Abstract
STUDY QUESTION
Do alterations in pro- and anti-angiogenic estrogen metabolites in follicular fluid (FF) contribute to the follicular growth arrest and anovulation associated with polycystic ovary syndrome (PCOS)?
SUMMARY ANSWER
FF of PCOS women with anovulation have reduced levels of pro-angiogenic estrogen metabolites (EMs) and vascular endothelial growth factor (VEGF) compared to that of fertile women with regular menstrual cycles, but exogenous gonadotropins increase the pro-angiogenic EMs and VEGF levels in PCOS women.
WHAT IS KNOWN ALREADY
PCOS is characterized by the arrest of follicular development that leads to chronic anovulation. Follicular arrest is generally associated with elevated plasma levels of luteinizing hormone (LH), androgens and anti-Mullerian hormone (AMH). There is also reduced angiogenesis in the follicles of PCOS women compared to those of normal cycling women. It is known that angiogenesis is a critical factor during follicular development. We and other investigators have explored the role of EMs in ovarian angiogenesis, particularly in human corpus luteum function, showing that 4-hydroxyestrone (4-OHE1) and 16-ketoestradiol (16-kE2) have pro-angiogenic effects while 2-methoxyestradiol (2-ME2) and 2-methoxyestrone (2-ME1) have anti-angiogenic effects. Additionally, 2-hydroxyestradiol (2-OHE2), which is produced in the ovary, has proliferative and pro-angiogenic properties. We hypothesized that EMs could be involved in angiogenesis necessary for ovarian follicular development in fertile women, and that dysregulation of these factors may contribute to follicular arrest in PCOS. The relationship between EMs, VEGF and AMH in the pathophysiology of follicular arrest in PCOS has not been previously studied at a follicular level in anovulatory women without ovulation induction.
STUDY DESIGN, SIZE, DURATION
This is a comparative experimental study of serum and FF collected from different sized follicles (antral ˂10 mm and dominant ˃16 mm) of women with and without ovarian stimulation. The study included women with regular menstrual cycles who were proven to be fertile (n = 20) and PCOS women with follicular arrest who were candidates for ovarian drilling (n = 17), as well as other patients requiring ovarian stimulation, i.e. control women undergoing IVF for male factor infertility (n = 12) and PCOS women undergoing IVF (n = 17). In vitro studies were carried out on granulosa-lutein cells (GCs) obtained from subsets of women undergoing IVF for male factor infertility (n = 6) and PCOS women undergoing IVF (n = 6). GCs were maintained in culture for up to 6 days.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Intrafollicular estradiol, estrone and EMs concentrations were determined by high performance liquid chromatography–mass spectrometry. Testosterone in serum was measured by RIA, and LH, FSH and sex hormone-binding globulin in serum were measured with IRMA kits. AMH was determined in serum and FF by enzyme linked immunosorbant assay (ELISA). VEGF levels were measured in FF and conditioned medium by ELISA. Conditioned medium were obtained from cultured GCs. The angiogenic potential was assessed by in vitro angiogenic assays.
MAIN RESULTS AND THE ROLE OF CHANCE
Pro-angiogenic EMs (4-OHE1, 16-kE2 and 2-OHE2) and VEGF were lower in FF of antral follicles of PCOS women with follicular arrest compared those of fertile women with ovulatory cycles (P < 0.05). In contrast, higher concentrations of AMH were found in FF of antral follicles from PCOS women with follicular arrest compared to those of fertile women with ovulatory cycles (P < 0.05). Exogenous gonadotropins used in IVF increased pro-angiogenic EMs and VEGF production in PCOS women, reaching similar profiles compared to control women receiving gonadotropins in their IVF treatment for male factor infertility. The pro-angiogenic EM 2-OHE2 increased the angiogenic potential and VEGF levels of GCs from PCOS women compared to the basal condition (P < 0.05). These findings suggest that there is a role for pro-angiogenic EMs in the control of follicular VEGF production.
LIMITATIONS, REASONS FOR CAUTION
The limitations include the possibility that in vitro analysis of GCs might not reflect the in vivo mechanisms involved in the pro-angiogenic action of 2-OHE2 since GCs obtained at the time of oocyte retrieval belong to a very early stage of the luteal phase and might not be representative of GCs during follicular growth. Therefore, our findings do not conclusively rule out the possibility that other in vivo mechanisms also account for defective angiogenesis observed in PCOS.
WIDER IMPLICATIONS OF THE FINDINGS
The present study highlights the significance of EMs, angiogenic factors and AMH and their interaction in the pathophysiology of follicular development in PCOS. This study provides new insights into the role of pro-angiogenic factors in follicular arrest in PCOS.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by CONICYT/FONDECYT 1140693 and NIH grant R01HD083323. All authors declare no conflict of interest.
TRIAL REGISTRATION NUMBER
N/A
Keywords: polycystic ovary syndrome, follicular growth arrest, 2-hydroxyestradiol, 16-ketoestradiol, 4-hydroxyestrone, VEGF, angiogenesis
Introduction
Polycystic ovary syndrome (PCOS) is a common disorder that affects 8–13% of reproductive aged women. This disorder is characterized by hyperandrogenism, oligo-anovulation and polycystic ovarian morphology (Azziz, 2006; Azziz et al., 2006; 2016; Costello et al., 2019). Follicular growth arrest is one of the main characteristics of PCOS, creating the classical ovarian morphology of multiple subcortical follicular ‘cysts’ that reach no more than 10 mm in diameter. This means that the selection of one dominant Graafian follicle (∼20 mm in diameter) is impaired, despite the presence of an excess number of selectable follicles (Valkenburg et al., 2009). The current understanding of the mechanisms underlying follicular growth arrest in PCOS is limited. It is thought that selection of a dominant follicle does not occur due to deficient secretion of FSH or impaired FSH action (Banaszewska et al., 2003; Broekmans et al., 2008). However, other factors are involved in the follicular growth arrest, including an hypothalamic-pituitary gonadotropin imbalance causing greater luteinizing hormone (LH) pulse amplitude and frequency. This leads to an altered LH:FSH ratio which increases thecal cell androgen secretion and increased anti-Mullerian hormone (AMH) expression by granulosa cells which impairs follicular development and estradiol secretion by inhibiting aromatase activity (Banaszewska et al., 2003; Webber et al., 2003; Weenen et al., 2004; Broekmans et al., 2008; Gleicher et al., 2011; Ikeda et al., 2014).
Angiogenesis in the normal ovary is critical for follicular growth and ovulation and the subsequent development and regression of the corpus luteum (Tamanini and De Ambrogi, 2004; Robinson et al., 2009). In contrast, follicular atresia is associated with inadequate development and/or regression of the thecal vasculature (Wulff et al., 2002). The resulting angiogenic factor dysregulation has adverse effects on oocyte maturation. The importance of vascular endothelial growth factor (VEGF) in ovarian function is well known (Kamat et al., 1995; Ferrara, 2001). VEGF expression is low during early ovarian development and becomes more pronounced in granulosa cells and theca cells through dominant follicle development (McGee et al., 1997; Wulff et al., 2002). It seems plausible that abnormal angiogenesis and/or vascularity is also involved in the follicular growth arrest and infertility in PCOS.
The secretion of estradiol throughout the ovarian cycle depends upon follicle recruitment and selection of a single dominant follicle followed by the LH/FSH surge, which ends the program of FSH-dependent steroidogenesis (Hillier et al., 1994). Estrogens may be metabolized in the ovary by diverse pathways including hydroxylation, glucoronidation, sulfonation and methylation to form estrogen metabolites (EMs) (Zhu and Conney, 1998; Rosenfeld and Wagner, 2001). EMs are thought to act through proteins other than the classical estradiol receptors.
Recently, we published a number of papers regarding the role of VEGF and EMs in ovarian angiogenesis, particularly in human corpus luteum function. We found that EMs, 4-hydroxyestrone (4-OHE1) and 16-ketoestradiol (16-kE2), have pro-angiogenic effects while 2-methoxyestradiol (2-ME2) and 2-methoxyestrone (2-ME1) have anti-angiogenic effects during development and regression of the corpus luteum (Kohen et al., 2013; Henríquez et al., 2016; Devoto et al., 2017). Another EM that is produced in the ovary is 2-hydroxyestradiol (2-OHE2), which has proliferative and pro-angiogenic characteristics (Landeros et al., 2017).
We hypothesized that EMs could be involved in angiogenesis necessary for ovarian follicular development in normal fertile women and that dysregulation of these factors may contribute to the follicular growth arrest in PCOS women. Although the roles of gonadotrophins, androgens, AMH and other intra-ovarian factors in follicular development have been clarified over the years, it is important to understand the relationship between EMs, VEGF and AMH in the pathophysiology of follicular growth arrest in PCOS, which has not been previously studied at a follicular level in humans.
Materials and methods
Human subjects
This study was approved by the institutional review board of San Borja-Arriaran Clinical Hospital and Faculty of Medicine of the University of Chile. Written informed consent was obtained from all participants.
Four groups of patients were included in this study. The women from Groups A and B did not undergo ovarian stimulation treatment, whereas women from Group C and D did receive treatment for ovarian stimulation. Further details are described below.
Women with regular menstrual cycles (A)
Ovulatory women, aged 33–38 years with normal BMI (26–33 kg/m2), who were multiparous and did not use hormonal treatment for at least 3 months prior to laparoscopy for benign gynecological disease (myoma, tubal ligation or hysterectomy) were included in Group A of the study.
Follicular development was monitored by serial vaginal ultrasounds (TVUs) that were performed at different times in the follicular phase. TVUs were performed until the follicle reached 10 mm (early follicular phase group) or 16 mm of diameter (late follicular phase group). Serum samples were collected on the same day as the surgical procedure. Follicular fluid (FF) was aspirated during the surgical procedures transvaginally using ultrasonographic specific guides and Kitazato IVM needles from antral (follicle <10 mm follicular diameter) and dominant (>16 mm follicular diameter) follicles.
PCOS women with follicular growth arrest (B)
PCOS women presented with amenorrhea because of chronic anovulation, hyperandrogenism (biochemical or clinical) and ultrasonographic morphology according to the Rotterdam criteria (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2016). They were included in this group when there was no response to clomiphene citrate (150 mg/day for 5 days) or Letrozol (5 mg/day for 5 days). After 2–3 months without any hormonal treatment and ultrasound monitoring of multiple follicles <10 mm, patients were invited to undergo a drilling procedure. Before ovarian drilling, aspiration of FF was performed under vaginal ultrasound guidance. Serum samples were collected at the same day as the ovarian drilling and FF was obtained from antral follicles (<10 mm follicular diameter).
Study groups with ovarian stimulation
Control women undergoing IVF for male factor infertility (C)
Ovulatory women, aged 28–38 years, with a normal antral follicular count (the number of follicles in both ovaries being >5 follicles and <12 follicles) and AMH levels (>1.2 ng/ml and <4.9 ng/ml) (Humaidan et al., 2016), and participating in our IVF program for male factor infertility, were invited to donate their FF and granulosa cells for research. FF was obtained by transvaginal ultrasound aspiration from antral follicles (<10 mm follicular diameter) and dominant (>16 mm follicular diameter) follicles during the IVF procedure.
The ovarian stimulation protocol included recombinant FSH-hMG (Human Menopausal Gonadotrophin) adjusted according to age, BMI, antral follicle count (AFC) and AMH levels. Mean days of stimulation were 11.2 ± 0.6 days. The mean daily FSH dose was 236.7 ± 52.4 UI. The maximum daily FSH dose was 300 UI/daily. A fixed GnRH antagonist (Cetrotide 0.25 mg/day, Merck Serono) protocol was used. The first dose was given on Day 6 of stimulation. The mean days of antagonist administration were 6.3 ± 0.7 days. A single dose of recombinant hCG (Ovidrell 250 μg, Merck Serono) was used to trigger the process of ovulation.
PCOS women undergoing IVF (D)
PCOS patients who presented with oligo- or anovulation, hyperandrogenism (biochemical or clinical) and ultrasonographic ovarian morphology with a large number of arrested antral follicles <10 mm (Rotterdam criteria), were invited to donate their FF and granulosa cells for research. The ovarian stimulation protocol included rFSH, GnRH antagonist and ovulation triggering with recombinant hCG. FF was obtained by transvaginal ultrasound aspiration from antral (<10 mm follicular diameter) and dominant (>16 mm follicular diameter) follicles during the IVF procedures.
Power analysis calculations
The minimum number of patient samples required for the experiments were determined by power analysis calculations. Sample size calculations were performed based on the evidence indicating that the factor determined in this study with the highest percentage of variability is AMH, with difference of the means of AMH in serum of 6.15 ng/ml and SD 4.21 ( Stracquadanio et al., 2018 ). To detect this difference with an α significance of 5% and a 1-β power of 88%, it was necessary to recruit at least seven women per study group. Sample size calculations were based on García-García et al. (2013).
Women with regular menstrual cycles (A)
A total of 20 subjects were enrolled in this study group, 10 women corresponding to the early follicular phase and 10 corresponding to the late follicular phase. We obtained a serum sample to determine endocrine parameters and an adequate volume of FF to determine estrogens metabolites, AMH and VEGF levels. FF (0.5–0.8 ml) was collected from five to eight antral follicles of <10 mm per patient and from a single dominant follicles (0.7 ml).
PCOS women with follicular growth arrest (B)
A total of 17 subjects were enrolled in this group. In 10 women with follicular growth arrest, we obtained enough serum to determine endocrine parameters and an adequate volume of FF to determine EMs, AMH and VEGF levels. From the remaining seven women, we only obtained adequate samples of serum and FF to determine AMH and VEGF levels. FF (0.5–0.8 ml) was collected from five to eight antral follicles of <10 mm per patient.
Control women undergoing IVF (C)
From 12 women undergoing IVF for male factor infertility, we obtained 12 samples of FF with adequate volume to determine estrogens metabolites, VEGF and AMH levels. In addition, to perform granulosa-lutein cell cultures, follicular aspirates were obtained from six women in this study group.
PCOS women undergoing IVF (D)
From the total of 17 PCOS women undergoing IVF enrolled, we obtained 17 samples of FF with adequate volumes to determine estrogens metabolites, VEGF and AMH levels. In addition, follicular aspirates were obtained from six women of this study group for granulosa-lutein cell cultures.
The calculation of sample size for cell culture experiments was based on published data on VEGF levels in conditioned medium of granulosa-lutein cell (GC) cultures of control woman (110.2 ± 5.5 pg/ml) and PCOS woman (60.6 ± 2.5 pg/ml) (Henríquez et al., 2016). To detect this difference with an α significance of 5% and a 1-β power of 88%, it was necessary to perform cell cultures on at least four women per study group, IVF control and IVF PCOS.
Measurement of testosterone, LH, FSH, sex hormone-binding globulin, AMH and VEGF
The hormonal profile assessments were performed on serum. Testosterone was measured RIA kits (DIAsource ImmunoAssays S.A., Louvain-La-Neuve, Belgium) with a sensitivity of 0.17 nmol/l, and intra- and inter-assay coefficients of variation (CV) was 4.1% and 5.5%, respectively.
LH and FSH were measured by IRMA (DIAsource ImmunoAssays S.A., Louvain-La-Neuve, Belgium). LH had a sensitivity of 0.2 mUI/ml, and intra- and inter-assay CVs of 2.65% and 5.7%, respectively. FSH had a sensitivity of 0.1 mUI/ml, and intra- and inter-assay CVs of 1.63% and 3.4%, respectively.
Sex hormone-binding globulin (SHBG) was measured by IRMA (Izotop, Institute of Isotopes Ltd., Budapest, Hungary) with a sensitivity of 0.22 nmol/l, and intra- and inter-assay CVs of 5.77% and 4.93%, respectively.
AMH was quantified with Gen II ELISA kits (Beckman Coulter, Inc., USA) with a sensitivity of 0.10 ng/ml and intra- and inter-assay CVs of 3.21% and 5.82%, respectively.
VEGF was quantified in FF from different diameter follicles by enzyme linked immunosorbant assay (ELISA) according the protocol provided by R&D Systems, Inc. (Minneapolis, MN, USA). The samples were assayed in duplicate. The sensitivity of the assay is 5.0 pg/ml. The intra-assay CV was 2.4–10.2% and the inter-assay CV was 5.1–8.7%. VEGF levels were also determined in conditioned medium collected from GC cultures obtained from normal and PCOS participants in the IVF program.
Analysis of estrogen metabolites
Estradiol, estrone and estrogen metabolites (2-ME1, 2-ME2, 4-OHE1, 2-OHE2 and 16KE2) were determined in FF from all women participating of study by high performance liquid chromatography (HPLC) and mass spectrometry (HPLC-ESI-MS) as described in Xu et al. (2007). Briefly, an aliquot of 300 µl of FF was added with 300 µl of freshly prepared enzymatic hydrolysis buffer containing 2 mg of l-ascorbic acid, 5 µl of β-glucuronidase/sulfatase and 300 µl of 0.15 M sodium acetate buffer (pH 4.1). After hydrolysis with glucuronidase and sulfatase, each sample underwent slow inverse extraction (RKVSD, ATR, Inc., Laurel, MD, USA) with 8 ml of dichloromethane for 30 min. After extraction, the organic solvent portion was dried at 60°C under nitrogen gas (Reacti-Vap III, Pierce, Rockford, IL, USA). To each dried sample, 100 µl of 0.1 M sodium bicarbonate buffer (pH at 9.0) and 100 µl of dansyl chloride solution (1 mg/ml in acetone) were added. Afterwards, samples were heated at 60°C (Reacti-Therm III Heating Module, Pierce, Rockford, IL, USA) to form the EM derivatives. Calibration standards and quality control samples were hydrolyzed, extracted and derivatized following the same procedure as that used for the unknown FF samples. All samples were analyzed by HPLC-ESI-MS using a Finnigan TSQ Quantum-AM triple quadrupole mass spectrometer coupled with a Surveyor HPLC system (ThermoFinnigan, San Jose, CA, USA). Quantitation of FF EMs was carried out using Xcalibur Quan Browser (ThermoFinnigan). The lower limit of quantitation for each EM in FF was 8 pg/ml. For an FF sample containing 8 pg/ml of each EM, the recovery of a known added amount ranged from 91% to 113%. The intra-batch precisions (CV), based on four replicate samples assayed in one batch, were 4.2–16.4% and the inter-batch precisions (CV), based on the means for four replicate samples assayed in each of four batches, were 6.9–14.6%.
Granulosa-lutein cell cultures
Granulosa cells were obtained from follicular aspirates of control (n = 6) and PCOS women (n = 6) participating in our IVF program. The cells were obtained after centrifugation of FF at 400g for 5 min. The cell pellet was re-suspended, and red blood cells and detritus were removed using a Histopaque gradient. Macrophages were eliminated by preplating for 30 min at 37°C and cultures were carried out as previously described (Henríquez et al., 2017). Granulosa cells were cultured for 96 h in growth medium (M199, Sigma-Aldrich, MO, USA) supplemented with 10% fetal bovine serum followed by 24 h in serum-free medium. After this, the granulosa cells were cultured for 24 h under basal conditions and in the presence of 2-OHE2 (0.05 µM). Cell cultures were performed with 2-OHE2 because this pro-angiogenic metabolite has the highest concentration in FF of women with regular menstrual cycles. The conditioned media of cultured cells were used to evaluate angiogenic activity and VEGF levels.
In vitro analysis of the angiogenic activity
Angiogenic activity was determined in conditioned media obtained from GCs of control and PCOS women participating in our IVF program, cultured in the absence and presence of 2-OHE2 (0.05 μM) for 24 h. The EA.hy926 cell line (ATCC® CRL-2922™), obtained by the hybridization of human umbilical vein endothelial cells with the A549/8 human lung carcinoma cell line, and which has maintained the endothelial phenotype and highly differentiated functions characteristic of human vascular endothelium (Edgell et al., 1983, 1990), was used to determine angiogenic activity; 40 000 cells were plated onto matrigel in the presence of 500 µl of the different conditioned media under investigation and cultured 8 h. EA.hy926 cells were photographed every 2 h within a period of 8 h to evaluate the angiogenic score (AS) and analyzed using an inverted phase contrast microscope as previously reported (Aranda and Owen, 2009). Ten representative images per well were recorded and transferred to a computer for image analysis for quantification of an in vitro AS:
Statistical analysis
FF and serum values are presented as means ± SEM. Data were analyzed by one-way ANOVA followed by a Tukey’s multiple comparison test. The statistical analysis of the angiogenic potential data from granulosa-lutein cell cultures was carried out using a non-parametric method: Kruskal–Wallis (one-way analysis of variance) followed by a Dunn’s Multiple Comparison post hoc test. GraphPad-Prism Software 4.0 (Inc., San Diego, CA, USA) was used to analyze data. Significance was defined as P < 0.05. The data were analyzed for normality with the Shapiro–Wilk test, P < 0.05.
Results
Clinical and endocrine features of women with regular cycles and PCOS women with follicular arrest
Table I presents the endocrinological profiles of the study subjects. There were no differences in the mean age and BMI between the ovulatory women with regular menstrual cycles and PCOS women with follicular arrest. The LH/FSH ratio, AMH and testosterone serum concentrations in PCOS were significantly higher than in ovulatory women. Conversely, SHBG levels were significantly lower in PCOS compared to ovulatory woman (P < 0.05). The diameters of follicles aspirated were similar in both groups (<10 mm) and correspond to the early follicular phase.
Table I.
Clinical and endocrine profiles of women with regular menstrual cycles during the early follicular phase and PCOS women with follicular arrest.
| Ovulatory (n = 10) | PCOS (n = 17) | |
|---|---|---|
| Age (year) | 32.61 ± 1.14 | 31.05 ± 2.47 |
| BMI (Kg/m2) | 31.73 ± 1.50 | 32.11 ± 2.64 |
| LH/FSH ratioa | 0.68 ± 0.07 | 1.45 ± 0.21* |
| AMH (ng/ml)a | 3.30 ± 0.59 | 9.89 ± 1.91* |
| Testosterone (nmol/l)a | 1.25 ± 0.17 | 2.92 ± 0.49* |
| SHBG (nmol/l)a | 62.04 ± 7.72 | 22.25 ± 2.74* |
Serum levels.
Statistical difference between ovulatory and PCOS women (P < 0.05). Values are mean ± SEM.
AMH, anti-Mullerian hormone; SHBG, Sex hormone-binding globulin.
FF concentrations of steroid hormones and EMs
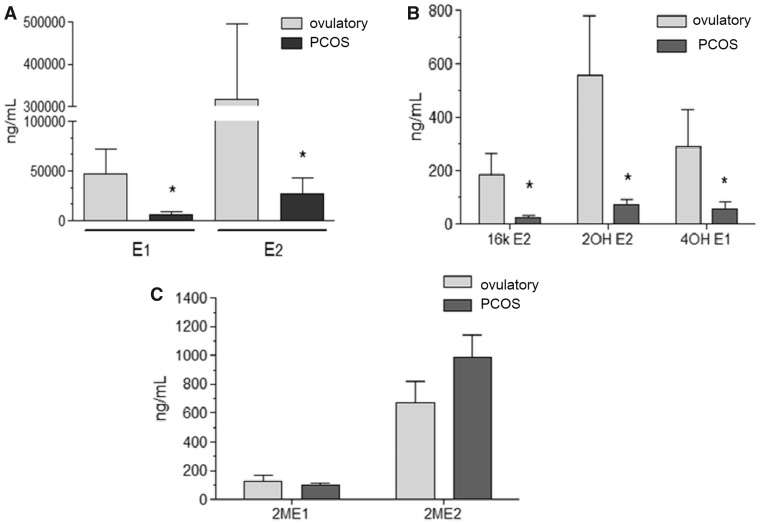
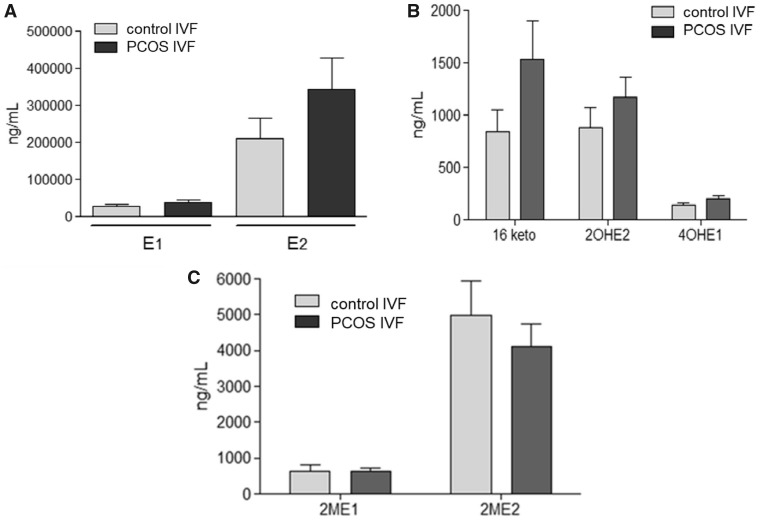
Figure 1A shows the FF concentrations of estrone (E1) and estradiol (E2) in the antral follicles (<10 mm) of women with regular menstrual cycles and PCOS women with follicular arrest. The highest FF concentrations of E1 and E2 were observed in ovulatory women compared to PCOS women (P < 0.001). Significantly lower levels of 2-OHE2, 4-OHE1 and 16-kE2 were detected in FF of antral follicles of PCOS women (P < 0.05) matched with similar size follicles of ovulatory women (Fig. 1B). There were no significant differences in the follicular levels of 2-ME1 and 2-ME2 (Fig. 1C) between follicles from the two groups of women. PCOS women who were undergoing ovarian stimulation had EMs levels similar to control women undergoing ovarian stimulation in our IVF program (Fig. 2A–C).
Figure 1.
Intrafollicular levels of E2, E1 and EMs in antral follicles of ovulatory women with regular menstrual cycles and PCOS women without ovarian stimulation. (A) E2 and E1 in ovulatory women and PCOS women (*P < 0.05). (B) 16-kE2, 2-OHE2 and 4-OHE1 in ovulatory women and PCOS women (*P <0 .05). (C) 2-ME2 and 2-ME1 in ovulatory women and PCOS women. Values are mean ± SEM, total n = 20.
Figure 2.
Intrafollicular levels of E2, E1, 16-kE2, 2-OHE2 and 4-OHE1 in antral follicle of control and PCOS women undergoing ovarian stimulation for IVF. (A) E2 and E1 in control and PCOS women undergoing IVF. (B) 16-kE2, 2-OHE2 and 4-OHE1 in control and PCOS women undergoing IVF. (C) 2-ME2 and 2-ME1 in control and PCOS women undergoing IVF. Values are mean ± SEM, PCOS n = 17, control n = 12.
Intrafollicular levels of EMs, VEGF and AMH of ovulatory women with regular menstrual cycles and PCOS women with follicular arrest
Table II presents the sum of FF pro-angiogenic EMs (16-ketoE2, 2-OHE2 and 4-OHE1), the sum of anti-angiogenic EMs (2-ME2 and 2-ME1) and ratio of ∑pro-angiogenic EMs/∑anti-angiogenic EMs in FF from different diameter follicles from women with regular menstrual cycles and PCOS women with follicular arrest. The intrafollicular levels of pro-angiogenic EMs were significantly higher in dominant and antral follicles of ovulatory women compared with follicles of PCOS women (P < 0.05).
Table II.
Intrafollicular levels of AMH, VEGF and estrogens metabolites (EMs) in antral and dominant follicle of normal women with spontaneous cycles and PCOS women with follicular arrest.
| Ovulatory (n = 10) (antral follicle) | PCOS (n = 10) (antral follicle) | Ovulatory (n = 10) (dominant follicle) | |
|---|---|---|---|
| Age (year) | 32.6 ± 1.14 | 29.6 ± 3.0 | 33.1 ± 2.0 |
| AMH (ng/ml)* | 213.2 ± 28.0 | 546.3 ± 16.7a | 2.9 ± 0.09b |
| VEGF (pg/ml)* | 503.2 ± 101.40 | 33.1 ± 5.9a | 6529.6 ± 514.3b |
| ∑ EMs pro-angiogenic (2-OHE2 + 4-OHE1 + 16-KE2) (ng/ml) | 1099.7 ± 128.1 | 441.9 ± 31.8a | 3658.5 ± 278.0b |
| ∑ EMs anti-angiogenic (2-ME2 + 2-ME1) (ng/ml) | 689.25 ± 111.4 | 1239.3 ± 177.7 | 3159.8 ± 604.3b |
| ∑ Pro-angio/∑ Anti-angio ratio | 1.59 | 0.35a | 1.15 |
| Follicular diameter (mm) | <10 | <10 | >16 |
Comparing the difference between antral follicles of ovulatory and PCOS women (P < 0.05). Values are mean ± SEM.
Comparing the difference between antral and dominant follicles of ovulatory women (P < 0.05). Values are mean ± SEM.
AMH and VEGF determination correspond to n = 17 FF samples of PCOS.
∑ EMs, sum of estrogen metabolites; VEGF, vascular endothelial growth factor.
The AMH levels in FF were significantly higher in antral follicles (<10 mm) of PCOS women compared to antral and dominant follicles (>16 mm) of ovulatory women (P < 0.05). The VEGF levels in FF were significantly lower in antral follicles of PCOS women compared with antral and dominant follicles of ovulatory women (P < 0.05).
The sum of FF levels of anti-angiogenic EMs was significantly higher in dominant follicles compared with that in antral follicles of PCOS and ovulatory women (P < 0.05). However no statistically significant differences were seen between the sum of FF levels of anti-angiogenic EMs in antral follicles of ovulatory women compared to that of PCOS women. Interestingly, the ratio between ∑pro-angiogenic EMs and ∑anti-angiogenic EMs was significantly lower in antral follicles of PCOS women compared with antral and dominant follicles of ovulatory women (P < 0.05).
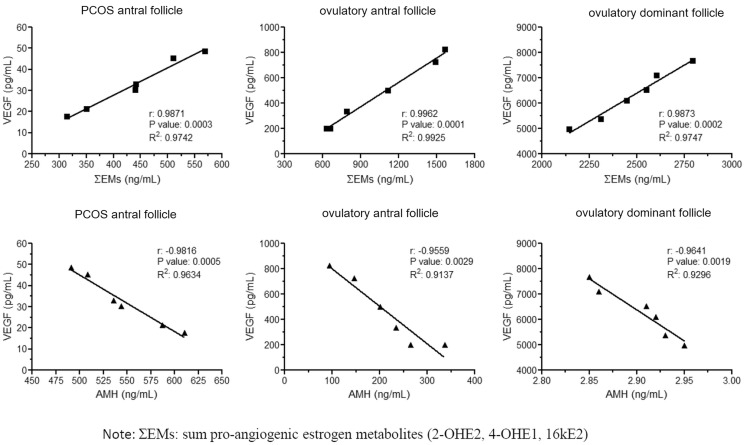
The pro-angiogenic EMs levels were directly correlated with the levels of VEGF found in these follicles (Pearson’s r = 0.98; r2 = 0.97; P < 0.0002) (Fig. 3A), while the AMH levels were inversely correlated with VEGF levels in antral follicles of PCOS women and in antral and dominant follicle of ovulatory women (Pearson’s r = −0.98; r2 = 0.96; P < 0.0005) (Fig. 3B).
Figure 3.
Linear regression between intrafollicular EMs, AMH and VEGF levels in different study groups. (A) Lineal regression between ΣEMs and VEGF. (B) Lineal regression between AMH and VEGF. ΣEMs: sum of pro-angiogenic estrogen metabolites (2-OHE2, 4-OHE1 and 16kE2).
Intrafollicular levels of AMH and VEGF of PCOS women compared to controls undergoing IVF
Table III presents the FF levels of AMH and VEGF in follicles of different diameters from control and PCOS women undergoing ovarian stimulation for IVF. The highest FF concentrations of VEGF were observed in dominant follicles of control and PCOS women compared to antral follicles (P < 0.05). No significant differences were found between FF AMH levels from follicles of different diameters between the two study groups. Interestingly, the ovarian stimulation protocol reduces AMH levels compared to those detected in dominant follicles and increases VEGF levels.
Table III.
Intrafollicular levels of AMH and VEGF in antral and dominant follicles of control and PCOS women undergoing ovarian stimulation for IVF.
| Control IVF (n = 12) (antral follicle) | Control IVF (n = 12) (dominant follicle) | PCOS IVF (n = 17) (antral follicle) | PCOS IVF (n = 17) (dominant follicle) | |
|---|---|---|---|---|
| Age (year) | 30.2 ± 1.3 | 30.2 ± 1.3 | 32.2 ± 1.3 | 32.2 ± 1.3 |
| AMH (ng/ml) | 2.5 ± 0.2 | 2.2 ± 0.3 | 2.5 ± 0.1 | 2.5 ± 0.3 |
| VEGF (pg/ml) | 1080.7 ± 66.8 | 3221.3 ± 335.6a | 1153.2 ± 42.0 | 1902.3 ± 50.0b |
| Follicular diameter (mm) | <10 | >16 | <10 | >16 |
Values are mean ± SEM.
P < 0.05 dominant follicles versus antral follicles in control women.
P < 0.05 dominant follicle versus antral follicle in PCOS women.
Effect of pro-angiogenic EMs on angiogenic activity of GCs
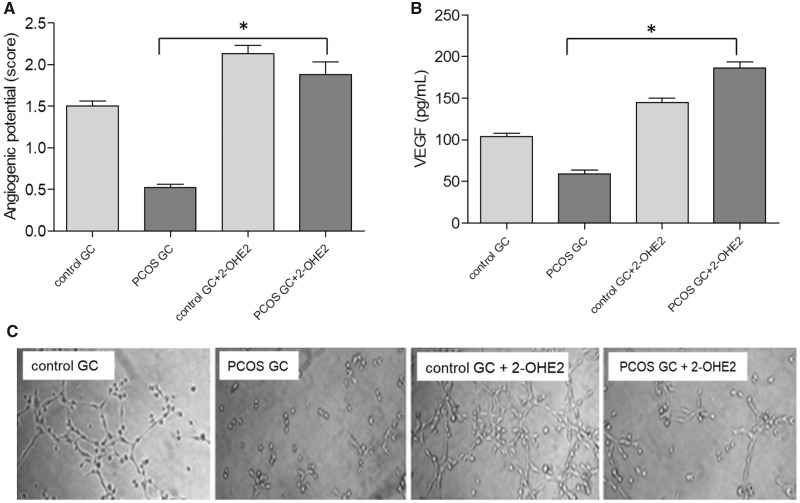
The pro-angiogenic metabolite, 2-OHE2, significantly increased angiogenic activity of GCs of control and PCOS women compared to basal conditions (P < 0.05) (Fig. 4A), as assessed by formation of capillary structures by the EA.hy926 cells. 2-OHE2 also significantly increased VEGF secretion from GCs cultures of control and PCOS women compared to basal conditions (P < 0.05) (Fig. 4B). These findings document the role of pro-angiogenic EMs in the control of follicular VEGF production.
Figure 4.
Effect of 2-OHE2 on angiogenic activity of granulosa cell cultures from control and PCOS women undergoing IVF. (A) The graph represents the angiogenic potential quantification of PCOS and control GCs cultures stimulated with 2-OHE2. *P < 0.05 PCOS GCs plus 2-OHE2 versus PCOS GCs in basal condition. (B) The graph represents the VEGF secretion of PCOS GCs cultures stimulated with 2-OHE2. *P < 0.05 PCOS GCs plus 2-OHE2 versus PCOS GCs in basal condition. (C) Photomicrograph of the angiogenic assay. Values are mean ± SEM, n = 6. GCs, granulosa-lutein cells.
Discussion
The present study reveals for the first time physiological and pathophysiological intrafollicular levels of EMs, VEGF and AMH in PCOS compared to ovulatory women with a regular menstrual cycle without ovulation stimulation, suggesting the participation of these factors in normal follicular development and follicular arrest in PCOS. The results show a diminished quantity of pro-angiogenic EMs (2-OHE2, 4-OHE1 and 16-kE2) in FF of antral PCOS follicles compared to FF from antral follicles of ovulatory women. The reduction in levels of pro-angiogenic EMs is associated with lower VEGF levels in antral follicles of PCOS women. Intrafollicular VEGF levels were higher in dominant follicles of ovulatory women. Our observations are consistent with previously published data that showed lower levels of 2-hydroxyestrogens in urine of PCOS women (Salih et al., 2007). On the other hand, no differences were found in the levels of anti-angiogenic EMs between antral follicles of PCOS and ovulatory women. However, the ratio of pro-angiogenic EMs to anti-angiogenic EMs was significantly lower in PCOS compared to ovulatory women. These results are consistent with a previous study that did not find genetic variation affecting the expression of catechol-O-methyltransferase (COMT), an enzyme in the pathway for the production of 2-methyoxyestradiol (2-ME2), an anti-angiogenic EM, in European-ancestry PCOS women (Hill et al., 2012). In conclusion, these data suggest that arrested follicles of PCOS produce lower levels of pro-angiogenic EMs, leading to an anti-angiogenic environment that contributes to lower vascularity of the PCOS follicles.
The antral follicles of PCOS women have high intrafollicular AMH levels compared to follicles of ovulatory women. AMH levels are markedly reduced in the dominant follicles of ovulatory women. AMH plays an inhibitory role in follicular development, preventing the premature recruitment and maturation of follicles (Weenen et al., 2004). When follicles reach 7–9 mm in diameter, AMH expression is downregulated and these follicles begin to respond to FSH and LH, leading to increased estrogen production, follicular selection and ovulation. AMH secretion is maximal at the antral stage in human follicles, and decreases in large follicle (Durlinger et al., 2002; Visser and Themmen, 2005).
PCOS ovaries have a higher number of preantral and antral follicles, indicating that follicular growth arrest occurs when AMH production is high (Pellatt et al., 2007). Multiple studies have documented that serum AMH concentrations are elevated in PCOS women compared to normally ovulating women (Laven et al., 2004). Moreover, other studies have suggested that AMH levels reflect the severity of PCOS (Jacob et al., 2017). There are significant differences in AMH levels among anovulatory PCOS women with oligo-amenorrhea compared with PCOS ovulatory women (Das et al., 2008). In anovulatory PCOS women, hypersecretion of AMH by granulosa cells of mature small antral follicles could impair follicular growth by reducing FSH sensitivity and blocking the conversion of androgens into estrogens by inhibiting the activity of aromatase, causing hyperandrogenism (Eldar-Geva et al., 2005; Piouka et al., 2009). Others found that follicular AMH levels are negatively correlated with FSH concentrations, indicating that AMH levels predict follicle responsiveness to FSH in ovulation induction cycles (Dumesic et al., 2009). AMH influences transcription of genes in granulosa cells through Smad proteins and regulates gene expression to maintain primordial follicles in their arrested state (Visser, 2006).
The results obtained in the present work show an inverse relationship between intrafollicular levels of AMH and VEGF that suggests an inhibitory role of AMH on angiogenesis. In support of this observation, it has been shown that AMH downregulates TGF beta signaling pathways leading to decreased cell differentiation and angiogenesis (Nilsson et al., 2007). Additionally, numerous publications show that FSH positively regulates angiogenesis, stimulating HIF-1α expression and VEGF secretion (Kuo et al., 2011; Stilley et al., 2014). This suggests that high AMH levels characteristic of PCOS, reduce sensitivity to FSH and are detrimental to follicular angiogenesis, resulting in follicular growth arrest. Interestingly, the recombinant FSH doses used in ovulation induction cycles in PCOS women stimulate angiogenesis and follicular growth.
Furthermore, our results from women undergoing IVF treatment show that ovarian stimulation increases the intrafollicular levels of pro-angiogenic EMs and VEGF, and reduces AMH levels in PCOS women, reaching levels found in FF from control women. Previously published data showed that PCOS women with elevated AMH levels (66%) after ovulation induction are predisposed to ovarian hyperstimulation syndrome (OHSS), while 16.5% of these women had normal AMH levels with low risk of OHSS: a result that is in agreement with our data, since we did not observe ovarian hyperstimulation in our study group (Stracquadanio et al., 2018).
These data suggest that there is a gonadotropin-regulated intrafollicular reduction of pro-angiogenic EMs in PCOS with follicular arrest. The in vitro results of this study suggest that PCOS GCs have lower angiogenic potential and VEGF levels due to the reduced concentrations of follicular pro-angiogenic EMs, and that these cells recover their angiogenic capacity when incubated with a pro-angiogenic EMs (2-OHE2).
Altogether, the present study strongly suggests that there is reduced angiogenic potential accompanied by high levels of AMH in the PCOS follicles that could explain, in part, the follicular growth arrest characteristic of this disorder. Notably, treatment with exogenous gonadotropins during IVF improved the production of pro-angiogenic EMs and VEGF in PCOS women.
Authors’ roles
S.H. and P.K. contributed to the conceptual formulation of the work, designed experiments, conducted experiments and helped in writing the manuscript. X.X. performed estrogen metabolite measurements. A.M. contributed to statistical and image analysis. A.G. and C.V. provided cells and follicular fluid. J.F.S. interpreted results and participated in writing the manuscript. L.D. contributed to the conceptual formulation of the work, designed experiments, provided cells, interpreted results and participated in writing the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by CONICYT/FONDECYT (Fondo Nacional de Desarrollo Cientifico y Tecnologico) grant N°1140693 and NIH (National Institutes of Health) grant N°R01HD083323.
Conflict of interest
All authors declare no conflict of interest.
References
- Aranda E, Owen GI.. A semi-quantitative assay to screen for angiogenic compounds and compounds with angiogenic potential using the EA.hy926 endothelial cell line. Biol Res 2009;42:377–389. [PubMed] [Google Scholar]
- Azziz R. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: the Rotterdam criteria are premature. J Clin Endocrinol Metab 2006;91:781–785. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO.. Polycystic ovary syndrome. Nat Rev Dis Primers 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E. et al. ; Androgen Excess Society. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 2006;91:4237–4245. [DOI] [PubMed] [Google Scholar]
- Banaszewska B, Spaczyński RZ, Pelesz M, Pawelczyk L.. Incidence of elevated LH/FSH ratio in polycystic ovary syndrome women with normo- and hyperinsulinemia. Rocz Akad Med Bialymst 2003;48:131–134. [PubMed] [Google Scholar]
- Broekmans F, Visser J, Laven J, Broer S, Themmen A, Fauser B.. Anti-Müllerian hormone and ovarian dysfunction. Trends Endocrinol Metab 2008;19:340–347. [DOI] [PubMed] [Google Scholar]
- Costello MF, Misso ML, Balen A, Boyle J, Devoto L, Garad RM. et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Hum Reprod Open 2019;1:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Gillott D, Saridogan E, Djahanbakhch O.. AMH is increased in follicular fluid from unstimulated ovaries in women with polycystic ovary syndrome. Hum Reprod 2008;23:2122–2126. [DOI] [PubMed] [Google Scholar]
- Devoto L, Henríquez S, Kohen P, Strauss JF 3rd.. The significance of estradiol metabolites in human corpus luteum physiology. Steroids 2017;123:50–54. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Lesnick TG, Stassart JP, Ball GD, Wong A, Abbott DH. Intrafollicular antimüllerian hormone levels predict follicle responsiveness to follicle-stimulating hormone (FSH) in normoandrogenicovulatory women undergoing gonadotropin releasing-hormone analog/recombinanthuman FSH therapy for in vitro fertilization embryo transfer . Fertil Steril 2009;92:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durlinger A, Visser J, Themmen A.. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction 2002;124:601–609. [DOI] [PubMed] [Google Scholar]
- Edgell CJ, Haizlip JE, Bagnell CR, Packenham JP, Harrison P, Wilbourn B, Madden VJ.. Endothelium specific Weibel-Palade bodies in a continuous human cell line, EA.hy926. In Vitro Cell Dev Biol 1990;26:1167–1172. [DOI] [PubMed] [Google Scholar]
- Edgell CJ, McDonald CC, Graham JB.. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA 1983;80:3734–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar-Geva T, Margalioth E, Gal M, Ben-Chetrit A, Algur N, Zylber-Haran E. et al. Serum anti-Mullerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum Reprod 2005;20:1814–1819. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 2001;280:1358-1366. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- García-García JA, Reding-Bernal A, López-Alvarenga JC.. Sample size calculation in education medical research. Inv Ed Med 2013;2:217–224. [Google Scholar]
- Gleicher N, Weghofer A, Barad DH.. The role of androgens in follicle maturation and ovulation induction: friend or foe of infertility treatment? Reprod Biol Endocrinol 2011;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henríquez S, Kohen P, Muñoz A, Godoy A, Orge A, Strauss JF III, Devoto L.. In-vitro study of gonadotrophin signaling pathways in human granulosa cells in relation to progesterone receptor expression. Reprod Biomed Online 2017;35:363–371. [DOI] [PubMed] [Google Scholar]
- Henríquez S, Kohen P, Xu X, Veenstra TD, Muñoz A, Palomino WA, Strauss JF 3rd, Devoto L.. Estrogen metabolites in human corpus luteum physiology: differential effects on angiogenic activity. Fertil Steril 2016;106:230–237. [DOI] [PubMed] [Google Scholar]
- Hill LD, Ewens KG, Maher BS, York TP, Legro RS, Dunaif A, Strauss JF 3rd.. Catechol-O-methyltransferase (COMT) single nucleotide polymorphisms and haplotypes are not major risk factors for polycystic ovary syndrome. Mol Cell Endocrinol 2012;350:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier SG, Whitelaw PF, Smyth CD.. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol 1994;100:51–54. [DOI] [PubMed] [Google Scholar]
- Humaidan P, Alviggi C, Fischer R, Esteves SC.. The novel POSEIDON stratification of ‘Low prognosis patients in Assisted Reproductive Technology’ and its proposed marker of successful outcome. F1000Res 2016;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Baba T, Morishita M, Honnma H, Endo T, Kiya T, Saito T.. Long-term treatment with dehydroepiandrosterone may lead to follicular atresia through interaction with anti-Mullerian hormone. J Ovarian Res 2014;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Field H, Calder N, Picton H, Balen A, Barth J.. Anti-Müllerian hormone reflects the severity of polycystic ovary syndrome. Clin Endocrinol (Oxf )2017;86:395–400. [DOI] [PubMed] [Google Scholar]
- Kamat BR, Brown LF, Manseau EJ, Senger DR, Dvorak HF.. Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa and theca lutein cells. Role in corpus luteum development. Am J Pathol 1995;146:157–165. [PMC free article] [PubMed] [Google Scholar]
- Kohen P, Henríquez S, Rojas C. et al. 2-Methoxyestradiol in the human corpus luteum throughout the luteal phase and its influence on lutein cell steroidogenesis and angiogenic activity. Fertil Steril 2013;100:1397–1404. [DOI] [PubMed] [Google Scholar]
- Kuo SW, Ke FC, Chang GD, Lee MT, Hwang JJ.. Potential role of follicle-stimulating hormone (FSH) and transforming growth factor (TGFβ1) in the regulation of ovarian angiogenesis. J Cell Physiol 2011;226:1608–1619. [DOI] [PubMed] [Google Scholar]
- Landeros RV, Jobe SO, Aranda-Pino G, Lopez GE, Zheng J, Magness R.. Convergent ERK1/2, p38 and JNK mitogen activated protein kinases (MAPKs) signalling mediate catecholoestradiol-induced proliferation of ovine uterine artery endothelial cells. J Physiol 2017;595:4663–4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laven J, Mulders A, Visser J, Themmen A, De Jong F, Fauser B.. Anti-Müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab 2004;89:318–323. [DOI] [PubMed] [Google Scholar]
- McGee E, Spears N, Minami S. et al. Preantral ovarian follicles in serum-free culture: suppression of apoptosis after activation of the cyclic guanosine 3’,5’-monophosphate pathway and stimulation of growth and differentiation by follicle-stimulating hormone. Endocrinology 1997;138:2417–2424. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Rogers N, Skinner M.. Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction 2007;134:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, Mason H.. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab 2007;92:240–245. [DOI] [PubMed] [Google Scholar]
- Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D.. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab 2009;296:E238–E243. [DOI] [PubMed] [Google Scholar]
- Robinso RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE.. Angiogenesis and vascular function in the ovary. Reproduction 2009;138:869–881. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Wagner JS, Roberts RM, Lubahn DB.. Intraovarian actions of oestrogen. Reproduction 2001;122:215–226. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- Salih S, Xu X, Veenstra TD, Duleba AJ, Fouad H, Nagamani M, Al-Hendy A.. Lower levels of urinary 2-hydroxyestrogens in polycystic ovary syndrome. J Clin Endocrinol Metab 2007;92:3285–3291. [DOI] [PubMed] [Google Scholar]
- Stilley JA, Guan R, Duffy DM, Segaloff DL.. Signaling through FSH receptors on human umbilical vein endothelial cells promotes angiogenesis. J Clin Endocrinol Metab 2014;99:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracquadanio M., Ciotta L., Palumbo MA. Relationship between serum anti-Mullerian hormone and intrafollicular AMH levels in PCOS women. Gynecol Endocrinol 2018;3:223–228. [DOI] [PubMed] [Google Scholar]
- Tamanini C, De Ambrogi M.. Angiogenesis in developing follicle and corpus luteum. Reprod Domest Anim 2004;39:206–216. [DOI] [PubMed] [Google Scholar]
- Valkenburg O, Uitterlinden A, Piersma D, Hofman A, Themmen A, Fauser B, Laven JS.. Genetic polymorphisms of GnRH and gonadotrophic hormone receptors affect the phenotype of polycystic ovary syndrome. Hum Reprod 2009;24:2014–2022. [DOI] [PubMed] [Google Scholar]
- Visser J. Role of anti-Müllerian hormone in follicle recruitment and maturation. J Gynecol Obstet Biol Reprod (Paris) 2006;35:2S30–2S34. [PubMed] [Google Scholar]
- Visser JA, Themmen AP.. Anti-Müllerian hormone and folliculogenesis. Mol Cell Endocrinol 2005;234:81–86. [DOI] [PubMed] [Google Scholar]
- Webber L, Stubbs S, Stark J, Trew G, Margara R, Hardy K, Franks S.. Formation and early development of follicles in the polycystic ovary. Lancet 2003;362:1017–1021. [DOI] [PubMed] [Google Scholar]
- Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA. et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 2004;10:77–83. [DOI] [PubMed] [Google Scholar]
- Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM.. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology 2002;143:2797–2807. [DOI] [PubMed] [Google Scholar]
- Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG.. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem 2007;79:7813–7821. [DOI] [PubMed] [Google Scholar]
- Zhu BT, Conney AH.. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis 1998;19:1–27. [DOI] [PubMed] [Google Scholar]