Abstract
STUDY QUESTION
Is circadian desynchrony a risk factor of male reproductive damage in semen parameters and/or reproductive hormones?
SUMMARY ANSWER
Circadian desynchrony correlates with decrease of sperm count, which was improved when circadian desynchrony was attenuated.
WHAT IS KNOWN ALREADY
Circadian desynchrony caused by work (shift work) and non-work-related reasons is prevalent worldwide and has been found to be associated with decreased female fertility, but whether it harms male reproductive health is unclear.
STUDY DESIGN, SIZE, DURATION
A hybrid research was conducted. (i) A cross-sectional study of 1346 Chinese men in 2007 was used to analyze the association between semen/hormone biomarkers and work-related circadian desynchrony, which was divided into rotating shift work and permanent shift work against non-shift work. (ii) A cohort of 796 Chinese undergraduates from 2013 to 2014 was used to analyzed the association between semen/hormone biomarkers and non-work-related circadian desynchrony (between school days and days off). (iii) The biomarker identified simultaneously in both populations was further validated in male C57BL/6J mice housed under conditions simulating circadian desynchrony.
PARTICIPANTS/MATERIALS, SETTING, METHODS
A total of 17 semen/hormone biomarkers were compared among rotating shift workers and permanent shift workers against non-shift workers in the 1346 reproductive-age Chinese men. A total of 14 semen/hormone biomarker was analyzed in the undergraduate cohort for correlation with non-work-related circadian desynchrony (measured by Munich Chronotype Questionnaire) in 2013 and 2014 and compared between the 2 years. Photoperiod-shifting method was used to establish the mouse model, in which the biomarker was examined and molecular mechanism was explored by apoptosis analysis, DNA content analysis, transcriptome sequencing, real-time PCR and western blotting.
MAIN RESULTS AND THE ROLE OF CHANCE
Among the semen/hormone biomarkers, sperm count was found to be lower in rotating shift workers, who had a higher risk of low sperm count defined by Chinese Ministry of Health (total sperm/ejaculate < 120 × 106) than non-shift workers (odds ratio = 1.26, 95% CI 1.05–1.52). This biomarker was replicated in the undergraduate cohort, where each hour of circadian desynchrony was associated with 1.16 (95% CI 1.02–1.31) fold odds of low sperm count, and sperm count increased during 2014 in men who reduced circadian desynchrony after 2013. A decrease of sperm count with circadian desynchrony and its recovery after removal of circadian desynchrony was also observed in the mouse model. During asynchrony, increased apoptosis was found in seminiferous tubules and the marker genes of post-spermatocyte stage cells were down-regulated. The most enriched functional pathway was homologous recombination, which happened during meiosis.
LIMITATIONS, REASONS FOR CAUTION
The study of human beings was observational while the animal study has potential difference in circadian desynchrony exposure and species susceptibility. Further researches are needed to clarify the causal relationship in men.
WIDER IMPLICATIONS OF THE FINDINGS
These findings provide novel insight to the effect of circadian desynchrony on male reproductive health and a potential strategy for prevention of reproductive damage.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by the National Key R&D Program of China [2017YFC1002001] and National Natural Science Foundation of China [81871208]. There are no conflicts of interest to declare.
TRIAL REGISTRATION NUMBER
NA.
Keywords: circadian desynchrony, chronobiology disorders, shift work, social jetlag, sleep disorders, Pittsburg sleep quality index, fertility, semen quality, sperm count, spermatogenesis
Introduction
The growing use of artificial light at night and the modern ‘24/7’ lifestyle have led individuals to adopt sleep/wake schedules that are discrepant against their inner biological clock, which is referred to as ‘circadian desynchrony’ (Roenneberg et al., 2019). This desynchrony may be related to rotating shift work (RSW), such as when clinical or security staff shift their working hours later or earlier than the prior work schedule every few days, or to permanent shift work (PSW), when workers always work shifts at odds with the typical 9-to-5 schedule. Circadian desynchrony can also have non-work-related causes, such as long-time use of mobile phone which may induce individuals to adopt different sleep/wake patterns on workdays and days off work (Liu et al., 2019). Circadian desynchrony is attracting public concern because it has been linked to cardiovascular, metabolic and other disruptions (Lunn et al., 2017; Kim et al., 2018; Crnko et al., 2019). In fact, the International Agency for Research on Cancer has listed shift work as a probable human carcinogen (Group 2A) (Stevens et al., 2011).
Circadian desynchrony may disrupt reproductive health, but most studies have focused on females and found that disruption of the circadian rhythm may affect follicular development and hormone secretion, alter the menstrual cycle and even cause a higher incidence of spontaneous abortion, spontaneous membrane rupture, pre-term birth and reduced breastfeeding success (Fernandez et al., 2016; Wang et al., 2016; Komada et al., 2019). One study of men in Egypt found that shift work was associated with nearly 4-fold higher risk of infertility (El-Helaly et al., 2010), and a US study found that shift work was associated with lower semen quality and abnormal levels of reproductive hormones (Kohn et al., 2017). However, another US study found no association between shift work and risk of infertility (Gracia et al., 2005), and two studies found no relationship between circadian desynchrony and semen quality (Wogatzky et al., 2012; Eisenberg et al., 2015). Another two studies found no relationship between circadian desynchrony of men and the time that their female partners need to achieve pregnancy (Bisanti et al., 1996; Tuntiseranee et al., 1998). These studies usually aggregate RSW and PSW in analyses, even though each type of work may affect reproductive health differently (Cheng and Cheng, 2017; Mancio et al., 2018). Further work is needed to examine whether work-related circadian desynchrony damages the male reproductive system; such work should carefully distinguish between RSW and PSW. Non-work-related circadian desynchrony has been associated with adverse reproductive phenotypes such as menstrual pain in women (Komada et al., 2019), but we are unaware of equivalent studies in men.
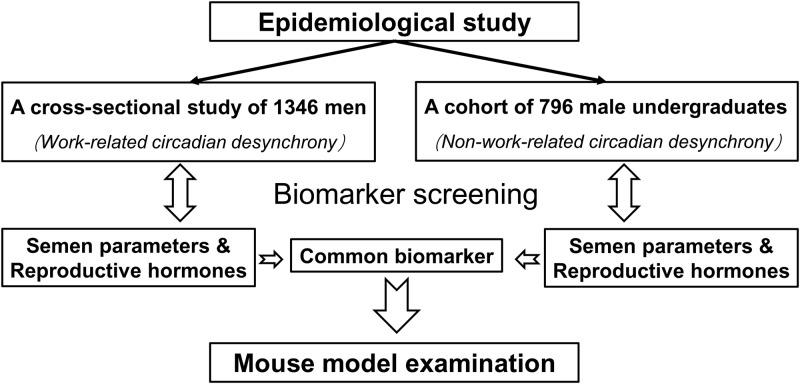
To clarify the effects of circadian desynchrony on male reproductive health, we investigated the relationship of semen/hormone biomarkers and circadian desynchrony in a hybrid research of epidemiological study and animal experiment (Fig. 1). A cross-sectional study of 1346 men was used to identify which biomarker was associated with work-related circadian desynchrony, which was divided into RSW and PSW against non-shift work. A cohort of 796 male undergraduates was used to identify which biomarker was associated with non-work-related circadian desynchrony between school days and days off. Then the biomarker identified simultaneously in the two populations was further examined in a mouse model of circadian desynchrony which was established by photoperiod-shifting method (McGowan and Coogan, 2013; Figueiro et al., 2017).
Figure 1.
Study design. A hybrid research was conducted to investigate which semen/hormone biomarker was associated with circadian desynchrony. (i) A cross-sectional study of 1346 men was used to analyse the association between semen/hormone biomarkers and work-related circadian desynchrony. (ii) A cohort of 796 undergraduates was used to analysed the association between semen/hormone biomarkers and non-work-related circadian desynchrony. (iii) The biomarker which was identified to be associated with circadian desynchrony simultaneously in both populations was further validated in mouse model.
Materials and methods
Ethics approval and consent to participate
The human procedures in this study were approved by the Ethics Committee of Third Military Medical University, while the animal procedures were approved by the Animal Care and Use Committee at the same institution.
A community population of Chinese adults
Potential correlation of RSW and PSW with semen parameters and reproductive hormone levels was analyzed in a cross-sectional study of 1346 Chinese men aged 20–41, which has been described previously (Li et al., 2009). Subjects were recruited in six districts along the Yangtze River in the Three Gorges Reservoir Region of Chongqing municipality in 2007. Information on demographic characteristics, working conditions and lifestyles were collected in face-to-face interviews. Subjects were asked ‘What is the type of your work during the past half year?’, to which they could answer, ‘permanent day work’, ‘permanent shift work’, ‘rotating shift work’ or ‘none of the above types’. Data were also collected on age (in years), education level (primary school and below; junior school; high school; university or higher), average monthly household income (<3000 RMB, 3000–8000 RMB; 8000–13 000 RMB; 13 000–18 000 RMB; ≥18 000 RMB), abstinence period prior to semen collection in the study (in days), tobacco smoking (never smoke; ever smoked; currently smoking) and alcohol drinking (never drink; ever drank; currently drinking). BMI was calculated by dividing body weight (kg) by height squared (m2), and individuals were categorized according to the recommendation for Chinese adults (<18.5; 18.5–23.9; 24–27.9; ≥28) (Zhou, 2002).
Peripheral blood (5 ml) was collected into vacuum blood tubes. Semen was collected by masturbation, after subjects had abstained for 2–7 days. The time points of bio-sample collection were recorded immediately.
A list of the semen parameters and reproductive hormones which were measured in the population was shown in Table I. Sperm concentration was measured by computer-aided sperm analysis, which was then accepted by the fifth edition of World Health Organization manual (World Health Organization, 2010). After thorough mixing, 10 μl of semen was put into a Macro Sperm Counting Chamber (Weili Ltd., Beijing, China), and scanned by WLJY-9000 Automatic Color Sperm Quality Analysis System (Weili Ltd., Beijing, China). With a total microscope magnification of ×400, at least six areas and 400 sperms were counted in each sample for estimation of sperm concentration. Only the sperms with tails were counted. Semen samples with concentrations over 50 × 106/ml were diluted to concentration between 2 × 106/ml and 50 × 106/ml with PBS rotating and PE as suggested (Ashok et al., 2016) and the fold of dilution was recorded to calculate the sperm concentration. The measurement was finished within 1 h since ejaculation. All semen samples were measured by one technician, who joined in the quality-control system under the supervision of the Chongqing Science and Technology Commission. Semen volume was measured by pipette, as recommended by the World Health Organization at the time of the study (World Health Organization, 1999). Total sperm count was calculated as sperm concentration multiplied by semen volume. The measurement methods of progressive motility, total motility and normal morphology have been described in details elsewhere (Li et al., 2009). Seminal fructose, α-glucosidase and zinc were assayed using commercial kits (Xindi Biopharmaceutical, Nanjing, China) following the manufacturer’s instructions. In a subgroup of 232 males, sperm apoptotic index was assessed in an annexin V assay, DNA damage was assessed using a comet assay (Han et al., 2011), and a radioimmunoassay was used to determine serum levels of testosterone, LH, FSH and estradiol (Han et al., 2014).
Table I.
Indices of male reproductive health measured in the community surveys of Chinese adults and undergraduates. a
| Reproductive index | Chinese adults (n = 1346) | Chinese undergraduates (n = 796) |
|---|---|---|
| Sperm concentration (million/ml) | √ | √ |
| Semen volume (ml) | √ | √ |
| Total sperm count (million) | √ | √ |
| Progressive motility (%) | √ | √ |
| Total motility (%) | √ | √ |
| Normal morphology (%) | √ | √ |
| High DNA stainability (%) | √ | |
| DNA fragmentation index (%) | √ | |
| Tail DNA (%) | √b | |
| Tail length (μm) | √b | |
| Tail distributed moment (μm) | √b | |
| Apoptotic index (%) | √ | |
| Testosterone (ng/ml) | √b | √ |
| LH (mIU/ml) | √b | √ |
| FSH (mIU/ml) | √b | √ |
| Estradiol (pg/ml) | √b | √ |
| Prolactin (ng/ml) | √ | |
| Progesterone (ng/ml) | √ | |
| Seminal fructose (g/l) | √ | |
| Seminal α-glucosidase (IU/ml) | √ | |
| Seminal zinc (μmol/ml) | √ |
See details of the surveys of adults and undergraduates in Methods section and in the corresponding original publications (Li et al., 2009; Yang et al., 2015).
Measured in a subgroup of 232 men.
A community cohort of Chinese undergraduates
Non-work-related circadian desynchrony, semen parameters and reproductive hormone levels were measured in the Male Reproductive Health in Chongqing College Students (MARHCS) cohort. The MARHCS cohort was built to longitudinally investigate relationships between male reproductive health and social/environmental factors (Yang et al., 2015). A total of 796 male undergraduates, none of whom was working a regular job in parallel with studies, were recruited in Chongqing in 2013, and 82.4% were followed up 1 year later. Of the variables examined in the study, no significant difference was found between subjects who were followed-up and those who were lost to follow-up (Chen et al., 2016). In each survey administration, extent of circadian desynchrony was estimated using the Chinese version of the Munich Chronotype Questionnaire (MCTQ) (Chen et al., 2016), which defines the extent of circadian desynchrony as the difference in ‘midsleep’ between ‘workdays’ (school days) and ‘days off’ (non-school days), where midsleep refers to the midpoint between the time of sleep onset and the time of waking up (Roenneberg et al., 2003). In the present study, we did not distinguish between whether midsleep was earlier or later on days off than on school days. During face-to-face interviews, the following additional information was collected: age (in years), abstinence period (in days), tobacco smoking (never smoke; ever smoked; currently smoking), alcohol drinking (never drink; ever drank; currently drinking), tea drinking (never drink; ever drank; currently drinking), cola drinking (never drink; ≤3 bottles/week; 3–6 bottles/week; >6 bottles/week) and coffee drinking (never drink; ≤3 cups/week; 3–6 cups/week; >6 cups/week). BMI was measured and categorized as in the study of the community sample of adults. Sleep duration was measured using the MCTQ and categorized from ≤6.5 h/day to >9 h/day in 0.5-h increments (Chen et al., 2016). At the follow-up survey, sleep quality was measured using the Pittsburg sleep quality index (PSQI) (Buysse et al., 1989). In each year, peripheral blood and semen samples were collected as in the study of the community sample of adults.
The semen parameters and reproductive hormones which were measured in the cohort are shown in Table I. The measurement of total sperm count, progressive motility, total motility and normal morphology was done as described by Yang et al. (2015). Total sperm count was calculated as sperm concentration multiplied by semen volume. The sperm concentration was measured by the Sperm Class Analyzer 5.3.00 (MICROPTIC S.L., Barcelona, Spain). After mixing thoroughly, 10 μl of semen was put into a Goldcyto counting chamber (Goldcyto, Spain), and scanned by the Sperm Class Analyzer. At least six areas and 400 sperms were counted for estimation of sperm concentration. Semen samples were diluted in the same way as in the community population study of Chinese adults if necessary. The measurement was finished within 1 h since ejaculation. All semen samples were measured based on the fifth edition of the World Health Organization manual (World Health Organization, 2010) by one technician. Semen volume was measured by weighing. DNA damage (DNA fragmentation index) and sperm maturity (high DNA stainability) were assessed using a sperm chromatin structure assay (Wang et al., 2018), while serum levels of testosterone, LH, FSH, estradiol, prolactin and progesterone were measured using a Unicel DXI 800 Immunoassay System (Beckman Coulter, Brea, CA, USA) (Chen et al., 2017).
Mouse model of circadian desynchrony
Male C57BL/6J mice, were housed in running wheel cages (Probcare, Wuhan, China) in light-tight, sound-attenuated cabinets maintained at a temperature of 20–22°C and 50–70% humidity. Throughout the study, animals were treated humanely and with the goal of minimizing their suffering. Food and water were provided ad libitum. The protocol and sample size were developed based on pilot studies.
After acclimation for at least 2 weeks on a 12-h light/12-h dark cycle, during which the lights were turned on at 8:00 a.m. and turned off at 8:00 p.m., mice 8–10 weeks old and weighing 15–22 g were randomly divided into two equal groups (Supplementary Fig. S1). The circadian desynchrony group was subjected alternately to a regime of lights on at 8 a.m. and lights off at 8 p.m. or of lights on at 8 p.m. and lights off at 8 a.m.; the light-dark regime was switched every 6 days for 24 days (four alternations). In parallel, control animals were maintained on a regime of lights on at 8 a.m. and lights off at 8 p.m. Zeitgeber time 0 (ZT0, Supplementary Fig. S1) was defined as 8 a.m.
This experiment was conducted with seven batches of mice. All completed the 24 day light schedule. The first six batches were sacrificed on Day 25 in darkness; the first batch at 8 a.m. (ZT0), and subsequent batches at 12 p.m. (ZT4), 4 p.m. (ZT8), 8 p.m. (ZT12), midnight (ZT16) and 4 a.m. (ZT20). Each of the first six batches was used for the comparison of sperm count and included seven mice from the circadian desynchrony group and seven control mice. One mouse in the ZT4 batch, one in the ZT 20 batch and six in the ZT8 batch were excluded because they did not complete the photoperiod schedule because of a logistic reason (e.g. breakdown of individual monitoring device). The seventh batch containing 28 animals was maintained on a fixed regime of lights on at 8 a.m. and lights off at 8 p.m. for an additional 35 days, and then they were sacrificed at 8 a.m.
The ZT0 batch contained additional 28 animals for molecular mechanism analysis (n = 14 for circadian desynchrony group and n = 14 for control group). Firstly, the testis tissue of each mouse was used for RNA isolation and transcriptome sequencing. Seven RNA samples of each group were randomly selected for RT-PCR validation. Secondly, testis tissue of five mice was randomly selected from each group for DNA content analysis. Thirdly, the testis tissue which was left was used for the apoptosis and western blot analyses. Both the experiments had eight samples of testis tissue for each group. The TUNEL analysis of epididymal sperm was done for all the mice and 13 results of each group were available except for a pair of samples which failed in preparation of slides. The results of the mechanism experiments except transcriptome sequencing were confirmed by independent replications.
Animal activity throughout the study was measured in terms of the revolutions of a running wheel recorded in 1-min bins and analyzed using ClockLab software (Actimetrics, Wilmette, IL, USA).
Effects of circadian desynchrony on total sperm count in mouse epididymides
After sacrifice, both epididymides of all circadian desynchrony and control mice were isolated, cleared of adhering tissues, chopped into pieces, and incubated in 800 μl of human tubal fluid (Merck, USA) at 37°C to allow sperm to spread into the medium. An aliquot of the mixture (10 μl) was analyzed for sperm concentration and progressive motility using a Sperm Class Analyzer (Microptic S.L., Barcelona, Spain). The sperm concentration per μl was multiplied by 800 to obtain total sperm count. Researchers performing these analyses were blinded to animal group allocation.
Effects of circadian desynchrony on apoptosis in mouse seminiferous tubules and epididymides
Paraffin-embedded sections of the testis and sperms were prepared from the circadian desynchrony and control mice of the ZT0 batch, and stained using a TdT-mediated dUTP nick-end labeling (TUNEL) kit (Roche, Basel, Switzerland) following the manufacturer’s instructions. TUNEL-positive cells (green) in seminiferous tubules were counted, and at least 100 seminiferous tubules from each testis were observed in randomly selected microscope fields at a magnification of 40×. A sperm apoptotic index was calculated by dividing the number of apoptotic sperm by the total number of sperm analyzed. At least 200 sperm were analyzed from each mouse.
Effects of circadian desynchrony on DNA content in mouse spermatogenic cells
After sacrifice, the tunica albuginea and then seminiferous tubules of the testes were removed from the circadian desynchrony and control mice of the ZT0 batch. Tubules were incubated in 10 ml of HBSS (Hyclone, Beijing, China) containing 100 U/ml of collagenase type I (Sangon Biotech, Shanghai, China) at 32°C for 25 min. The suspension was passed through a 40-μm nylon mesh to retain the tubules, which were then incubated at 32°C for 25 min in the same collagenase buffer as in the first step. The resulting suspension was filtered again through a 40-μm nylon mesh. After a wash in HBSS, cells passed through the filter were fixed in 70% ethanol for 12–24 h at 4°C. Fixed cells were then washed and stained with propidium iodide and RNAase (Beyotime, Shanghai, China) in PBS for 30 min at 37°C in the dark. Cells were analyzed using a FACSCalibur system (FACSAria, BD Biosciences, CA, USA), and 104 cells were counted in each sample. Testicular cell populations were classified according to their DNA content as 1n (corresponding to spermatids), 2n (somatic cells, spermatogonia and secondary spermatocytes) or 4n (primary spermatocytes and cells in G2/M phase).
Effects of circadian desynchrony on gene transcription in mice
After sacrifice, approximately 60 mg of the testes from the circadian desynchrony and control mice of ZT0 batch were ground up in liquid nitrogen in a 2-ml tube, resuspended in 1.5 ml Trizol reagent (Invitrogen, Carlsbad, CA, USA) for 2 min, and allowed to sit horizontally on ice for 5 min. The mixture was centrifuged for 5 min at 12 000g at 4°C, then the supernatant was transferred into a new tube containing 0.3 ml of chloroform/isoamyl alcohol (24:1) per 1.5 ml of Trizol reagent. The mixture was shaken vigorously for 15 s, then centrifuged at 12 000g for 10 min at 4°C. The aqueous phase was transferred to a new tube containing an equal volume of isopropyl alcohol, then centrifuged at 12 000g for 20 min at 4°C. The RNA pellet was washed twice with 1 ml of 75% ethanol, then the tube was centrifuged at 12 000g for 3 min at 4°C to collect residual ethanol, and the pellet was left to air-dry for 5–10 min in a biosafety cabinet. Finally, the RNA was dissolved in 25–100 µl of DEPC-treated water and quantified using a Nano Drop system and Agilent 2100 bioanalyzer (Thermo Fisher Scientific, MA, USA).
The isolated RNA were used for transcriptome sequencing. Sequencing libraries of mRNAs were generated using the rRNA-depleted RNA by NEBNext Ultra Directional RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, United States) following manufacturer’s recommendations. RNA-seq sequencing was performed using an Illumina HiSeq Xten (Illumina, San Diego, CA, USA) or a BGISEQ-500 (BGI-Shenzhen, China) at BGI-Tech, Shenzhen, China. Raw data (raw reads) of the fastq format were first processed using SOAPnuke (v 1.5.2). In this step, clean data (clean reads) were obtained by removing reads containing an adapter, reads containing poly-N, and low-quality reads from raw data. At the same time, Q20, Q30 and GC content of the clean data were calculated. The mRNA was sequenced by paired-end. Reads were aligned to the mouse genome with Tophat (v 2.0.9). Reads that were mapped to the mouse genome were assembled using HISAT2 (v 2.0.4) and Cufflinks (v 2.1.1). RSEM (v 2.2.5) was used to calculate the FPKMs (expected number of Fragments Per Kilobase of transcript sequence per Million base pairs sequenced) of protein-coding genes in each sample. Transcripts with P-values <0.05 were assigned as Difference. TPM normalization is used for estimating relative circRNAs and miRNAs production levels from RNA-seq data.
To validate the results obtained from this library analysis, total RNA (1 μg) was used to generate cDNA using the PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time, Takara, Dalian, China). PCR reactions (20 μl) contained 7 μl of nuclease-free water, 10 μl of 2X GoTaq Probe qPCR Master Mix, 1 μl of cDNA and 0.5 μl of each primer (the primer sequences for stage-specific markers of spermatogenic cells and several homologous recombination pathway genes is shown in Table II).
Table II.
The primer sequences for stage-specific markers of spermatogenic cells and some homologous recombination pathway genes.
| Name | Primer sequence |
|---|---|
| β-actin | F: 5′-CAGCTTCTTTGCAGCTCCTT-3′ |
| R: 5′-CACGATGGAGGGGAATACAG-3′ | |
| Hspa2 | F: 5′-GCGTGGGGGTATTCCAACAT-3′ |
| R: 5′-TGAGACGCTCGGTGTCAGT-3′ | |
| Magea4 | F: 5′-TCGGAGCCAAAGGGAGTTAGA-3′ |
| R: 5′-GGCTAGTATCACAAGGGGAGAG-3′ | |
| Tnp1 | F: 5′-GTCTTCAAACAACACGGGGC-3′ |
| R: 5′-CGAATTTCGTCACGACTGGC-3′ | |
| Prm1 | F: 5′-CCGCCGCTCATACACCATAA-3′ |
| R: 5′-TGTGGCGAGATGCTCTTGAA-3′ | |
| Prm2 | F: 5′-CTCCTCCAATCCAGGTCAGC-3′ |
| R: 5′-TCCTCGCGTTCATGGTCTTG-3′ | |
| Brca1 | F: 5′-GGCTGCTTTTGAGCTTGACA-3′ |
| R: 5′-CGCCTCCTCATTCAAACGC-3′ | |
| Rad54b | F: 5′-TGTAGGAAGGCGGGAAGCTC-3′ |
| R: 5′-TTCCCTGCACCTGACTTGGT-3′ | |
| Rad54L | F: 5′-TCAGACCTGGCTCAGTGGAACC-3′ |
| R: 5′-GAACGCTGGTGGAAGACGAAGG-3′ | |
| Nbn | F: 5′-AAAGCCAAGGATGGACGCAG-3′ |
| R: 5′-CAGTCAGCAGCAGTTTCCGT-3′ | |
| Mre11 | F: 5′-GGTCAATGTCGGTGGAGAAGGTTG-3′ |
| R: 5′-TGGAGCCTAAGCCGTACAGAGC-3′ |
Real-time PCR was performed using a Bio-Rad IQ5 system (Bio-Rad, USA). The tube lids were preheated to 105°C. Samples were subjected to 95°C for 5 min, followed by 40 cycles of 95°C for 10 s, 60°C for 30 s and 72°C for 5 min. Data were analyzed using the 2(−ΔΔCt) method and normalized to levels of β-actin cDNA.
Effects of circadian desynchrony on levels of key proteins in mice
After sacrifice, approximately 60 mg of the testes from the circadian desynchrony and control mice of ZT0 batch was ground into powder and lysed in RIPA buffer (Beyotime, China) on ice. Total protein content was estimated using the BCA Protein Assay Kit (Beyotime, China), and lysates were mixed with 5 × loading buffer (Beyotime, China), heated at 100°C for 5 min, fractionated on 6–15% SDS-PAGE gels, and transferred to polyvinylidene difluoride membranes (Merck, USA). Membranes were blocked for 1 h with 5% bovine serum albumin in Tris-buffered saline containing 0.1% (v/v)Tween-20 (TBST; pH 7.4), incubated with primary antibodies at 4°C for 12 h, washed in TBST, and finally incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000; Beyotime) for 1 h at room temperature. Immunoreactive bands were visualized using an enhanced chemiluminescent kit (Merck, USA). Protein levels were normalized to those of β-actin and expressed as a percentage of the levels in control animals.
Statistical analysis
The semen parameters and reproductive hormones levels were compared between RSW or PSW and day work by Mann–Whitney U test in the cross-sectional study. The linear association between circadian desynchrony and semen parameters and reproductive hormone levels was analyzed by Jonckheere–Terpstra test in the cohort. Stepwise linear regression and backward likelihood logistic regression were used to control for potential bias introduced by age, abstinence period, education level, average monthly household income, sampling time point, tobacco smoking, BMI, sleep duration, PSQI and drinking of alcohol, tea, cola and coffee. In the linear regression, variables showing a skewed distribution were analyzed on a logarithmic scale, and regression coefficients were back-transformed into percentages of change. Intra-individual differences in semen parameters or reproductive hormone levels between the first and second administrations of the MARHCS study were assessed for significance using the Wilcoxon test. Imputation was not used to compensate for missing data for the community sample of adults or undergraduates, since no more than 5% of data were missing in the major analyses. Multiple test correction was not implemented in the present research as the biomarker (semen parameter or reproductive hormone) identified simultaneously in the two populations was further examined in the animal experiment.
In the animal study, results were compared between circadian desynchrony and control groups using the t test for a single time point, or using two-way analysis of variance across different time points. Differentially expressed genes (DEGs) between circadian desynchrony and control conditions were analyzed for enrichment in certain gene ontology terms. DEGs with known biological functions were analyzed against the Kyoto Encyclopedia of Genes and Genomes (KEGG) database for enrichment in certain signal transduction pathways.
Results
Work-related circadian desynchrony, semen quality and reproductive hormone levels
Demographic characteristics of the 1346 adults were described in Table III. Among the subjects, 931 (69.2%) were dayworkers, 98 (7.3%) engaged in PSW and 139 (10.3%) engaged in RSW. The remaining respondents indicated that their employment did not fit any of these categories (143; 10.6%), or they failed to provide any information about their employment (35; 2.6%). There were differences of smoking behavior, educational level and family income among different work types (Table III). No other difference of demographic characteristics was found.
Table III.
Comparison of demographic characteristics of the Chinese adults with different work types.
| Demographic characteristics | Alla (n = 1346) | Day work (n = 931) | Permanent shift work (n = 98) | Rotating shift work (n = 139) | P-value |
|---|---|---|---|---|---|
| Age (years) | 34 (29–37) | 34 (29–37) | 34 (29–37) | 33 (27–37) | 0.152 |
| Abstinence period (day) | 4 (3–6) | 4 (3–6) | 4 (3–6) | 4 (3–6) | 0.303 |
| Sampling time point | 10 (9–10) | 10 (9–11) | 10 (9–10) | 10 (9–10) | 0.603 |
| Tobacco smoking | 0.005 | ||||
| Never | 484 (36.0%) | 363 (39.0%) | 21 (21.4%) | 44 (31.7%) | |
| Ever | 86 (6.4%) | 58 (6.2%) | 7 (7.1%) | 6 (4.3%) | |
| Current | 774 (57.6%) | 510 (54.8%) | 70 (71.4%) | 89 (64.0%) | |
| Alcohol drinking | 0.659 | ||||
| Yes | 855 (63.7%) | 595 (63.9%) | 67 (68.4%) | 88 (63.3%) | |
| No | 488 (36.3%) | 336 (36.1%) | 31 (31.6%) | 51 (36.7%) | |
| BMI | 22.0 (20.2–24.2) | 22.0 (20.2–24.2) | 22.3 (20.0–25.0) | 22.5 (20.5–24.3) | 0.469 |
| Education level | < 0.001 | ||||
| Primary school and below | 165 (12.4%) | 121 (13.1%) | 4 (4.2%) | 10 (7.2%) | |
| Junior school | 548 (41.0%) | 360 (38.9%) | 45 (46.9%) | 67 (48.2%) | |
| High school | 326 (24.4%) | 197 (21.3%) | 32 (33.3%) | 49 (35.3%) | |
| College and higher | 296 (22.2%) | 248 (26.8%) | 15 (15.6%) | 13 (9.4%) | |
| Family income (RMB/year) | 0.037 | ||||
| <3000 | 642 (48.8%) | 429 (46.8%) | 51 (54.8%) | 56 (41.2%) | |
| 3000–8000 | 378 (28.7%) | 283 (30.9%) | 21 (22.6%) | 34 (25.0%) | |
| 8000–13 000 | 193 (14.7%) | 136 (14.8%) | 11 (11.8%) | 30 (22.1%) | |
| >13 000 | 103 (7.8%) | 69 (7.5%) | 10 (10.8%) | 16 (11.8%) |
Values were presented as median (interquartile range) or frequency (percentage). Kruskal–Wallis test was used in the comparison of age, abstinence period, sampling time point and BMI. The χ2 test was used in the comparison of tobacco smoking, alcohol drinking, education level and family income.
One hundred and forty-three workers’ employment did not fit with one of the defined categories and 35 failed to complete the question.
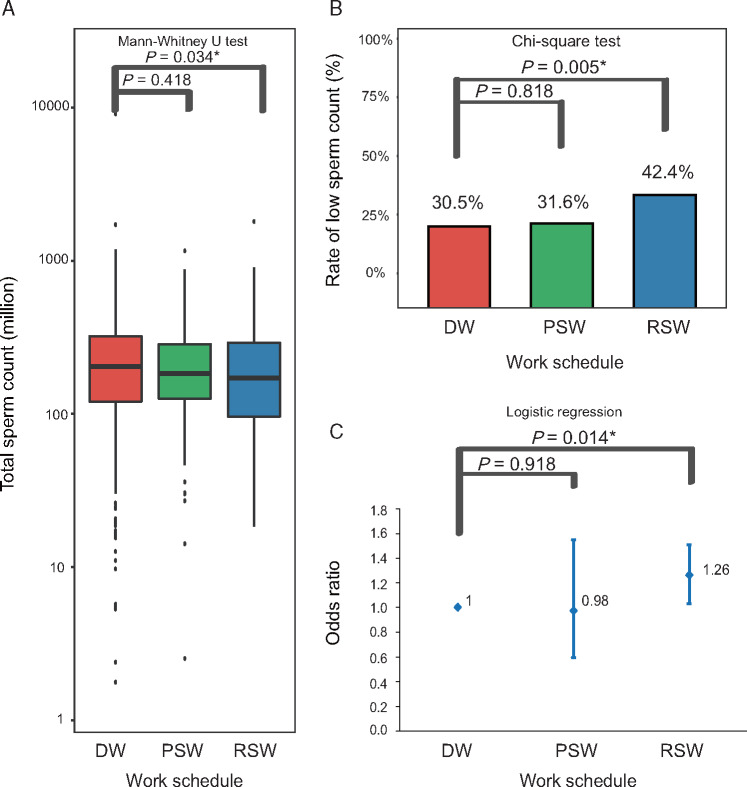
Among the semen parameters and reproductive hormone which were measured, total sperm count, a measure of the capability of the testes to produce spermatozoa, was significantly lower in RSW workers [median: 147.3 × 106, interquartile range (IQR) 80.7 × 106 to 255.3 × 106] than among day workers (median: 176.0 × 106, IQR 101.9 × 106 to 281.2 × 106; P = 0.034; Fig. 2A). The proportion of men with low total sperm count (≤120 × 106), as defined by the Chinese Ministry of Health (2004), was higher among RSW workers (42.4%) than among day workers (30.5%, P = 0.005; Fig. 2B). RSW was associated with significantly higher risk of low total sperm count after controlling for age, education level, average monthly household income, abstinence period, sampling time point, tobacco smoking, alcohol drinking and BMI [odds ratio (OR) 1.60, 95% CI 1.10–2.32, P = 0.014; Fig. 2C]. The logistic regression predicted that for the day workers and RSW workers, the probability to have low total sperm count was 30.8% and 43.4%, respectively. Other semen parameters and reproductive hormone levels did not differ significantly between RSW workers and day workers, except semen volume (P = 0.025, Supplementary Fig. S2), which was used to calculate total sperm count. Semen volume was mainly made up of secretions from accessory organs such as prostate and seminal vesicles. Semen volume itself had no clear association with fertility, except in the case that extremely low semen volume was caused by obstruction of the ejaculatory duct or congenital bilateral absence of the vas deferens, which had been excluded before recruitment.
Figure 2.
Association between work-related circadian desynchrony and total sperm count in Chinese community men. Data were taken from a community survey of Chinese adults taken in a single year (Li et al., 2009). Total sperm count was classified as low (≤120 × 106) or not according to the guidelines of the Chinese Ministry of Health (Chinese Ministry of Health, 2004). Comparison of total sperm count (A), rate of low sperm count (B) and multivariate-adjusted risk of low sperm count (C) among day workers (DW), permanent shift workers (PSW) and rotating shift workers (RSW) in the community sample of Chinese adults. *P < 0.05.
To see if smoking behavior, educational level and family income was mediator between circadian desynchrony and sperm count, stratified analysis for these demographic factors were performed. The results showed that work type was independently associated with risk of low sperm count from these factors (Supplementary Table SI).
Total sperm count in PSW workers was not significantly different from that of day workers (Fig. 2A). LH was the only parameter to differ between PSW workers and day workers (P = 0.044).
Non-work-related circadian desynchrony, semen quality and reproductive hormone levels
Demographic characteristics of the 796 undergraduates in the cohort were described in Table IV. The respondents showed that circadian desynchrony between schooldays and days off ranged from 0 to 9.6 h (Fig. 3A). There were difference of smoking behavior and cola intake among different levels of non-work-related circadian desynchrony in the population (Table IV). No other difference of demographic characteristics was found.
Table IV.
Comparison of demographic characteristics of the Chinese undergraduates with different levels of non-work-related circadian desynchrony.
| Demographic characteristics | All (n = 796) | ≤0.5 h/day (n = 269) | 0.5–1.0 h/day (n = 234) | 1.0–1.5 h/day (n = 153) | 1.5–2.0 h/day (n = 81) | >2.0 h/day (n = 56) | P-value |
|---|---|---|---|---|---|---|---|
| Age (years) | 20 (20–21) | 20 (20–21) | 20 (20–21) | 20 (20–21) | 20 (20–21) | 20 (19–21) | 0.086 |
| Abstinence period (day) | 4 (3–6) | 4 (3–6) | 4 (3–6) | 4 (3–6) | 4 (3–6) | 3 (3–4) | 0.373 |
| Sampling time point | 15.5 (11.4–17.9) | 16 (11–18) | 15 (11–18) | 15 (11–17) | 15 (12–17) | 15 (11–16) | 0.144 |
| Tobacco smoking | <0.001 | ||||||
| Never | 593 (74.7%) | 223 (82.9%) | 176 (75.2%) | 104 (68.0%) | 55 (67.9%) | 34 (60.7%) | |
| Ever | 30 (3.8%) | 6 (2.2%) | 13 (5.6%) | 7 (4.6%) | 4 (4.9%) | 0 (0.0%) | |
| Current | 171 (21.5%) | 40 (14.9%) | 45 (19.2%) | 42 (27.5%) | 22 (27.2%) | 22 (39.3%) | |
| Alcohol drinking | 0.092 | ||||||
| Never | 409 (51.4%) | 151 (56.1%) | 123 (52.6%) | 76 (50.0%) | 35 (43.2%) | 23 (41.1%) | |
| Ever | 10 (1.3%) | 1 (0.4%) | 5 (2.1%) | 1 (0.7%) | 1 (1.2%) | 2 (3.6%) | |
| Current | 374 (47.2%) | 117 (43.5%) | 106 (45.3%) | 75 (49.3%) | 45 (55.6%) | 31 (55.4%) | |
| BMI | 20.9 (19.6–22.7) | 21.0 (19.8–22.6) | 20.8 (19.4–22.5) | 21.1 (19.6–23.0) | 21.1 (19.6–22.3) | 20.4 (19.1–22.6) | 0.964 |
| Sleep duration | 7.8 (7.3–8.3) | 7.7 (7.2–8.3) | 7.9 (7.9–8.4) | 7.9 (7.3–8.4) | 7.8 (7.1–8.2) | 7.6 (7.6–8.4) | 0.409 |
| Tea intake | 0.304 | ||||||
| Never | 512 (64.5%) | 180 (66.9%) | 141 (60.3%) | 101 (66.0%) | 57 (70.4%) | 32 (57.1%) | |
| Ever | 123 (15.5%) | 33 (12.3%) | 48 (20.5%) | 22 (14.4%) | 10 (12.3%) | 10 (17.9%) | |
| Current | 159 (20.0%) | 56 (20.8%) | 45 (19.2%) | 30 (19.6%) | 14 (17.3%) | 14 (25.0%) | |
| Cola intake (bottles/w) | 0.004 | ||||||
| 0 | 273 (34.4%) | 117 (43.5%) | 68 (29.1%) | 50 (32.7%) | 25 (30.9%) | 12 (21.4%) | |
| <3 | 404 (50.9%) | 122 (45.4%) | 129 (55.1%) | 83 (54.2%) | 41 (50.6%) | 29 (51.8%) | |
| 3–6 | 100 (12.6%) | 26 (9.7%) | 35 (15.0%) | 15 (9.8%) | 12 (14.8%) | 12 (21.4%) | |
| >6 | 17 (2.1%) | 4 (1.5%) | 2 (0.9%) | 5 (3.3%) | 3 (3.7%) | 3 (5.4%) | |
| Coffee intake (cups/w) | 0.566 | ||||||
| 0 | 605 (76.2%) | 206 (76.6%) | 182 (77.8%) | 114 (74.5%) | 58 (71.6%) | 44 (78.6%) | |
| <3 | 154 (19.4%) | 50 (18.6%) | 44 (18.8%) | 29 (19.0%) | 22 (27.2%) | 9 (16.1%) | |
| 3–6 | 21 (2.6%) | 6 (2.2%) | 6 (2.6%) | 7 (4.6%) | 1 (1.2%) | 1 (1.8%) | |
| >6 | 14 (1.8%) | 7 (2.6%) | 2 (0.9%) | 3 (2.0%) | 0 (0.0%) | 2 (3.6%) |
Values were presented as median (interquartile range) or frequency (percentage). Jonckheere–Terpstra test was used in the comparison of age, abstinence period, sampling time point, BMI and sleep duration. The χ2 test was used in the comparison of tobacco smoking, tea intake and cola intake. Fisher’s exact test was used in the comparison of alcohol drinking and coffee intake.
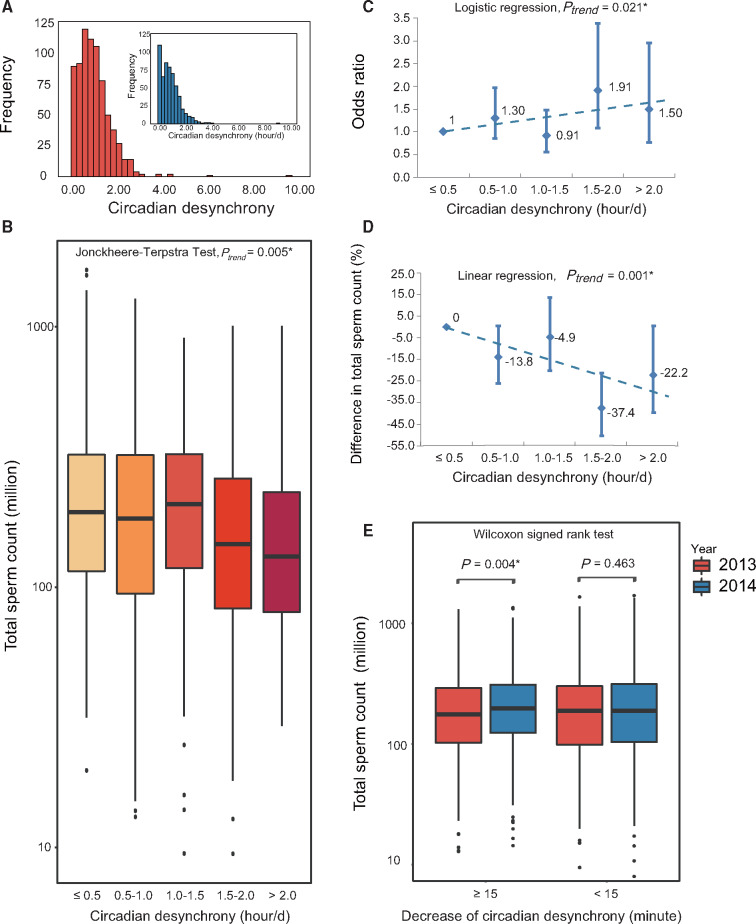
Figure 3.
Association between non-work-related circadian desynchrony and total sperm count in Chinese undergraduates. Data were taken from a cohort of Chinese undergraduates interviewed in two consecutive years (Yang et al., 2015). The analyses are shown for Chinese undergraduates differing in circadian desynchrony between school days and days off. (A) Circadian desynchrony at baseline (blue bars; median, 0.9 h) and 1-year follow-up (red bars; median, 0.8 h). (B) The comparison of total sperm count. (C) The risk of low sperm count in relation to circadian desynchrony at baseline, with adjustment for potential confounders. The regression result is shown as a dotted line. (D) The association of the decrease in total sperm count with circadian desynchrony at follow-up, with adjustment for sleep quality in addition to the confounders included at baseline. (E) The total sperm count at the end of 2013 and 2014 split according to the decrease in circadian desynchrony during the same period. *P < 0.05.
The extent of circadian desynchrony was inversely associated with total sperm count (P = 0.005, Fig. 3B). Total sperm count in men with ≤0.5 h circadian desynchrony was 194.2 (IQR 114.9–324.9) × 106, compared to 130.9 (IQR 79.0–241.2) × 106 for those with >2.0 h circadian desynchrony (P = 0.002). Analysis that controlled for age, abstinence period, sampling time point, tobacco smoking, sleep duration, BMI and drinking of alcohol, tea, cola and coffee showed that each hour of circadian desynchrony was associated with 5.9% lower total sperm count (95% CI 1.3–10.4%; P = 0.013) and with 1.16-fold higher risk of low total sperm count (95% CI 1.02–1.31, P = 0.021; Fig. 3C). If sleep duration and intake of tea, coffee and cola was not controlled as in the community population, the estimate remained largely unchanged (OR = 1.15, 95% CI 1.02–1.31, P = 0.023). This association remained significant after adjusting for PSQI score: in this case, each hour of circadian desynchrony was associated with 8.4% lower total sperm count (95% CI 3.6–12.9%, P = 0.001; Fig. 3D) and 1.17-fold higher risk of low total sperm count (95% CI 1.01–1.35; P = 0.032), although the subgroup with largest effect on outcome seemed to be slightly changed by the adjustment. The logistic regression predicted that for the subjects with circadian desynchrony of ≤0.5 h, 0.5–1.0 h, 1.0–1.5 h, 1.5–2.0 h and >2.0 h, the probability to have low total sperm count was 26.9%, 29.3%, 33.0%, 35.7% and 43.5%, respectively. Circadian desynchrony was not significantly associated with other semen parameters or reproductive hormone levels except sperm concentration (P = 0.041), which was an indirect measure of testicular sperm output and was used to calculate total sperm count (Supplementary Fig. S3).
To see if smoking behavior and cola intake was mediator between non-work-related circadian desynchrony and sperm count, stratified analysis for these demographic factors were performed. The results showed that non-work-related circadian desynchrony was independently associated with risk of low sperm count from these factors (Supplementary Table SII).
The fact that the Chinese undergraduates were surveyed twice at 1 year apart allowed us to examine whether decreased total sperm count in individuals suffering circadian desynchrony could recover if the circadian desynchrony was alleviated. Indeed, the change in circadian desynchrony between the two survey administrations tended to correlate inversely with the change of total sperm count during the same period (P = 0.054). In particular, among those whose circadian desynchrony decreased by at least 15 min during the same period, total sperm count increased significantly (P = 0.004, Fig. 3E).
Effects of circadian desynchrony on reproductive biomarker in male mice
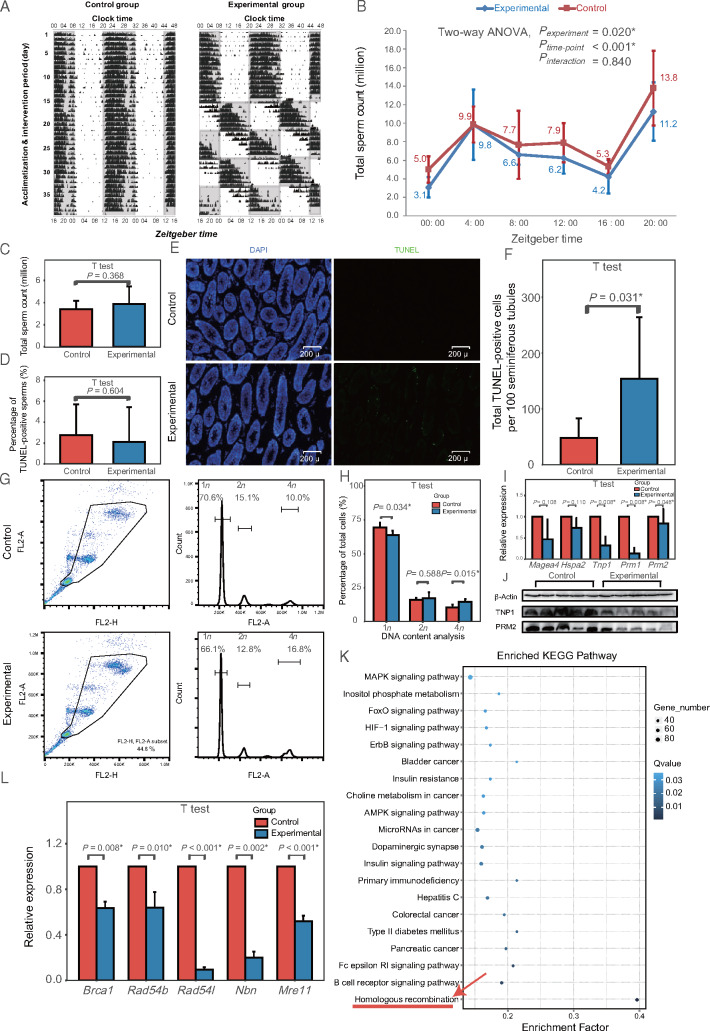
As both epidemiological studies identified total sperm count as the outcome associated with circadian desynchrony, we explored this biomarker in mice subjected to photoperiod shifting as a model of circadian desynchrony. As expected, photoperiod shifting adjusted diurnal mouse activity (Fig. 4A) without affecting body weight. Mean total sperm count across various times during a 24-h period was significantly lower in the circadian desynchrony mice (mean, 6.9 ± 0.4 × 106; range, 3.1 ± 1.1 × 106 to 11.2 ± 3.2 × 106) than in control mice (mean, 8.2 ± 0.4 × 106; range, 5.0 ± 1.4 × 106 to 13.8 ± 4.0 × 106) (P = 0.020, Fig. 4B). No significant interaction between photoperiod shifting and sampling time point was found (P = 0.840).
Figure 4.
Compromised spermatogenesis in a mouse model of circadian desynchrony. (A) Representative activity recorded by running wheel (double plot). Black bars indicate activity counts (in percentile) every 6 min. Gray squares represent darkness; white squares, light. (B) Comparison of total sperm count between experimental and control animals (n = 4–7 mice per group in each time point) at different zeitgeber time points. P-values are shown separately for group effect, time point effect and interaction of group and time point. (C) Comparison of total sperm count between control animals, maintained on a fixed light-dark cycle, and experimental animals after undergoing a 35-day recovery on the same light-dark cycle as control animals after the photoperiod shifting (n = 14 mice per group). (D) Comparison of apoptosis in epididymal sperm between experimental and control animals (n = 13 mice per group). (E) Representative photomicrographs comparing apoptosis in seminiferous tubules between experimental and control animals, using TUNEL staining of the testis. Apoptotic cells appear green. (F) Quantitation of assays shown in panel E (n = 8 mice per group). (G) Flow cytometric analysis of DNA content (1n, 2n or 4n) in cells in seminiferous tubules of experimental and control mice. (H) Quantitation of assays shown in panel G (n = 5 mice per group). (I) Real-time PCR analysis of spermatogenic stage markers differentially expressed in experimental animals (n = 7 mice per group). (J) Representative western blotting of markers of round/condensing spermatids (TNP1) and elongated/condensed spermatids and luminal sperm (PRM2) differentially expressed in experimental animals. (K) Pathway enrichment analysis of mRNAs differentially expressed in experimental mice testis, based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) (n = 14 mice per group). Homologous recombination (red arrow) pathway shows the greatest enrichment. (L) Real-time PCR validation of homologous recombination genes differentially expressed in experimental animals (n = 7 mice per group). *P < 0.05.
We further investigated whether total sperm count induced by circadian desynchrony could recover when circadian desynchrony was alleviated. One batch of mice were subjected first to photoperiod shifting and then to a constant photoperiod as at baseline. This ‘recovery’ period lasted 35 days, which corresponds to the duration of spermatogenesis. Total sperm count at the end of this recovery was similar to that in the control animals who were never subjected to photoperiod shifting (P = 0.368; Fig. 4C and Supplementary Fig. S1).
Using this validated mouse model, we found that circadian desynchrony was associated with a greater extent of apoptosis in seminiferous tubules but not epididymis (Fig. 4D–F) and with a lower proportion of testicular cells with 1n DNA content (corresponding to spermatids) and higher proportion of testicular cells with 4n DNA content (primary spermatocytes and cells in G2/M phase) (Fig. 4G and H). Analysis of DEGs showed that circadian desynchrony down-regulated genes expressed specifically in round/condensing spermatids, elongated/condensed spermatids and luminal sperm, without altering expression of genes expressed in spermatogonia and spermatocytes (Supplementary Table SIII). These transcriptome results were validated for specific genes using RT-PCR and Western blotting (Fig. 4I and J). The functional pathway most enriched among DEGs was homologous recombination, which was necessary for the progression from primary to secondary spermatocytes (4n to 2n; Fig. 4K). RT-PCR confirmed the down-regulation of key genes in homologous recombination (Fig. 4L).
Discussion
Our hybrid research of epidemiological study and mouse experiment showed that work-related circadian desynchrony and non-work-related circadian desynchrony were both associated with a decrease of total sperm count and similar changes could be induced by circadian desynchrony in animal model. Furthermore, an improvement of the decrease was observed when the circadian desynchrony was attenuated both in the human cohort and in the animal model. The present research may address a gap in the research literature by establishing a link between circadian desynchrony and reduced male reproductive health.
The cross-sectional data showed an association between RSW and reduced sperm count, which is in agreement with a study in the USA (Kohn et al., 2017). We did not, however, detect a significant association between PSW and total sperm count, which supports proposals that the two types of shift work have different effects (Cheng and Cheng, 2017; Mancio et al., 2018). It may be that the constant nature of PSW allows workers to ‘align’ their sleep/wake schedule on their days off with their schedule on workdays, thereby reducing circadian desynchrony. In addition, since permanent shifts include permanent morning, evening, or nights, some of non-night shift workers might be considered as permanent shift worker in the surveys, which might also reduce the circadian desynchrony exposure. These may help explain why some studies that did not differentiate between RSW and PSW failed to detect associations between circadian desynchrony and male reproductive outcomes (Eisenberg et al., 2015; Wogatzky et al., 2012). Future work should be careful to differentiate these types of work, such as by using a version of the MCTQ specifically developed for shift workers (Juda et al., 2013).
We found that non-work-related circadian desynchrony among unemployed Chinese undergraduates was associated with lower sperm count. There is a similar research of female reproductive health that found a correlation between non-work-related circadian desynchrony and menstrual pain as well as other adverse reproductive phenotypes (Komada et al., 2019). The exposure of work-related and non-work-related circadian desynchrony is not identical. But the irregularity of sleep behavior in undergraduates has been found to affect the inner clock biomarker (Phillips et al., 2017), which is similar to the phenomenon observed in RSW workers (Leung et al., 2016). It may provide additional support to the study hypothesis that circadian desynchrony may induce male reproductive damage. Further studies are needed to validate these findings and to answer whether inner clock was the common mediator between the two types of circadian desynchrony and the reproductive damage.
Using a well-established mouse model of circadian desynchrony based on photoperiod shifting, we also found that circadian desynchrony is associated with reduced sperm count. Several experiments of mechanism exploration suggest that circadian desynchrony arrests spermatocyte development at an early to middle stage. The fact that sperm count recovered in mice after a 35-day ‘resetting’ procedure seems to support this hypothesis. However, these preliminary results are far from adequate to clarify the mechanism clearly and in depth. More studies of mechanism are awaiting to be done.
Future studies should examine how circadian desynchrony interrupts spermatogenesis in testis. It is possible that circadian genes are involved, analogously to the way in which the circadian clock mediates other effects of circadian desynchrony (Bass and Lazar, 2016). Consistent with this idea, genes in mouse testis involved in ‘rhythmic processes’ are strongly differentially expressed in response to simulated circadian desynchrony (Supplementary Fig. S4). However, it is not clear whether human sperm count follows circadian rhythm (Boden et al., 2013), so this question should be explored further.
Our results should be interpreted carefully in light of several limitations. Firstly, the survey data were purely observational, highlighting the need for prospective studies that can clarify causal relationships between circadian desynchrony and male reproductive processes, preferably by sampling semen parameters at multiple times during a 24-h period. Secondly, healthy worker effect could not be ruled out in the analyses of shift workers. Thirdly, we did not analyze some indices of male reproductive health that may be relevant, such as anti-sperm antibody titer and sperm vitality. In addition, the photoperiod shifting of animal model was not completely identical to the exposure of the male subjects in terms of the source, the frequency and the phase of the shifting. Although decrease of sperm count was observed in the mice as in the Chinese populations, an animal model with exposure more closely equivalent to the real human world is needed in the future. Our animal studies were conducted during over six months, raising the possibility of seasonal effects; however, such effects likely contributed little to our results, since experimental and control animals were always processed in parallel and the diurnal pattern of total sperm count did not change in the experimental group.
Despite these limitations, our hybrid study shows strong evidence from epidemiological studies in men and molecular analyses of a mouse model that circadian desynchrony is associated with lower sperm count. This damage may be at least partially reversible when circadian desynchrony decreases. The importance of the problem is reflected in the fact that more than 10% of European and Chinese men of reproductive age engage in RSW, while at least 60% of the general population suffer some extent of non-work-related circadian desynchrony (Roenneberg et al., 2019). Circadian desynchrony may contribute to the decline of male fertility in these regions (Huang et al., 2011; Rolland et al., 2013).
Authors’ roles
J.C. and Q.C. formulated the overarching research goals and aims. K.L., G.H., X.W., H.C., F.S., C.L., X.Z., F.H., H.Y., N.Z., L.A., J.L., J.C. and Q.C. developed the methodology and conducted the research. Each authors joined in the writing of the article.
Funding
This work was supported by the National Key Research and Development Program of China [2017YFC1002001] and the National Natural Science Foundation of China [81871208].
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Material
References
- Ashok A, Sajal G, Rakesh S.. Andrological evaluation of male infertility In: Ashok A, Sajal G, Rakesh S. Semen Analysis Using Sperm Class Analyzer (SCA v5) for Computer Assisted Semen Analysis (CASA). Switzerland: Springer International Publishing, 2016,59-67. [Google Scholar]
- Bass J, Lazar MA.. Circadian time signatures of fitness and disease. Science 2016;354:994–999. [DOI] [PubMed] [Google Scholar]
- Bisanti L, Olsen J, Basso O, Thonneau P, Karmaus W.. Shift work and subfecundity: a European multicenter study. European Study Group on Infertility and Subfecundity. J Occup Environ Med 1996;38:352–358. [DOI] [PubMed] [Google Scholar]
- Boden MJ, Varcoe TJ, Kennaway DJ.. Circadian regulation of reproduction: from gamete to offspring. Prog Biophys Mol Biol 2013;113:387–397. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ.. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- Chen Q, Yang H, Zhou N, Sun L, Bao H, Tan L, Chen H, Ling X, Zhang G, Huang L. et al. Inverse U-shaped association between sleep duration and semen quality: longitudinal observational study (MARHCS) in Chongqing, China. Sleep 2016;39:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yang H, Zhou N, Sun L, Bao H, Tan L, Chen H, Ling X, Zhang G, Huang L. et al. Phthalate exposure, even below US EPA reference doses, was associated with semen quality and reproductive hormones: Prospective MARHCS study in general population. Environ Int 2017;104:58–68. [DOI] [PubMed] [Google Scholar]
- Cheng W-J, Cheng Y.. Night shift and rotating shift in association with sleep problems, burnout and minor mental disorder in male and female employees. Occup Environ Med 2017;74:483–488. [DOI] [PubMed] [Google Scholar]
- Chinese Ministry of Health. Basic standards and technical norms of human sperm banks. Chin J Reprod Health 2004;15:68–71. [Google Scholar]
- Crnko S, Du Pre BC, Sluijter JPG, Van Laake LW.. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol 2019;16:437–447. [DOI] [PubMed] [Google Scholar]
- Eisenberg ML, Chen Z, Ye A, Louis GMB.. Relationship between physical occupational exposures and health on semen quality: data from the Longitudinal Investigation of Fertility and the Environment (LIFE) study. Fertil Steril 2015;103:1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Helaly M, Awadalla N, Mansour M, El-Biomy Y.. Workplace exposures and male infertility—a case-control study. Int J Occup Med Environ Health 2010;23:331–338. [DOI] [PubMed] [Google Scholar]
- Fernandez RC, Marino JL, Varcoe TJ, Davis S, Moran LJ, Rumbold AR, Brown HM, Whitrow MJ, Davies MJ, Moore VM.. Fixed or rotating night shift work undertaken by women: implications for fertility and miscarriage. Semin Reprod Med 2016;34:74–82. [DOI] [PubMed] [Google Scholar]
- Figueiro MG, Radetsky L, Plitnick B, Rea MS.. Glucose tolerance in mice exposed to light-dark stimulus patterns mirroring dayshift and rotating shift schedules. Sci Rep 2017;7:40661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia CR, Sammel MD, Coutifaris C, Guzick DS, Barnhart KT.. Occupational exposures and male infertility. Am J Epidemiol 2005;162:729–733. [DOI] [PubMed] [Google Scholar]
- Han X, Cui Z, Zhou N, Ma M, Li L, Li Y, Lin H, Ao L, Shu W, Liu J. et al. Urinary phthalate metabolites and male reproductive function parameters in Chongqing general population, China. Int J Hyg Environ Health 2014;217:271–278. [DOI] [PubMed] [Google Scholar]
- Han X, Zhou N, Cui Z, Ma M, Li L, Cai M, Li Y, Lin H, Li Y, Ao L. et al. Association between urinary polycyclic aromatic hydrocarbon metabolites and sperm DNA damage: a population study in Chongqing, China. Environ Health Perspect 2011;119:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LP, Ya-Fei LI, Xiong HY, Cao J.. Trend of change in semen quality in Chinese healthy menover recent 25 years. Reprod Contracept 2011;31:122–133. [Google Scholar]
- Juda M, Vetter C, Roenneberg T.. The Munich chronotype questionnaire for shift-workers (MCTQ(shift)). J Biol Rhythms 2013;28:130–140. [DOI] [PubMed] [Google Scholar]
- Kim SM, Neuendorff N, Alaniz RC, Sun Y, Chapkin RS, Earnest DJ.. Shift work cycle-induced alterations of circadian rhythms potentiate the effects of high-fat diet on inflammation and metabolism. FASEB J 2018;32:3085–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn TP, Pastuszak AW, Pickett SM, Kohn JR, Lipshultz LI.. Shift work is associated with altered semen parameters in infertile men. J Urol 2017;197:E273–E274. [Google Scholar]
- Komada Y, Ikeda Y, Sato M, Kami A, Masuda C, Shibata S.. Social jetlag and menstrual symptoms among female university students. Chronobiol Int 2019;36:258–264. [DOI] [PubMed] [Google Scholar]
- Leung M, Tranmer J, Hung E, Korsiak J, Day AG, Aronson KJ.. Shift work, chronotype, and melatonin patterns among female hospital employees on day and night shifts. Cancer Epidemiol Biomarkers Prev 2016;25:830–838. [DOI] [PubMed] [Google Scholar]
- Li Y, Lin H, Ma M, Li L, Cai M, Zhou N, Han X, Bao H, Huang L, Zhu C. et al. Semen quality of 1346 healthy men, results from the Chongqing area of southwest China. Hum Reprod 2009;24:459–469. [DOI] [PubMed] [Google Scholar]
- Liu S, Wing YK, Hao Y, Li W, Zhang J, Zhang B.. The associations of long-time mobile phone use with sleep disturbances and mental distress in technical college students: a prospective cohort study. Sleep 2019;42:2. [DOI] [PubMed] [Google Scholar]
- Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, Hansen J, Nelson RJ, Panda S, Smolensky MH. et al. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ 2017;607–608:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancio J, Leal C, Ferreira M, Norton P, Lunet N.. Does the association of prostate cancer with night-shift work differ according to rotating vs. fixed schedule? A systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2018;21:337–344. [DOI] [PubMed] [Google Scholar]
- McGowan NM, Coogan AN.. Circadian and behavioural responses to shift work-like schedules of light/dark in the mouse. J Mol Psychiatr 2013;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJK, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW, Lockley SW, Klerman EB, Czeisler CA.. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep 2017;7:3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Pilz LK, Zerbini G, Winnebeck EC.. Chronotype and Social Jetlag: A (Self-) critical review. Biology 2019;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M.. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 2003;18:80–90. [DOI] [PubMed] [Google Scholar]
- Rolland M, Le Moal J, Wagner V, Royere D, De Mouzon J.. Decline in semen concentration and morphology in a sample of 26 609 men close to general population between 1989 and 2005 in France. Hum Reprod 2013;28:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, Castano-Vinyals G, Davis S, Frings-Dresen MH, Fritschi L. et al. Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup Environ Med 2011;68:154–162. [DOI] [PubMed] [Google Scholar]
- Tuntiseranee P, Olsen J, Geater A, Kor-anantakul O.. Are long working hours and shiftwork risk factors for subfecundity? A study among couples from southern Thailand. Occup Environ Med 1998;55:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen Q, Zou P, Liu T, Mo M, Yang H, Zhou N, Sun L, Chen H, Ling X. et al. Sleep duration is associated with sperm chromatin integrity among young men in Chongqing, China. J Sleep Res 2018;27:e12615. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gu F, Deng M, Guo L, Lu C, Zhou C, Chen S, Xu Y.. Rotating shift work and menstrual characteristics in a cohort of Chinese nurses. BMC Womens Health 2016;16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interactions, 4th edn Cambridge, UK: Cambridge University Press, 1999. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edn Geneva: World Health Organization, 2010. [Google Scholar]
- Wogatzky J, Wirleitner B, Stecher A, Vanderzwalmen P, Neyer A, Spitzer D, Schuff M, Schechinger B, Zech NH.. The combination matters - distinct impact of lifestyle factors on sperm quality: a study on semen analysis of 1683 patients according to MSOME criteria. Reprod Biol Endocrinol 2012;10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Chen Q, Zhou N, Sun L, Bao H, Tan L, Chen H, Zhang G, Ling X, Huang L. et al. Lifestyles associated with human semen quality: results from MARHCS cohort study in Chongqing, China. Medicine 2015;94:e1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B-F. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 2002;15:83–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.