Abstract
Advanced pancreatic cancer carries a poor prognosis and has traditionally been treated with chemotherapy. However, immunotherapy has made great strides in a subset of patients depending on mismatch repair/microsatellite status. We present a patient with locally advanced pancreatic cancer treated with neoadjuvant chemotherapy followed by surgery and additional adjuvant chemotherapy whose disease progressed while on adjuvant chemotherapy. Tumour testing showed a mismatch repair mutation and high microsatellite instability, making her eligible for treatment with immunotherapy. Germline genetic testing confirmed the clinical suspicion of Lynch syndrome. She has had isolated sites of progression treated with radiation but overall has been receiving immunotherapy for more than 3 years, highlighting the importance of tumour testing as it may allow for additional treatment options and improved survival.
Keywords: pancreatic cancer, therapeutic indications
Background
Pancreatic cancer is the fourth most common cause of cancer-related deaths in men and women in the USA with a 5-year survival rate of only 3% for metastatic disease. Associated risk factors include tobacco abuse, obesity, diabetes and chronic pancreatitis as well as genetic predispositions such as Lynch syndrome.1
Affected patients have a 9-fold to 11-fold increased risk of pancreatic cancer as well as other malignancies.2 Lynch syndrome occurs in about 5%–10% of pancreatic cancers and results from mutations in DNA mismatch repair (MMR) genes comprising of MLH1, MSH2, MSH6 or PMS2, which code proteins responsible for correcting errors in DNA base pairing. Failures in this system, referred to as MMR deficient (d-MMR), result in a higher mutation rate and cause microsatellite instability (MSI) with tumours being classified as MSI-high (MSI-H), MSI-low or MSI-stable.
Locally advanced disease is treated with initial chemotherapy and/or radiation in hopes of inducing sufficient tumour response to allow for successful margin-negative surgical resection, often followed by additional systemic therapy. Recurrent disease is also treated with chemotherapy unless the tumours are d-MMR/MSI-H, which allows for immunotherapy as another treatment option.
Case presentation
We present a 64-year-old woman with a medical history significant for endometrial cancer and limited stage colon cancer, both of which were treated with surgical resection. She had been disease free for 11 years when she presented with back pain. Workup revealed a pancreatic tail mass with no distant metastases; biopsy of this mass returned with pancreatic adenocarcinoma that was not graded. She was initially treated with neoadjuvant chemotherapy, given she had local disease and a markedly elevated CA 19-9 in the absence of jaundice. She received gemcitabine and nab-paclitaxel for three cycles followed by distal pancreatectomy and splenectomy. The final pathology reported post-treatment pathologic stage IIB pancreatic adenocarcinoma with 5/20 lymph nodes positive. Her CA 19-9 tumour marker had responded after chemotherapy but began rising postoperatively.
She received three additional cycles of adjuvant chemotherapy with folinic acid, 5-fluorouracil, irinotecan and oxaliplatin, but these treatments were complicated by severe toxicity. Furthermore, her CA 19-9 continued to increase and magnetic resonance cholangiopancreatography following her third cycle of chemotherapy revealed a 2 cm liver lesion consistent with metastatic disease. Given her poor tolerance of therapy at this point, her primary team recommended supportive care alone, prompting her to seek a second opinion.
Investigations
Given her significant history of endometrial, colon and now pancreatic cancer, we suspected Lynch syndrome and sent for both germline and tumour genetic testing. Tumour sequencing revealed an MSH2 mutation along with MSI-H, high tumour mutational burden and several other genetic alterations. Germline testing also found a pathogenic mutation in MSH2, confirming the diagnosis of Lynch syndrome.
Treatment
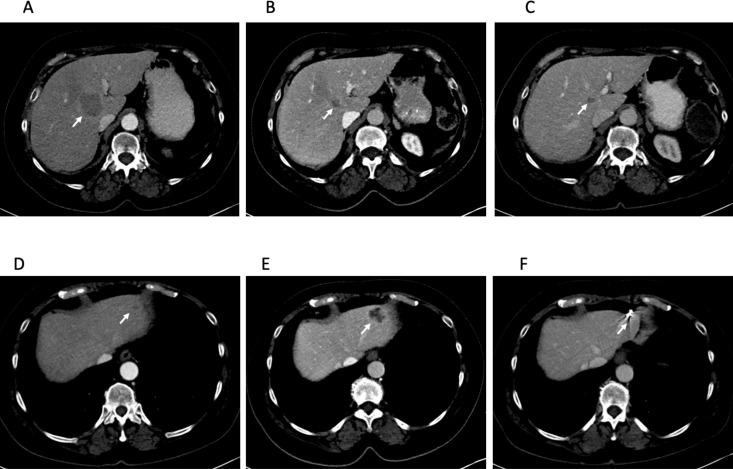
She began immunotherapy with pembrolizumab, and restaging scans 2 months later showed a 1.1 cm right lower lobe pulmonary nodule, multiple stable pulmonary nodules and 2 metastatic liver lesions. These lesions decreased in size (figure 1A–C), but 8 months later, a left liver lesion increased alongside an increasing CA 19-9 (figure 1D, E). This lesion was treated with stereotactic body radiation therapy (SBRT) with sustained improvement (figure 1F). She underwent SBRT again 18 months later to an enlarging paraaortic lymphadenopathy. CA 19-9 once again normalised after radiation. Repeat imaging also showed improvement in these lymph nodes.
Figure 1.
Central hepatic metastasis indicated by the arrow at 2 months (A), 10 months (B) and 35 months (C) after starting pembrolizumab. This lesion has had a sustained response to pembrolizumab. Left lateral hepatic metastasis 2 months (D), 10 months (E) and 35 months (F) after starting pembrolizumab. This lesion was treated with stereotactic body radiotherapy (SBRT) 11 months after starting pembrolizumab and has had a sustained response after SBRT. Fiducial placed for SBRT seen in (F).
Outcome and follow-up
She continued pembrolizumab during both of these periods of isolated progression and is still receiving it currently, now more than 3 years since beginning immunotherapy treatment. She has not required further local radiation and most recent scans show continued response (figure 1C–F).
Discussion
Chemotherapy has been the mainstay of treatment in many malignancies, including pancreatic cancer but, unfortunately, despite treatment, pancreatic cancer has continued to carry a poor prognosis due to its aggressive nature. However, immunotherapy has rapidly changed the landscape of oncology with many trials finding success in solid malignancies previously treated with only chemotherapy.
An early trial evaluated pembrolizumab in patients with treatment-refractory metastatic carcinoma with or without MMR deficiency. Pembrolizumab is an anti-programmed cell death-1 (PD-1) immune checkpoint inhibitor that binds to PD-1 on T cells, enhancing anti-tumour immune responses by reducing PD-1-mediated immune downregulation. The coprimary endpoint of 20-week immune-related progression-free survival was 78% and 71% in d-MMR colorectal and non-colorectal carcinomas, compared with 11% in MMR-proficient colorectal carcinomas. Other outcomes were also similar for d-MMR colorectal and non-colorectal carcinomas compared with MMR-proficient colorectal carcinomas, suggesting MMR status rather than cancer type was an important predictor of response to immunotherapy.3
In 2017, the Food and Drug Administration (FDA) granted accelerated approval for pembrolizumab for all MSI-H/d-MMR solid tumours that have progressed on prior treatment, making this the first FDA approval based solely on genetic features rather than cancer type. Recommendations for germline testing have also broadened from only patients with suspicious personal or family histories to now include germline genetic testing in all patients with confirmed pancreatic cancer due to increasing recognition that approximately 50% of patients found to have hereditary predisposition to pancreatic cancer do not have personal or family histories.4 The rate of germline mutation in pancreatic cancer is also higher than previously thought at about 4%–20%. This is especially important in light of additional treatment options and improved clinical outcomes with immunotherapy in patients with Lynch syndrome.
Immunotherapy has given our patient a durable response as well as better quality of life with fewer toxicities compared with chemotherapy. Isolated sites of progression were successfully treated with radiation, which can be safely incorporated as another treatment modality. Our case highlights the importance of tumour testing to identify additional treatment options in patients who otherwise would have very few options.
Learning points.
All patients with pancreatic cancer should have germline testing for hereditary cancer syndromes.
All patients with progressive metastatic cancer still eligible for treatment should have tumour testing to assess mismatch repair/microsatellite status as well as other genetic alterations that may be treated with targeted therapies.
Local therapies, including stereotactic body radiotherapy, can be used to treat isolated sites of disease progression in patients on immunotherapy.
Footnotes
Contributors: PN wrote the final draft and provided extensive edits to the previous drafts. MS wrote the majority of the initial drafts. AR made significant edits to all drafts and created the images.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Institute, N.C Cancer STAT factors: pancreatic cancer, 2019. Available: https://seer.cancer.gov/statfacts/html/pancreas.html
- 2.Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 2009;302:1790–5. 10.1001/jama.2009.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le DT, Uram JN, Wang H, et al. Pd-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoffel EM, McKernin SE, Brand R, et al. Evaluating susceptibility to pancreatic cancer: ASCO provisional clinical opinion. J Clin Oncol 2019;37:153–64. 10.1200/JCO.18.01489 [DOI] [PubMed] [Google Scholar]