Abstract
STUDY QUESTION
What is the semen quality in trans women at time of fertility preservation, prior to the start of gender-affirming hormone treatment?
SUMMARY ANSWER
Before the start of gender-affirming hormone treatment, semen quality in trans women was already strongly decreased compared to the general population.
WHAT IS KNOWN ALREADY
Hormone treatment for -trans women (birth-assigned males, female gender identity) consists of anti-androgens combined with estrogens in order to achieve feminization and it is accompanied by a loss of reproductive capability. Trans women can opt for semen cryopreservation prior to their medical transition to retain the possibility to parent genetically related offspring. Post-thaw semen parameters determine which ART can be used. Knowledge of semen quality and the factors negatively influencing semen parameters in trans women are important to improve semen quality before fertility preservation.
STUDY DESIGN, SIZE, DURATION
A retrospective cohort study was performed between 1972 and 2017. In total, 260 trans women were included for this study. Due to the study design, there was no loss to follow-up or attrition.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We studied the quality of the preserved semen in trans women, prior to their medical transition, who visited our gender clinic. Semen parameters were collected, as well as data on age, alcohol consumption, smoking, cannabis use, BMI, previous use of estrogens or anti-androgens and endocrine laboratory results. Semen parameters were categorized using reference values for human semen of the World Health Organization (WHO) and compared with data from the general population. Logistic regression analyses were performed to analyze the extent to which factors known to have a negative impact on semen quality in the general population explained the impaired semen quality in the cohort.
MAIN RESULTS AND THE ROLE OF CHANCE
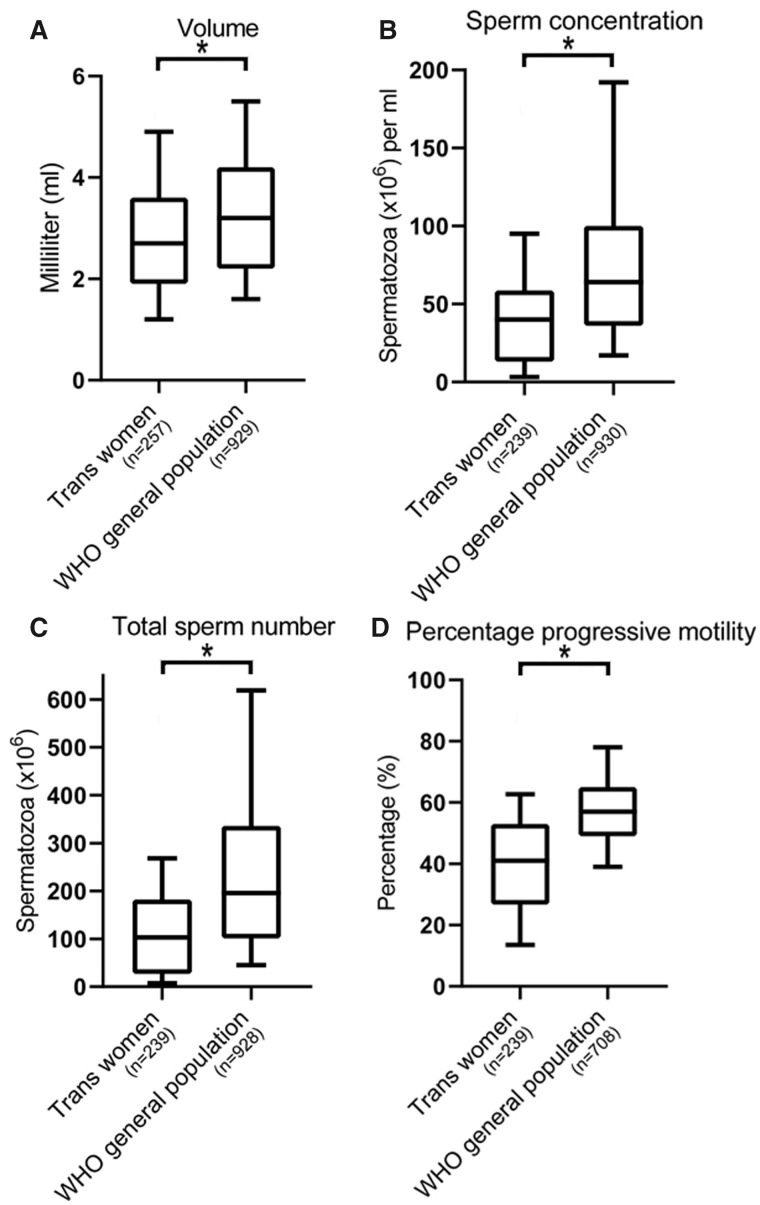
The cohort consisted of 260 trans women between the age of 16 and 52 years. Semen quality in trans women was significantly decreased compared to WHO data from the general population. In total, 21 trans women had an azoospermia and median semen parameters for the remaining trans women and the general population, respectively, were as follows: volume 2.7 and 3.2 ml (P < 0.05), sperm concentration 40 and 64 million/ml (P < 0.05), total sperm number 103 and 196 million (P < 0.05) and progressive motility 41% and 57% (P < 0.05). Smoking (odds ratio (OR) 2.35 (95% CI 1.06–5.21)) and a higher age at time of fertility preservation (OR 1.04 (95% CI 1.00–1.08)) were found to correlate with an impaired progressive motility. Twelve trans women reported to have used anti-androgens and estrogens, and all had discontinued for at least 3 months prior to the first attempt for semen cryopreservation. No correlation was found between previous gender-affirming hormone use and decreased semen parameters. The median post-thaw total motile sperm count was 1.0 million per vial (interquartile range 0.1–3.1) and in only 26.4% of thawed semen samples was the quality adequate for a minimally invasive IUI.
LIMITATIONS, REASONS FOR CAUTION
Limitations include the retrospective design and insufficient data on transgender-specific factors, such as bringing the testes into the inguinal position (tucking), wearing tight underwear and low masturbation frequency.
WIDER IMPLICATIONS OF THE FINDINGS
Semen quality in trans women was decreased compared to the general population, which could not be explained by known risk factors, such as BMI, alcohol consumption, cannabis use, gender-affirming hormone use or abnormal endocrine laboratory results. Although a negative impact of smoking was observed, it was insufficient to explain the overall decreased semen quality in this cohort. Since low pre-freeze semen quality results in an even lower post-thaw semen quality, the majority of trans women and their female partner or surrogate may need an invasive and burdensome treatment to establish a pregnancy.
STUDY FUNDING/COMPETING INTEREST(S)
For this study, no external funding was obtained and there were no competing interests.
TRIAL REGISTRATION NUMBER
NA.
Keywords: fertility / sperm quality / cryopreservation / andrology / transgender
Introduction
Gender dysphoria refers to the distress that results from a conflict between a person’s assigned gender at birth and one’s gender identity (APA, 2013). People assigned male at birth who identify as male are defined as cis men, and birth-assigned males who identify as female are defined as trans women. In the Netherlands, the prevalence of gender dysphoria in birth-assigned males is ∼1 in 2800 (Wiepjes et al., 2018). Transgender people may start medical treatment to align their physical characteristics with their gender identity, including gender-affirming hormone treatment and gender-affirming surgery. Hormone treatment for trans women consists of anti-androgens combined with estrogens in order to achieve feminization. However, hormone treatment is accompanied by a loss of reproductive capability, and while spermatogenesis might restore after discontinuation of prolonged treatment with anti-androgens and estrogens, it has not been well-studied (Schneider et al., 2017; Adeleye et al., 2018). After gender-affirming surgery, involving penectomy and bilateral orchiectomy combined with vaginoplasty, reproductive loss will certainly be permanent.
Many trans women desire to start a family and parent genetically related offspring, just like many other people of reproductive age. A recent study among trans women showed that 69.9% had an interest in having children in the future and 76.6% considered fertility preservation before starting a medical transition (Auer et al., 2018). For several years, scientific medical societies in the field of transgender health have emphasized the need to inform about the effect of the medical transition on fertility and the currently available options for fertility preservation (Coleman et al., 2012; Hembree et al., 2017). The equipoise of commencing medical transition, and fertility preservation as the only option for biological children, may be stressful.
In trans women, the option for fertility preservation is semen cryopreservation. In case of azoospermia or other anatomic variations or emotional concerns, testicular sperm extraction (TESE) is possible but not always successful. Once cryopreserved sperm has been stored, ART enable trans women to have genetically related children with their female partner or via a surrogate. Which technique is optimal is determined by the post-thaw semen parameters: semen of good quality can be used for minimally invasive and inexpensive IUI, while semen of low quality requires a more invasive and expensive technique such as IVF or ICSI (Ombelet et al., 2014).
Although data on semen characteristics in trans women show a high incidence of impaired semen quality, these studies report on a relatively low number of people (Hamada et al., 2015; Li et al., 2018; Marsh et al., 2019). The etiology could not be identified in these studies due to the small sample size and a lack of endocrine laboratory results and complete clinical data on certain lifestyle factors known to influence semen quality, such as age, obesity and cigarette smoking (Sermondade et al., 2013; Johnson et al., 2015; Sharma et al., 2016).
With knowledge of the factors negatively influencing semen parameters in trans women it could be possible to optimize counseling in order to improve semen quality before fertility preservation. The purpose of this study is to assess semen quality in our large cohort of trans women, to evaluate semen adequacy for the different types of ART and to identify life style factors influencing semen quality.
Materials and methods
Patient selection
All trans women seen at the gender identity clinic in the VU University Medical Center (VUmc) between 1972 and 2017 who provided at least one semen sample for cryopreservation before 2018 were identified. People who were under 16 years of age at the time of semen cryopreservation were excluded. This resulted in 260 trans women for the present analyses who, in total, provided 748 semen samples stored in 11 different fertility laboratories in the Netherlands.
Study design and clinical data selection
The medical charts from included trans women were used to obtain data about their medical history, medication use, prior gender-affirming hormone use, alcohol consumption, smoking, cannabis use, BMI and semen characteristics. For information on BMI, alcohol consumption, smoking and cannabis use in the general Dutch population of similar age, data were obtained from the online database of Statistics Netherlands (CBS, 2018).
The study protocol was assessed by the Ethical Review Board of the VU University Medical Center Amsterdam. It was concluded that the Medical Research Involving Human Subjects Act (WMO) did not apply to this study, and necessity for informed consent was waived because of the retrospective design and the large study population.
Laboratory tests
Endocrine laboratory data were collected from the hospital registries of VUmc where clinical data, obtained during regular patient care, are stored. The laboratory results included serum concentrations of testosterone, estradiol, LH, and FSH. Counseling and referral for fertility preservation took place before commencing hormonal treatment, thus results from blood obtained by venipuncture at the initiation of hormone treatment were used. Dates of sperm banking were compared with dates of initiation of hormone treatment, accepting an interval of fewer than 120 days for reliable coupling of semen parameters to endocrine data.
Semen quality
Semen cryopreservation was performed in ISO 15189 accredited fertility laboratories in the Netherlands, all following the Dutch guideline for sperm banks (KLEM, 2010).
All trans women were asked to provide semen samples via masturbation after 2–5 days of abstinence. The vast majority of trans women provided at least two semen samples for cryopreservation. Semen characteristics were manually assessed at time of semen cryopreservation, prior to the freezing process. Samples were kept at 37°C before analysis. The semen parameters measured were semen volume, sperm concentration and sperm motility. Volume was determined using a wide-bore volumetric pipette. To assess sperm concentration and motility, a Makler counting chamber (Sefi-Medical Instruments LTD, Haifa, Israel) and phase contrast microscope optics (200–400×) were used. Pre-freeze and post-thaw sperm motility was classified using a three-category scheme: progressive motile, motile and immotile.
Subsequently, ejaculates were diluted 1:1 and mixed thoroughly for at least 10 min with medium containing glycerol or egg yolk (TYB, Irvine Scientific) as cryoprotectant and put in 0.3 or 0.5 ml vials. Vials were put upright just above liquid nitrogen for gradual crystallization in nitrogen vapor. After total crystallization straws were stored in vapor phase nitrogen tanks. After 24–72 h, one vial was thawed and a semen analysis was performed, as described above. Some fertility laboratories assessed post-thaw semen quality of all semen samples, some assessed only one of the provided semen samples, and in two centers no assessment of post-thaw quality was performed.
The choice of the most appropriate ART (IUI, IVF or ICSI) is often determined by the total motile sperm count (TMSC) per vial, since it reflects sperm concentration and motility, as well as the effects of sperm processing. It is difficult to establish the most optimal cutoff values for the different types of reproductive techniques since a successful treatment is also dependent on other factors, such as the woman’s fertility, and therefore, recommended thresholds vary in literature (Rhemrev et al., 2001; van Weert et al., 2004). In this study, suitability for the most appropriate ART was determined using the following cutoff values: TMSC >2 million is suitable for IUI, TMSC >1 and <2 million is suitable for IVF and TMSC <1 million is suitable for ICSI.
Statistical analysis
Descriptive analyses were conducted to assess the distributions of semen parameters and patient characteristics, normally distributed data are presented as mean with SD, and non-normally distributed data as median with interquartile range (IQR). Qualitative data are presented as number with percentages. For trans women who preserved multiple semen samples, the collective semen parameters were averaged and the means were used for statistical analysis (Keel, 2006; Stokes-Riner et al., 2007). Semen quality was categorized in the following descriptive diagnoses, using reference values of the World Health Organization (WHO) for human semen; oligozoospermia (reduced sperm count), asthenozoospermia (reduced sperm motility), oligoasthenozoospermia (reduced sperm count and motility), azoospermia (no sperm in the ejaculate) or normozoospermia (normal semen parameters) (WHO, 2010). Post-thaw semen quality was assessed by calculating the TMSC per vial. Since volumes of vials differed between fertility laboratories a correction was performed to enable accurate comparison.
Wilcoxon signed-rank tests were performed on non-normally distributed semen parameters to compare with data from the general population of unscreened men (Cooper et al., 2010). Azoospermic trans women were excluded from analysis of sperm concentration, total sperm number and percentage progressive motility.
Predetermined factors (i.e. age at time of semen cryopreservation, alcohol consumption, smoking, cannabis use, BMI, previous feminizing hormone use, a history of inguinal hernia repair or cryptorchidism and a history of depression and anxiety) were used in logistic regression analyses to assess their impact on semen quality in our cohort. Semen parameters were dichotomized using WHO reference values to be able to compare impaired semen quality with normal semen quality (Cooper et al., 2010). Odds ratios (OR) with 95% CI were calculated.
STATA Statistical Software, version 15.1 (Statacorp, College Station, TX, USA) was used for statistical analyses.
Results
Description of study population
Our cohort consisted of 260 trans women who provided at least one semen sample for cryopreservation between August 1991 and December 2017. The median age at time of semen cryopreservation was 24.0 years (IQR 20.0–29.5). Alcohol consumption, smoking status, cannabis use and BMI were comparable to the general Dutch population and no abnormalities were found in the endocrine laboratory results (Table I) (CBS, 2018). The medical history of 13 trans women was positive for inguinal hernia repair or cryptorchidism. Anxiety or depression was reported in 34 trans women. In total, 12 trans women reported to have used anti-androgens and estrogens, and all had discontinued for at least 3 months prior to the first attempt for semen cryopreservation. The median number of semen samples provided per person was 3.0 (IQR 2.5–3.0), and in total 748 semen samples were included for analyses.
Table I.
Baseline characteristics of study cohort (n = 260 trans women).
| n | Mean (SD) or Median (IQR) | Percentage (n) | Dutch reference values* | |
|---|---|---|---|---|
| Age at time of fertility preservation (years) | 260 | 24.0 (IQR 20.0–29.5) | ||
| Testosterone (nmol/l) | 181 | 19.1 (SD 6.7) | 9–30 | |
| Estradiol (pmol/l) | 179 | 87.4 (SD 24.7) | 12–177 | |
| LH (U/l) | 177 | 3.5 (SD 2.1) | 1–8.4 | |
| FSH (U/l) | 18 | 4.1 (SD 4.0) | 1–10.5 | |
| BMI (kg/m2) | 200 | 22.7 (SD 3.8) | ||
| Underweight: <18.5 | 9.0% (18) | 3.9% | ||
| Normal weight: 18.5–25.0 | 65.5% (131) | 63.6% | ||
| Overweight: >25.0 | 25.5% (51) | 32.5% | ||
| Alcohol | 214 | |||
| Non drinker | 44.4% (95) | 14.6% | ||
| Current drinker | 55.6% (119) | 85.4% | ||
| Units/week | 100 | 2.0 (IQR 1.0–4.0) | 9.1 | |
| Smoking | 121 | |||
| Never | 52.9% (64) | 57.1% | ||
| Previous smoker | 9.9% (12) | 12.7% | ||
| Current smoker | 37.2% (45) | 30.2% | ||
| Cig/day | 45 | 10.0 (IQR 5.0–15.0) | 6.9 | |
| Cannabis | 170 | |||
| Never | 84.1% (143) | |||
| Weekly | 5.9% (10) | 9.9% | ||
| Sporadically | 10.0% (17) | 19.0% | ||
| Previous hormone use | 260 | |||
| Yes | 4.6% (12) | |||
| No | 95.4% (248) | |||
| History of anxiety or depression | 260 | |||
| Yes | 13.1% (34) | |||
| No | 86.9% (226) | |||
| History of inguinal hernia or cryptorchidism | 260 | |||
| Yes | 5.0% (13) | |||
| No | 95.0% (247) |
Data on BMI, alcohol consumption, smoking and cannabis use are obtained from the online database of Statistics Netherlands (CBS, 2018).
IQR, interquartile range.
Evaluation of semen variables and their determinants
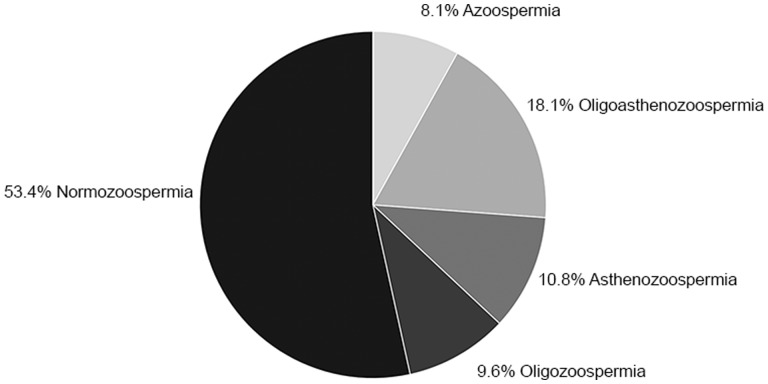
The median values of all semen parameters in our cohort (volume 2.7 ml (IQR 1.9–3.6), sperm concentration 40 million/ml (IQR 13–58.7), total sperm number 103 million (IQR 26.9–182.2) and progressive motility 41% (IQR 26.7–53)) were significantly lower than the WHO data on semen quality in the general population of unscreened men (Fig. 1) (Cooper et al., 2010). When applying WHO semen criteria, a substantial percentage of the study population did not meet the reference value for semen volume (<1.5 ml, 18.1%), total sperm number (<39 million, 35.8%), sperm concentration (<15 million/ml, 33.5%) and progressive motility (<32%, 36.9%). In Fig. 2, the classification of semen quality in our cohort is demonstrated using the descriptive diagnosis nomenclature.
Figure 1.
Box and whisker plots of semen analysis data. The data are semen volume, sperm concentration, total sperm number and percentage progressive motility from trans women compared with World Health Organization (WHO) values for unscreened men from the general population. The boxes represent the quartiles and the lines within them are the medians; the whiskers extend from the 10th to the 90th centiles. *P-value <0.05 using Wilcoxon signed-rank tests, for (A) analysis was performed on the entire cohort, for (B–D) azoospermic trans women were excluded from analysis.
Figure 2.
Classification of semen quality in the study sample of 260 trans women. The pie chart presents the descriptive diagnoses of trans women according to WHO reference values for human semen.
For 228 trans women, a post-thaw semen quality was assessed; the median TMSC was 1.0 million per vial (IQR 0.1–3.1). In only 26.4% of the post-thawed samples was the semen quality adequate for IUI, 13.4% was suitable for IVF and 60.2% required ICSI.
In total, 21 trans women had an azoospermia. Three of these individuals reported to have used gender-affirming hormones and stopped taking these 3 months prior to the first attempt of semen cryopreservation. Even 6 months after discontinuation of hormone treatment they still remained azoospermic. Seven hormone naïve trans women elected to undergo TESE, which resulted in cryopreserved spermatozoa in only three cases. Endocrine laboratory results for the azoospermic individuals were all in the normal range, except for FSH concentrations which were only available for three trans women but was elevated in one case (median 6.8 U/l, range 5.4–15.7 U/l).
Logistic regression analyses showed no effect of BMI, alcohol consumption or cannabis use on the semen parameters. Smoking was found to correlate with a progressive motility below 32% (OR 2.35; 95% CI 1.06–5.21) but within smokers no relation between the number of smoked cigarettes per day and semen parameters was found. A higher age at time of fertility preservation also correlated with an impaired progressive motility (OR 1.04 95% CI 1.00–1.08). The decreased semen quality in our cohort could not be explained by abnormal endocrine laboratory results, previous gender-affirming hormone use, a history of cryptorchidism or inguinal hernia repair and a history of anxiety or depression. Data for all logistic regression analyses are shown in Table II.
Table II.
Effect of patient-related factors on semen parameters.
| Semen volume | Sperm concentration | Total sperm number | Progressive motility | |
|---|---|---|---|---|
| <1.5 ml | <15 × 106 | <39 × 106 | <32% | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR(95% CI) | |
| Age at time of fertility preservation (years) | 0.96 (0.91–1.01) | 1.03 (0.99–1.07) | 1.00 (0.97–1.04) | 1.04 (1.00–1.08)* |
| BMI (kg/m²) | 1.01 (0.93–1.11) | 1.05 (0.97–1.13) | 1.04 (0.97–1.12) | 1.04 (0.97–1.12) |
| Alcohol—yes/no | 0.96 (0.47–1.89) | 0.59 (0.34–1.04) | 0.71 (0.41–1.24) | 0.85 (0.49–1.48) |
| Smoking—yes/no | 2.21 (0.95–5.17) | 1.33 (0.62–2.82) | 2.01 (0.95–4.27) | 2.35 (1.06–5.21)* |
| Cigarettes per day (in smokers) | 1.09 (0.98–1.22) | 1.03 (0.94–1.14) | 1.02 (0.93–1.13) | 1.11 (1.00–1.24) |
| Cannabis use—yes/no | ∞ | 0.80 (0.36–1.91) | 0.79 (0.34–1.85) | 0.40 (1.15–1.04) |
| Previous hormone use—yes/no | 1.55 (0.40–5.94) | 2.94 (0.91–9.55) | 2.64 (0.81–8.56) | 2.50 (0.77–8.11) |
| History of anxiety or depression—yes/no | 1.16 (0.47–2.83) | 1.83 (0.89–3.76) | 1.41 (0.69–2.91) | 0.88 (0.41–1.85) |
| History of inguinal hernia repair or cryptorchidism—yes/no | 0.85 (0.17–3.81) | 0.58 (0.16–2.17) | 0.52 (0.14–1.95) | 1.07 (0.34–3.37) |
P-value <0.05 using logistic regression analyses.
At time of writing, six trans women in our cohort had used their cryopreserved semen a median 6 years (range 2–18 years) after cryopreservation. One individual decided to donate two vials to a befriended couple. The other five trans women successfully used their semen with their female partner. Two couples conceived through IUI, one couple through IVF and the other two couples through ICSI.
Discussion
As far as we are aware, this is the largest cohort study showing an impaired semen quality in trans women at time of fertility preservation. In only 26.4% of the post-thawed samples was semen quality considered adequate for IUI. Although smoking and a higher age did affect progressive motility, it did not provide an explanation for the overall reduced quality in this cohort. Therefore, these results suggest the existence of one or more transgender-specific factors that negatively influence semen quality.
A high percentage of trans women had semen parameters below the WHO reference values when compared with a study on semen quality of young men from the general population, i.e. a sperm concentration below 15 million/ml (33.5% versus 17.5%) and progressive motility below 32% (36.9% versus 14.4%) (Hart et al., 2015). Furthermore, we observed a higher incidence of azoospermia (8.1% versus 0.8%) compared to the study of Hart et al. (2015).
Our findings are in line with previous studies on semen quality in trans women, which also showed a high incidence of azoospermia, oligozoospermia and asthenozoospermia (Hamada et al., 2015; Li et al., 2018; Marsh et al., 2019). Only one of these studies collected data on personal behavior, feelings of depression, anxiety and stress, as well as serum hormone concentrations. However, none of the characteristics were able to explain the reduced semen quality in trans women (Marsh et al., 2019). Despite the significantly larger sample size, our cohort study demonstrates similar observations.
Many trans women desire to start a family and prefer to use their gametes for this purpose (Auer et al., 2018). This is in direct conflict with their desire for medical transition, as anti-androgens, estrogens and gender-affirming surgery impair their reproductive function. Semen cryopreservation enables trans women to parent genetically related offspring later in life and they are strongly recommended to cryopreserve semen prior to hormone treatment as spermatogenesis might not restore, or only partially, after discontinuation. Furthermore, discontinuation of hormone treatment can be quite stressful for trans women due to the returning effects of testosterone. Therefore, trans women have to reflect on their reproductive wishes at a young age and early in the transition process.
Early banking of sperm enables trans women to have future reproductive options. However, a decreased semen quality limits the number of ART that can be used. A high post-thaw TMSC is preferred because semen can be used for IUI and the insemination can take place in the natural cycle of a cis woman. Therefore, it is an uncomplicated and noninvasive technique, with minimal monitoring and risks. IVF or ICSI, however, may have serious consequences and risks for the cis woman undergoing this treatment as it involves controlled ovarian stimulation and an oocyte retrieval procedure (Pennings and Ombelet, 2007). In our cohort, more than 70% of the thawed samples were only suitable for IVF or ICSI. Previous studies already demonstrated a high variation in the cryosurvival of semen, mainly depending on pre-freeze motility and total sperm count (Keel and Karow, 1980; Nallella et al., 2004; MacKenna et al., 2017). Although, because of the cryopreservation process, post-thaw motility is always decreased compared to pre-freeze values, it has been shown that higher pre-freeze sperm counts result in an increased potential to withstand cryopreservation. Conversely, lower pre-freeze semen quality results in an even lower post-thaw motility, underlining the importance of improving semen quality by improving certain lifestyle factors prior to the fertility preservation process (Keel and Karow, 1980).
The question arises of how we can counsel trans women to take the appropriate action to optimally cryopreserve their sperm. Multiple studies, performed in cis men, established the negative effect of a higher age, obesity, cigarette smoking and high levels of alcohol intake on semen quality (Sermondade et al., 2013; Johnson et al., 2015; Sharma et al., 2016; Ricci et al., 2017). In our cohort, some effects of these lifestyle factors were observed; smoking and a higher age correlated with an impaired progressive motility. However, as shown in Table I, compared with data from the general Dutch population of similar age, our cohort consisted of generally healthier individuals in terms of BMI, alcohol intake, smoking and cannabis use (CBS, 2018).
Other factors associated with a lower semen quality are psychological stress, depression and anxiety: previous studies showed a significantly negative effect of these factors on sperm concentration, motility and morphology (Nordkap et al., 2016; Li et al., 2011). Trans women desiring a medical transition might experience more stress compared to cis men but our data did not show a decreased semen quality in trans women who reported suffering from anxiety or depression. Hypothetically, semen quality in trans women is affected by transgender-specific factors, such as a low frequency of masturbation, gender-affirming hormone use, keeping the genitals tight against the body or even bringing the testicles into the inguinal canal (tucking) (Mieusset et al., 1985, 1987a,b; Tiemessen et al., 1996). Trans women with previous gender-affirming hormone use in our cohort had discontinued their medication for at least 3 months, and no negative effect on the semen parameters was observed. Lifestyle factors, such as tucking and wearing tight-fitting underwear, might increase scrotal temperatures which is associated with an impaired semen quality (Jung and Schuppe, 2007). In 1985, a study was performed to evaluate the influence of tucking on semen quality in order to provide a contraceptive method in cis men. They found an inhibition of spermatogenesis after 3 months of daily tucking and semen parameters were lowest after 6 months (Mieusset et al., 1985). Unfortunately, we were not able to reproduce this negative influence of tucking since it was not recorded in the medical files. Taking all these factors into account, we were not able to provide a clear explanation for the impaired semen quality in our cohort.
The major strengths of our study are the large cohort size, which is much bigger than previous studies on this topic, since ∼95% of all transgender people in the Netherlands are treated in our center, and the evaluation of potential influencing factors on semen quality obtained through access to the medical files.
However, owing to the retrospective design of the study, information on transgender-specific life style factors was only available for a few individuals and, for example, did not include the frequency and the duration of tucking or when tucking was last performed. As a result, we were unable to demonstrate that these habits might explain the observed reduced semen quality. Also, the retrospective design may lead to an underestimation of the prevalence of anxiety and depression in our cohort. Another limitation of the study is the absence of data on semen morphology as this was not recorded during the process of semen cryopreservation. Furthermore, although abstinence time was advised to be 2–5 days, exact abstinence time was not recorded and we were therefore unable the correct for this factor.
For future studies it would be worthwhile to prospectively obtain data on endocrine laboratory results and detailed information about lifestyle factors at time of fertility preservation in order to adequately determine the etiology of the impaired semen quality in trans women. Furthermore, more knowledge of the influence of gender-affirming hormone treatment on spermatogenesis and its restoration after discontinuation might help to establish the optimal strategy for trans women with a desire for genetically related offspring.
Conclusion
Fertility preservation has now become widely available in the Netherlands for most trans women and enables them to parent genetically related children after medical transition. Our findings show a high frequency of impaired semen parameters compared to the general population. Since low pre-freeze semen quality results in an even lower post-thaw semen quality, the majority of trans women and their female partner or surrogate may need an invasive and burdensome treatment to establish a pregnancy.
This cohort study shows that patient-related factors that are associated with a decreased semen quality in the general population do not seem to be responsible for the impaired quality in trans women. We have commenced a prospective cohort study on how transgender-specific factors, such as tucking, affect semen quality in trans women in order to optimize counseling. With this knowledge, we aim to help trans women in their wish to parent genetically related offspring by cryopreserving semen that will be suitable for the least burdensome strategy.
Acknowledgments
We thank the collaborating fertility laboratories for welcoming the referred trans women for semen cryopreservation.
Authors’ roles
I.d.N.—conception and design, acquisition of data, analysis and interpretation of data and drafting of manuscript. A.M.—acquisition of data and critical revision of the manuscript for intellectual content. E.H.K.—acquisition of data, administrative and technical support and critical revision of the manuscript for intellectual content. A.T.S.—acquisition of data, administrative and technical support and critical revision of the manuscript for intellectual content. I.A.C.V.-d.W.—acquisition of data, administrative and technical support. M.d.H.—conception and design, analysis and interpretation of data and critical revision of the manuscript for intellectual content. J.H.—conception and design and critical revision of the manuscript for intellectual content. N.M.v.M.—conception and design, analysis and interpretation of data and critical revision of the manuscript for intellectual content. All authors approved the final version of the manuscript.
Funding
For this study, no external funding was obtained.
Conflict of interest
For this study, there were no competing interests.
References
- Adeleye AJ, Reid G, Kao CN, Mok-Lin E., Smith JF. Semen parameters among transgender women with a history of hormonal treatment. Urology2018;124:136–141. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders 5th edn. American Psychiatric Association. Arlington, VA: Author. 2013.
- Auer MK, Fuss J, Nieder TO, Briken P., Biedermann SV, Stalla GK, Beckmann MW, Hildebrandt T.. Desire to have children among transgender people in Germany: a cross-sectional multi-center study. J Sex Med 2018;15:757–767. [DOI] [PubMed] [Google Scholar]
- CBS. Life Style and (Preventive) Health Examination; Personal Characteristics. Statistics Netherlands. The Hague, the Netherlands. 2018.
- Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, Fraser L, Green J, Knudson G, Meyer WJ et al. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int J Transgender Health 2012;13:165–232
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT. et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231–245. [DOI] [PubMed] [Google Scholar]
- Hamada A, Kingsberg S, Wierckx K, T'Sjoen G, De Sutter P, Knudson G, Agarwal A.. Semen characteristics of transwomen referred for sperm banking before sex transition: a case series. Andrologia 2015;47:832–838. [DOI] [PubMed] [Google Scholar]
- Hart RJ, Doherty DA, McLachlan RI, Walls ML, Keelan JA, Dickinson JE, Skakkebaek NE, Norman RJ, Handelsman DJ.. Testicular function in a birth cohort of young men. Hum Reprod 2015;30:2713–2724. [DOI] [PubMed] [Google Scholar]
- Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T'Sjoen GG.. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2017;102:3869–3903. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S.. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev 2015;19:22–33. [DOI] [PubMed] [Google Scholar]
- Jung A, Schuppe HC.. Influence of genital heat stress on semen quality in humans. Andrologia 2007;39:203–215. [DOI] [PubMed] [Google Scholar]
- Keel BA. Within- and between-subject variation in semen parameters in infertile men and normal semen donors. Fertil Steril 2006;85:128–134. [DOI] [PubMed] [Google Scholar]
- Keel BA, Karow AM Jr.. Motility characteristics of human sperm, nonfrozen and cryopreserved. Arch Androl 1980;4:205–212. [DOI] [PubMed] [Google Scholar]
- KLEM. Dutch Guideline for Sperm Banks. Klinische Chemie en Laboratoriumgeneeskunde en de Vereniging van Klinisch Embryologen. The Netherlands: KLEM. 2010.
- Li K, Rodriguez D, Gabrielsen JS, Centola GM, Tanrikut C.. Sperm cryopreservation of transgender individuals: trends and findings in the past decade. Andrology 2018;6:860‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lin H, Li Y, Cao J.. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril 2011;95:116–123. [DOI] [PubMed] [Google Scholar]
- MacKenna A, Crosby J, Huidobro C, Correa E, Duque G.. Semen quality before cryopreservation and after thawing in 543 patients with testicular cancer. JBRA Assist Reprod 2017;21:31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh C, McCracken M, Gray M, Nangia A, Gay J, Roby KF. Low total motile sperm in transgender women seeking hormone therapy. J Assist Reprod Genet 2019;36:1639-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieusset R, Bujan L, Mansat A, Pontonnier F, Grandjean H.. Hyperthermia and human spermatogenesis: enhancement of the inhibitory effect obtained by ‘artificial cryptorchidism’. Int J Androl 1987. a;10:571–580. [DOI] [PubMed] [Google Scholar]
- Mieusset R, Bujan L, Mansat A, Pontonnier F, Grandjean H.. Effects of artificial cryptorchidism on sperm morphology. Fertil Steril 1987. b;47:150–155. [PubMed] [Google Scholar]
- Mieusset R, Grandjean H, Mansat A, Pontonnier F.. Inhibiting effect of artificial cryptorchidism on spermatogenesis. Fertil Steril 1985;43:589–594. [DOI] [PubMed] [Google Scholar]
- Nallella KP, Sharma RK, Said TM, Agarwal A.. Inter-sample variability in post-thaw human spermatozoa. Cryobiology 2004;49:195–199. [DOI] [PubMed] [Google Scholar]
- Nordkap L, Jensen TK, Hansen AM, Lassen TH, Bang AK, Joensen UN, Blomberg Jensen M, Skakkebaek NE, Jorgensen N.. Psychological stress and testicular function: a cross-sectional study of 1,215 Danish men. Fertil Steril 2016;105:174–187.e1-2. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Dhont N, Thijssen A, Bosmans E, Kruger T.. Semen quality and prediction of IUI success in male subfertility: a systematic review. Reprod Biomed Online 2014;28:300–309. [DOI] [PubMed] [Google Scholar]
- Pennings G, Ombelet W.. Coming soon to your clinic: patient-friendly ART. Hum Reprod 2007;22:2075–2079. [DOI] [PubMed] [Google Scholar]
- Rhemrev JP, Lens JW, McDonnell J, Schoemaker J, Vermeiden JP.. The postwash total progressively motile sperm cell count is a reliable predictor of total fertilization failure during in vitro fertilization treatment. Fertil Steril 2001;76:884–891. [DOI] [PubMed] [Google Scholar]
- Ricci E, Al Beitawi S, Cipriani S, Candiani M, Chiaffarino F, Vigano P, Noli S, Parazzini F.. Semen quality and alcohol intake: a systematic review and meta-analysis. Reprod Biomed Online 2017;34:38–47. [DOI] [PubMed] [Google Scholar]
- Schneider F, Kliesch S, Schlatt S, Neuhaus N.. Andrology of male-to-female transsexuals: influence of cross-sex hormone therapy on testicular function. Andrology 2017;5:873–880. [DOI] [PubMed] [Google Scholar]
- Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, Van Wely M, Cao J, Martini AC, Eskandar M. et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update 2013;19:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Harlev A, Agarwal A, Esteves SC.. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur Urol 2016;70:635–645. [DOI] [PubMed] [Google Scholar]
- Stokes-Riner A, Thurston SW, Brazil C, Guzick D, Liu F, Overstreet JW, Wang C, Sparks A, Redmon JB, Swan SH.. One semen sample or 2? Insights from a study of fertile men. J Androl 2007;28:638–643. [DOI] [PubMed] [Google Scholar]
- Tiemessen CH, Evers JL, Bots RS.. Tight-fitting underwear and sperm quality. Lancet 1996;347:1844–1845. [DOI] [PubMed] [Google Scholar]
- van Weert JM, Repping S, Van Voorhis BJ, van der Veen F, Bossuyt PM, Mol BW.. Performance of the postwash total motile sperm count as a predictor of pregnancy at the time of intrauterine insemination: a meta-analysis. Fertil Steril 2004;82:612–620. [DOI] [PubMed] [Google Scholar]
- WHO. WHO Laboratory Manual for Examination and Processing of Human Semen. Switzerland: World Health Organization, 2010.
- Wiepjes CM, Nota NM, de Blok CJM, Klaver M, de Vries ALC, Wensing-Kruger SA, de Jongh RT, Bouman MB, Steensma TD, Cohen-Kettenis P. et al. The Amsterdam Cohort of Gender Dysphoria Study (1972-2015): trends in prevalence, treatment, and regrets . J Sex Med 2018;15:582–590. [DOI] [PubMed] [Google Scholar]