Abstract
Context
The use of anogenital distance (AGD) in clinical and epidemiological settings is increasing; however, sex-specific reference data on AGD and data on longitudinal changes in AGD in children is scarce.
Objective
To create age-, sex-, and method-related reference ranges of AGD in healthy boys and girls aged 0–24 months, to assess the age-related changes in AGD and to evaluate the 2 predominantly used methods of AGD measurement.
Design
The International AGD consortium comprising 4 centers compiled data from 1 cross-sectional and 3 longitudinal cohort studies (clinicaltrials.gov [NCT02497209]).
Setting
All data were collected from population-based studies, recruiting from 4 maternity or obstetric centers (United States, Cambridge [United Kingdom], Odense, and Copenhagen [Denmark]).
Subjects
This study included a total of 3705 healthy, mainly Caucasian children aged 0–24 months on whom 7295 measurements were recorded.
Main Outcome Measures
AGDAS (ano-scrotal), AGDAF (ano-fourchette), AGDAP (ano-penile), AGDAC (ano-clitoral), AGD body size indices (weight, body mass index [BMI], body surface area, and length), and intra- and interobserver biases.
Results
We created age-specific reference ranges by centers. We found that AGD increased from birth to 6 months of age and thereafter reached a plateau. Changes in AGD/BMI during the first year of life were minor (0–6% and 0–11% in boys and girls, respectively).
Conclusions
Reference ranges for AGD can be used in future epidemiological research and may be utilized clinically to evaluate prenatal androgen action in differences-in-sex-development patients. The increase in AGD during the first year of life was age-related, while AGD/BMI was fairly stable. The TIDES and Cambridge methods were equally reproducible.
Keywords: anogenital distance, reference ranges, endocrine disrupting chemicals, disorders of sexual development
Anogenital distance (AGD) is defined as the distance from the anus to the genitals and is considered a sensitive postnatal marker of in utero exposure to androgens in rodents as well as in humans (1, 2).
A wide spectrum of disorders at birth and later in life are linked to underandrogenization, including hypospadias, cryptorchidism, and reduced semen quality. These conditions have been united under the umbrella term “testicular dysgenesis syndrome” (3, 4), as they are believed to stem from testicular hypofunction during fetal life. Accumulating evidence from several studies links shorter-than-expected AGD in males to these phenotypes, thus providing further evidence of an etiologic role for insufficient androgen action in utero (2, 5–10). More severe phenotypes due to under-androgenization fall under the umbrella term “differences of sex development” (DSD), and although literature on AGD and DSD is scarce, shorter AGD would also be expected in underandrogenized males and, conversely, longer AGD would be expected in overandrogenized girls (11).
In the majority of mammals, male AGD is 50% to 100% longer than in females (12, 13). While the phenomenon is much less studied than male AGD is, a longer female AGD is likely to result from higher androgen levels in fetal life (14). Correspondingly, a longer AGD in adult females is associated with serum testosterone levels and ovarian follicle numbers as seen in women with PCOS (15, 16). Children born from mothers with PCOS also exhibit longer AGD, possibly because of the higher androgen levels in pregnancies complicated by PCOS (17, 18).
In animal, as well as human studies, AGD is associated with prenatal exposure to certain medications and environmental agents—for example, phthalates, mild analgesics, and antifungal agents (19–24). Anogenital distance is therefore identified as a valuable measure in the US Environmental Protection Agency guidelines for reproductive toxicity studies (25).
While the application of AGD in clinical and epidemiological settings is increasing, sex-specific reference data on AGD is scarce. The International AGD Consortium (IAC) was formed to address this need. Here, we present age- and sex-specific reference ranges in (primarily Caucasian) healthy boys and girls aged 0–2 years.
Methods
Populations
The IAC includes 4 research centers and was formed in order to create reference ranges and to identify the causes of variation in AGD measurements.
Center 1: The Infant Development and Environmental Study (TIDES) (26). Our analysis included a total of 757 children (male=371, female=386). Short and long AGD (described below) were measured at birth using the TIDES method (Fig. 1). Other aspects of the TIDES population and methods have previously been published, including data on AGD (27).
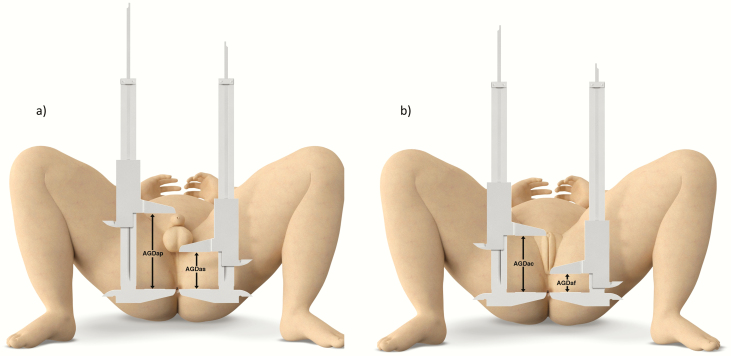
Figure 1.
Illustration of AGD measurements in boys (a) and girls (b), using the Cambridge method. Abbreviations: AGDAP, anogenital distance (ano-penile); AGDAS, anogenital distance (ano-scrotal); AGDAC, anogenital distance (ano-clitoral); AGDAF, anogenital distance (ano-fourchettal).
Center 2: The Cambridge Baby Growth Study (28). A total of 872 children were included (male=453, female=419). Short AGD was measured at birth and at 3, 12, 18, and 24 months of age using the Cambridge method (see below), amounting to a total of 3030 AGD measurements (male=1595, female=1435). Data on AGD from birth to 2 years of age in this cohort have been published previously (12).
Center 3: The Odense Child Cohort (29). In total, 1839 children were included in this study (male=993, female=846). Short and long AGD were measured at 3 and 18 months of age using the TIDES method, providing a total of 2560 AGD measurements (male=1428, female=1132). Anogenital distance data from this cohort have previously been published (13).
Center 4: The COPENHAGEN Minipuberty Study. A total of 236 children, recruited between 2016–2018 (male=123, female=113) were included in this study. Short and long AGD (TIDES method) and short AGD (Cambridge method) were measured 6 times during the first year of life: at birth and at 12 months of age, and either at 1, 3, 5, and 7 months or at 2, 4, 6, and 8 months of age, amounting to a total of 948 AGD measurements (male=501, female=447).
AGD measurements
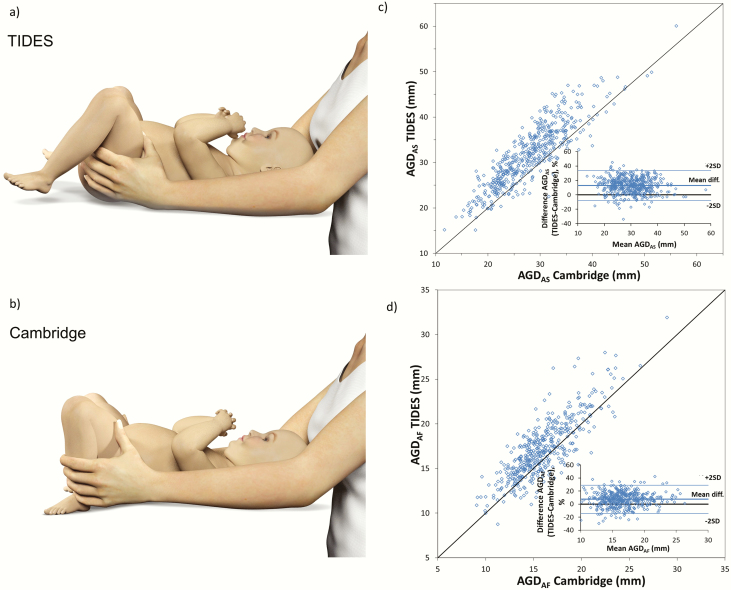
Short AGD refers to the distance from the anus to the perineo–scrotal junction (AGDAS) in boys and from the anus to the fourchette (AGDAF) in girls. Similarly, long AGD is the distance from the anus to the anterior insertion of the penis (AGDAP) in boys and from the anus to the clitoris (AGDAC) in girls (Fig. 1). While there was agreement on these definitions across centers, 2 different methods of positioning were used. The TIDES method places the infant in a supine position with the lower half of the body exposed and the legs lifted in a frog-like posture (with a 60–90° angle from the torso at the hip) and knees pulled back towards to shoulders (Fig. 2A) (26). The Cambridge method places the infant in a supine position with both hips flexed, feet placed on the surface, and light pressure exerted onto the thighs (Fig. 2B) (30).
Figure 2.
Illustration of child position in TIDES (a) and Cambridge methods (b). TIDES method versus Cambridge method, including Bland-Altman plot for short AGDAS (boys) (c) and short AGDAF (girls) (d). Center 4 only. Dots indicate individual values.
We created an AGD body size index by dividing the average AGD from each examination with body size measurements, that is, length (m), weight (kg), body mass index (BMI, kg/m2), and body surface area (BSA, m2). These indices were created using the longitudinal data from Center 4 only. Body mass index was calculated as BMI = kg/m2. Body surface area was calculated using the Du Bois’ formula: BSA = 0.007184 x (kg0.425) x (cm0.725).
Large- and small-for-gestational age (LGA and SGA, respectively) infants were defined as birth weight ≥ 2 standard deviations (SD) or ≤ 2 SD beyond the mean, adjusted for gestational age (GA) and sex (31).
Statistics
Reference ranges for short AGDAS (boys) and short AGDAF (girls), long AGDAP (boys) and long AGDAC (girls), as well as AGD body size indexes, were estimated using generalized additive models for location, scale, and shape. The LMS parameters were the age-dependent median (M), the approximate coefficient of variation (S), and the power in the Box-Cox transformation (L). All LMS-values are provided for the reader in a digital research material repository (32).
Longitudinal data from Center 4 was analyzed using Wilcoxon Signed Rank tests comparing (1) AGD between 3 time points: birth (< 1 month of age), 6 months (4.5–6.5 months of age), and 1 year (11–13 months of age); and (2) AGD body size indices at the first and last visits (weight, BMI, BSA, and length).
Intra- and interexaminer variation
Intraexaminer variation was evaluated using intraclass correlation coefficients (ICC), which were estimated using a 2-way random-effect model with absolute agreements between single measurements, based on data from Center 4 (948 measurements in 236 children).
Interexaminer ICCs were evaluated using 41 examinations from Center 4, performed by 6 different examiners on randomly selected children, who were repositioned between examiners. A 2-way random-effect model allowed for the quantification of the variation between measurements, that is, the contribution of (1) the variation between children, (2) the variation between examiners, and (3) the background variation that was not associated with examiners (including variation due to repositioning and caliper inaccuracy) between clinical measurements. Interexaminer ICC of 108 children in the TIDES study (Center 1) has previously been published (27).
All statistical analyses were performed using the R software/environment and IBM SPSS Statistics 25.0 (SPSS, Chicago, IL). P-Values < 0.05 were considered statistically significant.
Ethical considerations
The IAC is located at The Department of Growth and Reproduction in Rigshospitalet, Copenhagen, Denmark, and is registered at clinicaltrials.gov (NCT02497209). All necessary approvals from The Danish Data Protection Agency (i-suite 04241, jr. RH-2015–257, and transferal agreement 2012-58-0018 from the Region of Southern Denmark) have been obtained.
The required local approvals are outlined as follows: Center 1 (HS# 11-01240), Center 2 (LREC Ref. 00/325), Center 3 (the regional ethics committee [S-20090130] and the Danish Data Protection Agency [j.no. 2008-58-0035]), and Center 4 (regional ethics committees [H-15014876] and the Danish Data Protection Agency [RH-2015–210, i-suite nr. 04146]).
Results
Infant demographic characteristics, stratified by center, are available in a digital research material repository (33). In total, we included 7295 AGD measurements on 3705 mainly Caucasian children aged 0–24 months.
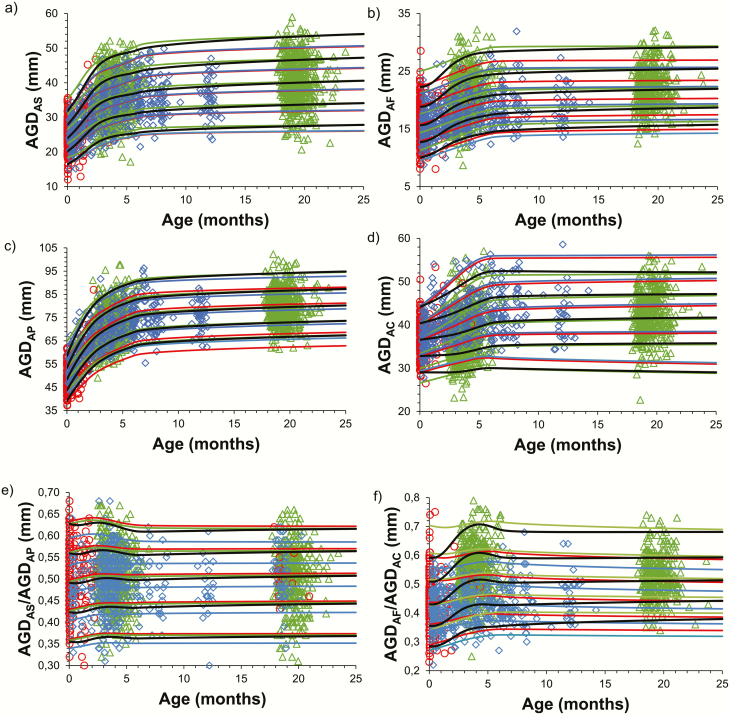
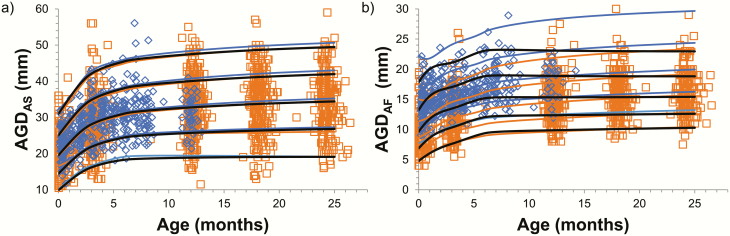
We created reference ranges according to age for each center using the TIDES method for short AGDAS (boys) and AGDAF (girls), long AGDAP (boys) and AGDAC (girls), as well as the short-to-long AGD ratio: AGDAS/AGDAP (boys) and AGDAF/AGDAC (girls) (Fig. 3). Similarly, we created reference ranges according to age using the Cambridge method for short AGDAS (boys) and AGDAF (girls) (Fig. 4).
Figure 3.
Method TIDES. Reference ranges according to age for short AGDAS (boys) (a), short AGDaf (girls) (b), long AGDap (boys) (c), long AGDac (girls) (d), short-to-long AGD ratio (AGDas/AGDap [boys]) (e), and short-to-long AGD ratio (AGDaf/AGDac [girls]) (f), according to Center 1 (red), Center 3 (green), and Center 4 (blue). Dots indicate individual values and lines indicate +2SD, +1SD, mean, -1SD, -2SD. Black lines indicate combined reference levels for all 3 centers.
Figure 4.
Cambridge method. Reference ranges according to age for short AGDas (boys) (a) and AGDAF (girls) (b) by center. Center 2 (orange) and center 4 (blue). Dots indicate individual values, lines indicate +2SD, +1SD, mean, -1SD, -2SD. Black lines indicate the combined reference levels for all 3 centers.
A systematic difference was found between the TIDES and Cambridge methods. Short AGDAS (boys) and AGDAF (girls) were on average 12.9% and 7.4% longer using the TIDES versus the Cambridge method, corresponding to a mean difference of 3.8 and 1.2 mm, respectively (Fig. 2C and 2D).
At birth, mean (±SD) short AGDAS (boys) and short AGDAF (girls) were 28 mm (±4.4) and 15.8 mm (±3.1), respectively, while long AGDAP (boys) and long AGDAC (girls) were 50.6 mm (±5.8) and 37. 6mm (±3.7), respectively, using the TIDES method (Centers 1 and 4 only). Both long and short AGD increased from birth to 6 months and thereafter appeared to be stable during the first 2 years of life. Likewise, the short-to-long AGD ratio using the TIDES method increased until 3 months of age, after which it gradually reached a plateau (Fig. 3E and 3F). Correspondingly, a significant increase in AGD from birth to 6 months of age (short and long AGD in both boys and girls evaluated by TIDES, as well as short AGD in boys and girls using Cambridge methods, all P < 0.001) was observed in longitudinal data from Center 4. Hereafter, no significant changes were noted from 6 months to 1 year of life, regardless of measurement method or AGD parameter (Table 1).
Table 1.
TIDES and Cambridge methods. Wilcoxon signed-rank test using 3 time points: birth (< 1 month of age), 6 months (4.5–6.5 months of age), and 1 year (11–13 months of age) for TIDES and Cambridge methods.
| n | Birth | 6 months | P-value | n | 6 months | 1 Year | P-value | ||
|---|---|---|---|---|---|---|---|---|---|
| TIDES | Short AGDAS (boys) | 75 | 25.5 (23.0–28.1) | 35.1 (32.5–40.9) | <0.001 | 40 | 35.9 (33.6–41.2) | 35.8 (31.2–40.2) | 0.076 |
| Short AGDAF (girls) | 62 | 15.0 (13.5–16.6) | 18.1 (16.6–20.3) | <0.001 | 31 | 18.3 (16.0–20.2) | 18.8 (16.8–21.0) | 0.422 | |
| Long AGDAP (boys) | 75 | 54.0 (50.9–57.3) | 73.6 (68.8–78.1) | <0.001 | 40 | 75.6 (70.6–79.2) | 76.2 (70.9–79.5) | 0.697 | |
| Long AGDAC (girls) | 62 | 36.8 (35.0–39.6) | 42.4 (39.5–47.4) | <0.001 | 30 | 42.6 (39.7–47.4) | 41.7 (37.5–44.8) | 0.111 | |
| CAMBRIDGE | Short AGDAS (boys) | 75 | 22.7 (20.6–24.9) | 31.0 (28.3–33.6) | <0.001 | 39 | 30.8 (26.7–32.5) | 31.5 (28.0–34.7) | 0.302 |
| Short AGDAF (girls) | 61 | 14.1 (12.7–15.7) | 16.3 (15.3–18.4) | <0.001 | 29 | 16.5 (15.4–18.2) | 19.0 (15.4–19.8) | 0.172 |
Data are given as medians and interquartile ranges. A P-value < 0.05 is considered significant.
Abbreviations: AGDAS, anogenital distance (ano-scrotal); AGDAF, anogenital distance (ano-fourchettal); AGDAP, anogenital distance (ano-penile); AGDAC anogenital distance (ano-clitoral).
Intraexaminer ICCs ranged from 0.91 to 0.98 (available in a digital research material repository) (34). In boys, short AGDAS, obtained using the TIDES method, showed the highest interexaminer ICC of 0.80 (0.67–0.90), while in girls, the highest interexaminer ICC was the long AGDAC measured by the TIDES method, that is, 0.64 (0.44–0.81). Long AGDAP (boys) using the TIDES method showed the lowest interexaminer ICC of 0.40 (0.15–0.65) as well as the highest examiner error SD at 5.16 mm (2.40–10.1). Aside from long AGDAP TIDES method, we found the remaining AGD variants to have lower examiner error SDs than background error SDs.
When comparing center medians (Centers 1 and 3) for the TIDES method at 6 months of age, with Center 4 as the reference center, variation was between 2% and 8%, except for AGDAF (girls), which was 15% (data not shown).
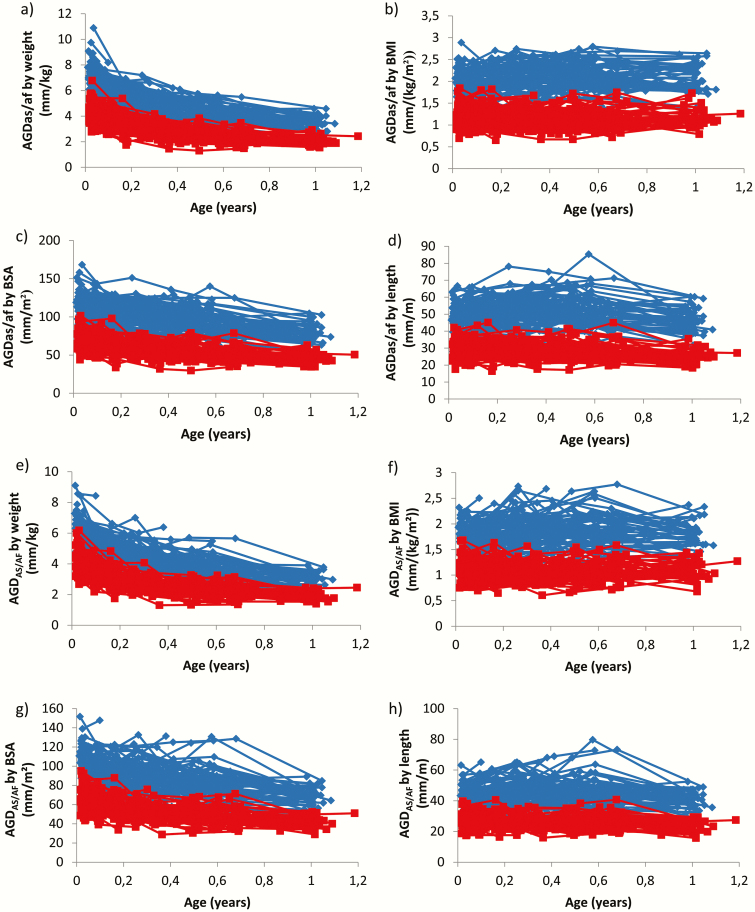
The overall change in AGD body size indices (weight, BMI, BSA, and length) from birth to 12 months of age were limited, ranging from 0% to 6% in boys and 0% to 11% in girls under both methods and all AGD variants (examples of AGD body size index for short AGDAS [boys] and AGDAF [girls] with the TIDES and Cambridge methods are illustrated in Fig. 5).
Figure 5.
TIDES and Cambridge methods. Longitudinal short AGDas (boys) and AGDaf (girls) body size indices for TIDES: weight (a), BMI (b), BSA (c), and length (d). Longitudinal short AGDas (boys) and AGDaf (girls) body size index for Cambridge: weight (e), BMI (f), BSA (g), and length (h). Center 4 only. Blue lines represent individual values for boys and red lines represent individual values for girls.
Discussion
In this study, we provide age-, sex-, and method-related reference ranges for AGD in a large cohort of 3705, mainly Caucasian, infants from birth to 2 years of age. Based on longitudinal data from a single cohort, we assess AGD dynamics during the first year of life. Short AGDAS (boys) was the most reproducible AGD measurement, followed by long AGDAC (girls) (both using TIDES method). The stable levels of AGD/BMI during infancy support the notion that AGD could be prenatally programmed and a permanent marker of in utero androgen exposure (35).
While data examining changes in AGD with age have been published (35–38), age- and sex-specific references are sparse.
Based on the reference ranges as well as longitudinal data presented in this study, we observed an increase in AGD in both boys and girls during the first 6 months of life, after which these changed minimally until 1 year of age. The plateau from 6 months to 1 year might persist even longer, since AGD has previously been reported to be stable for as long as 24 months of age (12). The increase in AGD during the first 6 months of life, as well as the transient increase in the short-to-long AGD ratio, is likely caused by the rapid overall growth during infancy.
Anogenital distance at birth in our study compares well to previously published studies of Caucasian newborns (39), but other studies with more ethnic diversity tend to report shorter AGD (36, 38–40). However, this could also be caused by variations in positioning infants and other methodological differences. Further research is necessary to explore the impact on ethnicity on AGD at birth.
No consensus exists on the positioning of the infant or on the use of specific anatomical landmarks when assessing AGD. In this study, we found that the TIDES methods resulted in systematically longer AGD measurements than the Cambridge method. This is consistent with a study of 17 children that reported a mean difference of 3 mm in short AGDAS (boys) when comparing the 2 methods (27). Positioning the child according to the TIDES method stretches the perineum slightly compared to the Cambridge method and increases the measurements made. The TIDES method is the most widely used, possibly because the TIDES study group was the 1st to widely apply AGD measurements in epidemiological studies and made their training material widely available. However, both methods are equally reproducible, and we therefore provide reference ranges for both methods.
Moreover, we found systematic differences in the reference ranges between centers. These could be due, in part, to the differences in methods and landmarks. However, the systematic differences were not deemed clinically relevant in any of the measures except for short AGDAF (girls). The landmark for AGDAF (girls), the female fourchette, can be difficult to identify and error in this measurement has the greater impact because it is the shortest of the AGD variants.
Interestingly, analyses from Center 4 indicate better replicability of short AGDAS (boys) using the TIDES method. While the interexaminer ICC for short AGDAS (boys) is comparable to what was reported from other studies, including the TIDES study (Center 1 in the present study) (27, 40), the remaining interexaminer ICCs in this study are lower. The poorer replicability of the long AGDAP (boys) measurement may be caused by difficulties identifying the anterior insertion of the penis compared to the perineo–scrotal junction used in the short AGDAS (boys). In line with previous studies, interexaminer ICCs indicate moderate replicability, regardless of AGD variant and method in girls, perhaps caused by the less distinct female landmarks (13, 39). As previously observed, the background error SD was higher than the examiner error SD, indicating that background error is a substantial source of overall measurement error (13, 39, 40). While overall AGD is a reproducible measurement, our findings emphasize the importance of training and correct positioning of the child, as these factors reduce background error.
Anogenital distance has been proposed as an important component of diagnosis and follow-up of children with ambiguous or atypical genitalia due to its reflection of the in utero androgen exposure (41). A recent study found that the short-to-long AGD ratio (AGDAS/AP) was significantly shorter in males with atypical genitalia than in healthy males (42). Our study provides reference ranges for the short-to-long AGD ratio to enable clinicians to apply this as a marker in DSD diagnostics and follow-up, although further studies are needed to confirm the applicability and usefulness of the ratios when evaluating DSD patients.
Anogenital distance has been extensively used in epidemiological studies because of evidence that it is a prenatally programmed direct readout of in utero androgen exposure, suggesting its clinical applicability (35, 43). While longitudinal data (Center 4 only) demonstrate slight changes during the first year of life, the overall change in the body size–adjusted measure from birth to 12 months of age is negligible. Thus, AGD/BMI is a stable postnatal marker and provides the theoretical rationale for applying AGD in epidemiological and clinical settings.
Large datasets from international collaborations are important to create a normative reference range to be used in clinical and epidemiological settings, as well to study variations related to the types of AGD measurements and techniques. However, such multicenter analyses also includes multiple examiners from different centers, which could cause an interobserver variability.
Conclusion
This multicenter study provides the largest AGD dataset to date on 3705 healthy infants, including longitudinal data on 236 children. We provide references for both the TIDES and Cambridge methods, applicable within clinical settings when evaluating DSD patients as well as for use in epidemiological research. The stability of AGD that these data demonstrate supports the conclusion that AGD is a stable marker of the fetal environment.
Acknowledgments
Financial Support: Center 1: S.H.S. would like to acknowledge the support of the following grants from the National Institute of Environmental Health Sciences: R01ES016863-04, R01 ES016863-02S4, and P30 ES023515. Center 2: This work was supported by a European Union Framework V programme, the World Cancer Research Fund International, the Medical Research Council (UK), Newlife the Charity for Disabled Children, the Mothercare Group Foundation, Mead Johnson Nutrition, and the National Institute for Health Research Cambridge Comprehensive Biomedical Research Centre. Center 3: This work was supported by the Danish Council for Independent Research medical sciences (4004-00352B_FSS and 8020-00123B_FSS), Odense University Hospital and Region of Southern Denmark, Municipality of Odense, the Mental Health Service of the Region of Southern Denmark, Odense University Hospital Research Foundation and Odense Patient data Explorative Network (OPEN), the Novo Nordisk Foundation (NNF15OC00017734 and NNF17OC0029404), Helsefonden and the foundation for research collaboration between Rigshospitalet and Odense University Hospital. Center 4: The COPENHAGEN minipuberty study has been funded by the research foundation of Rigshospitalet, research fund of the Capital Region of Copenhagen, the Danish Environmental Agency, and the International Research and Research Training Centre for Male Reproduction and Child Health (EDMaRC). M.L.L. has been funded by The Absalon Foundation.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability. All data generated or analyzed during this study are included in this published article or in the data repositories listed in the References.
References
- 1. Hsieh MH, Breyer BN, Eisenberg ML, Baskin LS. Associations among hypospadias, cryptorchidism, anogenital distance, and endocrine disruption. Curr Urol Rep. 2008;9(2):137–142. [DOI] [PubMed] [Google Scholar]
- 2. Dean A, Sharpe RM. Clinical review: anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J Clin Endocrinol Metab. 2013;98(6):2230–2238. [DOI] [PubMed] [Google Scholar]
- 3. Wohlfahrt-Veje C, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome: foetal origin of adult reproductive problems. Clin Endocrinol (Oxf). 2009;71(4):459–465. [DOI] [PubMed] [Google Scholar]
- 4. Jørgensen N, Rajpert-De Meyts E, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome comprises some but not all cases of hypospadias and impaired spermatogenesis. Int J Androl. 2010;33(2):298–303. [DOI] [PubMed] [Google Scholar]
- 5. Mendiola J, Stahlhut RW, Jørgensen N, Liu F, Swan SH. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect. 2011;119(7):958–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juul A, Almstrup K, Andersson AM, et al. Possible fetal determinants of male infertility. Nat Rev Endocrinol. 2014;10(9): 553–562. [DOI] [PubMed] [Google Scholar]
- 7. Cox K, Kyriakou A, Amjad B, et al. Shorter anogenital and anoscrotal distances correlate with the severity of hypospadias: a prospective study. J Pediatr Urol. 2017;13(1):57.e1–57.e5. [DOI] [PubMed] [Google Scholar]
- 8. Jain VG, Singal AK. Shorter anogenital distance correlates with undescended testis: a detailed genital anthropometric analysis in human newborns. Hum Reprod. 2013;28(9):2343–2349. [DOI] [PubMed] [Google Scholar]
- 9. Thankamony A, Lek N, Carroll D, et al. Anogenital distance and penile length in infants with hypospadias or cryptorchidism: comparison with normative data. Environ Health Perspect. 2014;122(2):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Priskorn L, Bang AK, Nordkap L, et al. Anogenital distance is associated with semen quality but not reproductive hormones in 1106 young men from the general population. Hum Reprod. 2019;34(1):12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Callegari C, Everett S, Ross M, Brasel JA. Anogenital ratio: measure of fetal virilization in premature and full-term newborn infants. J Pediatr. 1987;111(2):240–243. [DOI] [PubMed] [Google Scholar]
- 12. Thankamony A, Ong KK, Dunger DB, Acerini CL, Hughes IA. Anogenital distance from birth to 2 years: a population study. Environ Health Perspect. 2009;117(11):1786–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Priskorn L, Petersen JH, Jørgensen N, et al. Anogenital distance as a phenotypic signature through infancy. Pediatr Res. 2018;83(3):573–579. [DOI] [PubMed] [Google Scholar]
- 14. Barrett ES, Hoeger KM, Sathyanarayana S, et al. Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. J Dev Orig Health Dis. 2018;9(3):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sánchez-Ferrer ML, Mendiola J, Hernández-Peñalver AI, et al. Presence of polycystic ovary syndrome is associated with longer anogenital distance in adult Mediterranean women. Hum Reprod. 2017;32(11):2315–2323. [DOI] [PubMed] [Google Scholar]
- 16. Wu Y, Zhong G, Chen S, Zheng C, Liao D, Xie M. Polycystic ovary syndrome is associated with anogenital distance, a marker of prenatal androgen exposure. Hum Reprod. 2017;32(4):937–943. [DOI] [PubMed] [Google Scholar]
- 17. Caanen MR, Kuijper EA, Hompes PG, et al. Mass spectrometry methods measured androgen and estrogen concentrations during pregnancy and in newborns of mothers with polycystic ovary syndrome. Eur J Endocrinol. 2016;174(1):25–32. [DOI] [PubMed] [Google Scholar]
- 18. Glintborg D, Jensen RC, Schmedes AV, Brandslund I, Kyhl HB, Nielsen TK, Andersen MS. Ano-genital distance in children born of mothers with polycystic ovary syndrome - Odense Child Cohort. Endocr. Abstr. 2019;34(10):1–10. [Google Scholar]
- 19. Mogensen DM, Pihl MB, Skakkebæk NE, et al. Prenatal exposure to antifungal medication may change anogenital distance in male offspring: a preliminary study. Environ Health. 2017;16(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lind DV, Main KM, Kyhl HB, et al. Maternal use of mild analgesics during pregnancy associated with reduced anogenital distance in sons: a cohort study of 1027 mother-child pairs. Hum Reprod. 2017;32(1):223–231. [DOI] [PubMed] [Google Scholar]
- 21. Repouskou A, Panagiotidou E, Panagopoulou L, et al. Gestational exposure to an epidemiologically defined mixture of phthalates leads to gonadal dysfunction in mouse offspring of both sexes. Sci Rep. 2019;9(1):6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jensen TK, Frederiksen H, Kyhl HB, et al. Prenatal exposure to phthalates and anogenital distance in male infants from a low-exposed danish cohort (2010-2012). Environ Health Perspect. 2016;124(7):1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bornehag CG, Carlstedt F, Jönsson BA, et al. Prenatal phthalate exposures and anogenital distance in Swedish boys. Environ Health Perspect. 2015;123(1):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swan SH. Prenatal phthalate exposure and anogenital distance in male infants. Environ Health Perspect. 2006;114(2):A88–A89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. United States Environmental Protection Agency. No Title. July 1, 2020. https://www.epa.gov/. Accessed March 12, 2019.
- 26. Barrett ES, Sathyanarayana S, Janssen S, et al. ; TIDES Study Team Environmental health attitudes and behaviors: findings from a large pregnancy cohort study. Eur J Obstet Gynecol Reprod Biol. 2014;176:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sathyanarayana S, Grady R, Redmon JB, et al. ; TIDES Study Team Anogenital distance and penile width measurements in The Infant Development and the Environment Study (TIDES): methods and predictors. J Pediatr Urol. 2015;11(2):76.e1–76.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prentice P, Acerini CL, Eleftheriou A, Hughes IA, Ong KK, Dunger DB. Cohort Profile: the Cambridge Baby Growth Study (CBGS). Int J Epidemiol. 2016;45(1):35.a–35.g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kyhl HB, Jensen TK, Barington T, et al. The odense child cohort: aims, design, and cohort profile. Paediatr Perinat Epidemiol. 2015;29(3):250–258. [DOI] [PubMed] [Google Scholar]
- 30. Salazar-Martinez E, Romano-Riquer P, Yanez-Marquez E, Longnecker MP, Hernandez-Avila M. Anogenital distance in human male and female newborns: a descriptive, cross-sectional study. Environ Health. 2004;3(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843–848. [DOI] [PubMed] [Google Scholar]
- 32. Fischer MB. LMS values: reference levels for anogenital distance in healthy children.xlsx. Environ Health Perspec. 2020. doi: 10.6084/m9.figshare.12179190 [DOI] [Google Scholar]
- 33. Fischer MB. Supplementary table 1. 2020. doi: 10.6084/m9.figshare.12179811. [DOI] [Google Scholar]
- 34. Fischer MB. Supplementary table 2. 2020. doi: 10.6084/m9.figshare.12179817 [DOI] [Google Scholar]
- 35. Jain VG, Goyal V, Chowdhary V, et al. Anogenital distance is determined during early gestation in humans. Hum Reprod. 2018;33(9):1619–1627. [DOI] [PubMed] [Google Scholar]
- 36. Adekoya AO, Fetuga MB, Jarrett OO, Ogunlesi TA, Chanoine JP, Adekoya AO. Clitoral sizes and anogenital distances in term newborns in Nigeria. Int J Pediatr Endocrinol. 2019;2019:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arbuckle TE, MacPherson S, Barrett E, et al. Do stressful life events during pregnancy modify associations between phthalates and anogenital distance in newborns? Environ Res. 2019;177:108593. [DOI] [PubMed] [Google Scholar]
- 38. Nguyen TV, Monnier P, Muckle G, et al. Androgenic and estrogenic indices in human newborns and infants: the MIREC-ID study. J Dev Orig Health Dis. 2019;10(5):578–586. [DOI] [PubMed] [Google Scholar]
- 39. Papadopoulou E, Vafeiadi M, Agramunt S, et al. Anogenital distances in newborns and children from Spain and Greece: predictors, tracking and reliability. Paediatr Perinat Epidemiol. 2013;27(1):89–99. [DOI] [PubMed] [Google Scholar]
- 40. Romano-Riquer SP, Hernández-Avila M, Gladen BC, Cupul-Uicab LA, Longnecker MP. Reliability and determinants of anogenital distance and penis dimensions in male newborns from Chiapas, Mexico. Paediatr Perinat Epidemiol. 2007;21(3):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cools M, Nordenström A, Robeva R, et al. ; COST Action BM1303 working group 1 Caring for individuals with a difference of sex development (DSD): a Consensus Statement. Nat Rev Endocrinol. 2018;14(7):415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van der Straaten S, Springer A, Zecic A, et al. The External Genitalia Score (EGS): a European multicenter validation study. J. Clin. Endocrinol. Metab. 2019;105(3):e222-e230. doi: 10.1210/clinem/dgz142 [DOI] [PubMed] [Google Scholar]
- 43. Thankamony A, Pasterski V, Ong KK, Acerini CL, Hughes IA. Anogenital distance as a marker of androgen exposure in humans. Andrology. 2016;4(4):616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]