Abstract
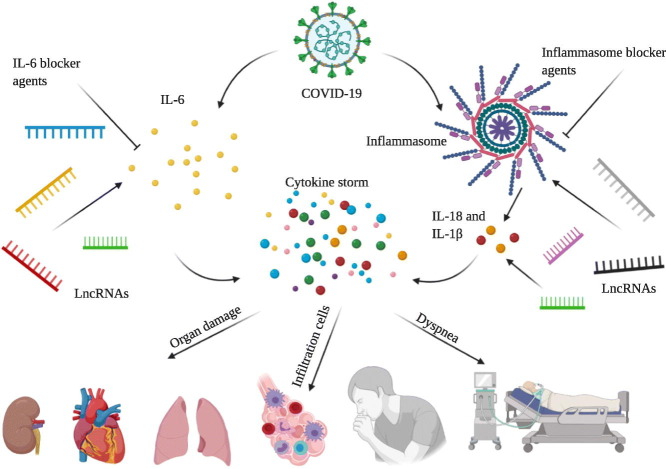
The world has witnessed a high morbidity and mortality caused by SARS-CoV-2, and global death toll is still rising. Exaggerated inflammatory responses are thought to be more responsible for infiltrated immune cells accumulation, organ damage especially lung, dyspnea, and respiratory failure rather than direct effect of viral replication. IL-6 and NLRP3 inflammasome are the major immune components in immune responses stimulation upon pathogen infection. It's noteworthy that the function and expression of these components are remarkably influenced by non-coding RNAs including long non-coding RNAs. Given the potential role of these components in organ damage and pathological manifestations of patients infected with COVID-19, their blockage might be a hopeful and promising treatment strategy. Notably, more study on long non-coding RNAs involved in inflammatory responses could elevate the efficacy of anti-inflammatory therapy. In this review we discuss the potential impact of IL-6 and NLRP3 inflammasome blocker drugs on inflammatory responses, viral clearance, and pathological and clinical manifestations. Collectively, anti-inflammatory strategy might pave the way to diminish clinical and pathological manifestations and thereby discharging patients infected with COVID-19 from hospital.
Keywords: SARS-CoV-2, COVID-19, Inflammation, Cytokine release syndrome, Inflammasome, Drug
Graphical abstract
1. Introduction
COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rapidly disseminate all around the world by 9,068,108 confirmed cases and 471,042 death until June, 22, 2020 [1]. COVID-19 is an enveloped virus that belongs to the Coronaviridae family containing a positive-sense RNA genome which encodes essential structural proteins including spike (S), envelope (E), membrane (M), and nucleocapsid (N) [2]. COVID-19s' cell entry strongly depends on S protein through interacting with angiotensin-converting enzyme (ACE) on the target tissues such as lung, kidney, heart, and gastrointestinal (Fig. 1 ) [3,4]. The inflammatory cascade is activated following sensing virus' RNA and its structural proteins by inflammatory sensors [5,6]. It seems that interleukin-6 (IL-6) and NOD-like receptor protein 3 (NLRP3) inflammasome are the major cause of inflammatory cytokine storm, and thereby clinical and pathological manifestations of patients infected with COVID-19 [7,8]. Correspondingly, infiltrated immune cells including macrophages and monocytes, minimal lymphocytes including CD4+ T cells, eosinophils and neutrophils were presented in lungs of patients who died of SARS-CoV-2 [9]. Its noteworthy that epigenetic modulations such as non-coding RNAs, DNA methylation, and histone acetylation are implicated in inflammatory cytokine storm and inflammatory complex including IL-6, tumor necrosis factor (TNF)-α, and NLRP3 inflammasome [10,11]. Therefore, designing anti-inflammatory drugs to target inflammatory cytokines especially IL-6 and inflammatory complex including inflammasome could be a promising strategy to deal with SARS-CoV-2 [12,13]. Patients with rheumatoid arthritis showed down-regulation of the levels of acute-phase reactants including prototypic C-reactive protein (CRP) upon administration of tocilizumab [14]. Also, glyburide is a food and drug administration (FDA) approved drug for treatment of type 2 diabetes able to block NLRP3 inflammasome activation through inhibiting ATP sensitive K+ (KATP) channels, caspase-1, IL-1β, and apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) assembly, thereby halts inflammation responses and organ damage [[15], [16], [17], [18]]. Furthermore, well recognition of non-coding RNAs involved in SARS-CoV-2-induced inflammation response could serve as new prognostic biomarkers and therapeutic targets in treatment of patients infected with COVID-19 [10]. Collectively, co-administration of anti-IL-6 and inflammasome blocker drugs might improve clinical manifestations of COVID-19 patients, and reduce morbidity and mortality through limiting COVID-19-mediated inflammation responses. In this review, we describe the mechanism of IL-6 and NLRP3 inflammasome in pathogenesis of SARS-CoV-2, and thereby clinical and pathological manifestations of the disease. Also, we review long non-coding RNAs implicated in IL-6 and NLRP3 inflammasome activation. Finally, we discuss mechanism and pharmacokinetic properties of some reported pharmacological inhibitors targeting these most important inflammatory components.
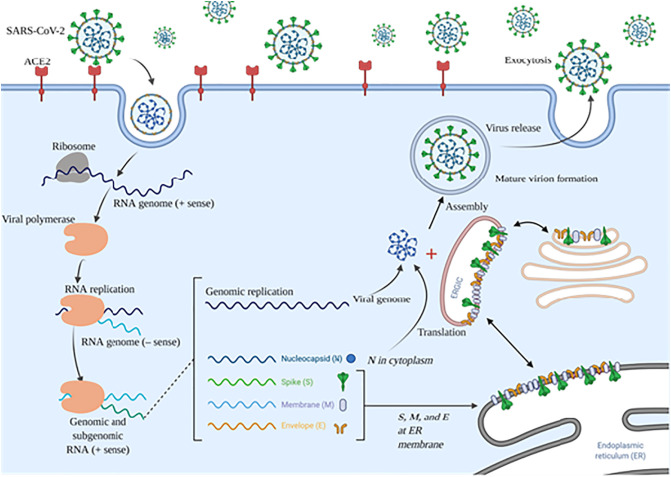
Fig. 1.
The mechanism of cell entry and life cycle of SARS-CoV-2 in host cell; SARS-CoV-2 life cycle initiation is mediated by S protein binding to the ACE2. Conformation change in S protein following binding to ACE2 promotes its fusion with cell membrane via endosomal pathway. Viral genomic RNA is released and translated into viral polymerase proteins that synthesize the negative (−) sense genomic RNA, and thereby produce a series of subgenomic mRNAs to translation and residing of essential, structural viral proteins including nucleocapsid (N), spike (S), membrane (M), envelope (E) into ER and further transport to the Golgi apparatus. Finally, viral RNA–N complex and S, M, and E proteins are assembled into virion and released out of the host cell. ACE2: angiotensin-converting enzyme 2; ER: endoplasmic reticulum; ERGIC: ER–Golgi intermediate compartment.
2. Mechanism of IL-6 secretion and inflammatory cascade formation mediated by SARS-CoVs' infection
SARS-CoV-induced inflammatory responses largely cause organ damage especially lung, and thereby high mortality and morbidity [7,19]. Inflammatory cytokines comprising IL-6, and TNF-α and inflammatory complexes including inflammasome were activated following ACE-mediated SARS-CoVs' cell entry [20,21]. Studies carried out on human and animal models infected with SARS-CoV suggest that SARS-CoV-mediated fatal pneumonia might be due to immunopathological events [[22], [23], [24]]. Also, human lung fibroblasts infected with MERS-CoV and HCoV-229E were shown to cause a delayed, strong increase in the levels of IL-1β, IL-6, IL-8, TNF-α, interferon (IFN)-β, and IFN-γ-induced protein (IP)-10. However, the levels of IL-6, IL-8, IFN- β, and IP-10 were significantly higher in HCoV-229E-infected cells relative to MERS-CoV-infected cells [25]. Moreover, the lungs' pathological study of patients who died of COVID-19 demonstrated the presence of infiltrated immune cells such as macrophages and monocytes, minimal lymphocytes including CD4+ T cells, eosinophils and neutrophils, alveolar exudative inflammation as well as interstitial inflammation (Fig. 2 ) [9]. Recent studies raised the possibility that inflammatory cytokine storm and inflammatory events may be responsible for the severe COVID-19 pathology [26,27].
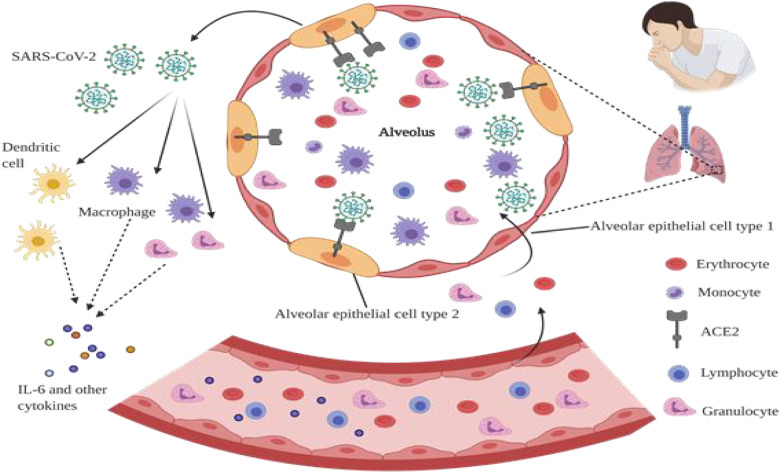
Fig. 2.
Possible mechanism of cytokine release syndrome in severe COVID-19 patients. SARS-CoV-2 infects alveolar epithelial type 2 cells through ACE2 receptor, leading to destruction and permeability of epithelial cells, and thereby virus release. Releasing of virus strongly activates innate and adaptive immune cells including macrophages, granulocytes, lymphocytes, monocytes, dendritic cells, and also a large number of cytokines including IL-6. Furthermore, following the stimulation of inflammatory factors, a large number of inflammatory cells and erythrocytes enter the alveoli, and cause dyspnea and respiratory failure.
2.1. IL-6 secretion mediated by SARS-CoVs' infection
IL-6 is a potent pro-inflammatory cytokine that plays a crucial role in inflammatory responses, autoimmune diseases, cancers, and viral infections [28]. Also, recently IL-6 have been identified in development of SARS-CoV-2-induced inflammatory responses, and further affected patient's clinical manifestations [29]. Relatively, recent studies illustrated the remarkable, higher levels of IL-6 in patients with COVID-19 in comparison with those in control group. Also, the levels of IL-6 were strongly correlated with severity of patients' clinical manifestation, serum SARS-CoV-2 viral load (RNAaemia), and mechanical ventilation requirement [14,[30], [31], [32]]. Moreover, the levels of IL-6 were increased up to 7-fold (P = 0.016) following human peripheral blood mononuclear cells (PBMCs) treatment with SARS-CoVs' spike protein through activation of nuclear factor kappa B (NF-κB) pathway [33]. Correspondingly, treatment of murine macrophages cell line (RAW264.7) with SARS-CoV spike protein led to NF-κB-induced IL-6 and TNF-α up-regulation [34]. IL-6 exerts its effects on target cells through three different mechanisms comprising binding directly to the membrane-bound glycoprotein 130 (gp130), also known as classical signal transduction; forming a complex with its receptor IL-6R and then binding to gp130; and binding to soluble gp130 in a trans presentation manner [35,36]. IL-6 influences immune processes via activating multiple downstream signaling pathways including janus kinase/signal transducers and activators of transcription (JAK-STATA) (STAT1,3, and 5) [37,38], RAS-rapidly accelerated fibrosarcoma (RAS-RAF) [37,39], SRC-yes-associated protein(YAP) -neurogenic locus notch homolog (NOTCH) [40], phosphatidylinositol 3-kinase (PI3K)-AKT [41,42]. IL-6 has a pivotal role in regulating the immune system and inflammatory pathways through several ways including promoting B lymphocytes proliferation and differentiation to induce antibody production (Fig. 3 ) [43], cytotoxic T lymphocyte (CTL) activating [44], inducing hepatocytes-mediated acute phase reactive proteins secretion [45], and hematopoietic stem cells differentiation [46]. Moreover, IL-6 could induce T helper-17 (Th-17) cells, is a pro-inflammatory cytokine involved in pathogenesis of several inflammatory diseases, via recruiting the transforming growth factor beta (TGF-β) [47,48]. It's noteworthy that IL-6 is also known as a major inducer of acute phase reactive protein that lead to acute phase reactive proteins secretion comprising serum amyloid A (SAA) and CRP from hepatocytes [45,49]. Recently, it was shown that IL-6 is a core regulator of vascular endothelial growth factor (VEGF) expression, and vessel permeability in alveolar epithelial cells [50,51].
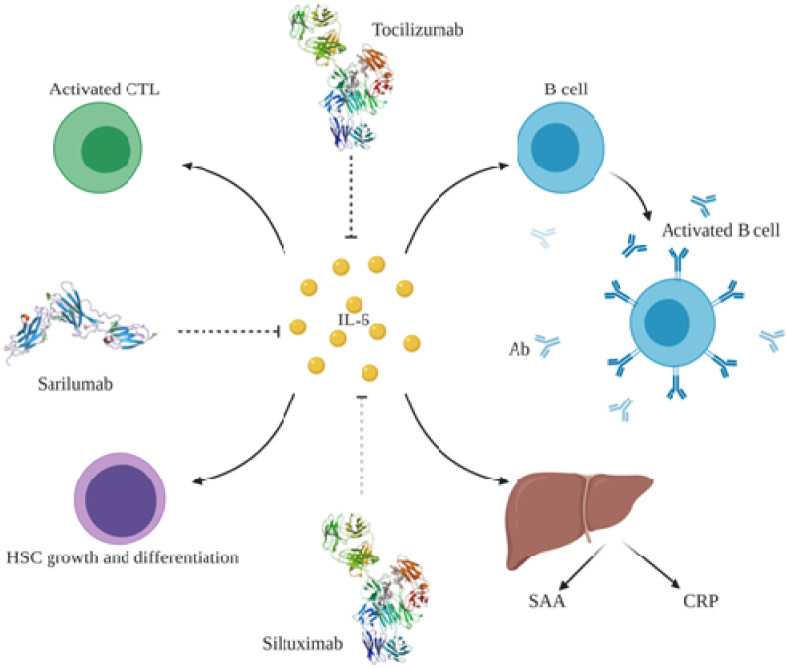
Fig. 3.
IL-6 is involved in inflammation through activating different pathways. IL-6 influences both B and T lymphocyte cells to induce antibody production and CTL activation, respectively. IL-6 promotes HSC growth through enhancing the differentiation of blood cells and promoting their colony formation. Moreover, IL-6 influences acute phase reactive protein production such as SAA and CRP from hepatocytes. CTL: cytotoxic T lymphocyte; Ab: antibody; CRP: C-reactive protein; SAA: serum amyloid A, HSC: hematopoietic stem cells.
2.2. NLRP3 inflammasome formation upon SARS-CoVs' infection
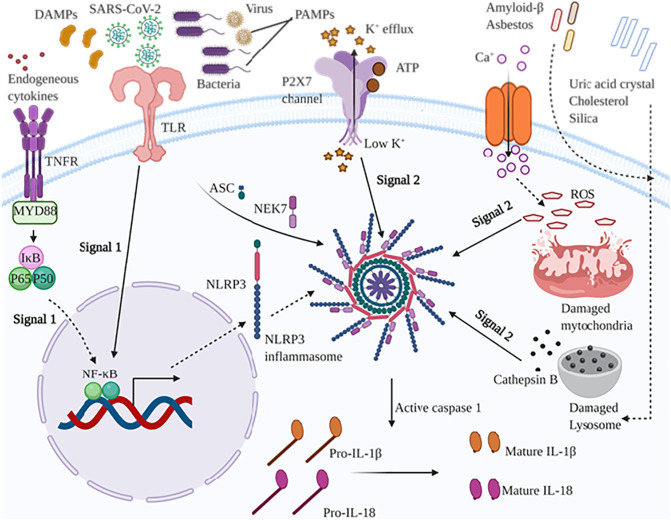
SARS-CoV-2 induces IL-1 family members including IL-1β and IL-18 through activating an inflammatory protein complex named NLRP3 inflammasome [21,52]. Activation of NLRP3 inflammasome occurs via two signaling mechanisms (Fig. 4 ). The first one also called priming signal is triggered via microbial agents sensing with toll like receptors (TLRs) or cytokines to NF-kB-induced pro-IL-1β and NLRP3 up-regulation. The second one is triggered by pathogen associated molecular patterns (PAMPs), and danger-associated molecular patterns (DAMPs) resulting in ASC and pro-caspase-1 assembly, and thereby activation of NLRP3 inflammasome [53,54]. Its noteworthy that NLRP3 inflammasome is also activated by ATP and K+ efflux in a P2X7 receptor and pannexin-1 dependent manner [54]. Inflammasome is a central inflammatory multimeric complex including pro-caspase-1, ASC, and NLRP3 protein [55]. Inflammasome is activated via nucleotide-binding oligomerization domain–like receptors (NLRs) upon sensing a wide stimuli spectrum comprising PAMPs, DAMPs, and reactive oxygen species (ROS) [56,57]. Several types of NLRs, innate cytosolic receptors, including NLRP1–7, and NLRP12 could promote inflammasome assembly; nonetheless NLRP3 is more studied than other types [58,59]. Multiple lines of evidence illustrated that NLRP3 interacts with the pyrin domain (PYD) of ASC following sensing stimulus-mediated oligomerization, and then ASC recruits pro-caspase-1 via a caspase recruitment domain (CARD) [60]. Consequently, upon autocatalysis, activated caspase-1 cleaves pro-interleukin (IL)-1β and pro-IL-18 into IL-1β and IL-18 respectively, resulting in inflammatory responses [61]. Recent in vitro study have illustrated that SARS-Coronavirus open reading frame-8b interacts with leucine-rich repeat (LRR) domain of NLRP3 to activating NLRP3 inflammasome, leading to increasing levels of IL-1β and IL-18 in macrophages and probably lung epithelial cells [52]. Surprisingly, another recent study revealed that SARS-CoV open reading frame-3a protein activates both required signaling mechanisms to inflammasome activation independent of ion channel activity. Therefore, open reading frame-3a either activates NF-κB and thereby IL-1β transcription through inducing ubiquitination of p105 in a TRAF3-dependent manner, or it interacts with TRAF3 to induce K63-linked ubiquitination of ASC and consequently promotes caspase 1 activation and IL-1β maturation [62]. SARS-CoV 3a protein acts as a viroporin to NLRP3 inflammasome formation, and thereby IL-1β and IL-18 secretion through K+ channel activity and mitochondrial ROS induction [63]. SARS-CoV E protein ion channel activity is associated to IL-1β secretion through acting as Ca+ channel, and consequently forms NLRP3 inflammasome compartment [21]. Correspondingly, creation of N15A and V25F mutations in the transmembrane domain of SARS-CoV E protein abrogated the Ca+ flux as well as K+, Na+, and Cl− transportation [21,64]. Animals infected with SARS-CoV without E protein ion channel activity have shown decreased inflammasome-induced IL-1β highlighting the pivotal role of E protein in inflammasome formation. Consistently, mice infected with SARS-CoV containing E protein ion channel activity displayed swollen alveoli walls and leukocyte infiltration whereas those infected with SARS-CoV lacking E protein ion channel activity presented moderate swollen lung epithelia and leukocyte infiltration [64].
Fig. 4.
NLRP3 inflammasome pathway is activated through signal 1 and signal 2. Signal 1 is mediated trough sensing microbial and virus ligands (PAMPs), DAMPS, and cytokines such as TNF-α by TLR and TNFR, respectively. Activation of signal 1 leads to NF-kB pathway activation, and thereby up-regulates pro-IL-1β, pro-IL-18, and NLRP3 protein levels. Signal 2 is primed by extracellular ATP and K+ efflux leading to the activation of NLRP3 inflammasome via the P2X7 receptor. Also, calcium influx activates NLRP3 inflammasome by damaging mitochondria, and consequently releases the mitochondrial ROS. Different endogenous and exogenous agents including amyloid β, asbestos, uric acid crystal, cholesterol, silica crystal cause lysosome damage and cathepsin B release from lysosome into cytosol, promoting NLRP3 activation. Collectively, NLRP3 inflammasome activation leads to caspase 1 activation, and thereby converting pro-IL-1β and pro-IL-18 into mature form. TNFR: tumor necrosis factor receptor; TLR: toll-like receptors; DAMPs: damage-associated molecular patterns; PAMPs: pathogen associated molecular patterns; IκB: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3: NACHT, LRR, and PYD domains-containing protein 3; P2X7: P2X purinoceptor 7; IL: interleukin.
3. Role of LncRNAs implicated in IL-6 and NLRP3 inflammasome signaling pathway
LncRNAs are a novel class of non-coding transcripts with longer than 200 nucleotides length, and play a crucial role in a broad spectrum of disorders [[65], [66], [67]]. A growing number of studies have indicated that ncRNAs play a crucial role in inflammatory disease progression [[68], [69], [70]]. LncRNAs have been strongly implicated in regulation of NLRP3 inflammasome and IL-6-assocciated inflammatory signaling [10,71,72].
3.1. LncRNAs involved in IL-6 secretion
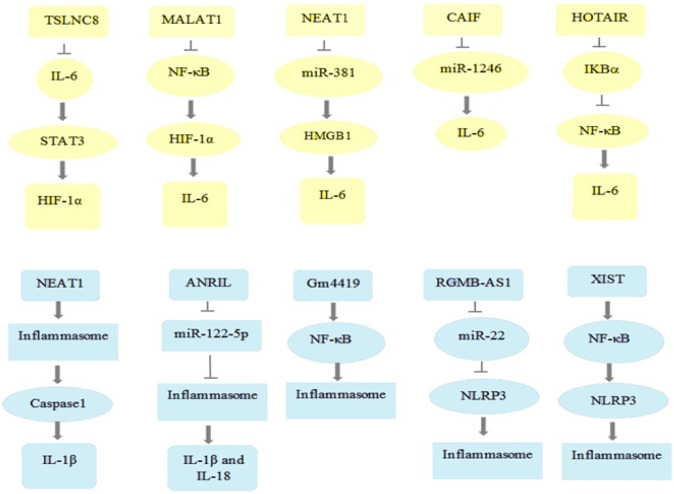
There is some evidence about the key role of lncRNAs in both down- and up-regulation of IL-6 [10]. As listed in Table 1 , and Fig. 5 lncRNAs may regulate IL-6 expression via several pathways like JAK/STAT, NF-κB, HIF-1α, and MAPK. LncRNA/IL-6/STAT is one of the well-studied pathways involved in multiple malignancies such as gastric cancer, hepatocellular carcinoma, non-small cell lung cancer, etc. [[73], [74], [75]]. Correspondingly, lncRNA down-regulated in liver cancer stem cells (lnc-DILC) suppresses IL-6 transcription and its downstream pathway, JAK2/STAT3, and also reduces spheroid formation by binding directly to IL-6 promoter in lnc-DILC overexpressing cells. Moreover, subcutaneous inoculation of hepatoma cells knocked down for lnc-DILC to mice resulted in a greater xenograft tumor growth, size, and weight, highlighting the fundamental role of lnc-DILC in restraining liver cancer stem cells and hepatocellular carcinoma progression. Also, patients with higher levels of lnc-DILC showed a lower risk of hepatocellular carcinoma recurrence and better survival following surgical resection [76]. Another regulator lncRNA is tumor-suppressive lncRNA on chromosome 8p12 (TSLNC8) that remarkably suppresses proliferation, migration, invasion, and autophagy, and induces apoptosis in non-small cell lung cancer through inactivating IL-6/STAT3/hypoxia-inducible factor 1-alpha (HIF-1a) axis [77]. Contrary to lnc-DILC and TSLNC8, lncRNA regulating IL-6 transcription (LNRRIL6) demonstrated an oncogenic role by promoting IL-6/STAT3 axis and its downstream molecules including cell division cycle 25 A (CDC25A), cyclin D1, survivin, and B-cell lymphoma 2 (BCL2). Relatively, injection of LNRRIL6 overexpression cells into athymic nude mice induced tumor growth [78]. Strikingly, lncRNA metastasis associated lung adenocarcinoma transcript 1 (MALAT1), also known as NEAT2, has indicated a dual role regarding inflammation responses and cytokine secretion especially IL-6 in different signaling pathways. Increased MALAT1 levels induced by acute kidney injury and cobalt chloride-induced hypoxia in mice suggested that it might have an anti-inflammatory role in acute kidney injury. Also, knocking down MALAT1 in HK2 cells led to NF-κB and HIF-1α activation, and consequent increase of many inflammatory cytokines such as IL-6 and TNF-a to promoting inflammatory cell infiltration and tissue damage [79]. Moreover, the inflammatory role of MALAT1 has been revealed upon its overexpression, and thereby inflammatory cytokines (IL-6, TNF-α, and IL-1β) up-regulation in LPS-induced acute lung injury. Correspondingly, histopathological investigation of the lung sections of MALAT1 knocked down rat demonstrated that LPS-induced lung injury was strongly diminished in comparison with the control group [80]. Furthermore, IL-6 up-regulation was shown to be tightly linked to T-cell proliferation blockage, accumulation of infiltration neutrophils in ovarian cancer tissue, and ovarian tumor growth through recruitment by lncRNA HOXA transcript at the distal tip (HOTTIP). In view of these, NOD/SCID mice were injected by HOTTIP overexpressing SKOV3 cells demonstrated a higher tumor volume in comparison with those injected with normal SKOV3 cells [81]. Surprisingly, IL-6 was shown to be also regulated through NF-κB signaling following HOX antisense intergenic RNA (HOTAIR)-mediated nuclear translocation and activation of NF-κB in LPS-induced macrophages. In the light of this result, the levels of inflammatory response and cytokines induced by LPS potentially decreased upon HOTAIR knock down in RAW264.7 cells [82]. Another oncogenic lncRNA that might be implicated in inflammatory cytokines regulation including IL-6, IL-1β, and TNF-α, and consequently neuropathic pain development in chronic constriction injury (CCI) rats model is lncRNA nuclear-enriched abundant transcript 1 (NEAT1). Consistently, neuropathic pain has ameliorated in NEAT1 knocked down rat upon considerable down-regulation of inflammatory cytokines such as IL-6, IL-1β, and TNF-α which suggested the crucial role of NEAT1 in regulation of IL-6 expression [83]. Current studies regarding osteoarthritis pathogenesis suggest that lncRNA cardiac autophagy inhibitory factor (CAIF) could restrain osteoarthritis progression through blocking miR-1246, and IL-6. Also, it was shown that the IL-6 and miR-1246 levels were remarkably up-regulated in synovial fluid of osteoarthritis patients whereas CAIF was significantly down-regulated, highlighting the potential role of IL-6 in osteoarthritis as an inflammatory disease [84].
Table 1.
Most relevant lncRNAs involved in dysregulated IL-6 signaling pathway and their target molecules.
| LncRNA | Target(s) | Tissue or cell type | Effect | Disease | References |
|---|---|---|---|---|---|
| DILC | IL-6/STAT | Liver cancer stem cells (in vitro) and tumor xenografts in mice | Suppresses IL-6 expression, spheroid formation, and JAK2/STAT3 signaling | Hepatocellular carcinoma | [76] |
| TSLNC8 | IL-6/STAT3/HIF-1a | A549 cells | Inhibits proliferation, migration, invasion, and autophagy and induces apoptosis | Non-small cell lung cancer | [77] |
| LNRRIL6 | IL-6/STAT3 | Colorectal cancer tissue (in vitro) and athymic nude mice (in vivo) | Promotes CRC cell survival and tumor growth | Colorectal cancer cells | [78] |
| UICC | IL-6/STAT3 | Caski, SiHa, HeLa, Ms751 and C33a cells (in vitro) and xenograft tumors grown from HeLa cells overexpressing lnc-UICC (in vivo) | Promotes tumor growth and metastasis | Cervical cancer | [85] |
| MALAT1 | NF-κB and MAPK pathways | Acute lung injury rats (in vivo) and rat pulmonary microvascular endothelial cells (in vitro) | Induces inflammatory responses and cytokine release including IL-6 and TNF-α | Acute lung injury | [86] |
| MALAT1 | NF-κB/HIF-1α/IL-6 | Acute kidney injury mice (in vivo) and HK2 cells (in vitro) | Anti-inflammatory effect | Acute kidney injury | [79] |
| MALAT1 | miR-146-a | LPC-induced acute lung injury rats (in vivo) | Induces inflammatory responses and cytokine release including IL-6 and TNF-α | Acute kidney injury | [80] |
| HOTTIP | STAT3/PD-L1 | NOD/SCID mice (in vivo) and ovarian cancer cells (in vitro) | Promotes inflammatory responses | Ovarian cancer cells | [81] |
| HOTAIR | NF-κB | LPS-induced macrophages (in vitro) | Inflammatory response and cytokines release | Inflammation | [82] |
| NEAT1 | miR-381/HMGB1 | Chronic constriction injury rats (in vivo) | Neuropathic pain development | Chronic constriction injury | [83] |
| CAIF | miR-1246/IL-6 | Synovial fluid and CHON-001 cells (in vitro) | Promotes inflammatory responses | Osteoarthritis | [84] |
| MEG3 | miR-203 | LPS-induced ATDC5 cells injury (in vitro) | Promotes inflammatory injury | Osteoarthritis | [87] |
| PVT1 | TNF-α/JNK NF-κB pathway |
LPS-induced HK-2 cells | Promote inflammatory response | Septic acute kidney injury | [88] |
Fig. 5.
Different potential lncRNAs and their targets involved in IL-6 and NLRP3 inflammasome pathway. IL: interleukin; STAT: signal transducers and activators of transcription; HIF-1α: hypoxia-inducible factor 1-alpha; IκB-α: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3: NACHT, LRR, and PYD domains-containing protein 3.
3.2. LncRNAs implicated in NLRP3 inflammasome formation
Inflammasome, a multiprotein complex, is a core inflammatory component involved in innate immunity and inflammation responses upon induction with various stimuli [89]. Accumulating evidence has indicated that lncRNAs are implicated in inflammasome formation followed by severe disorders promotion (Table 2 , Fig. 5) [72,90]. Nuclear enriched abundant transcript 1 (NEAT1) has been associated with malignancy in multiple types of cancer including non-small cell lung, ovarian, cervical, and breast cancer suggesting its potential role in cell proliferation and tumor growth [91]. Moreover, murine immortalized bone marrow-derived macrophages (iBMDMs) induced by LPS facilitated NEAT1 translocation from nucleus to the cytoplasm to inflammasome assembly, and thereby caspase 1 activation and inflammatory cytokine release, highlighting the inflammatory role of NEAT1. Accordingly, treatment of Neat1 knocked out mice (Neat −/−) with LPS resulted in reduced caspase 1 activation and IL-1β secretion in comparison with wild type mice (Neat +/+). Surprisingly, hypoxia-induced iBMDMs indicated that Nlrp3, Nlrc4, and absent in melanoma 2 (Aim2) up-regulation are triggered by Hif-1α which might be mediated by Hif-2α-induced Neat1 up-regulation [92]. Intriguingly, antisense non-coding RNA in the INK4 locus (ANRIL) has been closely linked to inflammation responses through acting as a competing endogenous RNA (ceRNA). ANRIL up-regulates the BRCA1-BRCA2-containing complex subunit 3 (BRCC3) and NLRP3 inflammasome, and consequently promotes uric acid nephropathy by sponging miR-122-5p. In this regard, histological study of kidney section of Anril knocked down rat indicated that the inflammatory cells infiltration, collagen fibers, and renal injury were more prominent than those in control group. Also, HK-2 cells transfected with ANRIL showed that NLRP3, IL-1β, and IL-18 were remarkably up-regulated whereas siRNA-medicated ANRIL silencing in HK-2 cells showed that NLRP3, IL-1β, and IL-18 were suppressed [93]. One of the tumor suppressive lncRNAs that is downregulated in a wide variety of cancers is lncRNA growth arrest-specific transcript 5 (GAS5) [94]. In this context, inducing GAS5 overexpression in nude mice with ovarian cancer led to tumor growth inhibition via promoting ASC and caspase 3 expression to activation of inflammasome, and further increasing IL-1β secretion. Also, GAS5 knock down in 3AO cell line demonstrated that GAS5 exerts its antitumor activity via inflammasome-induced inflammatory cytokine release [95]. Another lncRNA that serves as a tumor suppressor in a NLRP3 inhibition manner is XLOC_000647 that is implicated in pancreatic cancer pathogenesis. Correspondingly, subcutaneous injection of pancreatic cancer cell lines including MIA-PaCa-2 and BxPC-3 expressing XLOC_000647 into nude mice resulted in significantly lower tumor weight in comparison with control group [96]. Long intergenic noncoding RNA (LincRNA)-Gm4419 is another regulatory lncRNA related to inflammation events through NF-κB pathway. Gm4419 facilitates inflammation in diabetic nephropathy through NF-κB-mediated NLRP3 inflammasome activation by binding to p50 (NF-κB subunit), in a positive feedback manner. Also, Gm4419 knock down in mesangial cells with high glucose expression resulted in inflammation, fibrosis, and proliferation down-regulation whereas Gm4419 overexpression with low glucose reversed these phenotypes [71].
Table 2.
Most relevant lncRNAs involved in dysregulated NLRP3 inflammasome signaling pathway and their target molecules.
| LncRNA | Target(s) | Tissue or cell type | Effect | Disease | References |
|---|---|---|---|---|---|
| NEAT 1 | Caspase1 | LPS-induced murine immortalized bone marrow-derived macrophages (in vitro) and mice treated with LPS | Inflammasome activation and IL-β secretion | Inflammation | [92] |
| ANRIL | miR-122-5p/BRCC3 | Kidney section of rat and HK-2 cells | Uric acid nephropathy and inflammation responses | Uric acid nephropathy | [93] |
| GAS5 | ASC and Caspase3 | Nude mice with ovarian cancer (in vivo) and 3AO cell line | Suppresses ovarian cancer | Ovarian cancer | [95] |
| GAS5 | NLRP3 | Cardiac fibrosis induced in Sprague-Dawley rats (in vivo) and cardiac fibroblast tissue (in vitro) | Inhibits cardiac fibrosis | Cardiac fibrosis | [11] |
| XLOC_000647 | NLRP3 | Nude mice | Suppresses pancreatic cancer | Pancreatic cancer | [96] |
| Gm4419 | NF-κB | Mesangial cells | Promotes inflammation in diabetic nephropathy | Diabetic nephropathy | [71] |
| RGMB-AS1 | miR-22/NLRP3 | Hep-2 and AMC-HN-8 cells (in vitro) and tumor xenograft in nude mice (in vitro) | Tumor growth and poor prognosis | Laryngeal squamous cell carcinoma | [97] |
| XIST | NF-κB/NLRP3 inflammasome | Mammary alveolar cell-T (MAC-T) infected with E. coli and S. aureus | Promotes cell proliferation, viability and apoptosis of inflammatory MAC-T | Inflammation in bovine mammary epithelial cells | [98] |
4. IL-6 and NLRP3 inflammasome blocker drugs are promising strategy to combat COVID-19
High morbidity and mortality caused by current SARS-CoV-2 pandemy created an urgent need for developing effective therapeutic strategies to combat SARS-CoV-2 pathogenesis and thereby its outbreak [1]. Accumulating evidence suggest that the inflammatory cytokine storm and inflammatory responses might be responsible for the severe COVID-19 pathology and clinical manifestation deterioration [27,99]. Accordingly, recent studies carried out on patients infected with COVID19 showed that inflammatory cytokines including IL-1β, IL-18, IL-6, and TNF-α were remarkably higher in comparison with subjects in control group [30,33,100]. Also, there is some evidence about the potential role of NLRP3 inflammasome in SARS-CoV-induced inflammatory cytokine responses modulation [21,63]. The probable role of inflammatory cytokines especially IL-6 and NLRP3 inflammasome in SARS-CoV-2 pathogenesis have raised the possibility that blockage of inflammatory cytokines and NLRP3 inflammasome might be a hopeful strategy to cope with COVID-19 [101]. Previous studies have indicated the efficiency of several anti-NLRP3 inflammasome and anti-cytokine small molecules in management of inflammatory, and autoinflammatory diseases [18,102].
4.1. IL-6 blocker drugs
Elevated plasma levels of inflammatory cytokines especially IL-6 in patients infected by SARS-CoV-2 shed some light on efficacy of therapeutic strategy of IL-6 blockers in ameliorating of severe clinical manifestations induced by SARS-CoV-2 infectious [14,29] (Fig. 3, Table 3, Table 4 ). Tocilizumab, a humanized monoclonal antibody also known as actemra, inhibited the IL-6 receptor (IL-6R), and thereby significantly decreased serum acute phase reactants such as CRP, and SAA in patients who were injected intravenously with 2, 4, or 8 mg/kg biweekly for 6 weeks [[103], [104], [105]]. Given the indirect inhibitory mechanism of tocilizumab through abrogating IL-6 signaling by binding to its receptor, unbound IL-6 serum levels are increased after initial drug administration but are gradually down-regulated upon immune activation abrogation [105,106]. Notably, after several clinical trial studies intravenous administration of tocilizumab was approved in Japan (2008), Europe (2009), and USA (2010) and further subcutaneous injection was also approved in USA (2013) and in Europe (2014) [107,108]. BML-111, a lipoxin receptor agonist, has an anti-inflammatory effect through several mechanisms. BML-111 potentially decreases TNF-α, inflammatory cells infiltration, NF-κB/DNA binding activity, and P65 nuclear translocation whereas it promotes IκB-α expression and consequently suppresses inflammation in rats with haemorrhagic shock-induced acute lung injury [109]. It also can alleviate inflammatory responses and inflammatory cells infiltration in acute lung injury by blocking MAPK/AP-1 pathways and interfering with IL-6, IL-8, AP-1/DNA interaction [110]. In this view, BML-111 was shown to cause a significant increase in MALAT1 levels which is down-regulated in rats with acute lung injury, and consequently MALAT1 reduces activation of NF-κB, MAPK, and expression of inflammatory factors including monocyte chemoattractant protein-1 (MCP-1) and IL-6 [86,109].
Table 3.
Potential mechanisms of several IL-6 and NLRP3 inflammasome inhibitors.
| Agent | Target (s) | Potential mechanism | Disease | References |
|---|---|---|---|---|
| Tocilizumab | Interleukin-6 receptor | Inhibits interleukin-6 receptor consequently decreased serum acute phase reactants such as CRP, and SAA | Rheumatoid arthritis | [103,105] |
| Sarilumab | Interleukin-6 receptor | Inhibits interleukin-6 receptor consequently decreased serum acute phase reactants such as CRP | Rheumatoid arthritis | [118] |
| Siltuximab | Interleukin-6 receptor | Inhibits interleukin-6 receptor consequently decreased serum acute phase reactants such as CRP and circulating free IL-6 | Metastatic renal cell cancer | [119] |
| BML-111 | Interleukin-6 receptor | -Upregulates lncRNA MALAT levels to decreases reduced activation of NF-κB, MAPK, and IL-6 -Disrupts IL-6, IL-8, AP-1/DNA interaction -Inhibits NF-κB and suppresses inflammatory responses |
Acute lung injury | [86] [110] [109] |
| Glyburide | NLRP3 (indirect) | Blocks ATP sensitive K+ (KATP) channels, inhibits capase-1, disrupts ASC complex forming | Inflammatory disease | [15,17,18] |
| MCC950 | NLRP3 | Inhibits caspase-1, ASC oligomerization, blocks the ATPase domain of NLRP3, and thereby inhibits of canonical and non-canonical NLRP3 inflammasome activation | Inflammation, Parkinson's | [18,115,120] |
| OLT1177 | NLRP3 | Disrupts in recruiting of ASC and caspase-1 by NLRP3 and blocks NLRP3 inflammasome activation | Cryopyrin-associated periodic syndrome | [117] |
| Fc11a-2 | NLRP3 | Suppresses TNF-a, IL-1β, IL-18, IL17A and IFN-g | Dextran sulfate sodium-induced model of experimental colitis in mice | [121] |
| BOT-4-one | NLRP3 | Impairs ATPase activity of NLRP3 by alkylation of NLRP3 | Peritonitis in mice | [122] |
| Parthenolide | NLRP3 | Inhibits ATPase activity of the NLRP3 inflammasome via alkylation of NLRP3, inhibits NF-κB, phosho-p38MAPK, and caspase-1 | Rat stroke model | [102,123] |
| BAY 11-7082 | NLRP3 | Inhibits ATPase activity of the NLRP3 inflammasome independent of their NF-κB inhibitory activity. | Rat stroke model | [102,123] |
| INF39 | NLRP3 | Inhibits ATPase activity of NLRP3 | Colitis in rat | [124] |
| JC-171 | NLRP3 | Inhibits NLRP3 activation and IL-1β secretion | Multiple sclerosis | [125] |
| 16673-34-0 | NLRP3 | Inhibits NLRP3 inflammasome formation | Myocardial injury following ischemia-reperfusion in the mouse | [126] |
| Colchicine | NLRP3 (indirect) | Prevents NLRP3 assembly through blocking of ASC oligomerization, lysosome damage and P2X7 receptor activity | Acute myocardial infarction in mouse model | [127] |
| AZD9056 | NLRP3 (indirect) | P2X7 inhibitor | Rheumatoid arthritis | [128] |
| GSK1070806 | IL-18 | Inhibition of IL-18 | Type 2 diabetes mellitus | [129] |
| Canakinumab | IL-1β | INHIBITS IL-1β | Lung cancer | [130] |
Table 4.
Three dimensional or chemical structure of most effective IL-6 and NLRP3 inflammasome blockers.
| Name | Chemical or 3D structure | Name | Chemical or 3D structure |
|---|---|---|---|
| Tocilizumab |  |
Parthenolide | 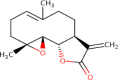 |
| Sarilumab | 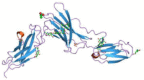 |
BAY 11-7082 | 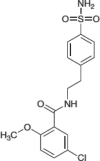 |
| Siltuximab | 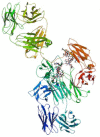 |
INF39 | 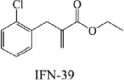 |
| Glyburide | 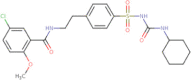 |
JC-171 | 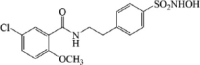 |
| MCC950 | 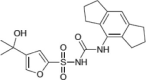 |
16673-34-0 | 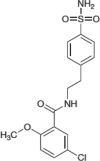 |
| OLT1177 | 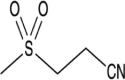 |
Colchicine | 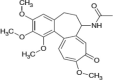 |
4.2. NLRP3 inflammasome blockers
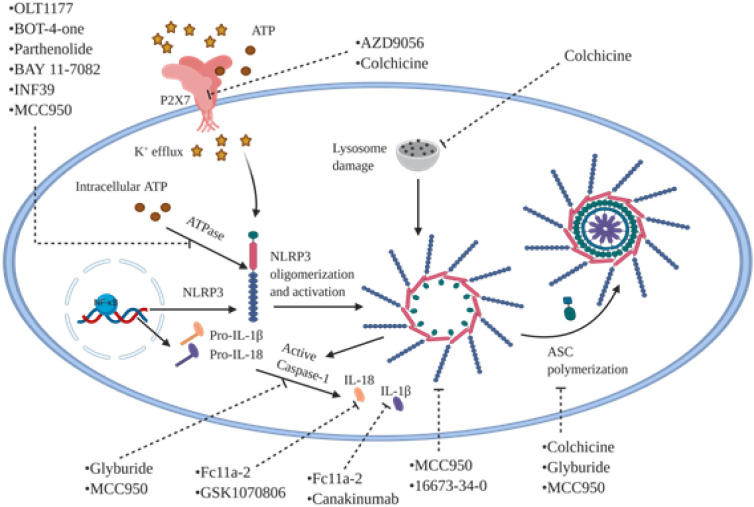
A growing body of evidence highlit the potential role of inflammatory components such as NLRP3 inflammasome in inflammatory responses mediated by SARS-CoV-2's infection. Correspondingly, it's becoming increasingly evident that anti-NLRP3 inflammasome drugs could diminish the inflammatory responses, and consequently alleviate clinical manifestations of patients with inflammatory disorders [[111], [112], [113]] (Fig. 6 , Table 3, Table 4). Glyburide, also known as glibenclamide, have been long used for type 2 diabetes treatment by blocking ATP sensitive K+ (KATP) channels [16,17]. It potentially could inhibit caspase-1 and IL-1β activation following treatment of human trophoblasts with nigericin [15]. It also partially prevents ASC complex from aggregation, but doesn't show any effect on NLRC4 or NLRP1 [18]. Furthermore, glyburide impedes PAMP, DAMP, and crystal-mediated NLRP3 inflammasome activation in bone marrow-derived macrophages [114]. MCC950 is another small molecule that inhibits both canonical and non-canonical NLRP3 inflammasome activation through interacting with a wide spectrum of components. Moreover, treating of bone marrow-derived macrophages with MCC950 caused inhibition of IL-1β secretion through abrogating of caspase-1 [18] or disrupting ASC oligomerization [18]. It's noteworthy that MCC950 blocks ATP hydrolysis ability of NLRP3, an essential process to activation of inflammasome, by direct binding to Walker B motif located in NLRP3 NACHT domain [115]. Notably, MCC950 couldn't influence NLRC4, AIM2, and NLRP3 or TLR signaling [18]. OLT1177 is a β-sulfonyl nitrile drug which is being investigated under phase II clinical trial for the treatment of acute gouty arthritis [116]. In this regard, murine macrophages cell line J774A.1 treated with OLT1177 was shown to cause 50% decrease of IL-1β secretion induced by LPS/ATP stimulation. However, the IL-1β and IL-18 secretion were diminished by 60% and 70%, respectively following human monocyte derived macrophages treatment with OLT1177. Also, it reduced IL-1β secretion via disrupting ASC and caspase-1 recruitment by NLRP3, thereby blocking NLRP3 inflammasome formation. However, OLT1177 doesn't have any effect on NLRC4, AIM2, TNF-α, and ion flux. It's noteworthy that OLT1177 also decreased IL-6 by 44% in mice with LPS-induced systemic inflammation [117]. Taken together, anti-inflammatory agents raise hope for diminishing pathological and clinical manifestations, and consequently morbidity and mortality induced by SARA-CoV-2 through targeting the main components involved in cytokine storm including IL-6 and NLRP3 inflammasome.
Fig. 6.
Mechanism of action of most potential pharmacological inhibitors for NLRP3 inflammasome blockage. Several chemical agents have been studied with inhibitory effects on different component of NLRP3 inflammasome pathway. OLT1177, BOT-4-one, parthenolide, BAY 11-7082, INF39, and MCC950 could inhibit ATPase activity of NLRP3. MCC950 and 16673-34-0 are able to inhibit NLRP3 oligomerization. Colchicine, glyburide, and MCC950 block NLRP3 inflammasome activation through disruption of ASC oligomerization. Colchicine could also halt lysosome damage and P2X7 receptor activity as well as AZD9056, consequently prevents from NLRP3 inflammasome activation. GSK1070806 similar to Fc11a-2 suppress IL-18 secretion whereas canakinumab as well as Fc11a-2 block IL-1β. NLRP3, NACHT, LRR, and PYD domains-containing protein 3; P2X7, P2X purinoceptor 7; IL: interleukin; ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain.
5. Discussion
The ongoing high morbidity and mortality caused by SARS-CoV-2s' pandemy pose a threat to global public health. SARS-CoV-2 outbreak has generated an urgent requirement to highly efficient with low side effect agents to combat it [131]. Recent studies have shown that exaggerated inflammatory responses and inflammatory cytokine storm might be the main cause of COVID-19 pathogenesis, and thereby fatality [27,99,132]. The potential role of IL-6 and NLRP3 inflammasome in immune response upon SARS-CoV-2 infection have emerged the hypothesis that blocking of these components could be a hopeful strategy to cope with it [14,20,21,133]. However, concerns have been raised about the probable role of epigenetic modulations including DNA methylation, histone modification, and ncRNAs such as micro RNA, lncRNA, and circular RNA (circRNA) in controlling IL-6 and inflammasome expression [10,134]. Therefore, designing and administration of potential drugs to target the wide spectrum molecules involved in IL-6 and inflammasome-associated epigenetic mechanisms along with IL-6 and inflammasome blockers may improve the efficacy of SARS-CoV-2s' treatment [12,13]. On the other hand, some of these ncRNAs have an anti-inflammatory effect through blocking IL-6 and inflammasome components whereas others promote inflammatory responses [77,135]. Recently, it was reported that circRNA_100782, as an oncogene, promotes pancreatic ductal adenocarcinoma BxPC3 cells proliferation by counteracting inhibitory effects of miR-124 in IL6-JAK2-STAT3 signaling pathway. Correspondingly, circRNA_100782 knocked down BxPC3 cells injected into BALB/c nude mice suppressed cell growth and IL6/STAT3 signaling pathway [136]. Contrary, circulating circ-DLGAP4 has an anti-inflammatory effect through sponging miR-143, and consequently decreases significantly CRP as well as inflammation cytokines including serum TNF-α, IL-6, IL-8 and IL-22 without affecting ESR, IL-1β, and IL-17 serum levels [137]. Furthermore, down-regulated miR-149 in osteoarthritis chondrocytes, exacerbates osteoarthritis progression via increasing inflammatory cytokines including TNF-α, IL-6, and IL-1β [138]. Notably, GAS5 is widely down-regulated by epigenetic mechanisms resulting in tumor progression in various malignancies [94]. Also, GAS5 down-regulation following DNMT1-mediated methylation of its promoter promotes pyroptosis-related proteins caspase1 and NLRP3 up-regulation, and thereby cardiac fibrosis progression. In this view, inducing cardiac fibrosis in rats led to Gas5 down-regulation and Nlrp3, caspase1, and Dnmt1 up-regulation [11]. Besides, some anti-inflammatory agents including emodin exert their effect via recruiting ncRNAs. Emodin, suppresses LPS-induced murines' ATDC5 cells apoptosis and inflammation by up-regulating lncRNA taurine-upregulated gene 1(Tug1), and thereby blocks NF-κB signaling and inflammatory cytokines especially Il-6 [139]. Discrepant results from several clinical trials about the efficacy of antiviral drugs including lopinavir/ritonavir (LPV/r) or arbidol in restraining COVID-19 infection and patients' manifestations raise the possibility that the pathological and clinical manifestations of infected patients might be due to virus-induced inflammatory cytokine storm and not only to virus replication [140]. Obtained results from a clinical trial including administration of tocilizumab for 20 patients with acute COVID-19 showed that 19 patients recovered from hospital within two weeks [141]. Tiziana Life Sciences (TZLS-501) is another fully-human anti-IL6R monoclonal antibody able to bind to both the membrane-bound and soluble forms of IL-6R, and thereby reduces circulating IL-6 levels in the blood and lung damage [142]. TJM2, a neutralizing antibody, is a promising agent to treatment of SARS-CoV-2 patients through targeting human granulocyte-macrophage colony stimulating factor (GM-CSF), and consequently diminishes inflammatory cytokine storm [141]. Furthermore, glyburide might be a useful drug to combat SARS-CoV-2 through blocking the wide spectrum of molecules related to inflammatory cascade including KATP channels, ASC oligomerization, caspase-1 and IL-1β, and it also could inhibit PAMP, DAMP, and crystal-mediated NLRP3 inflammasome activation [[15], [16], [17], [18]]. Strikingly, lungs' pathological postmortem examination of SARS-CoV-2 patients have shown elevated infiltrating immune cells including macrophages and monocytes, minimal lymphocytes including CD4+ T cells, eosinophils, and neutrophils, highlighting the probable role of inflammatory cells in deterioration of patients' clinical manifestations [9]. Correspondingly, elevated IL-6 serum levels in patients infected with SARS-CoV-2 suggested that the IL-6 serum levels is remarkably associated with severity of patients' clinical manifestations, serum SARS-CoV-2 viral load (RNAaemia), and mechanical ventilation requirement [14,[30], [31], [32]]. Moreover, results from recent studies shed some light on the importance of IL-6 serum levels as a diagnostic and prognostic biomarker in patients infected with SARS-CoV-2 [30,143]. Taken together, it seems that antiviral drugs and anti-inflammatory agent's co-administration might be more efficient to reducing SARS-CoV-2 patient's clinical manifestations and inflammatory responses-induced organ damage. Further clinical trials should be performed to evaluate the efficiency and safety of anti-inflammatory agents targeting IL-6 and NLRP-inflammasome and also, identify COVID-19 patients that may benefit from anti-inflammatory therapy.
Acknowledgments
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Authors declare no conflict of interest.
References
- 1.2020 [Available from: https://www.worldometers.info/coronavirus/.
- 2.Siu Y., Teoh K., Lo J., Chan C., Kien F., Escriou N., et al. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008;82(22):11318–11330. doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pieruzzi F., Abassi Z.A., Keiser H.R. Expression of renin-angiotensin system components in the heart, kidneys, and lungs of rats with experimental heart failure. Circulation. 1995;92(10):3105–3112. doi: 10.1161/01.cir.92.10.3105. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y., Shan K., Qian W. 2020. Asians and Other Races Express Similar Levels of and Share the Same Genetic Polymorphisms of the SARS-CoV-2 Cell-entry Receptor. [Google Scholar]
- 5.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., et al. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim Y.X., Ng Y.L., Tam J.P., Liu D.X. Human coronaviruses: a review of virus–host interactions. Diseases. 2016;4(3):26. doi: 10.3390/diseases4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tufan A., GÜLER A.A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turkish Journal of Medical Sciences. 2020;50(SI-1):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao X., Li T., He Z., Ping Y., Liu H., Yu S., et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua bing li xue za zhi= Chinese journal of pathology. 2020;49:E009–E. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J., Chu M. Targeting of IL-6-relevant long noncoding RNA profiles in inflammatory and tumorous disease. Inflammation. 2019:1–8. doi: 10.1007/s10753-019-00995-2. [DOI] [PubMed] [Google Scholar]
- 11.She Q., Shi P., Xu S.-S., Xuan H.-Y., Tao H., Shi K.-H., et al. DNMT1 methylation of LncRNA GAS5 leads to cardiac fibroblast pyroptosis via affecting NLRP3 axis. Inflammation. 2020:1–12. doi: 10.1007/s10753-020-01191-3. [DOI] [PubMed] [Google Scholar]
- 12.Zahid A., Li B., Kombe J.K., Jin T., Tao J. Pharmacological inhibitors of the NLRP3 inflammasome. Front. Immunol. 2019;10:2538. doi: 10.3389/fimmu.2019.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell B., Moss C., George G., Santaolalla A., Cope A., Papa S., et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111:1–8. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura K., Ishikawa G., Yoshie M., Ohneda W., Nakai A., Takeshita T., et al. Glibenclamide inhibits NLRP3 inflammasome-mediated IL-1β secretion in human trophoblasts. J. Pharmacol. Sci. 2017;135(2):89–95. doi: 10.1016/j.jphs.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 16.Riddle M.C. Oxford University Press; 2003. Sulfonylureas Differ in Effects on Ischemic Preconditioning—Is It Time to Retire Glyburide? [DOI] [PubMed] [Google Scholar]
- 17.Ashcroft F.M. ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Invest. 2005;115(8):2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coll R.C., Robertson A.A., Chae J.J., Higgins S.C., Muñoz-Planillo R., Inserra M.C., et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015;21(3):248. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dandekar A.A., Perlman S. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005;5(12):917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34(2):1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 21.Nieto-Torres J.L., Verdiá-Báguena C., Jimenez-Guardeño J.M., Regla-Nava J.A., Castaño-Rodriguez C., Fernandez-Delgado R., et al. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Channappanavar R., Perlman S., editors. Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology. Seminars in Immunopathology. Springer; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockx B., Baas T., Zornetzer G.A., Haagmans B., Sheahan T., Frieman M., et al. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J. Virol. 2009;83(14):7062–7074. doi: 10.1128/JVI.00127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau S.K., Lau C.C., Chan K.-H., Li C.P., Chen H., Jin D.-Y., et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J. Gen. Virol. 2013;94(12):2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 26.Zumla A., Hui D.S., Azhar E.I., Memish Z.A., Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395(10224):e35–e36. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020;55:1–6. doi: 10.1016/j.ijantimicag.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019;20(23) doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gubernatorova E., Gorshkova E., Polinova A., Drutskaya M. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020;53:13–24. doi: 10.1016/j.cytogfr.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y., Li T., Han M., Li X., Wu D., Xu Y., et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020;92(7):791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv. 2020:1–6. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herold T., Jurinovic V., Arnreich C., Hellmuth J.C., von Bergwelt-Baildon M., Klein M., et al. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.04.01.20047381. [DOI] [Google Scholar]
- 33.Dosch S.F., Mahajan S.D., Collins A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142(1–2):19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W., Ye L., Ye L., Li B., Gao B., Zeng Y., et al. Up-regulation of IL-6 and TNF-α induced by SARS-coronavirus spike protein in murine macrophages via NF-κB pathway. Virus Res. 2007;128(1–2):1–8. doi: 10.1016/j.virusres.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015;16(5):448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 36.Jones S.A., Jenkins B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018;18(12):773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 37.Zegeye M.M., Lindkvist M., Fälker K., Kumawat A.K., Paramel G., Grenegård M., et al. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Communication and Signaling. 2018;16(1):1–10. doi: 10.1186/s12964-018-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson D.E., O’Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15(4):234. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villarino A.V., Kanno Y., O’Shea J.J. Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat. Immunol. 2017;18(4):374. doi: 10.1038/ni.3691. [DOI] [PubMed] [Google Scholar]
- 40.Taniguchi K., Wu L.-W., Grivennikov S.I., De Jong P.R., Lian I., Yu F.-X., et al. A gp130–Src–YAP module links inflammation to epithelial regeneration. Nature. 2015;519(7541):57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada O., Ozaki K., Akiyama M., Kawauchi K. JAK–STAT and JAK–PI3K–mTORC1 pathways regulate telomerase transcriptionally and posttranslationally in ATL cells. Mol. Cancer Ther. 2012;11(5):1112–1121. doi: 10.1158/1535-7163.MCT-11-0850. [DOI] [PubMed] [Google Scholar]
- 42.Thiem S., Pierce T.P., Palmieri M., Putoczki T.L., Buchert M., Preaudet A., et al. mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. J. Clin. Invest. 2013;123(2) doi: 10.1172/JCI65086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasukawa K., Hirano T., Watanabe Y., Muratani K., Matsuda T., Nakai S., et al. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J. 1987;6(10):2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones B.E., Maerz M.D., Buckner J.H. IL-6: a cytokine at the crossroads of autoimmunity. Curr. Opin. Immunol. 2018;55:9–14. doi: 10.1016/j.coi.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt-Arras D., Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J. Hepatol. 2016;64(6):1403–1415. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Campard D., Vasse M., Rose-John S., Poyer F., Lamacz M., Vannier J.P. Multilevel regulation of IL-6R by IL-6–sIL-6R fusion protein according to the primitiveness of peripheral blood-derived CD133+ cells. Stem Cells. 2006;24(5):1302–1314. doi: 10.1634/stemcells.2005-0173. [DOI] [PubMed] [Google Scholar]
- 47.Kimura A., Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur. J. Immunol. 2010;40(7):1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 48.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., et al. Reciprocal developmental pathways for the generation of pathogenic effector TH 17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 49.Fraunberger P., Wang Y., Holler E., Parhofer K.G., Nagel D., Walli A.K., et al. Prognostic value of interleukin 6, procalcitonin, and C-reactive protein levels in intensive care unit patients during first increase of fever. Shock. 2006;26(1):10–12. doi: 10.1097/01.shk.0000215319.06866.bd. [DOI] [PubMed] [Google Scholar]
- 50.Maloney J.P., Gao L. Proinflammatory cytokines increase vascular endothelial growth factor expression in alveolar epithelial cells. Mediat. Inflamm. 2015;2015 doi: 10.1155/2015/387842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen T., Nahari D., Cerem L.W., Neufeld G., Levi B.Z. Interleukin 6 induces the expression of vascular endothelial growth factor. J. Biol. Chem. 1996;271(2):736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 52.Shi C.-S., Nabar N.R., Huang N.-N., Kehrl J.H. SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Dis. 2019;5(1):1–12. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019;20(13):3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jo E.-K., Kim J.K., Shin D.-M., Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016;13(2):148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rathinam V.A., Vanaja S.K., Fitzgerald K.A. Regulation of inflammasome signaling. Nat. Immunol. 2012;13(4):333. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 57.Pétrilli V., Dostert C., Muruve D.A., Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 2007;19(6):615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Gurung P., Kanneganti T.-D. Novel roles for caspase-8 in IL-1β and inflammasome regulation. Am. J. Pathol. 2015;185(1):17–25. doi: 10.1016/j.ajpath.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Connor W., Harton J.A., Zhu X., Linhoff M.W., Ting J.P.-Y. Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1.1 is an inducible inflammatory mediator with NF-κB suppressive properties. J. Immunol. 2003;171(12):6329–6333. doi: 10.4049/jimmunol.171.12.6329. [DOI] [PubMed] [Google Scholar]
- 60.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 61.He Y., Hara H., Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016;41(12):1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siu K.-L., Yuen K.-S., Castaño-Rodriguez C., Ye Z.-W., Yeung M.-L., Fung S.-Y., et al. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019;33(8):8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen I.-Y., Moriyama M., Chang M.-F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nieto-Torres J.L., DeDiego M.L., Verdia-Baguena C., Jimenez-Guardeno J.M., Regla-Nava J.A., Fernandez-Delgado R., et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10(5) doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X., Wu Z., Fu X., Han W. lncRNAs: insights into their function and mechanics in underlying disorders. Mutation Research/Reviews in Mutation Research. 2014;762:1–21. doi: 10.1016/j.mrrev.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Niland C.N., Merry C.R., Khalil A.M. Emerging roles for long non-coding RNAs in cancer and neurological disorders. Front. Genet. 2012;3:25. doi: 10.3389/fgene.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grammatikakis I., Panda A.C., Abdelmohsen K., Gorospe M. Long noncoding RNAs (lncRNAs) and the molecular hallmarks of aging. Aging (Albany NY) 2014;6(12):992. doi: 10.18632/aging.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pearson M.J., Jones S.W. Long noncoding RNAs in the regulation of inflammatory pathways in rheumatoid arthritis and osteoarthritis. Arthritis & rheumatology (Hoboken, NJ) 2016;68(11):2575. doi: 10.1002/art.39759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mirza A.H., Berthelsen C.H., Seemann S.E., Pan X., Frederiksen K.S., Vilien M., et al. Transcriptomic landscape of lncRNAs in inflammatory bowel disease. Genome medicine. 2015;7(1):39. doi: 10.1186/s13073-015-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mathy N.W., Chen X.-M. Long non-coding RNAs (lncRNAs) and their transcriptional control of inflammatory responses. J. Biol. Chem. 2017;292(30):12375–12382. doi: 10.1074/jbc.R116.760884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yi H., Peng R., Zhang L.-y., Sun Y., Peng H.-m., Liu H.-d., et al. LincRNA-Gm4419 knockdown ameliorates NF-κ B/NLRP3 inflammasome-mediated inflammation in diabetic nephropathy. Cell Death Dis. 2017;8(2):e2583–e. doi: 10.1038/cddis.2016.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu S.-y., Dong B., Tang L., Zhou S.-h. LncRNA MALAT1 sponges miR-133 to promote NLRP3 inflammasome expression in ischemia-reperfusion injured heart. Int. J. Cardiol. 2018;254:50. doi: 10.1016/j.ijcard.2017.10.071. [DOI] [PubMed] [Google Scholar]
- 73.Wu J., Zhang J., Shen B., Yin K., Xu J., Gao W., et al. Long noncoding RNA lncTCF7, induced by IL-6/STAT3 transactivation, promotes hepatocellular carcinoma aggressiveness through epithelial-mesenchymal transition. J. Exp. Clin. Cancer Res. 2015;34(1):116. doi: 10.1186/s13046-015-0229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen W., Yuan Y., Zhao M., Li J., Xu J., Lou G., et al. Novel long non-coding RNA GACAT3 promotes gastric cancer cell proliferation through the IL-6/STAT3 signaling pathway. Tumor Biol. 2016;37(11):14895–14902. doi: 10.1007/s13277-016-5372-8. [DOI] [PubMed] [Google Scholar]
- 75.Li S., Mei Z., Hu H.B., Zhang X. The lncRNA MALAT1 contributes to non-small cell lung cancer development via modulating miR-124/STAT3 axis. J. Cell. Physiol. 2018;233(9):6679–6688. doi: 10.1002/jcp.26325. [DOI] [PubMed] [Google Scholar]
- 76.Wang X., Sun W., Shen W., Xia M., Chen C., Xiang D., et al. Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J. Hepatol. 2016;64(6):1283–1294. doi: 10.1016/j.jhep.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 77.Fan H., Li J., Wang J., Hu Z. Long non-coding RNAs (lncRNAs) tumor-suppressive role of lncRNA on chromosome 8p12 (TSLNC8) inhibits tumor metastasis and promotes apoptosis by regulating interleukin 6 (IL-6)/signal transducer and activator of transcription 3 (STAT3)/hypoxia-inducible factor 1-alpha (HIF-1α) signaling pathway in non-small cell lung cancer. Med. Sci. Monit. 2019;25:7624. doi: 10.12659/MSM.917565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J., Zhou J., Jiang C., Zheng J., Namba H., Chi P., et al. LNRRIL 6, a novel long noncoding RNA, protects colorectal cancer cells by activating the IL-6–STAT 3 pathway. Mol. Oncol. 2019;13(11):2344–2360. doi: 10.1002/1878-0261.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tian H., Wu M., Zhou P., Huang C., Ye C., Wang L. The long non-coding RNA MALAT1 is increased in renal ischemia-reperfusion injury and inhibits hypoxia-induced inflammation. Ren. Fail. 2018;40(1):527–533. doi: 10.1080/0886022X.2018.1487863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dai L., Zhang G., Cheng Z., Wang X., Jia L., Jing X., et al. Knockdown of LncRNA MALAT1 contributes to the suppression of inflammatory responses by up-regulating miR-146a in LPS-induced acute lung injury. Connect. Tissue Res. 2018;59(6):581–592. doi: 10.1080/03008207.2018.1439480. [DOI] [PubMed] [Google Scholar]
- 81.Shang A., Wang W., Gu C., Chen C., Zeng B., Yang Y., et al. Long non-coding RNA HOTTIP enhances IL-6 expression to potentiate immune escape of ovarian cancer cells by upregulating the expression of PD-L1 in neutrophils. J. Exp. Clin. Cancer Res. 2019;38(1):411. doi: 10.1186/s13046-019-1394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Obaid M., Udden S.N., Deb P., Shihabeddin N., Zaki M.H., Mandal S.S. LncRNA HOTAIR regulates lipopolysaccharide-induced cytokine expression and inflammatory response in macrophages. Sci. Rep. 2018;8(1):1–18. doi: 10.1038/s41598-018-33722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xia L.X., Ke C., Lu J.M. NEAT1 contributes to neuropathic pain development through targeting miR-381/HMGB1 axis in CCI rat models. J. Cell. Physiol. 2018;233(9):7103–7111. doi: 10.1002/jcp.26526. [DOI] [PubMed] [Google Scholar]
- 84.Qi K., Lin R., Xue C., Liu T., Wang Y., Zhang Y., et al. Long non-coding RNA (LncRNA) CAIF is downregulated in osteoarthritis and inhibits LPS-induced interleukin 6 (IL-6) upregulation by downregulation of MiR-1246. Med. Sci. Monit. 2019;25:8019. doi: 10.12659/MSM.917135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Su K., Zhao Q., Bian A., Wang C., Cai Y., Zhang Y. A novel positive feedback regulation between long noncoding RNA UICC and IL-6/STAT3 signaling promotes cervical cancer progression. Am. J. Cancer Res. 2018;8(7):1176. [PMC free article] [PubMed] [Google Scholar]
- 86.Li H., Shi H., Ma N., Zi P., Liu Q., Sun R. BML-111 alleviates acute lung injury through regulating the expression of lncRNA MALAT1. Arch. Biochem. Biophys. 2018;649:15–21. doi: 10.1016/j.abb.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 87.Wang Z., Chi X., Liu L., Wang Y., Mei X., Yang Y., et al. Long noncoding RNA maternally expressed gene 3 knockdown alleviates lipopolysaccharide-induced inflammatory injury by up-regulation of miR-203 in ATDC5 cells. Biomed. Pharmacother. 2018;100:240–249. doi: 10.1016/j.biopha.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 88.Huang W., Lan X., Li X., Wang D., Sun Y., Wang Q., et al. Long non-coding RNA PVT1 promote LPS-induced septic acute kidney injury by regulating TNFα and JNK/NF-κB pathways in HK-2 cells. Int. Immunopharmacol. 2017;47:134–140. doi: 10.1016/j.intimp.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 89.Davis B.K., Wen H., Ting J.P.-Y. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xue Z., Zhang Z., Liu H., Li W., Guo X., Zhang Z., et al. lincRNA-Cox2 regulates NLRP3 inflammasome and autophagy mediated neuroinflammation. Cell Death Differ. 2019;26(1):130–145. doi: 10.1038/s41418-018-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dong P., Xiong Y., Yue J., Hanley S.J., Kobayashi N., Todo Y., et al. Long non-coding RNA NEAT1: a novel target for diagnosis and therapy in human tumors. Front. Genet. 2018;9:471. doi: 10.3389/fgene.2018.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang P., Cao L., Zhou R., Yang X., Wu M. The lncRNA Neat1 promotes activation of inflammasomes in macrophages. Nat. Commun. 2019;10(1):1–17. doi: 10.1038/s41467-019-09482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu J., Wu H., Wang D., Yang Z., Dong J. LncRNA ANRIL promotes NLRP3 inflammasome activation in uric acid nephropathy through miR-122-5p/BRCC3 axis. Biochimie. 2019;157:102–110. doi: 10.1016/j.biochi.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 94.Ji J., Dai X., Yeung S.J., He X. The role of long non-coding RNA GAS5 in cancers. Cancer Manag. Res. 2019;11:2729–2737. doi: 10.2147/CMAR.S189052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J., Yang C., Li Y., Chen A., Li L., You Z. LncRNA GAS5 suppresses ovarian cancer by inducing inflammasome formation. Biosci. Rep. 2018;38(2) doi: 10.1042/BSR20171150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu H., Wang Y., Ding X., He Y., Lu Z., Wu P., et al. Long non-coding RNA XLOC_000647 suppresses progression of pancreatic cancer and decreases epithelial-mesenchymal transition-induced cell invasion by down-regulating NLRP3. Mol. Cancer. 2018;17(1):18. doi: 10.1186/s12943-018-0761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu Z., Xi K. LncRNA RGMB-AS1 promotes laryngeal squamous cell carcinoma cells progression via sponging miR-22/NLRP3 axis. Biomed. Pharmacother. 2019;118:109222. doi: 10.1016/j.biopha.2019.109222. [DOI] [PubMed] [Google Scholar]
- 98.Ma M., Pei Y., Wang X., Feng J., Zhang Y., Gao M.Q. LncRNA XIST mediates bovine mammary epithelial cell inflammatory response via NF-κB/NLRP3 inflammasome pathway. Cell Prolif. 2019;52(1) doi: 10.1111/cpr.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Felsenstein S., Herbert J.A., McNamara P.S., Hedrich C.M. COVID-19: immunology and treatment options. Clinical immunology (Orlando, Fla) 2020;215 doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020:1–6. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin. Immunol. 2020;214:1–5. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Juliana C., Fernandes-Alnemri T., Wu J., Datta P., Solorzano L., Yu J.-W., et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 2010;285(13):9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nishimoto N., Yoshizaki K., Maeda K., Kuritani T., Deguchi H., Sato B., et al. Toxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody MRA in rheumatoid arthritis. Phase I/II clinical study. J. Rheumatol. 2003;30(7):1426–1435. [PubMed] [Google Scholar]
- 104.Nishida S., Hagihara K., Shima Y., Kawai M., Kuwahara Y., Arimitsu J., et al. Rapid improvement of AA amyloidosis with humanised anti-interleukin 6 receptor antibody treatment. Ann. Rheum. Dis. 2009;68(7):1235–1236. doi: 10.1136/ard.2008.099267. [DOI] [PubMed] [Google Scholar]
- 105.Nishimoto N., Terao K., Mima T., Nakahara H., Takagi N., Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti–IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112(10):3959–3964. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- 106.Peruzzi B., Cappariello A., Del Fattore A., Rucci N., De Benedetti F., Teti A. c-Src and IL-6 inhibit osteoblast differentiation and integrate IGFBP5 signalling. Nat. Commun. 2012;3(1):1–10. doi: 10.1038/ncomms1651. [DOI] [PubMed] [Google Scholar]
- 107.Ogata A., Hirano T., Hishitani Y., Tanaka T. Safety and efficacy of tocilizumab for the treatment of rheumatoid arthritis. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders. 2012;5 doi: 10.4137/CMAMD.S7371. (CMAMD. S7371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ogata A., Morita T., Yoshida Y., Tanaka T. Subcutaneous formulation of tocilizumab for treatment of rheumatoid arthritis. Ther. Deliv. 2015;6(3):283–295. doi: 10.4155/tde.14.118. [DOI] [PubMed] [Google Scholar]
- 109.Gong J., Guo S., Li H.-B., Yuan S.-Y., Shang Y., Yao S.-L. BML-111, a lipoxin receptor agonist, protects haemorrhagic shock-induced acute lung injury in rats. Resuscitation. 2012;83(7):907–912. doi: 10.1016/j.resuscitation.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 110.Li H.-B., Wang G.-Z., Gong J., Wu Z.-Y., Guo S., Li B., et al. BML-111 attenuates hemorrhagic shock-induced acute lung injury through inhibiting activation of mitogen-activated protein kinase pathway in rats. J. Surg. Res. 2013;183(2):710–719. doi: 10.1016/j.jss.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 111.Shah A. Novel coronavirus-induced NLRP3 inflammasome activation: a potential drug target in the treatment of COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dorfleutner A., Chu L., Stehlik C. Inhibiting the inflammasome: one domain at a time. Immunol. Rev. 2015;265(1):205–216. doi: 10.1111/imr.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mangan M.S., Olhava E.J., Roush W.R., Seidel H.M., Glick G.D., Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018;17(8):588. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 114.Lamkanfi M., Mueller J.L., Vitari A.C., Misaghi S., Fedorova A., Deshayes K., et al. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J. Cell Biol. 2009;187(1):61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coll R.C., Hill J.R., Day C.J., Zamoshnikova A., Boucher D., Massey N.L., et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 2019;15(6):556–559. doi: 10.1038/s41589-019-0277-7. [DOI] [PubMed] [Google Scholar]
- 116.Toldo S., Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 2018;15(4):203. doi: 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- 117.Marchetti C., Swartzwelter B., Gamboni F., Neff C.P., Richter K., Azam T., et al. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. 2018;115(7):E1530–E1539. doi: 10.1073/pnas.1716095115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huizinga T.W., Fleischmann R.M., Jasson M., Radin A.R., van Adelsberg J., Fiore S., et al. Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann. Rheum. Dis. 2014;73(9):1626–1634. doi: 10.1136/annrheumdis-2013-204405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rossi J., Negrier S., James N., Kocak I., Hawkins R., Davis H., et al. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br. J. Cancer. 2010;103(8):1154–1162. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fu Q., Li J., Qiu L., Ruan J., Mao M., Li S., et al. Inhibiting NLRP3 inflammasome with MCC950 ameliorates perioperative neurocognitive disorders, suppressing neuroinflammation in the hippocampus in aged mice. Int. Immunopharmacol. 2020;82 doi: 10.1016/j.intimp.2020.106317. [DOI] [PubMed] [Google Scholar]
- 121.Liu W., Guo W., Wu J., Luo Q., Tao F., Gu Y., et al. A novel benzo [d] imidazole derivate prevents the development of dextran sulfate sodium-induced murine experimental colitis via inhibition of NLRP3 inflammasome. Biochem. Pharmacol. 2013;85(10):1504–1512. doi: 10.1016/j.bcp.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 122.Shim D.-W., Shin W.-Y., Yu S.-H., Kim B.-H., Ye S.-K., Koppula S., et al. BOT-4-one attenuates NLRP3 inflammasome activation: NLRP3 alkylation leading to the regulation of its ATPase activity and ubiquitination. Sci. Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-15314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dong L., Qiao H., Zhang X., Zhang X., Wang C., Wang L., et al. Parthenolide is neuroprotective in rat experimental stroke model: downregulating NF-B, phospho-p38MAPK, and caspase-1 and ameliorating BBB permeability. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/370804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cocco M., Pellegrini C., Martínez-Banaclocha H., Giorgis M., Marini E., Costale A., et al. Development of an acrylate derivative targeting the NLRP3 inflammasome for the treatment of inflammatory bowel disease. J. Med. Chem. 2017;60(9):3656–3671. doi: 10.1021/acs.jmedchem.6b01624. [DOI] [PubMed] [Google Scholar]
- 125.Guo C., Fulp J.W., Jiang Y., Li X., Chojnacki J.E., Wu J., et al. Development and characterization of a hydroxyl-sulfonamide analogue, 5-chloro-N-[2-(4-hydroxysulfamoyl-phenyl)-ethyl]-2-methoxy-benzamide, as a novel NLRP3 inflammasome inhibitor for potential treatment of multiple sclerosis. ACS Chem. Neurosci. 2017;8(10):2194–2201. doi: 10.1021/acschemneuro.7b00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marchetti C., Chojnacki J., Toldo S., Mezzaroma E., Tranchida N., Rose S.W., et al. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury following ischemia-reperfusion in the mouse. J. Cardiovasc. Pharmacol. 2014;63(4):316. doi: 10.1097/FJC.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leung Y.Y., Yao Hui L.L., Kraus V.B. Colchicine—update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015;45(3):341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Keystone E.C., Wang M.M., Layton M., Hollis S., McInnes I.B., Team D.S. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann. Rheum. Dis. 2012;71(10):1630–1635. doi: 10.1136/annrheumdis-2011-143578. [DOI] [PubMed] [Google Scholar]
- 129.McKie E.A., Reid J.L., Mistry P.C., DeWall S.L., Abberley L., Ambery P.D., et al. A study to investigate the efficacy and safety of an anti-interleukin-18 monoclonal antibody in the treatment of type 2 diabetes mellitus. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Crossman D., Rothman A.M. Interleukin-1 beta inhibition with canakinumab and reducing lung cancer—subset analysis of the canakinumab anti-inflammatory thrombosis outcome study trial (CANTOS) Journal of thoracic disease. 2018;10(Suppl. 26):S3084. doi: 10.21037/jtd.2018.07.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Word Health organization 2020. https://www.who.int/ Available from.
- 132.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J. Inf. Secur. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sanman L.E., Qian Y., Eisele N.A., Ng T.M., van der Linden W.A., Monack D.M., et al. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death. eLife. 2016;5 doi: 10.7554/eLife.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ning B., Yu D., Yu A.-M. Advances and challenges in studying noncoding RNA regulation of drug metabolism and development of RNA therapeutics. Biochem. Pharmacol. 2019;169:1–8. doi: 10.1016/j.bcp.2019.113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou Z., Sun B., Huang S., Zhao L. Roles of circular RNAs in immune regulation and autoimmune diseases. Cell Death Dis. 2019;10(7):1–13. doi: 10.1038/s41419-019-1744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen G., Shi Y., Zhang Y., Sun J. CircRNA_100782 regulates pancreatic carcinoma proliferation through the IL6-STAT3 pathway. OncoTargets and therapy. 2017;10:5783. doi: 10.2147/OTT.S150678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhu X., Ding J., Wang B., Wang J., Xu M. Circular RNA DLGAP4 is down-regulated and negatively correlates with severity, inflammatory cytokine expression and pro-inflammatory gene miR-143 expression in acute ischemic stroke patients. Int. J. Clin. Exp. Pathol. 2019;12(3):941. [PMC free article] [PubMed] [Google Scholar]
- 138.Santini P., Politi L., Dalla Vedova P., Scandurra R., d’Abusco A.S. The inflammatory circuitry of miR-149 as a pathological mechanism in osteoarthritis. Rheumatol. Int. 2014;34(5):711–716. doi: 10.1007/s00296-013-2754-8. [DOI] [PubMed] [Google Scholar]
- 139.Liang Z., Ren C. Emodin attenuates apoptosis and inflammation induced by LPS through up-regulating lncRNA TUG1 in murine chondrogenic ATDC5 cells. Biomed. Pharmacother. 2018;103:897–902. doi: 10.1016/j.biopha.2018.04.085. [DOI] [PubMed] [Google Scholar]
- 140.Li Y., Xie Z., Lin W., Cai W., Wen C., Guan Y., et al. An exploratory randomized, controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI) MedRxiv. 2020 doi: 10.1101/2020.03.19.20038984. [DOI] [Google Scholar]
- 141.Abd El-Aziz T.M., Stockand J.D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2)-an update on the status. Infect. Genet. Evol. 2020 doi: 10.1016/j.meegid.2020.104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.5/2020 [Available from: https://www.pharmiweb.com/press-release/2020-03-11/tiziana-life-sciences-plc-to-expedite-development-of-its-fully-human-anti-interleukin-6-receptor-monoclonal-antibody-a-potential-treatment-of-certain.
- 143.Ulhaq Z.S., Soraya G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med. Mal. Infect. 2020;83 doi: 10.1016/j.meegid.2020.104327. [DOI] [PMC free article] [PubMed] [Google Scholar]