011). Additionally, around 11% of ectopic pregnancies result in serious complications, such as blood transfusion and sterilization (Stulberg et al., 2016). Most ectopic pregnancy implantations (>98%) occur in the fallopian tube; this occurs when changes to the tubal environment impair embryo-tubal transport (including smooth muscle contraction and ciliary beating) resulting in early implantation (Halbert et al., 1976; Walker, 2007; Shaw et al., 2010). Although several risk factors for ectopic pregnancy have been identified, such as pelvic infections, use of reproductive technology, intrauterine devices, smoking and increased age, approximately half of women who have an ectopic pregnancy do not have any known risk factors (Kamwendo et al., 2000; Bouyer 2003; Backman et al., 2004; Barnhart et al., 2006; Li et al., 2015; Du et al., 2017; Mikhail et al., 2018). A potential but unstudied risk factor for ectopic pregnancy is benzodiazepine use. Benzodiazepine use could affect muscle contraction in the fallopian tube through two mechanisms—through the central relaxation of smooth muscle and the direct effect on GABA receptors in the fallopian tube (French et al., 1989; László et al., 1989).
Benzodiazepine use has increased substantially in the USA in the past few decades and is commonly prescribed for indications such as anxiety disorder, insomnia, acute alcohol withdrawal and seizures (Lembke et al., 2018). Based on national data from 2008 in the USA, ~3.6% of women between ages 18 and 35 years had a prescription for benzodiazepine in a 1-year period (Olfson et al., 2015). While the use of benzodiazepines during pregnancy has been found to be associated with miscarriage, adverse birth outcomes and child development outcomes (Kieviet et al., 2013; Yonkers et al., 2017; Sheehy et al., 2019), it is unknown whether their use affects ectopic pregnancy. We conducted a cohort study using a large national database of privately insured women in the USA to assess whether women who are prescribed benzodiazepines before conception are at increased risk of ectopic pregnancy.
Materials and Methods
We conducted a cohort study of pregnant women identified in the IBM® MarketScan® Research Databases (MarketScan). MarketScan is a large administrative claims database including information on a portion of the US population covered by employer-sponsored insurance (IBM Corporation, 2018). This database allows for linkages at the individual level of inpatient services, outpatient services, outpatient pharmacy prescription claims and healthcare plan enrollment information.
Pregnancies were identified using International Classification of Diseases (ICD)-9 diagnosis and procedure codes, Diagnosis-Related Group codes, Current Procedural Terminology codes and Healthcare Common Procedure Coding System codes from inpatient and outpatient services, and National Drug Codes from outpatient pharmaceutical claims. Codes used to define pregnancies are consistent with previous research (Scholes et al., 2011; Ailes et al., 2016). Intrauterine pregnancies (i.e. those ending in live birth, stillbirth, miscarriage and abortion) were identified using a set of claims codes identified by Ailes et al., (2016); there had to be at least a 2-month gap between pregnancies for codes to be deemed valid (Ailes et al., 2016). Ectopic pregnancies were identified using a validated algorithm developed by Scholes et al., (2011); this algorithm required there to be at least two ectopic pregnancy-related diagnoses or procedures, or an ICD-9 diagnosis code for a tubal pregnancy (633.10, 633.11), or at least one ectopic pregnancy-related diagnosis or procedure code followed by a methotrexate dispensation or injection (Scholes et al., 2011).
Gestational age is not available in the claims data; therefore, we used an algorithm to estimate the date of last menstrual period, which was then used to estimate conception as 14 days after the last menstrual period (Ailes et al., 2016). This algorithm estimates ectopic pregnancy to end at 56 days of gestation and intrauterine pregnancies to end between 56- and 301-day gestation. For intrauterine pregnancies, information in the codes used to identify pregnancies was used to estimate the last menstrual period. For example, if a live birth was identified with an ICD-9 code of 765.14 (other preterm infant, 1000–1259 grams), the last menstrual period was estimated to be 29 weeks before the birth. Detailed information on identification of pregnancies in the MarketScan database is provided in Supplementary Table SI and Supplementary File S1.
Benzodiazepine prescriptions were identified from outpatient pharmacy files through national drug codes and generic drug names. Drugs included are alprazolam, chlordiazepoxide, clobazam, clonazepam, clorazepate, diazepam, estazolam, flurazepam, lorazepam, oxazepam, quazepam, temazepam and triazolam (National Center for Injury Prevention and Control, 2017). Benzodiazepines are generally prescribed to be taken as needed, and not to be taken on a daily basis. Thus, to capture women who are most likely to be taking benzodiazepines before conception, we defined exposure as having at least two benzodiazepine prescriptions totaling at least 10 days’ supply in the 90 days before their estimated conception date.
The cohort included pregnancies identified in the MarketScan databases between 1 November 2008 and 30 September 2015 among women aged 15 to 44. Not all women included in the MarketScan database had insurance that included prescription drug coverage; therefore, we excluded those whose insurance plan did not include this coverage. We excluded women who did not have continuous enrollment in the year before conception—this ensured complete claims information in the 9-month baseline and the 90-day exposure periods. We also excluded pregnancies that had a prescription for benzodiazepine in the exposure period, but did not meet our exposure criteria; this included women who had only one prescription or had more than one prescription but having fewer than 10 days’ supply of benzodiazepines in the 90 days before conception. Our final cohort included 1 691 366 pregnancies (Fig. 1).
Figure 1.
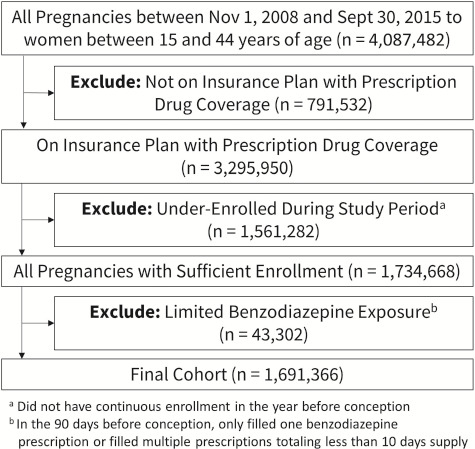
Cohort selection process.
Statistical analyses
The relative risk (RR) of ectopic pregnancy in pregnancies with benzodiazepine prescriptions in the exposure period compared with pregnancies without benzodiazepine prescriptions in the exposure period was estimated using log-linked binomial models. To reduce the threat to validity from confounding variables, we used a multistep algorithm to apply high-dimensional propensity score adjustments (Schneeweiss et al., 2009). Unlike traditional propensity score methods, this algorithm identifies potential confounders from a database by selecting variables correlated to both the exposure and outcome, prioritizing covariates by prevalence and potential for bias (Schneeweiss et al., 2017). Covariates were drawn from three data dimensions during the 9 months prior to the start of the exposure period, which include (i) inpatient and outpatient diagnostic codes (3-digit ICD-9-CM codes), (ii) inpatient and outpatient procedure codes (4-digit ICD-9-CM codes) and (iii) outpatient prescription medication claims (11-digit NDCs) (Rassen et al., n.d.; Schneeweiss et al., 2009, 2017). The inclusion of near-instruments in the high-dimensional propensity score model may be amplifying bias (Myers et al., 2011); thus, we excluded pre-exposure period benzodiazepine prescriptions the inverse probability of treatment weight analysis. In addition to the health variables obtained from these data, we also included the year (2007–2011, 2012–2015) and women’s age at the end of the pregnancy (15–19, 20–24, 25–29, 30–34, 35–39, 40–44), and five pre-defined conditions identified as being risk factors for ectopic pregnancy. These conditions are pelvic infections, sexually transmitted infections, assisted reproductive technology, intrauterine devices and smoking in the 6 months before the exposure period (Kamwendo et al., 2000; Bouyer, 2003; Backman et al., 2004; Du et al., 2017); Supplementary Table SII lists the codes used to identify these conditions.
For each model, high-dimensional propensity scores were estimated using demographic and predefined conditions, as well as the top 500 covariates selected from the health databases. Inverse probability of treatment weights was derived from the propensity scores and stabilized. To minimize the influence of outlier weights, inverse probability of treatment weights were truncated beyond the pre-specified range of 0.10 and 10 (Harder et al., 2010). Unweighted and weighted standardized differences of baseline characteristics by exposure are presented for each model in the Supplementary Tables SIII–SXI; we consider a covariate to be balanced if the standardized difference is <0.25 (Harder et al., 2010).
Confounding by indication is important to consider when examining the effect of a particular treatment because clinical indications for being prescribed a particular medication could also affect the outcome (Kyriacou and Lewis, 2016). To identify potential confounding by indication, we examined risk of ectopic pregnancy among pregnancies for which the woman had one of the two most common indications for benzodiazepine prescription—anxiety and insomnia; codes used to define these conditions are provided in Supplementary Table SII. Unadjusted and inverse probability of treatment-weighted log-linked binomial regression models were first conducted on a cohort limited to pregnancies that had at least one anxiety diagnosis in the year before conception (n = 90 479) and then conducted on a cohort limited to pregnancies that had at least one insomnia diagnosis in the year before conception (n = 21 802).
We conducted several sensitivity analyses to examine how our exposure definition affected the results of this study. In our first sensitivity analysis, we used a less strict definition of exposure, in which all women who filled at least one benzodiazepine prescription in the 90 days before conception were defined as exposed. This increased our cohort to 1 734 668 pregnancies, of which 61 292 were exposed to benzodiazepines before conception. In our second sensitivity analysis, we used a stricter definition of exposure. While we can identify women who filled a prescription, we are unable to identify whether women ingested the benzodiazepines. To increase the likelihood of capturing only women who took benzodiazepines right before conception, we required at least two prescriptions to be filled within the 90-day exposure period totaling at least 10 days’ supply, and one of these prescriptions to be filled in the 30 days before conception. In this analysis, pregnancies with at least two prescriptions totaling at least 10 days supply, where both were filled before 30 days before their last menstrual period (n = 4177), were defined as ‘questionable benzodiazepine exposure’ and excluded from the analysis, resulting in a cohort of 1 687 189 pregnancies. All data management, programming and analyses were performed using SAS, version 9.4 (SAS Institute Inc., 2013).
Ethical approval
The study was approved by the institutional review board of Stanford University and the need for informed consent was waived.
Results
Our study included 1 691 366 pregnancies, of which 30 046 (1.78%) were ectopic and 17 990 (1.06%) were to women who had a benzodiazepine prescription before conception. Women who were older at the time of their pregnancy, those who had a sexually transmitted infection, pelvic infection, intrauterine device, used assisted reproductive technology and those who had a smoking-related diagnosis or procedure in the 9 months before the exposure period were more likely to be have a benzodiazepine prescription before conception (Table I).
Table I.
Comparison of baseline characteristics among women with and without benzodiazepine prescriptions before conception (n = 1 691 366).
| Characteristic | Had a benzodiazepine prescription a (n = 17 990) | Did not have any benzodiazepine prescriptions (n = 1 673 376) |
|---|---|---|
| No. (%) | No. (%) | |
| Age | ||
| 15–19 | 427 (2.37) | 84 219 (5.03) |
| 20–24 | 2330 (12.95) | 199 522 (11.92) |
| 25–29 | 4183 (23.25) | 449 669 (26.87) |
| 30–34 | 5901 (32.80) | 561 771 (33.57) |
| 35–39 | 3856 (21.43) | 298 499 (17.84) |
| 40–44 | 1293 (7.19) | 79 696 (4.76) |
| Year | ||
| 2008–2011 | 7578 (42.12) | 760 223 (45.43) |
| 2012–2015 | 10 412 (57.88) | 913 153 (54.57) |
| Medical conditions in the 9 months before the exposure period b | ||
| Sexually transmitted infection | 280 (1.56) | 17 166 (1.03) |
| Assisted reproductive technology | 119 (0.66) | 7135 (0.43) |
| Pelvic infection | 163 (0.91) | 5973 (0.36) |
| Intrauterine device | 585 (3.25) | 42 018 (2.51) |
| Smoking | 1451 (8.07) | 26 828 (1.60) |
aHad at least two benzodiazepine prescriptions totaling at least 10 days’ supply in the 90 days before conception
bExposure period is the 90 days before conception
There were 80 excess ectopic pregnancy per 10 000 pregnancies among pregnancies exposed to benzodiazepines compared to pregnancies not exposed to benzodiazepine prescriptions before conception (Table II). After IPT weighting, pregnancies with benzodiazepine prescriptions had 1.47 (95% CI 1.32–1.63) times higher risk of ectopic pregnancy than pregnancies without benzodiazepine prescriptions.
Table II.
Frequency of pregnancies and relative risk of ectopic pregnancies between women with and without a benzodiazepine prescription.
| Exposure | Had a benzodiazepine prescription | Did not have any benzodiazepine prescriptions | Risk differenceb (95% CI) | Relative risk (95% CI) | |||
|---|---|---|---|---|---|---|---|
| N | Eventsa (Riskb) | N | Eventsa (riskb) | Crude | IPT-weightedb | ||
| Any benzodiazepine | 17 990 | 462 (257) | 1 673 376 | 29 584 (177) | 80 (57, 103) | 1.45 (1.33, 1.59) | 1.47 (1.32, 1.63)d |
| Specific benzodiazepine c | |||||||
| Alprazolam | 9261 | 237 (256) | 1 682 105 | 29 809 (177) | 79 (46, 111) | 1.44 (1.27, 1.64) | 1.51 (1.31, 1.73)e |
| Clonazepam | 5527 | 126 (228) | 1 685 839 | 29 920 (177) | 50 (11, 90) | 1.28 (1.08, 1.53) | 1,19 (0.96, 1.48)f |
| Lorazepam | 3568 | 93 (261) | 1 687 798 | 29 953 (177) | 83 (31, 135) | 1.47 (1.20, 1.80) | 1.51 (1.20, 1.90)g |
| Diazepam | 1847 | 55 (298) | 1 689 519 | 29 991 (178) | 120 (43, 198) | 1.68 (1.29, 2.18) | 1.49 (1.08, 2.07)h |
Abbreviations: IPT = inverse probability of treatment
aNumber of ectopic pregnancies
bPer 10 000 pregnancies
cWomen can have a prescription for more than one type of benzodiazepine
dSee Supplementary Table SIII for unweighted and IPT-weighted standardized differences of baseline characteristics
eSee Supplementary Table SIV for unweighted and IPT-weighted standardized differences of baseline characteristics
fSee Supplementary Table SV for unweighted and IPT-weighted standardized differences of baseline characteristics
gSee Supplementary Table SVI for unweighted and IPT-weighted standardized differences of baseline characteristics
hSee Supplementary Table SVII for unweighted and IPT-weighted standardized differences of baseline characteristics
The most commonly used benzodiazepines are alprazolam, clonazepam, lorazepam and diazepam. Other than clonazepam, the weighted RR of ectopic pregnancy remained substantially higher for women taking any of these benzodiazepines compared to women not taking these benzodiazepines, with IPT-weighted RR ranging from 1.19 (95% CI 0.96, 1.48) for women taking clonazepam, to 1.51 (1.20, 1.90) for women taking lorazepam.
When limiting the cohort to women who had an anxiety diagnosis in the year before the start of their pregnancy, the IPT-weighted RR of ectopic pregnancy among women who had a benzodiazepine prescription remained significantly higher (weighted RR = 1.33, 95% CI 1.17, 1.51). The unweighted RR of ectopic pregnancy remained significantly higher for women with a benzodiazepine prescription when limiting the cohort to women who had an insomnia diagnosis in the year before conception (RR = 1.36, 95% CI 1.06, 1.5). However, the IPT-weighted RR of ectopic pregnancy was not significantly higher among women exposed to benzodiazepines than women not exposed to benzodiazepines (weighted RR = 1.28, 95% CI 0.99, 1.68) (Table III).
Table III.
Frequency of pregnancies and relative risk of ectopic pregnancies between women with and without a benzodiazepine prescription, by indication.
| Indication | N | Eventsa (riskb) | Risk differenceb (95% CI) | Relative risk (95% CI) | |
|---|---|---|---|---|---|
| Crude | IPT-weighted | ||||
| Anxiety (n = 90 479) | |||||
| No benzodiazepine Prescription | 81 291 | 1730 (213) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Benzodiazepine prescription | 9188 | 249 (2.71) | 58 (24, 93) | 1.27 (1.12, 1.45) | 1.33 (1.17, 1.51)c |
| Insomnia (n = 21 802) | |||||
| No benzodiazepine prescription | 19 588 | 442 (226) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Benzodiazepine prescription | 2214 | 68 (307) | 81 (7, 156) | 1.36 (1.06, 1.75) | 1.28 (0.99, 1.68)d |
Abbreviations: IPT = inverse probability of treatment
aNumber of ectopic pregnancies
bPer 10 000 pregnancies
cSee Supplementary Table SVIII for unweighted and IPT-weighted standardized differences of baseline characteristics
dSee Supplementary Table SIX for unweighted and IPT-weighted standardized differences of baseline characteristics
Sensitivity analyses
We conducted two sensitivity analyses, the first using a less strict definition of benzodiazepine exposure (at least one prescription in the 90 days before conception), and the second using a stricter definition of benzodiazepine exposure (at least two prescriptions in the 90 days before conception totaling 10 days’ supply, with at least one of these prescriptions being filled in the 30 days before conception). When using the less strict definition, we found that 61 292 (3.5%) of the 1 734 668 pregnancies in the cohort were exposed to benzodiazepines before conception (Table IV). For this cohort, the association was not as strong as what we saw in our original analysis (weighted RR = 1.27, 95% CI 1.20–1.34). When using the stricter definition of benzodiazepine exposure, we found that 13 813 (0.8%) of the 1 687 189 pregnancies in the cohort were exposed to benzodiazepines before conception. In this analysis, the association was stronger than what we saw in our original analysis (weighted RR = 1.54, 95% CI 1.37–1.73).
Table IV.
Frequency of pregnancies and relative risk of ectopic pregnancies between women with and without a benzodiazepine prescription, by exposure definition.
| Benzodiazepine exposure definition | Had a benzodiazepine prescription | Did not have any benzodiazepine prescriptions | Risk differenceb (95% CI) | Relative risk (95% CI) | |||
|---|---|---|---|---|---|---|---|
| N | Eventsa (Riskb) | N | Eventsa (riskb) | Crude | IPT-weightedb | ||
| Less strict* definition | 61 292 | 1555 (254) | 1 673 376 | 29 584 (177) | 77 (64, 90) | 1.44 (1.36, 1.51) | 1.27 (1.20, 1.34)c |
| Stricter** definition | 13 813 | 354 (258) | 1 673 376 | 29 584 (177) | 81 (55, 108) | 1.46 (1.32, 1.62) | 1.54 (1.37, 1.73)d |
Abbreviations: IPT = inverse probability of treatment
*At least one benzodiazepine prescription in the 90 days before conception
**At least two benzodiazepine prescriptions in the 90 days before conception totaling at least 10 days supply, with at least one benzodiazepine prescription in the 30 days before conception
aNumber of ectopic pregnancies
bPer 10 000 pregnancies
cSee Supplementary Table SX for unweighted and IPT-weighted standardized differences of baseline characteristics
dSee Supplementary Table SXI for unweighted and IPT-weighted standardized differences of baseline characteristics
Discussion
In this study of pregnancies using a large cohort covered through employer-based insurance in the USA between 2008 and 2015, we found an increased risk of ectopic pregnancy among women who filled at least two benzodiazepine prescriptions totaling at least 10 days’ supply in the 90 days before conception compared to women who did not have a benzodiazepine prescription in this time period. Although attenuated, the risk of ectopic pregnancy remained significantly higher among women who had an anxiety diagnosis in the 9 months before conception. The attenuation of results when limiting our cohort to women with specific indications for benzodiazepine use suggests that at least some of the association between benzodiazepine use and ectopic pregnancy can be explained by the conditions that are being treated with benzodiazepines. This study adds valuable information on the safety of benzodiazepines for women who may become pregnant and has important clinical and public health implications.
Previous studies on the use of benzodiazepine and reproductive outcomes have focused on benzodiazepine use have reported increased risks for spontaneous abortion, preterm birth, low birth weight and congenital malformations (Morgan and Winship, 2007; Wikner et al., 2007; Bellantuono et al., 2013; Sheehy et al., 2019). No previous studies have examined the use of benzodiazepines before conception and risk of ectopic pregnancy; however, there is biologic plausibility for a potential relationship (Zhou et al., 2010). Embryo-tubal transport is facilitated through smooth muscle contraction and ciliary beating; thus, changes to these mechanisms could impair transportation (Halbert et al., 1976; Walker, 2007; Shaw et al., 2010). Binding of GABA to GABA-A receptors in the fallopian tube is known to alter fallopian contractility (Erdö, 1986). In the normal pregnant oviduct, concentrations of GABA are reduced but the expression of GABA-A receptors is increased (László et al., 1989). The action of benzodiazepines, which bind to the GABA-A receptor, could result in excessive GABA-A-mediated transmission, reducing fallopian contractility. This could result in failure of the ovum to progress along the oviduct and increase risk for fertilization and implantation within the tube.
This study provides important information regarding the potential effects of benzodiazepines on women’s reproductive health. For women who filled at least two prescriptions for benzodiazepines totaling at least 10 days’ supply in the 90 days before conception, particularly if they also have other risk factors for ectopic pregnancy, providers could provide early pelvic ultrasound to assess for intrauterine (normal) vs. ectopic (potentially life-threatening) pregnancy, allowing them to identify and treat ectopic pregnancy in order to avoid tubal rupture (Cacciatore et al., 1994; Barnhart, 2009).
The use of the MarketScan database is a significant strength of our study, as it provides a large cohort, objective assessment of potential drug exposure, and a wide range of potential confounders through linkages to clinical records. Our analysis also has several limitations. We used an algorithm to identify pregnancy outcomes and last menstrual period and were unable to validate these estimates using data from medical records or birth certificates. This approach could result in misclassification; however, previous studies have validated similar algorithms, demonstrating good to excellent agreement on designation and dating of pregnancy outcomes (Hornbrook et al., 2006). Ectopic pregnancies are generally identified between 42 and 84 days after a pregnant woman’s last menstrual period; given this small range in dates and the accuracy of the algorithm in identifying the last menstrual period before an ectopic pregnancy, we are confident that our exposure window includes their conception date (Matcho et al., 2018).
Relying on outpatient prescription data to identify medication use could result in over- or under-estimation of actual benzodiazepine consumption. Not all dispensed benzodiazepines are consumed, which would result in overestimation of exposure. Underestimation of exposure could result from benzodiazepines consumed during inpatient hospitalizations, benzodiazepines paid for out-of-pocket and benzodiazepines obtained from sources other than a prescription for the woman. Further, benzodiazepines are often consumed on an acute basis; women may start and stop use when symptoms appear or reappear and may be using benzodiazepines long after the dispensing date. In our sensitivity analyses, the association between benzodiazepine exposure and ectopic pregnancy was weaker when using a less strict definition, and stronger when using a stricter definition of exposure to benzodiazepines before conception, increasing our confidence in our findings.
The use of inpatient and outpatient claims allowed us to adjust for a wide range of potential confounders; however, this approach cannot remove confounding by variables that are not well captured in the data and for which there are no suitable proxies. For example, it is known that smoking status is underestimated in claims data (Desai et al., 2016). The data also had limited information on sociodemographic characteristics. Benzodiazepine use during pregnancy is higher among women who have had previous miscarriages, and those with higher parity (Wikner et al., 2007); these factors could affect both benzodiazepine use and ectopic pregnancy. Lastly, our cohort included only women with employer-based medical insurance coverage. We suspect that the mechanism responsible for the observed association is biological; therefore, we expect our findings to be generalizable to women not covered through employer-based insurance.
A lack of information on the safety of drug treatment during pregnancy is a serious public health concern (Adam et al., 2011). Most studies that have examined the effects of drug treatment in pregnancy have focused on pregnancy complications and fetal development; fewer have examined the potential effects of these treatments on the risk of ectopic pregnancy and other early pregnancy loss. Given the serious consequences associated with ectopic pregnancy, it is important to better understand risk factors, which could help women and their care providers make more fully informed decisions about benzodiazepine use.
Authors’ roles
E.W.: conception and design, analysis and interpretation of data, drafting the article and critical revision, funding and approval of the final manuscript. T.R.: conception and design, revising article for important intellectual content and approval of the final manuscript. D.L.: conception and design, revising article for important intellectual content and approval of the final manuscript. R.M.: conception and design, revising article for important intellectual content and approval of the final manuscript. R.P.: conception and design, revising article for important intellectual content and approval of the final manuscript. S.C.: data acquisition, conception and design, revising article for important intellectual content and approval of the final manuscript.
Funding
This study was funded through a Banting Postdoctoral Fellowship (Elizabeth Wall-Wieler) and a Stanford Maternal and Child Health Research Institute Postdoctoral Award (Elizabeth Wall-Wieler). Dr Platt holds the Albert Boehringer I Chair in Pharmacoepidemiology and is supported by a Foundation Grant from CIHR (FDN-143927) and by the Canadian Network for Observational Drug Effect Studies (CNODES, CIHR grant DSE-111845). Data access for this project was provided by the Stanford Center for Population Health Sciences Data Core. The PHS Data Core is supported by a National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085) and internal Stanford funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
None to declare.
Supplementary Material
References
- Adam MP, Polifka JE, Friedman JM. Evolving knowledge of the teratogenicity of medications in human pregnancy. Am J Med Genet Part C Semin Med Genet 2011;157:175–182. [DOI] [PubMed] [Google Scholar]
- Ailes EC, Simeone RM, Dawson AL, Petersen EE, Gilboa SM. Using insurance claims data to identify and estimate critical periods in pregnancy: an application to antidepressants. Birth Defects Res Part A - Clin Mol Teratol 2016;106:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman T, Rauramo I, Huhtala S, Koskenvuo M. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol 2004;190:50–54. [DOI] [PubMed] [Google Scholar]
- Barnhart KT. Ectopic pregnancy. N Engl J Med 2009;361:379–387. [DOI] [PubMed] [Google Scholar]
- Barnhart KT, Sammel MD, Gracia CR, Chittams J, Hummel AC, Shaunik A. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril 2006;86:36–43. [DOI] [PubMed] [Google Scholar]
- Bellantuono C, Tofani S, Sciascio G. Di Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013;35:3–8. [DOI] [PubMed] [Google Scholar]
- Bouyer J. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol 2003;157:185–194. [DOI] [PubMed] [Google Scholar]
- Cacciatore B, Stenman U-H, Ylostalo P. Early screening for ectopic pregnancy in high-risk symptom-free women. Lancet 1994;343:517–518. [DOI] [PubMed] [Google Scholar]
- Chang J, Elam-Evans L, Berg C, Herndon J, Flowers L, Seed K, Syverson C. Pregnancy-related mortality surveillance - United States, 1991-1999. MMWR Surveill Summ 2003;52:1–8. [PubMed] [Google Scholar]
- Creanga AA, Shapiro-Mendoza CK, Bish CL, Zane S, Berg CJ, Callaghan WM. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol 2011;117:837–843. [DOI] [PubMed] [Google Scholar]
- Curtin SC, Abma JC, Ventura SJ, Henshaw SK. Pregnancy rates for U.S. women continue to drop. NCHS data brief, no 136 Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- Desai R, Solomon D, Shadick N, Iannaccone C, Kim S. Identification of smoking using Medicare data - a validation study of claims-based algorithms. Pharmacoepidemiol Drug Saf 2016;25:472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine S, West S, Andrews E, Tennis P, Hammad TA, Eaton S, Thorp J, Olshan A. The identification of pregnancies within the general practice research database. Pharmacoepidemiol Drug Saf 2010;19:45–50. [DOI] [PubMed] [Google Scholar]
- Du T, Chen H, Fu R, Chen Q, Wang Y, Mol BW, Kuang Y, Lyu Q. Comparison of ectopic pregnancy risk among transfers of embryos vitrified on day 3, day 5, and day 6. Fertil Steril 2017;108:108–116.e1. [DOI] [PubMed] [Google Scholar]
- Erdö S. GABAergic mechanisms and their possible role in the oviduct and uterus In: Erdö S, Bowery N (eds). GABAergic Mech Mamm Peripher. New York: Raven Press, 1986,205–217. [Google Scholar]
- French J, Rapoport R, Matlib M. Possible mechanism of benzodiazepine-induced relaxation of vascular smooth muscle. J Cardiovasc Pharmacol 1989;14:405–411. [DOI] [PubMed] [Google Scholar]
- Halbert S, Tabm P, Blandau R. Egg transport in the rabbit oviduct: the roles of cilia and muscle. Science (80-) 1976, 191;4231:1052–1053. [DOI] [PubMed] [Google Scholar]
- Harder V, Stuart E, Anthony J. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 2010;15:234–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover K, Tao G, Kent C. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstet Gynecol 2010;115:495–502. [DOI] [PubMed] [Google Scholar]
- Hornbrook MC, Dietz PM, Williams SB, Gold R, Bruce FC, Whitlock EP, Callaghan WM, Berg CJ, Bachman DJ. Development of an algorithm to identify pregnancy episodes in an integrated health care delivery system. Health Serv Res 2006;42:908–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Watson Health. IBM MarketScan Research Databases for life sciences researchers. Somers NY: IBM Corporation; 2019. [Google Scholar]
- Kamwendo F, Forslin L, Bodin L, Danielsson D. Epidemiology of ectopic pregnancy during a 28 year period and the role of pelvic inflammatory disease. Sex Transm Infect 2000;76:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieviet N, Dolman KM, Honig A. The use of psychotropic medication during pregnancy: how about the newborn? Neuropsychiatr Dis Treat 2013;9:1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriacou D, Lewis R. Confounding by indication in clinical research. JAMA 2016;316:1818–1819. [DOI] [PubMed] [Google Scholar]
- László Á, Villányi P, Zsolnai B, Erdö SL. Gamma-aminobutyric acid, its related enzymes and receptor-binding sites in the human ovary and fallopian tube. Gynecol Obstet Invest 1989;28:94–97. [DOI] [PubMed] [Google Scholar]
- Lembke A, Papac J, Humphreys K. Our other prescription drug problem. N Engl J Med 2018;378:693–695. [DOI] [PubMed] [Google Scholar]
- Li C, Zhao W-H, Zhu Q, Cao S-J, Ping H, Xi X, Qin G-J, Yan M-X, Zhang D, Qiu J et al. Risk factors for ectopic pregnancy: a multi-center case-control study. BMC Pregnancy Childbirth 2015;15:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus EV, Guérin A, Yu AP, Wu EQ, Yang M, Chao J, Mulani PM. Increased risks of developing anxiety and depression in young patients with Crohn’s disease. Am J Gastroenterol 2011;106:1670–1677. [DOI] [PubMed] [Google Scholar]
- MacDonald SC, Cohen JM, Panchaud A, McElrath TF, Huybrechts KF, Hernández-Díaz S. Identifying pregnancies in insurance claims data: methods and application to retinoid teratogenic surveillance. Pharmacoepidemiol Drug Saf 2019;28:1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AS, Monsour M, Kissin DM, Jamieson DJ, Callaghan WM, Boulet SL. Trends in severe maternal morbidity after assisted reproductive technology in the United States, 2008-2012. Obstet Gynecol 2016;127:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcho A, Ryan P, Fife D, Gifkins D, Knoll C, Friedman A. Inferring pregnancy episodes and outcomes within a network of observational databases. PLoS One 2018;13:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee DL, Hu Z, Stahlman S. Incidence and sequelae of acute pelvic inflammatory disease among active component females, U.S. Armed Forces, 1996–2016. MSMR 2018;25:2–8. [PubMed] [Google Scholar]
- Mikhail E, Salemi JL, Schickler R, Salihu HM, Plosker S, Imudia AN. National rates, trends and determinants of inpatient surgical management of tubal ectopic pregnancy in the United States, 1998–2011. J Obstet Gynaecol Res 2018;44:730–738. [DOI] [PubMed] [Google Scholar]
- Morgan S, Winship C. Counterfactuals and Causal Inference: Methods and Principles for Social Research. New York: Cambridge University Press, 2007. [Google Scholar]
- Myers JA, Rassen JA, Gagne JJ, Huybrechts KF, Schneeweiss S, Rothman KJ, Joffe MM, Glynn RJ. Effects of adjusting for instrumental variables on bias and precision of effect estimates. Am J Epidemiol 2011;174:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control. CDC Compilation of Benzodiazepines, Muscle Relaxants, Stimulants, Zolpidem, and Opioid Analgesics with Oral Morphine Milligram Equivalent Conversion Factors, 2017 Version. Atlanta: Centers for Disease Control and Prevention, 2017. Available from:https://www.cdc.gov/drugoverdose/resources/data.html. (15 October 2019, date last accessed). [Google Scholar]
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiat 2015;72:136–142. [DOI] [PubMed] [Google Scholar]
- Prabhu M, Garry EM, Hernandez-Diaz S, MacDonald SC, Huybrechts KF, Bateman BT. Frequency of opioid dispensing after vaginal delivery. Obstet Gynecol 2018;132:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassen J, Doherty M, Huang W, Schneeweiss S. Pharmacoepidemiology Toolbox . Boston, MA: Brigham and Women’s Hospital, 2013. Available from:http://www.hdpharmacoepi.org (19 November 2019, date last accessed). [Google Scholar]
- For the Record. Coding for insomnia. Rec 2012a; Vol. 24 No. 12 (P.27); Available from: https://www.fortherecordmag.com/archives/032612p27.shtml. (15 October 2019, date last accessed). [Google Scholar]
- For the Record. Coding for STDs. Rec 2012b;24:27. [Google Scholar]
- SAS Institute Inc SAS 9.4. 2013; Cary, NC.
- Schneeweiss S, Eddings W, Glynn R, Patorno E, Rassen J, Franklin J. Variable selection for confounding adjustment in high-dimensional covariate spaces when analyzing healthcare databases. Epidemiology 2017;28:237–248. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S, Rassen J, Glynn R, Avorn J, Mogun H, Brookhart M. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009;20:512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes D, Yu O, Raebel MA, Trabert B, Holt VL. Improving automated case finding for ectopic pregnancy using a classification algorithm. Hum Reprod 2011;26:3163–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JLV, Dey SK, Critchley HOD, Horne AW. Current knowledge of the aetiology of human tubal ectopic pregnancy. Hum Reprod Update 2010;16:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy O, Zhao J-P, Bérard A. Association between incident exposure to benzodiazepines in early pregnancy and risk of spontaneous abortion. JAMA Psychiat 2019;76:948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulberg DB, Cain L, Dahlquist IH, Lauderdale DS. Ectopic pregnancy morbidity and mortality in low-income women, 2004-2008. Hum Reprod 2016;31:666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. Ectopic pregnancy. Clin Obstet Gynecol 2007;50:89–99. [DOI] [PubMed] [Google Scholar]
- Webb L. Capturing all necessary codes for IUD insertion and removal can be challenging. JustCoding News Outpatient 2009; Available from:http://www.hcpro.com/HIM-240086-8160/Capturing-all-necessary-codes-for-IUD-insertion-and-removal-can-be-challenging.html. (7 May 2019, date last accessed). [Google Scholar]
- Wikner B, Stiller C, Bergman U, Asker C, Kallen B. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformationsy. Pharmacoepidemiol Drug Saf 2007;16:1203–1210. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Gilstad-Hayden K, Forray A, Lipkind HS. Association of panic disorder, generalized anxiety disorder, and benzodiazepine treatment during pregnancy with risk of adverse birth outcomes. JAMA Psychiat 2017;74:1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Sun H, Li X, Li Y, Zhao S, Zhang D, Yao Z, Li J. A local GABAergic system is functionally expressed in human fallopian tube. Biochem Biophys Res Commun 2010;398:237–241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


