Abstract
A tonic pupil, without other features of an oculomotor neuropathy, is due to a lesion in the ciliary ganglion or short ciliary nerves. Here, we present a case of a tonic pupil in a woman with radiation-treated adenoid cystic carcinoma of the nasopharynx with perineural spread and skull base involvement. This a rare case of a tonic pupil caused by direct invasion of the ciliary ganglion or postradiation effects.
Keywords: cranial nerves, neuroopthalmology, pupil, radiology
Background
Adenoid cystic carcinoma (ACC) is a malignancy of exocrine glands that most often arises from the salivary glands, with 2.4%–3.7% of head and neck cases originating in the nasopharynx.1 Perineural spread is common via branches of the trigeminal nerve. Skull base involvement can occur through the trigeminal nerve, internal carotid artery or Eustachian tube.2
A tonic pupil is due to a lesion in the ciliary ganglion or short ciliary nerves; it is usually unilateral and displays light-near dissociation with minimal reactivity to light, retained constriction to near, and slow redilation to distance. The most common cause of an isolated tonic pupil is Adie syndrome—a benign condition associated with loss of reflexes.3 Tonic pupils arising from a direct tumour or treatment-related effects of the ciliary ganglion are uncommon. Pupillary involvement in ACC has been reported in only a few cases.2
Here, we present a case of tonic pupil and abducens nerve palsy, which were likely due to direct ACC neural invasion or side effects of radiotherapy (RT).
Case presentation
The patient is a woman in her 50s with a history of stage IVA (T4 N0) ACC of the nasopharynx. She originally presented to outside physicians with a 2-year to 3-year history of chronic congestion, runny nose, left ear pain, occipital headache and left facial droop.
An MRI at the time of initial diagnosis showed tumour infiltration of the left parapharyngeal space, deep masticator space and pterygopalatine fossa. There was perineural spread into the cranium, involving the maxillary (V2) and mandibular (V3) nerves via the foramen rotundum and foramen ovale to involve Meckel’s cave and the cavernous sinus. There was associated extensive sclerosis of the central and left skull base. No intraorbital involvement was noted.
Surgical resection was not a viable option due to diffuse involvement of critical structures. The patient completed a course of intensity-modulated radiation therapy (IMRT) of 6600 cGy delivered in 33 fractions, with gross disease receiving 200 cGy daily and areas of the tumour near the inferior orbit and left optic nerve receiving 180 cGy daily. Additionally, she received concurrent chemotherapy, initially with cisplatin and later with carboplatin.
Twenty months after completing radiation and chemotherapy treatment, the patient noticed blurry vision on left gaze and a left head turn in photographs. She denied diplopia, changes in visual acuity, facial pain, numbness or paresthesias. Her past ocular and medical history were unremarkable. Old photographs did not reveal an evident anisocoria.
Her family history was significant for a father with prostate cancer and melanoma, a sister with breast cancer diagnosed at the age 30 with a negative BRCA mutation and a sister who passed away from leukaemia at age 4.
Investigations
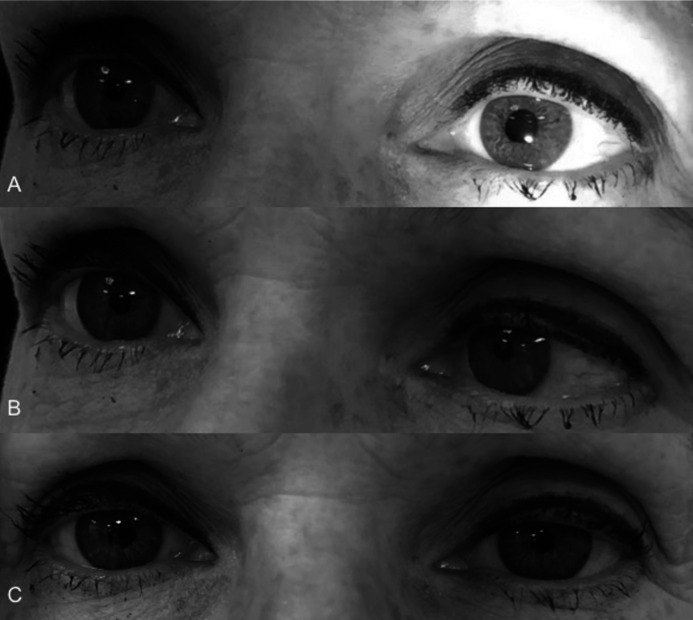
A complete neuro-ophthalmic examination was performed for her new symptoms. Physical examination revealed a visual acuity of 20/15-2 oculus dexter (OD) and 20/15-1 oculus sinister (OS). She accurately identified 6/6 AO-HRR colour plates OU and had unremarkable Amsler grid testing. Her pupil exam revealed anisocoria: left mydriasis with greater asymmetry in a brightly lit room. There was brisk constriction to light OD and imperceptible reaction to light OS (figure 1A), with OS constriction to near greater than OD (figure 1B) and tonic redilation OS on refixation at a distance. Thirty minutes after 0.125% pilocarpine in both eyes, her right pupil was no different and the left had constricted to be the smaller pupil, thus confirming the diagnosis of a left tonic pupil (figure 1C). To exclude a concomitant Horner syndrome on either side, apraclonidine 0.5% was applied to both eyes on another visit, and the anisocoria did not change. Slit-lamp examination revealed sectoral palsies of her left pupil sphincter.
Figure 1.
Anisocoria with brisk constriction to light OD and imperceptible reaction to light OS (A), with OS constriction to near greater than OD (B). 0.125% pilocarpine test demonstrates 3.75 mm OD and 3.25 mm OS, confirming the diagnosis of a left tonic pupil (C). OD, oculus dexter; OS, oculus sinister.
Motility testing revealed 35 prism diopters of esotropia in primary gaze, >50 in left gaze, 7 in right gaze, 14 in upgaze and 9 in downgaze with no vertical deviation. Intraocular pressure was 14 OU, and a dilated fundus examination was unremarkable.
Trigeminal sensation to light touch, pain and temperature were intact and symmetric in V1, V2 and V3. There were no signs of cranial nerve III or VII aberrant regeneration. Knee and ankle reflexes were 2+ bilaterally. Humphrey 24-2 visual field testing was unremarkable.
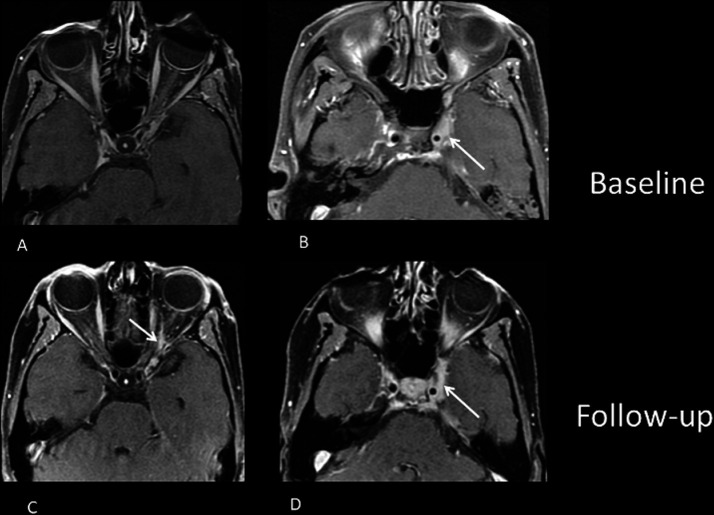
An MRI was obtained for further assessment, which demonstrated a new enhancement in the posterior orbit 1 cm anterior to the optic canal between the optic nerve and lateral rectus corresponding with the expected location of the ciliary ganglion. The degree of enhancement was slightly less intense than that of the existing tumour (figure 2A–D). Also noted was volume loss of the left lateral rectus muscle and unchanged enhancement along the left abducens nerve.
Figure 2.
New enhancement in the posterior orbit 1 cm anterior to the optic canal between the optic nerve and lateral rectus corresponding with the expected location of the ciliary ganglion (C), which previously did not demonstrate enhancement (A). The degree of enhancement is slightly less intense than that of the existing tumour (B, D).
Differential diagnosis
This patient had anisocoria greater in bright light than dim light, indicating a left mydriasis rather than a right miosis. The absence of other features of an oculomotor palsy, light-near dissociation and reversal of anisocoria following dilute pilocarpine in both eyes confirmed the diagnosis of a left tonic pupil. Restricted abduction of the left eye with esotropia worse on left gaze were consistent with a left abducens palsy.
Given her history of skull base invasive cancer, local ciliary ganglion involvement was determined to be the cause. This was confirmed with imaging. Given that the intensity of enhancement was different than that of the tumour and that there were no other sites of tumour progression, the findings are favoured to represent postradiation effects and less likely tumour invasion, although it cannot be excluded. The left abducens palsy was explained by tumour in the cavernous sinus and orbital apex, although radiation side effects could also be a factor in the presentation nearly 2 years after treatment. Other common differential causes for a tonic pupil or abducens palsy were not considered relevant in this case.
Treatment
The patient was recommended to be fit for prism glasses, and a repeat MRI was suggested to differentiate between tumour spread and radiation scarring. She was also referred to a strabismus specialist. There was no indication for biopsy of this lesion given the critical site of involvement and as sequential imaging would likely determine the need for further treatment of her malignancy.
Outcome and follow-up
The patient will be followed up with routine imaging as this may discern between tumour spread and radiation side effects.
Discussion
The ciliary ganglion is the affected site in tonic pupils.4 5 Most cases of idiopathic tonic pupils are unilateral, benign and classically seen in young women following a viral infection.6 They have also been seen in association with a variety of conditions such as sarcoidosis,7 trauma, iatrogenic damage or diabetic neuropathy.4 There is one reported case of a tonic pupil caused by direct malignant involvement of the ciliary ganglion, specifically a lacrimal gland ACC with spread to the lacrimal nerve, V1 in the cavernous sinus, the nasociliary nerve and the ciliary ganglion.8 The few additional reported cases of tonic pupils associated with malignancy in the literature have not been associated with direct ciliary ganglion involvement. For example, a previous report describes two patients with small cell lung cancer who developed bilateral tonic pupils. However, in both cases, there was no orbital pathology found on MRI, and since the patients were both found to have positive anti-Hu antibodies in the blood and cerebrospinal fluid, a paraneoplastic aetiology was favored.9 An additional report suggests an association among Hirschsprung disease, neuroblastoma and tonic pupils due to the common neural crest derivation of ganglion cells in the colon, ciliary ganglion and certain cells of the adrenal medulla.10 In the only report of direct neuroblastoma involvement of the ciliary ganglion, there is no note of pupillary involvement. The patient in this report was a 9 month old who presented with proptosis and a lateral rectus palsy and was thought to have a primary intraorbital neuroblastoma that originated in the ciliary ganglion and extended to the lateral rectus muscle.11
In a systematic review of 32 cases of ACC involving the cavernous sinus, an abducens palsy was reported in 45.2% of cases and pupillary abnormalities were reported in 15.6% of cases. However, there were no cases of tonic pupils.2
It is challenging to separate inflammation, radiation effects and diffuse tumour infiltration on MRI findings alone. The timing of symptoms 1–2 years after treatment is consistent with radiation effects. A biopsy is usually the preferred investigation to clarify these possibilities, but would be hazardous in the posterior orbit. In our patient, since the intensity of enhancement within the ciliary ganglion is different than that of the baseline tumour, and since there were no other sites of tumour progression, postradiation effects were favoured over direct tumour invasion.
In theory, a close look at the radiation paradigm that a patient receives could add insight and favour a specific aetiology. High radiation doses ranging between 60 and 70 Gy are usually recommended for the control of ACC given its dose–response relationship often cited in the literature. IMRT, which uses computer-controlled linear accelerators to deliver radiation dose more precisely than conventional RT, is nowadays favoured for ACC. IMRT allows achieving this high dose to the tumour while maintaining the dose to surrounding critical structures below their dose tolerance limits. Doses can furthermore be fractionated. Fractionated IMRT has been shown to be better at local control for ACC compared with conventional RT, achieving progression-free survival rates of 38% at 3 years.12 In our patient, IMRT was fractionated to small doses over 33 days. Areas of the tumour near the inferior orbit and left optic nerve received a maximum dose of 1.80 Gy daily, which is within their recommended tolerance dose. While radiation side effects were minimised by using a fractionated IMRT paradigm compared with conventional RT, radiation effects on healthy tissues, in this case, the ciliary ganglion, can still occur. This was likely the case in our patient.
Learning points.
A truly isolated tonic pupil is usually a benign finding. The dilated pupil will be more evident in a bright room, reacts poorly to light but briskly to accommodation. The patient must also be evaluated for an oculomotor nerve palsy. In isolated cases with reduced tendon reflexes, this is Adie syndrome.
Patients with a tonic pupil should be assessed for history or signs that might suggest other lesions in the orbit or brain. Here, the history of malignancy and abducens palsy both mandated imaging for a structural cause in the area of the ciliary ganglion.
Separating diffuse tumour infiltration from radiation effects can be difficult with MRI, and when a tissue biopsy is not safe, serial imaging can be important to watch for tumour growth and spread.
Footnotes
Contributors: MLMY wrote the case report. MLMY and JO contributed to patient evaluation. ELP helped in revising it critically for important intellectual content. JO and GM helped in final approval of the version to be published. JO, GM, ELP and MLMY agree to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Soprani F, Armaroli V, Venturini A, et al. A rare case of adenoid cystic carcinoma of the nasopharynx manifesting as Horner's syndrome: discussion and review of the literature. Acta Otorhinolaryngol Ital 2007;27:216–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Dumitrascu OM, Costa RMS, Kirsch C, et al. Cavernous sinus syndrome resulting from contiguous spread of adenoid cystic carcinoma: a systematic analysis of reported cases. Neuro-Ophthalmology 2009;33:300–7. 10.3109/01658100903226208 [DOI] [Google Scholar]
- 3.Kelly-Sell M, Liu GT. “Tonic” but not “Adie” Pupils. J Neuroophthalmol 2011;31:393–5. 10.1097/WNO.0b013e318219fbdf [DOI] [PubMed] [Google Scholar]
- 4.Harriman DG, Garland H. The pathology of Adie's syndrome. Brain 1968;91:401–18. 10.1093/brain/91.3.401 [DOI] [PubMed] [Google Scholar]
- 5.Adler FH, Scheie HG. The site of the disturbance in tonic pupils. Trans Am Ophthalmol Soc 1940;38:183–92. [PMC free article] [PubMed] [Google Scholar]
- 6.Bremner FD, Smith SE. Bilateral tonic pupils: Holmes Adie syndrome or generalised neuropathy? Br J Ophthalmol 2007;91:1620–3. 10.1136/bjo.2007.118968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowie EM, Givre SJ. Tonic pupil and sarcoidosis. Am J Ophthalmol 2003;135:417–9. 10.1016/S0002-9394(02)01959-1 [DOI] [PubMed] [Google Scholar]
- 8.Tse DT, Benedetto P, Morcos JJ, et al. An atypical presentation of adenoid cystic carcinoma of the lacrimal gland. Am J Ophthalmol 2006;141:187–9. 10.1016/j.ajo.2005.08.070 [DOI] [PubMed] [Google Scholar]
- 9.Wabbels BK, Elflein H, Lorenz B, et al. Bilateral tonic pupils with evidence of anti-Hu antibodies as a paraneoplastic manifestation of small cell lung cancer. Ophthalmologica 2004;218:141–3. 10.1159/000076151 [DOI] [PubMed] [Google Scholar]
- 10.Lambert SR, Yang LL, Stone C. Tonic pupil associated with congenital neuroblastoma, Hirschsprung disease, and central hypoventilation syndrome. Am J Ophthalmol 2000;130:238–40. 10.1016/S0002-9394(00)00480-3 [DOI] [PubMed] [Google Scholar]
- 11.Latchaw RE, L'Heureux PR, Young G, et al. Neuroblastoma presenting as central nervous system disease. AJNR Am J Neuroradiol 1982;3:623–63. [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen AD, Nikoghosyan A, Hinke A, et al. Combined treatment of adenoid cystic carcinoma with cetuximab and IMRT plus C12 heavy ion boost: ACCEPT [ACC, Erbitux® and particle therapy]. BMC Cancer 2011;11:70. 10.1186/1471-2407-11-70 [DOI] [PMC free article] [PubMed] [Google Scholar]