Abstract
Background
The coronavirus pandemic has provoked discussions among healthcare providers how to manage cancer patients when faced with the threat of severe acute respiratory syndrome related coronavirus 2 (SARS-CoV-2) infection. Immune checkpoint inhibitor (ICI) containing regimens are standard of care in the majority of metastatic clear cell renal cell carcinoma (mccRCC) patients. It remains unclear whether therapies should be modified in response to the COVID-19 pandemic.
Methods
We performed an online survey among physicians involved in the treatment of mccRCC, and 41 experts responded. Questions focused on criteria relevant for treatment decision outside the pandemic and the modifications of systemic therapy during COVID-19.
Findings
For the majority of experts (73%), the combination of International metastatic renal cell carcinoma Database Consortium (IMDC) risk category and patient fitness are two important factors for decision-making. The main treatment choice in fit, favourable risk patients outside the pandemic is pembrolizumab/axitinib for 53%, avelumab/axitinib, sunitinib or pazopanib for 13% of experts each. During the pandemic, ICI-containing regimens are chosen less often in favour of a tyrosine kinase inhibitors (TKI) monotherapy, mainly sunitinib or pazopanib (35%).
In fit, intermediate/poor-risk patients outside the pandemic, over 80% of experts choose ipilimumab/nivolumab, in contrast to only 41% of physicians during COVID-19, instead more TKI monotherapies are given. In patients responding to established therapies with ICI/ICI or ICI/TKI combinations, most participants modify treatment regimen by extending cycle length, holding one ICI or even both.
Conclusion
mccRCC treatment modifications in light of the coronavirus pandemic are variable, with a shift from ICI/ICI to ICI/TKI or TKI monotherapy.
Keywords: renal cell carcinoma, COVID-19, pandemic, treatment pattern, decision criteria
Key questions.
What is already known about this subject?
The COVID-19 pandemic has substantial impact on public life and health care delivery all over the world. Among others, the benefit/risk ratio of cancer treatment needs to be reconsidered. Immune checkpoint inhibitor (ICI) containing regimens are standard of care in the majority of metastatic clear cell renal cell carcinoma (mccRCC) patients. It remains unclear whether and how mccRCC therapies should be modified in response to the pandemic.
What does this study add?
We performed an online survey among kidney cancer experts with the aim to ascertain their treatment algorithm outside and during the coronavirus pandemic. The degree of impact on each health system is variable as the infection struck countries at different times and may have caused resource constraints. Hence, attitudes towards mccRCC treatment modifications diverge. The most common adaptations in response to the pandemic are avoidance of one or two ICI and use of a tyrosine kinase inhibitor (TKI) monotherapy instead. In patients responding to established therapies with ICI/ICI or ICI/TKI combinations most experts change treatment regimens by extending cycle length, holding one ICI or even both.
How might this impact on clinical practice?
The results of our survey may provide some guidance in the context of mccRCC treatment and SARS-CoV-2. In particular, we would like to raise awareness to the many uncertainties on the interplay of ICI and viral infections, outcome of cancer patients with SARS-CoV-2 and whether modifications in systemic therapy during the pandemic alter long term mccRCC patient outcome.
Introduction
The coronavirus pandemic has substantial impact on public life all over the world. Since the onset of the pandemic,1 reports have been published on the adverse outcome of cancer patients with COVID-19.2 3 This has provoked discussions among healthcare providers how to manage cancer patients when faced with the threat of severe acute respiratory syndrome related coronavirus 2 (SARS-CoV-2) infection and strategies were proposed to mitigate the hazard. In response to the COVID-19 pandemic, oncological societies have issued practice information and guidance.4 ESMO recommends discussion of the benefits and risks of palliative therapy in the setting of the COVID-19 pandemic and local constraints, weighing in all relevant factors: disease prognosis, patient comorbidities and preferences, probability and risks from COVID-19 infection. Considerations should be given to drug holidays, regimens and schedules that reduce the number of hospital visits during the pandemic (once weekly as opposed to thrice or twice weekly, oral or subcutaneous alternatives as opposed to intravenous administration). In addition, ESMO issued specific priorities for several cancer types5 and published management-adapted and treatment-adapted recommendations for renal cell carcinoma.6
Treatment of metastatic clear cell renal cell carcinoma (mccRCC) has advanced substantially during the past decade. Multiple tyrosine kinase inhibitors (TKI) and the monoclonal antibody bevacizumab, targeting either the vascular endothelial growth factor (VEGF) and VEGF receptors (VEGF-R), or the mammalian target of rapamycin pathways have been shown to significantly improve progression-free survival and/or even overall survival (OS), respectively.7–12 Four randomised trials have recently established combination treatment of either two immune checkpoint inhibitors (ICI) or an ICI/VEGF/VEGF-R-targeted combination: ipilimumab and nivolumab in CheckMate 214,13 pembrolizumab and axitinib in KEYNOTE-426,14 avelumab and axitinib in JAVELIN Renal 10115 and atezolizumab in combination with bevacizumab in IMmotion151.16 So far, an improvement in OS has been demonstrated for ipilimumab/nivolumab and pembrolizumb/axitinib versus sunitinib. In summary, ICI-containing regimens are considered standard of care in the majority of mccRCC patients today.
To explore the immediate response of physicians treating patients with mccRCC to the COVID-19 pandemic, we performed an online survey. The aim of this study is to assess if and how experts in the field modify their first-line treatment in light of the COVID-19 pandemic and to reflect about these changes.
Method
We constructed a short online questionnaire. The survey was sent to 85 medical oncologists and urologists who were selected from peer-reviewed publications in the field of mccRCC, site leads of the International Metastatic Renal Cell Carcinoma (mRCC) Database Consortium (IMDC) and previous personal collaborations.17 Questions were derived from practical discussions and considerations when preparing oncology services for the coronavirus pandemic. Participants were asked introductory questions about affiliation and number of new mccRCC patients seen per year. Participants were asked about the importance of the following criteria for mccRCC first-line treatment decisions: IMDC risk category (favourable vs intermediate/poor risk), programmed death-ligand 1 (PD-L1) status (PD-L1 high/positive, PD-L1 low/negative) and patients’ fitness (fit=performance status (PS) 0–1, unfit=PS >1). Depending on the chosen criteria, a set of tailored questions followed on treatment selection outside and during the COVID-19 pandemic. Predefined therapy options included: ipilimumab/nivolumab, pembrolizumab/axitinib, avelumab/axitinib, pazopanib or sunitinib, tivozanib and cabozantinib. Finally, we explored possible treatment modifications in patients responding to previously chosen mccRCC drugs.
Data collected were descriptively summarised by frequency and proportions. Paired samples were compared using McNemar’s test. Exact p value was reported and statistical significance was set at p value <0.05. SAS V.9.4 (SAS Institute) was used for analyses.
Results
From 4 April 2020 to 15 April 2020, a total of 85 medical experts were contacted by e-mail and asked to participate in this survey. Forty-one of 85 experts responded. Not every question was answered by each participant. Twenty-nine participants (71%) are based in Europe, 10 (24%) in North America and 1 (3%) in Asia Pacific and Israel, respectively. Eighteen (44%) physicians treat more than 50 new patients with mRCC per year, 10 (24%) treat 20–50 patients, 12 (29%) treat 10–20 patients and 1 (2%) treats 0–10 patients per year.
IMDC risk category is considered relevant by 38 (93%), patients’ fitness is relevant for 36 (88%) and PD-L1 status is relevant only for 6 (15%) experts. All three factors are used by six participants, while none of these factors influences their treatment choice for two physicians.
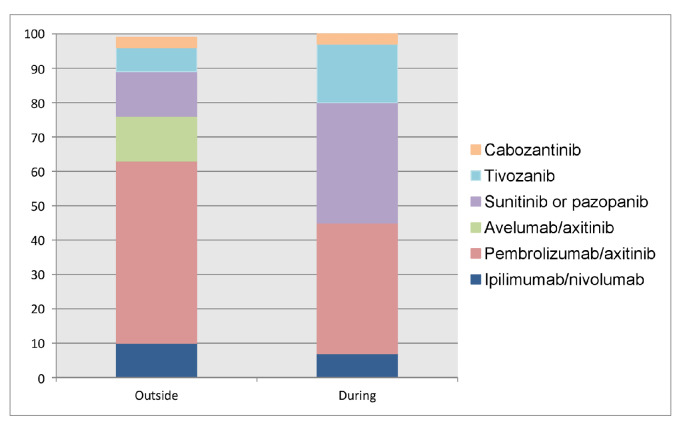
For the majority, the combination of IMDC risk category and patient fitness are two important factors for decision-making (30, 73%). The treatment choice in fit and favourable risk patients outside the coronavirus pandemic is pembrolizumab/axitinib for 16 (53%), avelumab/axitinib and sunitinib or pazopanib for 4 (13%) each, ipilimumab/nivolumab for 3 (10%), tivozanib for 2 (7%) participants and cabozantinib for 1 (3%), respectively. During the COVID-19 pandemic, ICI-containing regimens are chosen less often (11 (38%) pembrolizumab/axitinib, 2 (7%) ipilimumab/nivolumab) in favour of a TKI monotherapy (19 (35%) sunitinib or pazopanib, 5 (17%) tivozanib and 1 (4%) cabozantinib) (figure 1).
Figure 1.
Preferred treatment choice in fit, IMDC favourable risk patients outside and during the COVID-19 pandemic. IMDC, International Metastatic Renal Cell Carcinoma Database Consortium.
The difference in use of sunitinib or pazopanib outside and during COVID-19 is statistically significant (p<0.001).
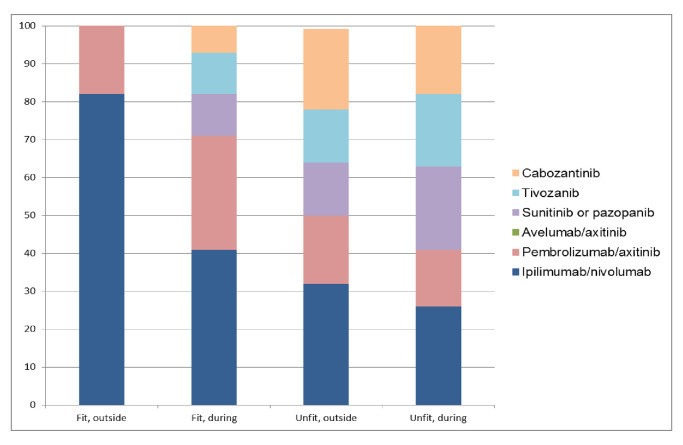
In fit and intermediate/poor-risk patients outside the coronavirus pandemic, over 80% of experts (eighteen) choose ipilimumab/nivolumab and 18% (five) choose pembrolizumab/axitinib. During the pandemic, only 11 physicians (41%) still treat with ipilimumab/nivolumab. Pembrolizumab/axitinib use increases to eight (30%), and a TKI monotherapy is recommended by one-third of physicians: three (11%) sunitinib or pazopanib, three (11%) tivozanib and two (7%) cabozantinib. The change with respect to ipilimumab/nivolumab use outside and during the pandemic is statistically significant (p<0.001). In unfit and intermediate/poor-risk patients, treatment recommendations are diverse: ipilimumab/nivolumab 32% (9 experts), cabozantinib 21% (6), pembrolizumab/axitinib 18% (5) and sunitinib/pazopanib or tivozanib 14% (4), respectively. Only small changes are seen during coronavirus pandemic (figure 2).
Figure 2.
Preferred treatment choice in fit and unfit, IMDC intermediate/poor-risk patients outside and during the COVID-19 pandemic. IMDC, International Metastatic Renal Cell Carcinoma Database Consortium.
The group of participants who consider all three criteria relevant for treatment choice (IMDC, fitness and PD-L1) treat favourable risk patients mainly with pembrolizumab/axitinib, some with ipilimumab/nivolumab and one with avelumab/axitinib. During the pandemic, a TKI monotherapy is used more often. In intermediate/poor-risk patients, only ipilimumab/nivolumab (mainly in patients with high PD-L1) or pembrolizumab/axitinib (mainly in patients with low PD-L1) is chosen. During the pandemic, ipilimumab/nivolumab is often being replaced by either pembrolizumab/axitinib, avelumab/axitinib, tivozanib or cabozantinib.
For two participants, none of the given criteria are relevant for treatment decision, neither is the coronavirus pandemic. One expert prescribes ipilimumab/nivolumab, and the other prescribes pembrolizumab/axitinib irrespective of the situation.
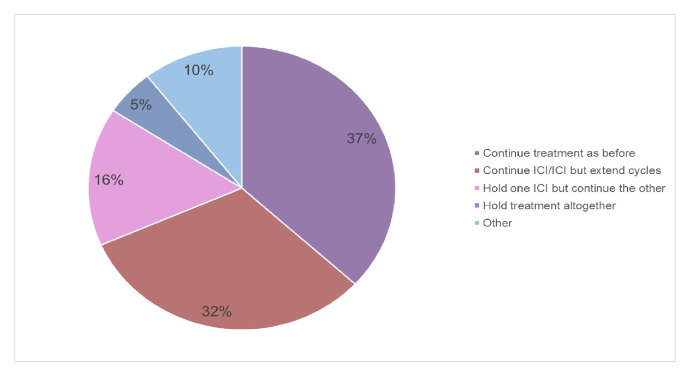
During COVID-19, in patients already responding to an ICI/ICI combination 14 (37%) continue treatment as before. Twenty-four experts adapt their treatment in the following way: 32% (12 experts) continue ICI but extend cycle length, 16 (6) % hold one ICI but continue the other and 5% (2) hold treatment altogether. For the remaining participants (10%, 4), this is a case-by-case decision and could not be generalised (figure 3).
Figure 3.
Treatment adaptation during COVID-19 pandemic in patients responding to ICI/ICI. ICI, immune checkpoint inhibitor.
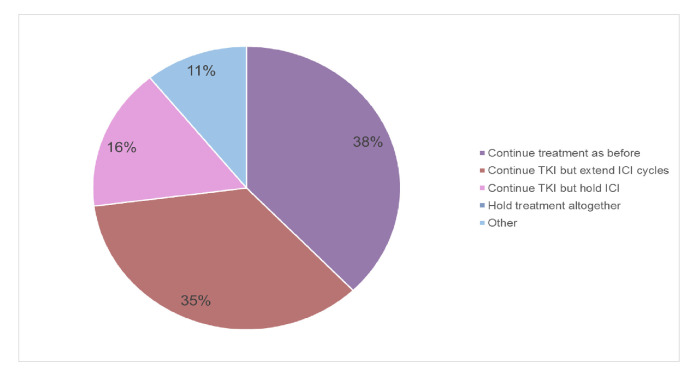
During the pandemic, in patients responding to ICI/TKI 14 experts (37%) continue treatment as earlier, 13 (34%) continue the TKI but extend ICI cycle length and 6 (16%) continue TKI but hold ICI altogether. For the five remaining participants (13%), it is again a case-by-case decision. One expert holds TKI but continues ICI (figure 4).
Figure 4.
Treatment adaptation during COVID-19 pandemic in patients responding to TKI/ICI. ICI, immune checkpoint inhibitor; TKI, tyrosine kinase inhibitor.
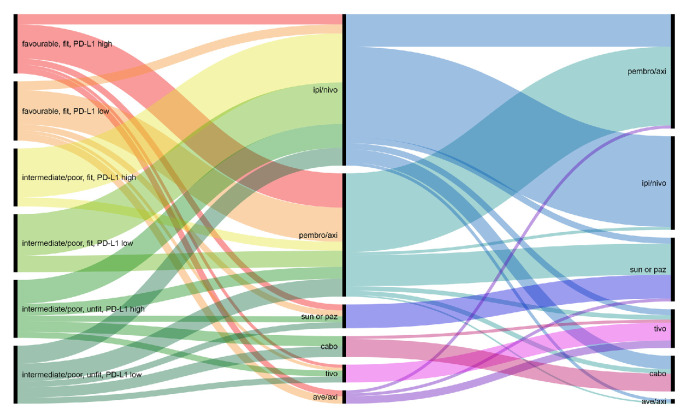
An overview of all treatment recommendations is depicted in figure 5.
Figure 5.
Overview of all treatment recommendations. Left column: decision criteria; middle column: outside COVID-19 and right column: during COVID-19. Ave/axi, avelumab/axitinib; cabo, cabozantinib; ipi/nivo, ipilimumab/nivolumab; PD-L1, programmed death-ligand 1; pembro/axi, pembrolizumab/axitinib; sun or paz, sunitinib or pazopanib; tivo, tivozanib.
Discussion
This is the first report on mccRCC systemic treatment modifications facing the COVID-19 pandemic. We performed an online survey among medical oncologists and urologists with expertise in kidney cancer treatment.
During a 12-day period, 48% of experts answered the survey. The majority of participants regard IMDC risk category and patient fitness as relevant for decision-making, while only a minority also takes PD-L1 status into account. Two experts chose treatment irrespective of these factors. The use of IMDC risk category as distinction complies with international guidelines (eg, ESMO,18 NCCN19). Nevertheless, some drugs or drug combinations can be considered across all IMDC risk groups. Concerning patient fitness, it has been shown that elderly patients experience more adverse events than their younger counterparts when receiving targeted therapy of mRCC and this may be more pronounced when using combination treatment.20 The discordance among experts concerning use of PD-L1 expression for treatment selection may be explained by the fact that a post-hoc analysis of CheckMate 214 showed longer OS and a higher overall response rate (ORR)with ipilimumab/nivolumab than with sunitinib among intermediate-risk and poor-risk patients across tumour PD-L1 expression levels.13 Though, partial responders and complete responders to ipilimumab/nivolumab both had higher baseline tumour PD-L1 expression than non-responders.21
Most common recommendations for treatment modifications in response to the coronavirus pandemic are avoidance of one or two ICI and use of a TKI monotherapy instead: half the participants abstain from using ipilimumab/nivolumab for fit and intermediate/poor-risk patients. Some choose pembrolizumab/axitinib instead, and one-third opt for a TKI monotherapy with sunitinib, pazopanib, tivozanib and cabozantinib. For patients responding to ICI/ICI, more than 50% of experts modify the treatment regimen by either extending cycle length, holding one ICI or even both. Similarly, for patients responding to ICI/TKI the majority of experts extend cycle length or only continue TKI or ICI. Some physicians decide on a case-by-case basis.
In addition to therapy modifications, some experts observe patients with asymptomatic disease and attempt to postpone treatment initiation in the current situation.
There are early data from China on the high COVID-19 lethality rate of patients with an active cancer diagnosis: in this admittedly small retrospective case study, cancer patients showed deteriorating conditions and poor outcomes.22 An Italian study assessing the case fatality of COVID-19 found that among 355 patients who died and underwent detailed chart review, 72 (20%) had active cancer.23 Importantly, at this time it remains unclear whether patients with mccRCC are at higher risk of COVID-19 infection. The published data may represent a selection bias in which patients with COVID-19 and cancer are more likely to be hospitalised.24 It is possible that anticancer treatment itself poses additional risks in inducing an immune response and causing overlapping toxicities. Cancer is usually associated with a blunted immune status, characterised by overexpressed immunosuppressive cytokines, suppressed induction of proinflammatory danger signals, impaired dendritic cell maturation and enhanced functional immunosuppressive leucocyte populations.25 Drugs blocking the PD-1/PD-L1 interaction may on the one hand enhance CD8 T-cell response and thereby decrease viral load, on the other hand they can exaggerate primary T-cell response and worsen acute infections.26 27
Apart from possible negative interference of ICI in the pathogenesis of COVID-19, controversies about SARS-CoV-2 and anticancer treatment with ICI are also driven by concerns about potential overlap between the coronavirus-related interstitial pneumonia and lung toxicity from anti-PD-1/PD-L1 agents.28 Outside the pandemic, the overall incidence rate of ICI-related pneumonitis ranges from 2.5%–5% with anti-PD-1/PD-L1 monotherapy to up to 7%–10% with cytotoxic t-lymphocyte-associated protein 4 (CTLA-4)/anti-PD-1 combination therapy in trial participants and likely the rate is significantly higher in the real-world setting.29 To our knowledge, there is currently no data on the pneumonitis rate since the onset of the pandemic.
Interestingly, data from Thoracic Cancers International COVID-19 Collaboration presented during the 2020 American Society of Clinical Oncology Virtual Scientific Program suggests that prior administration of chemotherapy as unique modality or in combination with ICI is associated with increased risk of death in patients treated for COVID-19, while immunotherapy and TKI are not.30
In addition to the apprehension of direct adverse drug effects, reasons for mccRCC treatment modifications entail efforts to maintain physical distancing and reduce hospital visits. In this line, participants may choose TKI monotherapy in favourable risk patients and omit an ICI altogether or postpone its use until after the pandemic.
Several physicians suggest reducing the frequency of radiological assessment of treatment status or treatment response. Some experts put favourable risk patients on active surveillance instead of starting active treatment. And some participants offer best supportive care to patients with IMCD poor risk and PS≥2. We would like to point to the recent update of KEYNOTE-426: the hazard ratio for pembrolizumab/axitinib versus sunitinib with respect to OS in patients with IMDC favourable risk is 1.06 (95% CI, 0.60–1.86) after a median follow-up of 27 months.31 This could support the argument of starting a TKI and adding the ICI drug when the pandemic subsides. The data need to be interpreted with caution as it is a subgroup analysis.
Nivolumab monotherapy is active in treatment-naïve mccRCC across all IMDC risk groups. However, only a minority of patients could be rescued by the addition of ipilimumab at the time of disease progression.32 Hence, compromising drug exposure in the initial treatment phase when an ICI/ICI combination is chosen needs careful balancing.
What is apparent from this survey: most experts feel unease in continuing treatment in mccRCC patients as earlier. This pandemic is an unprecedented situation and the degree of impact on each health system is variable as the infection struck countries at different times and may have caused resource constraints.33 Also, national preparation and coping strategies were manifold.34 35 Hence, it is no surprise that attitudes towards mccRCC treatment choices diverge. Some participants clearly state that they have not changed anything in their practice as of yet due to the lack of data and uncertainty regarding the interference of ICI and SARS-CoV-2 and vice versa.
There are several limitations to this analysis. A bias was introduced through selection of survey recipients. In addition, a physician with a case load of only 0–10 patients per year may not be considered a medical expert on RCC. However, we believe that international collaboration in large phase 3 trials and/or databases may be a qualification per se. Due to the small number of responses, the conclusions may not be representative and do not gather opinions from all regions of the world. For most questions only one answer could be selected, preventing participants from choosing several options. In drafting our survey, we focused on patients in imminent need of systemic treatment and therefore did not include active surveillance or best supportive care. Nevertheless, some physicians specifically mentioned these as preferred options.
There is also an ethical dimension of forgoing optimal treatment and many questions remain open. Should patients continue to receive mccRCC therapy, which has been proven to have the best long-term outcome? Or should physicians refrain from using active drugs with the presumed risk of aggravating COVID-19 and rendering patients in need of intensive care? Furthermore, in case of shortage of ventilators, presence of a chronic cancer will probably have impact on treatment allocation.36
In summary, no solid recommendation can be given of withholding ICI drugs. We would rather propose a careful decision-making process for every individual patient, weighing all short-term and long-term pros and cons. Our aim is to highlight the challenges within the management of mccRCC patients, where life expectancy and quality of life have been improved with novel anticancer therapies such as immunotherapy.
A study on the impact of major non-pharmaceutical interventions across 11 European countries for the period from the start of COVID-19 until 4 May 2020, when lockdowns started to be lifted, estimates that measures have been sufficient to drive the reproduction number below 1, achieve epidemic control and avert about 3.1 million deaths.37 Yet, far from reaching herd immunity, there is a real risk of a second COVID-19 wave.38 This raises the question on how physicians anticipate such a scenario and prepare for it. In many settings precautions taken during the initial phase of the pandemic, such as physical distancing and wearing masks, remain in place. In addition, real-world data from international collaborative projects such as ESMO-CoCARE and the COVID-19 and Cancer Consortium (CCC19) cohort study may hopefully make it possible to rapidly accumulate knowledge, which will allow to disseminate information and guidance for patients and physicians in the future.39
With this manuscript, we hope not only to gather valuable opinions from experts in the field, but also to stimulate discussion of the theoretical and practical rationales of mccRCC treatments during the coronavirus pandemic and to show the scarce current level of evidence and the varying consequences thereof. We also want to raise questions most urgently needed to be addressed in the future:
Further investigation is warranted on the interplay of ICI and viral infections, outcome of cancer patients with SARS-CoV-2 and whether treatment modifications during pandemic alter long-term mccRCC patient outcome.
Conclusion
In this online survey among medical experts treating mccRCC, a shift away from use of ipilimumab/nivolumab was shown in response to the COVID-19 pandemic and increased use of TKI monotherapy. In patients responding to established therapies with ICI/ICI or ICI/TKI combinations, most participants modify the treatment regimen by either extending cycle length, holding one ICI or even both. However, strategies in response to SARS-CoV-2 differ substantially. It is crucial to find a balance between delivering high quality and efficacious treatment while limiting exposure to coronavirus and possible overlapping toxicities due to systemic cancer treatment.
Acknowledgments
Atkins Michael, USA; Axel Heidenreich, Germany; Bearz, Alessandra, Italy; Bedke Jens, Germany; Bellmunt Joaquim, Spain; Boleti Ekaterini, UK; Cathomas Richard, Switzerland; Elizabeth Plimack, USA; Finek Jindřich, Czech Republic; Furness Andrew, UK; Gallardo Enrique, Spain; Georg A Bjarnason, USA; Heng, Daniel, Canada; Koutsoukos Konstantinos, Greece; Lorch Anja, Switzerland; Ouard Stephane, France; Powels Tom, UK; Ravaud Alain, France; Staehler Michael, Germany; Stenner Frank, Switzerland; Torgrim Tandstad, Norway; Vogelzang Nicholas, USA; Vogl Ursula, Switzerland; Wheater Matthew, UK; Wood Lori, USA; Yuasa Takeshi, Japan.
Footnotes
Twitter: @CPRT65
Contributors: SA and CR were involved in planning and conduct of the study. All authors have reviewed the data analyses, contributed to data interpretation, contributed to drafting the work and revising the publication for important intellectual content, approved the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SA: MSD (C/A), Sanofi-Genzyme (C/A) recipient: my institution. TE: Personal: AstraZeneca (RF, E, OI), Bayer (RF), Pfizer (RF), Roche (E, OI); Institution: AstraZeneca (RF), Roche (RF). BE: Pfizer (C/A), BMS (C/A), Ipsen (C/A), Roche (C/A), Oncorena (C/A), Aveo (C/A). SF: Bayer (TS), Astellas (RF, TS). VG: Astra Zeneca (C/A, H, OI, RF), Bristol-Myers Squibb (C/A, H, OI, RF), Roche Pharma AG (C/A, H), MSD Oncology (C/A, H, OI, RF), Ipsen (C/A, H, RF), Bayer (H, RF), Merck Serono (C/A, H), Janssen Cliag (C/A, H), Pfizer (C/A, H), Lilly (C/A, H), PharmaMar (H), EUSAPharm (C/A, H), Novartis (C/A, H, RF), EISAI (H), Onkowissen (C/A). JML: Achilles Therapeutics (C/A, grant support), Bristol-Myers Squibb (C/A, grant support), Merck Sharp & Dohme (C/A, grant support), Nektar (C/A, grant support), Novartis (C/A, grant support), Pfizer (C/A, grant support), Roche–Genentech (C/A, grant support), Immunocore (C/A, grant support), AstraZeneca (C/A), Boston Biomedical (C/A), Eisai (C/A), EUSA Pharma (C/A), GlaxoSmithKline (C/A), Ipsen (C/A), Imugen (C/A), Incyte (C/A), iOnctura (C/A), Kymab (C/A), Merck Serono (C/A), Pierre Fabre (C/A), Secama (C/A), Vitaccess (C/A), Covance (C/A), Aveo (C/A), Pharmacyclics (C/A). DM: BMS (H, C/A), Pfizer (H, C/A), Merck (H, C/A), Alkermes, Inc. (H, C/A). AO: Personal: Astellas (TS), Bayer (TS), Sanofi (TS), Janssen (TS). Instituional: Astellas (C/A, SB), Bayer (C/A, SB), Sanofi (C/A), Roche (C/A), Janssen (C/A, RF, SB), MSD (C/A), Molecular Partners (C/A), Teva (RF). CP: Bristol-Myers Squibb (personal fees), Merck Sharpe & Dohme (personal fees), Novartis (personal fees), Ipsen (personal fees), EUSA (personal fees), Eisai (personal fees), Janssen (personal fees), AstraZeneca (personal fees), General Electric (personal fees), Pfizer (grants and personal fees). BR: Merck (C/A), BMS (C/A), AVEO (C/A), Pfizer (C/A), Roche(C/A), Pfizer (RF), Merck (RF), BMS (RF), AVEO (RF), Astra-Zeneca (RF), Roche (RF). MS: Pfizer, BMS, Ipsen, MSD, Merck, Exelixis, EISAI, EUSA, Roche, Novartis, Alkermes. CS: Pfizer (C/A), MSD (C/A), Merck (C/A), AstraZeneca (C/A), Astellas (C/A), Sanofi-Genzyme (C/A), Roche-Genentech (C/A), Incyte (C/A). CR: Pfizer (C/A), Bristol-Myers Squibb (C/A), Roche Pharma AG (C/A), MSD Oncology (C/A), Merck (Schweiz) AG (C/A) recipient for all: my institution. Astellas Pharma (RF) recipient: my institution. Legend: (C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (TS) Travel Support; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board, Speaker Bureau (SB).
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to the study are included in the article.
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 2020. 10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 Provider & Practice Information. Available: https://www.asco.org/asco-coronavirus-information/provider-practice-preparedness-covid-19
- 5.Cancer patient management during the COVID-19 pandemic. Available: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=mail-op&utm_campaign=OncologyPRO&utm_source=hs_email&utm_medium=email&utm_content=86335874&_hsenc=p2ANqtz-9iByssoZG0oduWLWSghjTWjFy0MfOQyeRNkaju4U8hDA1bcrgYWJIFeFO_5hC2Q0J-pbDT5ZWjTDFjZUKQMl2sHw652n9Iu1neQ5jfMSJ5M-UhiE&_hsmi=86335874
- 6.Renal cell cancer management in challenging environments and health care systems. Available: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/genitourinary-cancers-renal-cell-cancer-in-the-covid-19-era
- 7.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24. 10.1056/NEJMoa065044 [DOI] [PubMed] [Google Scholar]
- 8.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061–8. 10.1200/JCO.2009.23.9764 [DOI] [PubMed] [Google Scholar]
- 9.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–81. 10.1056/NEJMoa066838 [DOI] [PubMed] [Google Scholar]
- 10.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007;370:2103–11. 10.1016/S0140-6736(07)61904-7 [DOI] [PubMed] [Google Scholar]
- 11.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol 2017;35:591–7. 10.1200/JCO.2016.70.7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer RJ, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol 2013;31:3791–9. 10.1200/JCO.2012.47.4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116–27. 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1103–15. 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019;393:2404–15. 10.1016/S0140-6736(19)30723-8 [DOI] [PubMed] [Google Scholar]
- 17.Global sites. Available: https://www.imdconline.com/globalsites
- 18.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:706–20. 10.1093/annonc/mdz056 [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Jonasch E, Michaelson MD, et al. NCCN guidelines insights: kidney cancer, version 2.2020. J Natl Compr Canc Netw 2019;17:1278–85. 10.6004/jnccn.2019.0054 [DOI] [PubMed] [Google Scholar]
- 20.Panian J, Lin X, Simantov R, et al. The impact of age and gender on outcomes of patients with advanced renal cell carcinoma treated with targeted therapy. Clin Genitourin Cancer 2020. 10.1016/j.clgc.2020.03.010. [Epub ahead of print: 16 Mar 2020]. [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019;20:1370–85. 10.1016/S1470-2045(19)30413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol 2020;31:894–901. 10.1016/j.annonc.2020.03.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onder G, Rezza G, Brusaferro S. Case-Fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020. 10.1001/jama.2020.4683. [Epub ahead of print: 23 Mar 2020]. [DOI] [PubMed] [Google Scholar]
- 24.Cannistra SA, Haffty BG, Ballman K. Challenges faced by medical journals during the COVID-19 pandemic. J Clin Oncol 2020;38:JCO.20.00858. 10.1200/JCO.20.00858 [DOI] [PubMed] [Google Scholar]
- 25.Xia Y, Jin R, Zhao J, et al. Risk of COVID-19 for cancer patients. 21. The lancet oncology, 2020: e180. 10.1016/S1470-2045(20)30150-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682–7. 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- 27.Schönrich G, Raftery MJ. The PD-1/PD-L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol 2019;9:207. 10.3389/fcimb.2019.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy 2020;12:269–73. 10.2217/imt-2020-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw 2020;20:e9. 10.4110/in.2020.20.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horn L, Whisenant JG, Torri V, et al. Thoracic cancers international COVID-19 collaboration (TERAVOLT): impact of type of cancer therapy and COVID therapy on survival. JCO 2020;38:LBA111. 10.1200/JCO.2020.38.18_suppl.LBA111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plimack ER, Rini BI, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced renal cell carcinoma (RCC): updated analysis of KEYNOTE-426. JCO 2020;38:5001. 10.1200/JCO.2020.38.15_suppl.5001 [DOI] [Google Scholar]
- 32.Atkins MB, Jegede O, Haas NB, et al. Phase II study of nivolumab and salvage nivolumab + ipilimumab in treatment-naïve patients (PTS) with advanced renal cell carcinoma (RCC) (HCRN GU16-260). JCO 2020;38:5006. 10.1200/JCO.2020.38.15_suppl.5006 [DOI] [Google Scholar]
- 33.COVID-19 Dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU). Available: https://coronavirus.jhu.edu/map.html
- 34.Pan A, Liu L, Wang C, et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA 2020. 10.1001/jama.2020.6130. [Epub ahead of print: 10 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer AJ, Morley EJ, Henry MC. Staying ahead of the wave. N Engl J Med 2020;382:e44. 10.1056/NEJMc2009409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Truog RD, Mitchell C, Daley GQ. The Toughest Triage - Allocating Ventilators in a Pandemic. N Engl J Med 2020;382:1973–5. 10.1056/NEJMp2005689 [DOI] [PubMed] [Google Scholar]
- 37.Flaxman S, Mishra S, Gandy A, et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 2020. 10.1038/s41586-020-2405-7. [Epub ahead of print: 08 Jun 2020]. [DOI] [PubMed] [Google Scholar]
- 38.Wise J. Covid-19: risk of second wave is very real, say researchers. BMJ 2020;369:m2294. 10.1136/bmj.m2294 [DOI] [PubMed] [Google Scholar]
- 39.ESMO-COCARE registry. Available: https://www.esmo.org/covid-19-and-cancer/collaborating-on-registries-studies-and-surveys/esmo-cocare-registry