Abstract
BACKGROUND
Hemiablation is a less morbid treatment alternative for appropriately selected patients with unilateral prostate cancer (PCa). However, to the authors’ knowledge, traditional diagnostic techniques inadequately identify appropriate candidates. In the current study, the authors quantified the accuracy for identifying hemiablation candidates using contemporary diagnostic techniques, including multiparametric magnetic resonance imaging (mpMRI) and MRI-fusion with complete systematic template biopsy.
METHODS
A retrospective analysis of patients undergoing MRI and MRI-fusion prostate biopsy, including full systematic template biopsy, prior to radical prostatectomy in a single tertiary academic institution between June 2010 and February 2018 was performed. Hemiablation candidates had unilateral intermediate-risk PCa (Gleason score [GS] of 3+4 or 4+3, clinical T classification ≤T2, and prostate-specific antigen level <20 ng/dL) on MRI-fusion biopsy and 2) no contralateral highly or very highly suspicious Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) MRI lesions. Hemiablation candidates were inappropriately selected if pathologists identified contralateral GS ≥3+4 or high-risk ipsilateral PCa on prostatectomy. The authors tested a range of hemiablation inclusion criteria and performed multivariable analysis of preoperative predictors of undetected contralateral disease.
RESULTS
Of 665 patients, 92 met primary hemiablation criteria. Of these 92 patients, 44 (48%) were incorrectly identified due to ipsilateral GS ≥3+4 tumors crossing the midline (21 patients), undetected distinct contralateral GS ≥3+4 tumors (20 patients), and/or ipsilateral high-risk PCa (3 patients) on prostatectomy. The rate of undetected contralateral disease ranged from 41% to 48% depending on inclusion criteria. On multivariable analysis, men with anterior index tumors were found to be 2.4 times more likely to harbor undetected contralateral GS ≥3+4 PCa compared with men with posterior lesions (P < .05).
CONCLUSIONS
Clinicians and patients must weigh the risk of inadequate oncologic treatment against the functional benefits of hemiablation. Further investigation into methods for improving patient selection for hemiablation is necessary.
Keywords: focal therapy, hemiablation, magnetic resonance imaging, patient selection, prostatectomy, prostatic neoplasms, unilateral
INTRODUCTION
Radical prostatectomy and radiotherapy are standard whole-gland treatments among patients with organ-confined prostate cancer (CaP) requiring definitive intervention.1 Despite favorable oncologic outcomes, these treatments cause significant genitourinary side effects.2 Due to the largely indolent nature of these tumors, the treatment of choice for patients with low-risk CaP and select patients with intermediate-risk CaP and a limited life expectancy is active surveillance.3–5
In the past decade, urologists worldwide have widely adopted multiparametric magnetic resonance imaging (mpMRI) of the prostate into the management of patients with CaP. Patients and clinicians are increasingly interested in alternative treatment modalities, such as high-intensity focused ultrasound, cryotherapy, photodynamic therapy, and radiofrequency ablation, which may provide reasonable oncologic outcomes with superior side effects in select individuals.6–9 Focal therapy targets only areas of known cancer, whereas hemiablation limits tissue damage to the prostate lobe harboring known clinically significant CaP (csCaP).7,10
The multifocal nature of CaP and limitations in tumor detection and localization make patient selection for subtotal treatments challenging. Appropriate candidates for hemiablation have csCaP limited to 1 prostate lobe. Previous studies have reported low concordance between disease laterality on transrectal ultrasound (TRUS)–guided prostate biopsy and prostatectomy pathology.11–17 Although there is strong clinical evidence that prostate imaging with mpMRI and targeted biopsy improves csCaP detection on the patient level,18–23 to our knowledge the detection sensitivity for mpMRI for individual CaP foci remains moderate, particularly in smaller, nonindex, multifocal lesions.24,25
Although experts agree that mpMRI is essential for selecting hemiablation candidates in combination with targeted and systematic biopsy, to our knowledge the accuracy of these contemporary CaP diagnostic techniques in the identification of patients with unilateral CaP is unknown. Therefore, we investigated the rate of undetected contralateral csCaP and high-grade ipsilateral CaP in radical prostatectomy whole-mount pathology (WMP) specimens in hemiablation candidates. In addition, we explored pretreatment predictors of laterality discordance on final pathology.
MATERIALS AND METHODS
Study Population
The study population was derived from a database of 665 men who underwent 3-tesla mpMRI, prostate biopsy (TRUS and/or MRI-fusion using the transrectal Artemis [Eigen, Grass Valley, California] or UroNav [Invivo, Gainesville, Florida] systems), and radical prostatectomy at a single tertiary academic institution. Pathologists processed all prostatectomy specimens using WMP. Research staff recorded patients’ demographic, clinical, MRI, and histopathologic information. The institutional review board approved this study.
Candidacy for Hemiablation
For the primary analysis, candidates met the following preoperative criteria: 1) unilateral intermediate-risk csCaP (defined as a Gleason score [GS] of 3+4 or 4+3 [Gleason grade group (GG) 2 or 3], clinical stage of disease ≤T2, and prostate-specific antigen [PSA] level ≤20 ng/mL)26; and 2) no midline contralateral Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) 4 to 5 lesions on mpMRI. Hemiablation candidates must have undergone combined MRI-fusion and complete systematic template biopsy. We included patients with GS 3+4 and 4+3 pathology from targeted and/or systematic core needle biopsies provided it was unilateral. We determined clinical stage according to digital rectal examination and mpMRI findings. We included patients with contralateral low-risk tumors (GS 3+3) and/or contralateral PI-RADSv2 3 lesions in our primary analysis. We excluded patients with mpMRI prostate volume ≥60 cm3, PI-RADSv2 4 to 5 lesions described as “midline,” incomplete systematic template (<10 nontargeted biopsy core needle biopsy specimens), or cognitive MRI-fusion biopsy. At least 2 members of the research staff (D.C.J., J.J.Y., D.E.B., A.P., T.S., and R.J.) independently identified hemiablation candidates by manual chart review. A total of 92 patients met the primary eligibility according to clinical reports (Fig. 1). One author (D.C.J.) compared independent candidacy assessments and adjudicated discrepancies (12 patients). In addition, a software algorithm, written in Matlab 2015a (MathWorks, Natick, Massachusetts), was used to measure exact spatial coordinates of radiographic regions of interest (ROIs), core needle biopsy specimens, and prostate contours to generate 3-dimensional renderings using the Artemis in MRI-fusion biopsy system. This technique identified additional patients with tumor crossing the sagittal midline. The research team (D.C.J., S.Z., A.M.P., and S.N.) verified these findings visually (Fig. 2) and cross-referenced the patients’ radiology report to confirm accuracy. We performed sensitivity analyses that excluded these cases to assess alternative radiographic, pathologic, and biopsy criteria for hemiablation candidacy.
Figure 1.
Hemiablation eligibility. 3D indicates 3-dimensional; 3-T, 3-tesla; ECE, extracapsular extension; GG, Gleason grade group; MRI, magnetic resonance imaging; PI-RADSv2, Prostate Imaging Reporting and Data System version 2; PSA, prostate-specific antigen; TRUS, transrectal ultrasonography.
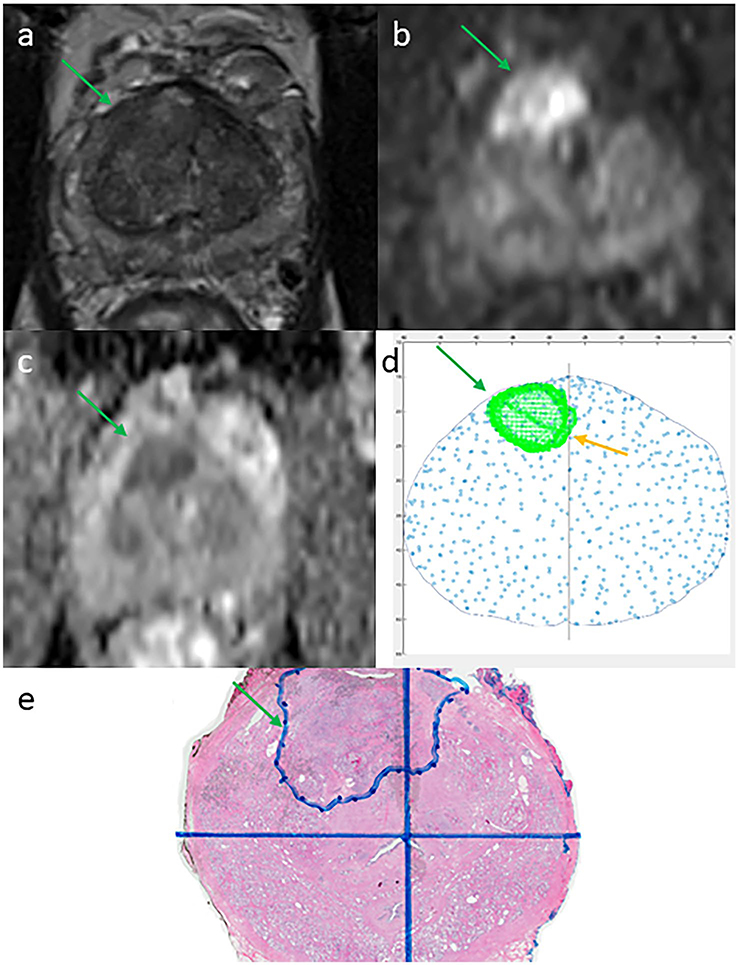
Figure 2.
Example of a magnetic resonance imaging (MRI) region of interest reported as unilateral but which 3-dimensional (3D) reconstruction for MRI-fusion biopsy identified as bilateral clinically significant prostate cancer in a 62-year-old man with a prostate-specific antigen level of 10.6 ng/mL. A Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) 5 target lesion (green arrow) was reported as on the right side only on 3-tesla multiparametric MRI. (a) T2-weighted image. (b) Diffusion-weighted image. (c) Apparent diffusion coefficient map. (d) Section of 3D reconstruction using spatial coordinates demonstrating tumor crossover (yellow arrow). (e) Confirmation of bilateral disease on whole-mount pathology.
Multiparametric MRI and MRI-Fusion Biopsy
Patients underwent 3-tesla mpMRI (Siemens Magnetom Trio, Skyra, or Verio; Siemens Medical Systems, Malvern, Pennsylvania) according to standardized protocols with pelvic external phased array coils, with or without endorectal coil. All images were read preliminarily by an abdominal imaging fellow (postgraduate year 6) and confirmed by 1 of 3 attending abdominal radiologists with 600, 2000, and 3000 prior prostate mpMRI reads, respectively. Radiologists were not blinded to clinical details. MRI-fusion biopsies were performed by 4 urologic oncologists using the Artemis or UroNav systems (82 of 92 biopsies [89%] were performed by L.S.M. using the Artemis system).
Whole-Mount Processing of Prostatectomy Specimens
Two fellowship-trained genitourinary pathologists (with 4 years and 12 years, respectively, of CaP experience) assessed each whole-mount slide. This process involves axial sectioning of each prostate specimen from the inked basal margin to the apex in 4-mm to 5-mm intervals and mounting the sections on large slides. A genitourinary radiologist and pathologist reviewed each case individually to confirm the location, including laterality, of all radiographic and pathologic lesions at a separate monthly multidisciplinary meeting. Research staff prospectively entered results into the database.
Definition of Inappropriate Hemiablation Candidacy at Radical Prostatectomy
We defined discordant laterality in hemiablation candidates as any contralateral csCaP (GS ≥3+4) in the prostatectomy specimen. Discordant csCaP consisted of distinct contralateral tumors and/or an ipsilateral tumor crossing the midline (Fig. 3 Top and Bottom). We also considered patients with ipsilateral high-grade tumor (GS ≥4+3 with tertiary pattern 5) on WMP as having been incorrectly selected for hemiablation. We assumed hemiablation adequately treats ipsilateral extracapsular extension. We characterized the pathology of unidentified contralateral csCaP on WMP.
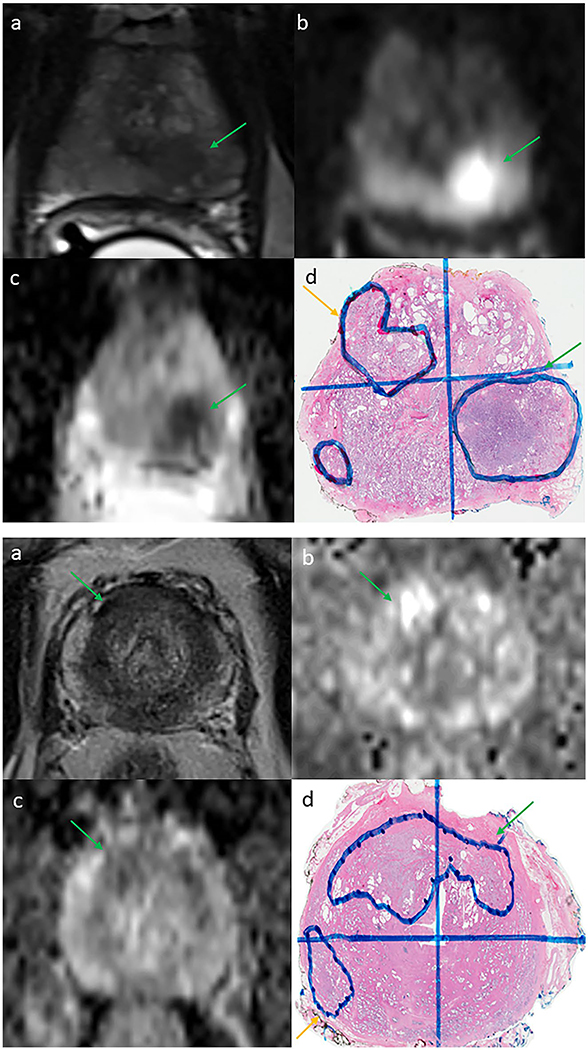
Figure 3.
(Top) Example of a missed discrete contralateral tumor in a 55-year-old man with a prostate-specific antigen (PSA) level of 7.5 ng/mL with a unilateral Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) 5 target lesion (green arrow) with no evidence of a contralateral lesion on 3-tesla (3-T) multiparametric magnetic resonance imaging (mpMRI) or biopsy. (a) T2-weighted image. (b) Diffusion-weighted image. (c) Apparent diffusion coefficient map. (d) Detected (Gleason grade group 3 [GG3], green arrow) and missed (GG2, yellow arrow) contralateral prostate cancer lesions on final whole-mount histopathology slide. (Bottom) Example of a missed crossover tumor in a 54-year-old man with a PSA level of 5.3 ng/mL and a unilateral PI-RADS 3 target lesion (green arrow) with no evidence of contralateral extension on 3-T mpMRI. (a) T2-weighted image. (b) Diffusion-weighted image. (c) Apparent diffusion coefficient map. (d) Right-sided lesion with undetected contralateral extension (GG2, green arrow) and missed insignificant (GG1) prostate cancer on final whole-mount histopathology slide.
Statistical Analysis
We compared continuous variables using the Student t test (or nonparametric Wilcoxon rank sum test and the median test for nonnormal variables) and a chi-square test (or Fisher exact test if necessary) for categorical Boolean variables between patients with concordant and discordant laterality. We also conducted a multivariate logistic regression to find clinical and biopsy-related covariates associated with discordant laterality, including age, GS, PSA density (<0.15 vs ≥0.15), ROI location (anterior vs not), ROI zone (transition vs peripheral), focality (solitary vs multifocal), percentage positive core needle biopsy specimens (in quartiles), maximum cancer percentage in a core needle biopsy specimen (<50% vs ≥50%), endorectal coil use, and risk of capsular involvement. We conducted all analyses using SAS statistical software (version 9.4; SAS Institute Inc, Cary, North Carolina).
RESULTS
Percentage of Patients With Undetected csCaP
Table 1 describes the characteristics of the 92 primary candidates for hemiablation. Pathologists determined that 44 of these 92 preoperative candidates (48%) ultimately were ineligible for hemiablation on prostatectomy: 41 patients had discordant laterality of csCaP (21 patients with tumor crossing the midline and 20 patients with undetected distinct contralateral tumors) and 3 patients had ipsilateral upgrading (GS 4+3 with tertiary pattern 5 [1 patient], GS 4+4 [1 patient], and GS 4+5 [1 patient]). Of the 41 patients with unidentified contralateral csCaP, 10 (24%) had tumors containing ≥GS 3+4 with tertiary pattern 5 pathology. Table 2 includes additional details regarding missed contralateral tumors.
TABLE 1.
Characteristics of Hemiablation Candidates in the Primary Analysis
| Total N = 92 | Discordant N = 44 | Concordant N = 48 | P | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Patient characteristics Mean age (SD), y | 61.7 (5.5) | 62.3 (5.3) | 61.2 (5.7) | .34 |
| PSA density, ng/mL/cm3a | ||||
| <0.15 | 40% (37) | 46% (20) | 35% (17) | .33 |
| ≥0.15 | 60% (55) | 55% (24) | 65% (31) | |
| MRI-fusion biopsy characteristics | ||||
| MRI of ipsilateral radiographic ROI | ||||
| Negative | 15% (14) | 11% (5) | 19% (9) | .59 |
| 3 | 24% (22) | 25% (11) | 23% (11) | |
| 4–5 | 60% (55) | 64% (28) | 57% (27) | |
| Size of ipsilateral radiographic ROI, cm | N = 40 | N = 38 | ||
| Mean (SD) | - | 1.4 (0.5) | 13 (0.6) | .20b |
| Median (IQR) | - | 1.4 (1.1–1.6) | 1.2 (0.8–1.5) | .37c |
| Anterior location of index tumord | ||||
| Yes | 48% (44) | 61% (27) | 35% (17) | .01 |
| No | 52% (48) | 39% (17) | 65% (31) | |
| Index tumor location (zone) | ||||
| Peripheral | 59% (54) | 45% (20) | 71% (34) | .04 |
| Transition | 30% (28) | 39% (17) | 23% (11) | |
| Both | 11% (10) | 16% (7) | 5% (3) | |
| Capsular involvemente | ||||
| Low risk | 69% (63) | 68% (30) | 69% (33) | .80f |
| Intermediate risk | 21% (19) | 18% (8) | 23% (11) | |
| High risk | 10% (9) | 11% (5) | 8% (4) | |
| Endorectal coil | ||||
| Yes | 11% (10) | 14% (6) | 8% (4) | .51f |
| No | 89% (82) | 86% (38) | 92% (44) | |
| Biopsy pathology: highest Gleason grade group | ||||
| 2 | 78% (72) | 77% (34) | 79% (38) | .83 |
| 3 | 22% (20) | 23% (10) | 21% (10) | |
| Biopsy type with highest Gleason grade group | ||||
| Systematic | 21% (19) | 20% (9) | 21% (10) | .41 |
| Targeted | 43% (40) | 50% (22) | 38% (18) | |
| Both | 36% (33) | 30% (13) | 42% (20) | |
| Median % positive core needle biopsy specimens (IQR) | 32% (23%−46%) | 29% (21%−41%) | 36% (26%−50%) | .06c |
| No. of pathologic foci | ||||
| Solitary | 32% (29) | 30% (13) | 33% (16) | .70 |
| Multifocal | 68% (63) | 70% (31) | 67% (32) | |
| Maximum cancer in core needle biopsy specimen, % | ||||
| <50% | 30% (28) | 32% (14) | 29% (14) | .98 |
| ≥50% | 70% (64) | 68% (30) | 71% (34) | |
| Location of CaP | ||||
| In target only | 10% (9) | 12% (5) | 8% (4) | .77f |
| Outside target only | 7% (6) | 4% (2) | 8% (4) | |
| Both | 84% (77) | 84% (37) | 83% (40) | |
| Location of csCaP | ||||
| In target only | 39% (36) | 45% (20) | 33% (16) | .48 |
| Outside target only | 16% (15) | 14% (6) | 19% (9) | |
| Both | 45% (41) | 41% (18) | 48% (23) | |
| Final whole-mount pathology characteristics | ||||
| Gleason grade group of pathologic index tumor | ||||
| 1 | 4% (4) | 0% (0) | 8% (4) | .07f |
| 2 | 74% (68) | 70% (31) | 77% (37) | |
| 3 | 15% (14) | 18% (8) | 13% (6) | |
| >3g | 7% (6) | 11% (5) | 2% (1) | |
| Median size of csCaP (IQR), cm | 2.0 (1.9–2.5) | 1.9 (1.2–2.5) | 2.0 (1.8–2.4) | .53c |
Abbreviations: CaP, prostate cancer; csCaP, clinically significant prostate cancer; IQR, interquartile range; MRI, magnetic resonance imaging; PI-RADSv2, Prostate Imaging and Data Reporting System Version 2; PSA, prostate-specific antigen; ROI, region of interest.
Based on MRI estimate of prostate size.
Derived using the Wilcoxon rank sum test.
Derived using the median test.
Target lesion if PI-RADS 3 to 5 lesion on MRI or location of biopsy with highest pathology if negative MRI.
Low risk indicates does not abut capsule, may abut capsule, or abuts <1 cm. Intermediate risk indicates abuts >1 cm, capsular bulge, and broad capsular involvement. High risk indicates macroscopic extracapsular extension, involves anterior fibromuscular stroma, and irregular.
Derived using the Fisher exact test.
Includes Gleason grade group 3 with tertiary pattern 5.
TABLE 2.
Details of Missed Contralateral Tumors (N = 41)
| No. | Mean Size, cm | Whole-Mount Tumor Pathology | Index Tumor Location on Biopsy/MRI, No. | Location of Crossover csCaP, No. | |
|---|---|---|---|---|---|
| Crossover | 21 | 2.6 | GG2 (≤10% pattern 4): 6 | Zone | Anterior: 11 |
| GG2 (20%−40% pattern 4): 9 | PZ only: 10 | Posterior: 8 | |||
| GG3: 4 | PZ plus AFS: 1 | Anterior plus posterior: 2 | |||
| GG3 (tertiary 5): 1 | PZ plus TZ: 6 | ||||
| GG4: 1 | PZ plus CZ: 1 CZ only: 1 TZ only 2 |
||||
| Location Apex: 18 Midline: 16 Base: 7 |
Crossover from: Right to left: 13 Left to right: 8 |
||||
| Distinct | 20 | 1.4 | GG2 (≤10% pattern 4): 9 | Zone | Laterality of missed csCaP |
| GG2 (20%−40% pattern 4): 7 | PZ only: 16 | Left: 11 | |||
| GG2, tertiary 5: 1 | PZ plus AFS: 1 | Right: 9 | |||
| GG3: 3 | PZ plus TZ: 1 TZ only: 1 CZ only: 1 |
||||
| Location Apex: 9 Mid: 15 Base: 2 |
Abbreviations: AFS, anterior fibromuscular stroma; csCaP, clinically significant prostate cancer; CZ, central zone; GG, Gleason grade group; MRI, magnetic resonance imaging; PZ, peripheral zone; TZ, transition zone.
Sensitivity Analyses
Figure 1 describes different inclusion and exclusion criteria for hemiablation. The percentage of inappropriately selected hemiablation candidates ranged from 41% to 48% (see Supporting Table 1). The highest discordant rate (48%) occurred in the primary cohort in the current study. Even applying the most stringent criteria (no contralateral GS 3+3 on biopsy and no contralateral PI-RADSv2 3 lesions), >40% of patients had unidentified contralateral csCaP. Our computerized algorithm using spatial coordinates and 3-dimensional reconstructions to identify tumor crossover ruled out 20 additional potential hemiablation candidates. Of these 72 patients, 30 (42%) would have been incompletely treated with hemiablation (16 patients with crossover tumors and 14 patients with distinct contralateral tumors). Compared with the 72 patients without crossover on digital renderings, the 20 patients who were identified as ineligible due to tumor crossover using the digital renderings were more likely to have anterior tumors (75% vs 40%; P = .006) limited to the transition zone only (60% vs 22%; P = .002). In addition, we evaluated a separate subset of patients (68 patients) with unilateral csCaP based on mpMRI with TRUS biopsy only (no MRI-fusion biopsy). It is interesting to note that the laterality discordance was lower in this cohort compared with the MRI-fusion biopsy cohort (32% vs 48%; P = .05).
Predictors of Undetected Contralateral csCaP
We explored predictors of undetected contralateral csCaP in hemiablation candidates. These factors included age; PSA density; biopsy GS; multifocal tumors; presence of tumor outside of the ROI; and location, size, and PI-RADSv2 score of the ROI. On multivariable analysis, an anterior index tumor location on biopsy and/or mpMRI was the only factor found to predict undetected contralateral csCaP at prostatectomy. Men with anterior tumors were more likely to have missed contralateral csCaP compared with men without anterior tumors (odds ratio, 2.4; 95% CI, 1.0–5.8 [P < .05]). In addition, although men with lesions identified exclusively in the peripheral zone were more likely to have undetected contralateral tumors, this variable was no longer statistically significant when adjusted for anterior location.
DISCUSSION
Using what to our knowledge is the largest prospectively maintained database of patients with preoperative 3-tesla mpMRI, MRI-fusion biopsy including full systematic template, and WMP specimens, we found that hemiablation would have inadequately treated nearly one-half of preoperative hemiablation candidates based on unrecognized contralateral csCaP or ipsilateral high-grade CaP noted on WMP. This discordance rate was robust across a range of inclusion criteria, with the most conservative inclusion criteria still missing contralateral csCaP in >40% of cases. Finally, we found that anterior index tumor location portends a nearly 3-fold higher risk of undetected contralateral csCaP, primarily manifested by unidentified anterior tumor crossover.
In prior studies of patients with predominantly low-risk, unilateral CaP diagnosed by TRUS biopsy only without mpMRI, approximately 65% to 79% of patients actually were found to harbor bilateral CaP on prostatectomy.11–18 Two of these studies reported on the rate of bilateral csCaP on WMP (33% and 50%, respectively) in patients with only unilateral disease on TRUS biopsy alone.14,17 In the current study, we reported similar discordance rates in our subset analysis of patients undergoing TRUS biopsy with the addition of mpMRI. That our discordance rates were comparable to those of the prior series without mpMRI may be attributed to 2 offsetting factors: although mpMRI presumably reduces undetected contralateral csCaP, the higher risk biopsy criteria for hemiablation in the current study (all GS 3+4 or GS 4+3) may have increased the likelihood of unrecognized contralateral csCaP.
Somewhat unexpectedly, we found that the discordant laterality rate on prostatectomy was lower in patients undergoing TRUS biopsy only compared with those undergoing MRI-fusion with systematic template biopsy. However, these 2 patient cohorts were systematically different, which offers possible explanations. First, by definition, those patients undergoing MRI-fusion biopsy had undergone MRI prior to biopsy, when their cancer status was unknown. Conversely, approximately 88% of patients diagnosed by TRUS underwent mpMRI after biopsy, which introduces interpretation bias, particularly with equivocal lesions. Compared with pathologic tumor size, radiologists underestimated matching ROIs in the MRI-fusion cohort to a significantly greater degree (−8 mm vs −5 mm; P = .05), further suggesting interpretation bias when radiologists are aware of CaP. We previously reported the degree to which MRI underestimated pathologic size using patient-specific prostate molds.27 With this knowledge, it is possible that radiologists draw more generous tumor boundaries when they are aware of a cancer diagnosis. Finally, we performed mpMRI with endorectal coil in a higher percentage of patients who underwent TRUS compared with MRI-fusion biopsy (77% vs 13%; P < .001). However, although the use of endorectal coil is associated with improved tumor detection, we previously concluded that after accounting for the timing of biopsy in relation to MRI, endorectal coil does not independently predict tumor detection.25 Whether this contributed to the apparent bias between these cohorts remains unclear.
In a prior cohort of 1184 prostatectomy specimens, pathologists identified unilateral cancer in approximately 20%, making these patients potential candidates for hemiablation.28 However, a separate study demonstrated that preoperative TRUS biopsy inadequately identified unilaterality.15 Furthermore, clinicopathologic factors including family history,29 percentage of positive core needle biopsy specimens,15 percentage of malignant tissue in core needle biopsy specimens,16 and PSA density16 were found to have minimal and/or inconsistent predictive ability.11,13,14,17
A study of 59 patients undergoing mpMRI, MRI-fusion biopsy, and systematic template biopsies prior to radical prostatectomy demonstrated only 11 patients (19%) with residual csCaP if treated by hemiablation on the side of the suspicious MRI lesion.30 These results likely differ from those of the current study for 2 major reasons: 1) hemiablation candidates in the current study could only have a single radiographic ROI, which had to correspond to a positive biopsy; and 2) approximately 25% of patients had GS 3+3 disease only on biopsy, representing a patient cohort at lower risk. Finally, the current study used PI-RADSv1 as opposed to the contemporary PI-RADSv2 system.
The current study addressed several shortcomings of prior analyses. To our knowledge, no prior study has reported on the rate of undiagnosed contralateral csCaP using mpMRI and MRI-targeted plus complete systematic template biopsy with WMP specimens as the reference standard. We specifically designed our multidisciplinary approach to accurately localize, confirm, and record the location and laterality of all radiographic and pathologic lesions. Furthermore, we verified the laterality assignment by both redundant, independent chart reviews and a computerized algorithm. Second, the current study cohort represents a contemporary prostatectomy population with 95% of individuals found to harbor intermediate-risk disease on final pathology, whereas prior studies evaluated primarily patients with low-risk CaP who were appropriate for active surveillance.11–17 Third, the 4 studies reporting oncologic outcomes after hemiablation reported that repeat TRUS biopsy identified contralateral CaP in approximately 10% to 22% of patients.31–34 In a similar baseline patient population, contralateral CaP was detected in 65% to 79% of prostatectomy specimens.11–17,33 This finding highlights the potential for biopsy sampling error to overestimate the true efficacy of hemiablation, a shortcoming that we avoided by using WMP as the reference standard.
The current study had limitations. Selection bias inherent to a prostatectomy cohort is inevitable. However, we identified hemiablation candidates using the same information available to clinicians and patients when making treatment decisions, and the fact that these patients underwent prostate cancer surgery had no bearing on the objective requirements for hemiablation candidacy. Therefore, we believe our primary cohort accurately represents potential hemiablation patients. Second, to our knowledge, the clinical significance of untreated contralateral tumors remains unknown. Proponents of focal therapy cite the theory regarding the monoclonal origin of lethal metastatic CaP to argue that index tumor ablation may effectively prevent distant or recurrent disease.35–37 However, we currently lack the ability to localize the lethal metastatic clone to a single cancer foci, and therefore it is impossible to be sure the lethal clone lies within the index lesion. Furthermore, we must assume that residual malignant cells in an incompletely treated index tumor harbor biologic potential similar to that of the lethal clone. The finding that clinically significant index tumors with unrecognized contralateral extension comprised approximately one-half of discordant cases in the current series argues that this represents inadequate oncologic treatment. Although the clinical significance of a small amount of residual contralateral tumor is unknown, we must assume that any amount of residual csCaP represents treatment failure. This is particularly true considering that the active surveillance protocol is largely unchanged after focal therapy.38 Third, the distinction between a unilateral and bilateral/midline tumor on WMP and mpMRI is subjective and relies on the reports of the pathologists and radiologists. Although radiologists may interpret these midline lesions differently within the clinical context of hemiablation planning, for the purposes of the current study they did not redraw radiographic ROIs. Although the 3-dimensional digital reconstruction method identified crossover in an additional 20 of 92 patients (22%), this only improved patient selection from 48% to 42%. The radiology department at the study institution is characterizing the missed crossover lesions further and investigating methods with which to improve detection. Finally, we made 2 assumptions that rendered our estimates conservative. First, although the clinical significance of untreated, large-volume GS 3+3 CaP in young, healthy patients is debatable, we defined csCaP on prostatectomy as GS ≥3+4.26 Finally, we assumed that hemiablation adequately treats patients with extracapsular extension.
Conclusions
The current study quantifies the significant likelihood of untreated, contralateral csCaP remaining after hemiablation, even after the use of mpMRI with MRI-fusion and complete systematic template biopsies. Clinicians must inform patients about this risk and include this estimate in the informed consent process. The results of the current study support the need for a more in-depth analysis of radiographic ROI and MRI-fusion biopsy geometry to improve patient selection for subtotal prostate treatments, which we currently are undertaking. In addition, we are investigating whether novel imaging techniques, such as restriction spectrum imaging, more accurately predict tumor crossover, and/or whether template-mapping biopsy is necessary for adequate patient selection.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
Funded by the Department of Radiology and Pathology Integrated Diagnostics (IDx) program and the specialized program of research excellence (SPORE) in prostate cancer and the Veteran Affairs Office of Academic Affiliations through the VA/National Clinician Scholars Program and the David Geffen School of Medicine at the University of California at Los Angeles.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Leonard S. Marks is the cofounder of Avenda Health. The other authors made no disclosures.
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Heidenreich A, Bastian PJ, Bellmunt J, et al. ; European Association of Urology. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent–update 2013. Eur Uro l 2014;65:124–137. [DOI] [PubMed] [Google Scholar]
- 2.Barocas DA, Alvarez J, Resnick MJ, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA. 2017;317:1126–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dall’Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976–983. [DOI] [PubMed] [Google Scholar]
- 4.Moschini M, Carroll PR, Eggener SE, et al. Low-risk prostate cancer: identification, management, and outcomes. Eur Urol. 2017;72:238–249. [DOI] [PubMed] [Google Scholar]
- 5.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–277. [DOI] [PubMed] [Google Scholar]
- 6.Barret E, Ahallal Y, Sanchez-Salas R, et al. Morbidity of focal therapy in the treatment of localized prostate cancer. Eur Urol. 2013;63:618–622. [DOI] [PubMed] [Google Scholar]
- 7.Tay KJ, Scheltema MJ, Ahmed HU, et al. Patient selection for prostate focal therapy in the era of active surveillance: an International Delphi Consensus Project. Prostate Cancer Prostatic Dis. 2017;20:294–299. [DOI] [PubMed] [Google Scholar]
- 8.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. [DOI] [PubMed] [Google Scholar]
- 9.Crouzet S, Chapelon JY, Rouviere O, et al. Whole-gland ablation of localized prostate cancer with high-intensity focused ultrasound: oncologic outcomes and morbidity in 1002 patients. Eur Urol. 2014;65:907–914. [DOI] [PubMed] [Google Scholar]
- 10.Valerio M, Cerantola Y, Eggener SE, et al. New and established technology in focal ablation of the prostate: a systematic review. Eur Urol. 2017;71:17–34. [DOI] [PubMed] [Google Scholar]
- 11.Tareen B, Godoy G, Sankin A, Temkin S, Lepor H, Taneja SS. Can contemporary transrectal prostate biopsy accurately select candidates for hemi-ablative focal therapy of prostate cancer? BJU Int. 2009;104:195–199. [DOI] [PubMed] [Google Scholar]
- 12.Mayes JM, Mouraviev V, Sun L, Tsivian M, Madden JF, Polascik TJ. Can the conventional sextant prostate biopsy accurately predict unilateral prostate cancer in low-risk, localized, prostate cancer? Urol Oncol. 2011;29:166–170. [DOI] [PubMed] [Google Scholar]
- 13.Scales CD Jr, Presti JC Jr, Kane CJ, et al. ; SEARCH Database Study Group. Predicting unilateral prostate cancer based on biopsy features: implications for focal ablative therapy–results from the SEARCH database. J Urol. 2007;178(4 pt 1):1249–1252. [DOI] [PubMed] [Google Scholar]
- 14.Fumado L, Cecchini L, Juanpere N, Ubre A, Lorente JA, Alcaraz A. Twelve core template prostate biopsy is an unreliable tool to select patients eligible for focal therapy. Urol Int. 2015;95:197–202. [DOI] [PubMed] [Google Scholar]
- 15.Gallina A, Maccagnano C, Suardi N, et al. Unilateral positive biopsies in low risk prostate cancer patients diagnosed with extended transrectal ultrasound–guided biopsy schemes do not predict unilateral prostate cancer at radical prostatectomy. BJU Int. 2012;110(2 pt 2):E64–E68. [DOI] [PubMed] [Google Scholar]
- 16.Sfoungaristos S, Perimenis P. Parameters predicting postoperative unilateral disease in patients with unilateral prostate cancer in diagnostic biopsy: a rationale for selecting hemiablative focal therapy candidates. Can Urol Assoc J. 2013;7:E82–E87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung JW, Lee BK, Choi WS, et al. Combination of clinical characteristics and transrectal ultrasound–guided biopsy to predict lobes without significant cancer: application in patient selection for hemiablative focal therapy. Prostate Int. 2014;2:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonn GA, Margolis DJ, Marks LS. Target detection: magnetic resonance imaging–ultrasound fusion-guided prostate biopsy. Urol Oncol. 2014;32:903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance–ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valerio M, Donaldson I, Emberton M, et al. Detection of clinically significant prostate cancer using magnetic resonance imaging–ultrasound fusion targeted biopsy: a systematic review. Eur Urol. 2015;68:8–19. [DOI] [PubMed] [Google Scholar]
- 21.Tonttila PP, Lantto J, Paakko E, et al. Prebiopsy multiparametric magnetic resonance imaging for prostate cancer diagnosis in biopsy-naive men with suspected prostate cancer based on elevated prostate-specific antigen values: results from a randomized prospective blinded controlled trial. Eur Urol. 2016;69:419–425. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. ; PROMIS study group. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–822. [DOI] [PubMed] [Google Scholar]
- 23.Kasivisvanathan V, Emberton M, Moore CM. MRI-targeted biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;379:589–590. [DOI] [PubMed] [Google Scholar]
- 24.Le JD, Tan N, Shkolyar E, et al. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol. 2015;67:569–576. [DOI] [PubMed] [Google Scholar]
- 25.Johnson DC, Raman SS, Mirak SA, et al. Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging [published online November 30, 2018]. Eur Urol doi: 10.1016/j.eururo.2018.11.031 [DOI] [PubMed] [Google Scholar]
- 26.Kryvenko ON, Epstein JI. Improving the evaluation and diagnosis of clinically significant prostate cancer. Curr Opin Urol. 2017;27:191–197. [DOI] [PubMed] [Google Scholar]
- 27.Priester A, Natarajan S, Khoshnoodi P, et al. Magnetic resonance imaging underestimation of prostate cancer geometry: use of patient specific molds to correlate images with whole mount pathology. J Urol. 2017;197:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouraviev V, Mayes JM, Sun L, Madden JF, Moul JW, Polascik TJ. Prostate cancer laterality as a rationale of focal ablative therapy for the treatment of clinically localized prostate cancer. Cancer. 2007;110:906–910. [DOI] [PubMed] [Google Scholar]
- 29.Polascik TJ, Mayes JM, Schroeck FR, et al. Patient selection for hemiablative focal therapy of prostate cancer: variables predictive of tumor unilaterality based upon radical prostatectomy. Cancer. 2009;115:2104–2110. [DOI] [PubMed] [Google Scholar]
- 30.Kenigsberg AP, Llukani E, Deng FM, Melamed J, Zhou M, Lepor H. The use of magnetic resonance imaging to predict oncological control among candidates for focal ablation of prostate cancer. Urology. 2018;112:121–125. [DOI] [PubMed] [Google Scholar]
- 31.Albisinni S, Aoun F, Bellucci S, et al. Comparing high-intensity focal ultrasound hemiablation to robotic radical prostatectomy in the management of unilateral prostate cancer: a matched-pair analysis. J Endourol. 2017;31:14–19. [DOI] [PubMed] [Google Scholar]
- 32.Bahn D, de Castro Abreu AL, Gill IS, et al. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol. 2012;62:55–63. [DOI] [PubMed] [Google Scholar]
- 33.Feijoo ER, Sivaraman A, Barret E, et al. Focal high-intensity focused ultrasound targeted hemiablation for unilateral prostate cancer: a prospective evaluation of oncologic and functional outcomes. Eur Urol. 2016;69:214–220. [DOI] [PubMed] [Google Scholar]
- 34.Rischmann P, Gelet A, Riche B, et al. Focal high intensity focused ultrasound of unilateral localized prostate cancer: a prospective multicentric hemiablation study of 111 patients. Eur Urol. 2017;71: 267–273. [DOI] [PubMed] [Google Scholar]
- 35.Liu W, Laitinen S, Khan S, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed HU. The index lesion and the origin of prostate cancer. N Engl J Med. 2009;361:1704–1706. [DOI] [PubMed] [Google Scholar]
- 37.Stamey TA, McNeal JM, Wise AM, Clayton JL. Secondary cancers in the prostate do not determine PSA biochemical failure in untreated men undergoing radical retropubic prostatectomy. Eur Urol. 2001;39(suppl 4):22–23. [DOI] [PubMed] [Google Scholar]
- 38.Tay KJ, Amin MB, Ghai S, et al. Surveillance after prostate focal therapy. World J Urol. 2019;37:397–407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.