Abstract
Electron Paramagnetic Resonance (EPR) spectroscopy coupled with spin traps/probes enables quantitative determination of reactive nitrogen and oxygen species (RNOS). Even with numerous studies using spin probes, the methodology has not been rigorously investigated. The autoxidation of spin probes has been commonly overlooked. Using the spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine hydrochloride (CMH), the present study has tested the effects of metal chelators, temperature, and oxygen content on the autoxidation of spin probes, where an optimized condition is refined for cell studies. The apparent rate of CMH autoxidation under this condition is 7.01 ± 1.60 nM/min, indicating low sensitivity and great variation of the CMH method and that CMH autoxidation rate should be subtracted from the generation rate of CMH-detectable oxidants (simplified as oxidants below) in samples. Oxidants in RAW264.7 cells are detected at an initial rate of 4.0 ± 0.7 pmol/min/106 cells, which is not considered as the rate of basal oxidants generation because the same method has failed to detect oxidants generation from the stimulation of phorbol-12-mysirate-13-acetate (PMA, 0.1 nmol/106 cells) in cells (2.5 ± 0.9 for PMA vs. 2.1 ± 1.5 pmol / min / 106 cells for dimethyl sulfoxide (DMSO)-treated cells). In contrast, spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), which exhibits minimal autoxidation, reveals differences between PMA and DMSO treatment (0.26 ± 0.09 vs. −0.06 ± 0.12 pmol / min / 106 cells), which challenges previous claims that spin probes are more sensitive than spin traps. We have also found that low temperature EPR measurements of frozen samples of CMH autoxidation provide lower signal intensity and greater variation compared to RT measurements of fresh samples. The current study establishes an example for method development of RNOS detection, where experimental details are rigorously considered and tested, and raises questions on the applications of spin probes and spin traps.
Keywords: Electron paramagnetic resonance spectroscopy (EPR); reactive oxygen species (ROS); reactive nitrogen species (RNS); spin probe; spin trap; 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine hydrochloride (CMH); 5,5-dimethyl-1-pyrroline-N-oxide (DMPO); phorbol-12-mysirate-13-acetate (PMA); RAW264.7 cell
Graphical Abstract

Introduction
Reactive species derived from nitric oxide, oxygen, and subsequent reactions, usually referred to as reactive nitrogen and oxygen species (RNOS) [1, 2], are implicated in virtually every physiological and pathophysiological process [3, 4]. Detection of RNOS and subsequent quantitation remain a technical challenge [5–9]. Chemiluminescent and fluorescent probes have been widely harnessed to measure RNOS [5, 10]. However, major concerns and problems about redox cycling and specificity have been neglected, resulting in erroneous statements [1, 11, 12]. Quantitative method of separating fluorescent products derived from specific species has been established [10], although, is still controversial [13].
Electron paramagnetic resonance spectroscopy (EPR) reveals positive signals only when free radicals (molecules with an unpaired electron) are present in samples. This is ideal for detection of those RNOS in the form of radicals. Nevertheless, most of the radicals are reactive and short-lived. Scavengers must be added to form relatively stable products that are also EPR positive [14, 15]. Spin traps (nitrones) are those scavengers that have been discovered and developed earlier [16]. Spin traps react with superoxide and hydroxyl radical forming distinct adducts that present distinct EPR spectra [16]. For example, the classic spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) forms DMPO-OOH• and DMPO-OH•, respectively, which have spectra with different hyperfine structure (Scheme 1) [17]. The formation of the covalent bond between DMPO and the oxidant establishes the capacity for immuno-spin trapping[18]. It appears that this can be a specific method for simultaneous measurement of superoxide and hydroxyl radical; unfortunately, the superoxide adduct is not stable and rapidly decomposes to the hydroxyl radical adduct (Scheme 1) [16]. It has also been reported that the superoxide adduct can be reduced to EPR silent products by ascorbate or ferric hemeproteins under certain conditions [16].
Scheme 1.
DMPO reacts with superoxide and hydroxyl radical forming products with distinct EPR spectra.
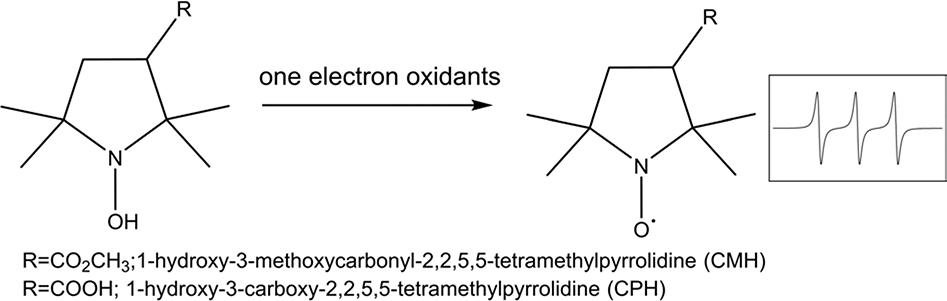
Spin probes (most commonly the cyclic hydroxylamines) are the other type of RNOS scavengers for EPR detection [19]. As shown in Scheme 2 for pyrrolidine hydroxylamines, spin probes undergo one-electron oxidation producing the nitroxide radical that can be detected by EPR [20]. They react with RNOS at a rate constant (k) of 103-104 M−1s−1, much faster than spin traps (k = 35–75 M−1s−1), thus application at much lower concentrations is feasible [20]. Spin probes have been claimed to be more sensitive than spin traps [15, 20, 21]. On the other hand, hydrogen peroxide (H2O2) can be generated from the reaction of spin probe and superoxide [22]. Although H2O2 does not directly react with spin probes [23, 24], it can exacerbate spin probe oxidation in the presence of transition metals or heme via Fenton chemistry [24]. It is worth emphasizing that various one-electron oxidants could oxidize spin probes to form EPR detectable radicals. Unlike spin trap forming covalent bond with reactant radical generating spectra identity, spin probe is oxidized to the same nitroxide radical (always giving the 3-line spectrum derived from nitrogen) regardless of the nature of the oxidant. Overall, neither spin traps nor spin probes are specific for certain RNOS; although, since EPR only measures paramagnetic species (radicals), one can infer that the measurement using spin traps or spin probes coupled with EPR is contaminated with fewer nonspecific species than that using fluorescence or chemiluminescence. Moreover, unlike fluorescent or chemiluminescent measurements that usually provide arbitrary readings of signals for qualitative comparison, EPR coupled with spin traps/probes can provide quantitative RNOS measurements without separation of products.
Scheme 2.
Spin probe pyrrolidine hydroxylamines react with one-electron oxidants forming nitroxide with universal EPR spectrum.
Despite numerous studies using spin probes, the methodology has not been rigorously investigated. Various protocols and reagents have been applied in studies. Buffers have been Krebs-Henseleit buffer [25], Krebs-Hepes buffer (KHB) [20, 21, 26–31], 10 mM HEPES containing various components [32], PBS [15] containing 0.1 – 0.3 M phosphate [33–40] or other components [28], or other mixtures [41]. Metal chelators vary from 0.1 – 2 mM diethylenetriaminepentaacetic acid (DTPA) [15, 21, 26, 28, 33, 35–37, 39–41], 2.5 – 5 μM diethyldithiocarbamate (DETC) [20, 27, 29, 31], 20 – 50 μM deferoxamine mesylate (DFO) [20, 25, 27, 29, 31, 34, 38], 1 mM ethylenediaminetetraacetic acid (EDTA) [40], to pretreatment with Chelex® [28, 37, 41]. Sometimes metal chelators are applied in combination, or no metal chelator is indicated [30, 42]. Incubations at room temperature [20, 25, 32], 37°C [15, 20, 26, 29–31], or on ice [39] have been performed, or the incubation temperature is not specified [21, 27, 28, 33–38, 40, 41]. Oxygen has been occasionally removed from the stock solution of spin probes [26–28, 31, 33, 34, 41]; however, this protocol has not been consistently applied. All of these variations could impact experimental results and there has not been an established rationale for using specific conditions reported. Moreover, the spontaneous oxidation of the spin probe to its oxidized radical form in a solution containing oxygen, so called autoxidation, is well known [20, 25, 38, 40]. Autoxidation could significantly diminish the sensitivity of the method; however, there have not been reports on its kinetics.
1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine hydrochloride (CMH) reacts the fastest amongst the spin probes with superoxide (k = 1.2 × 104 M−1s−1) producing CM• [20]. It has been widely utilized to detect both intra- and extra-cellular RNOS [20, 26, 29, 31]. The oxidation product of the spin probe 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine (CPH), CP• (Scheme 2), has the same EPR spectrum (g-factor and hyperfine structure) as CM•. Also due to its commercial availability, it has been commonly used as standards for quantitation of CM• concentration [20, 26, 29, 31]. In the current study, using CP• as standards for quantitation, we have tested the effects of various experimental parameters (metal chelators, temperature and oxygen, etc.) on CMH autoxidation and established an optimized protocol/method for minimized CMH autoxidation. We further implemented our method to quantitate the rate of RNOS generation from unstimulated and stimulated RAW264.7 cells. RAW264.7 cells are chosen based on their ease of maintenance and longevity; they have also been frequently used for redox related studies [15, 21, 26, 29, 43–61]. Phorbol-12-mysirate13-acetate (PMA) is used as the stimulator since it activates NADPH oxidase via protein kinase C, which subsequently induce the production of superoxide (predominately) [62].
Some definitions must be clarified for accuracy. First, as described above, CMH does not detect a specific RNOS, but any one-electron oxidants that can oxidize it. In other words, this method detects CMH-reducible oxidants. Second, the rate of CM• formation can substitute for the rate of RNOS generation only under the assumption that the generation of the CMH-reducible oxidants is the rate-limiting step. In other words, the generation of the CMH-reducible oxidants is much slower than the penetration of CMH into the cells, and as soon as the oxidants are generated, they are scavenged by CMH and CM• is produced. In addition, so called “RNOS generation” includes not only the original RNOS generated from cells, but also likely the CMH-reducible intermediates from the initial reactions, for instance, the RNOS generation from downstream reactions of H2O2 that is produced from CMH reaction with superoxide as described above. Third, due to the presence of tremendous amounts of antioxidants inside of cells, what we obtained is the net production of the CMH-reducible oxidants that are generated after subtracting those eliminated by antioxidants in cells. With that being said, we define that this method detects CMH-detectable oxidants, which is simplified as “oxidants” as follows.
Methods
Materials
Diethylenediamine pentaacetic acid (DTPA, cat# D6518), deferoxamine mesylate (DFO, cat# D9533), diethyldithiocarbamate (DETC, cat# 228680), phorbol-12-mysirate13-acetate (PMA, cat# P8139), and dimethyl sulphoxide (DMSO, cat# D2650) were purchased from Sigma-Aldrich (St. Louis, MO). 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine hydrochloride (CMH, cat# ALX-430–117), 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine (CP•, cat# ALX-400–018), and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO, cat# ALX-430–090) were from Enzo Life Sciences (Farmingdale, NY). Dublecco’s Modification of Eagles Medium (DMEM, cat# 10–013-CV) and phosphate buffered saline (PBS, cat# 21–031-CV) were from Corning Cellgro (Corning, NY), fetal bovine serum (FBS, cat# AM-SM-001) from Amizona (Birmingham, AL), and 10,000 units/mL penicillin and 10,000 μg/mL streptomycin (cat# 15140–122) from Thermo Fisher (Waltham, MA).
Cell Culture
RAW264.7 murine macrophage cell line was purchased from ATCC (Manassas, VA) and were cultured in DMEM supplemented with 10% FBS as well as 100 units/mL penicillin and 100 μg/mL streptomycin.
Sample Preparation
Buffer was added to the weighed solid to directly achieve the concentration of 0.2 or 0.5mM for CMH and of 50mM for DMPO. For the metal chelator effects, 0.5M CMH stock solution was made in PBS first, and then immediately diluted in PBS containing respective metal chelators to a final concentration of 0.5mM. A timer was started when the buffer was added to solid. Cells were washed twice with PBS and collected via scrapping in PBS containing 1mM DTPA, pH 7.4 (PBSD). Following centrifugation at 300 × g for 5 minutes, the cell pellet was resuspended in PBSD containing CMH or DMPO. For the experiments involving stimulation, PMA or the same volume of DMSO (<0.5‰ of volume) was added to cell resuspension. Cell concentration and viability were determined before and after the experiment by trypan blue exclusion and TC20 automated Cell Counter (Bio-Rad, Hercules, CA).
Samples were kept at room temperature (RT) (or, on ice or at 37°C for temperature effect), and open to the air (or sparged with nitrogen for at least 20 min for oxygen effect). Samples containing cells were mixed from time to time by gently inverting the container upside down. Aliquots were either injected into Aqua-X sample cell for EPR measurement at RT, or transferred into EPR quartz tubes (cat#: 727-SQ-250M, from Wilmad-LabGlass, Vineland, NJ), frozen in liquid nitrogen, and stored at −80°C until analyzed at 150K. For fresh samples, the time was recorded when the EPR run started; for frozen samples, when the EPR tube was merged into liquid nitrogen.
EPR Measurements
EPR spectra were recorded by Elexsys E500 (Bruker, Billerica, MA) equipped with 4122SHQE-W1 resonator. The instrumental settings were the same as those have been extensively applied in previous studies using spin probes [20, 29]. For RT measurements [20] they were: 2 G modulation amplitude, 40.96 ms time constant, 81.92 ms conversion time, 20 mW power and 1 scan. For measurements at 150K, the temperature was maintained by variable temperature control unit, and the instrumental settings [20, 29] were: 5 G modulation amplitude, 40.96 ms time constant, 81.92 ms conversion time, 2.0 mW power, and 4 scans were accumulated.
Data analysis
Spectra were analyzed using the XEPR software bundled with the instrument. Since the EPR spectrum is the first derivative of the actual absorption spectrum, spectra were first integrated to obtain the absorption spectra. This was followed by baseline correction and subsequent second integration that yielded the area under curve (AUC) in arbitrary units (AU). The AUC was then used to calculate the concentration of CM• or DMPO-OH• based on the CP• standard curve. As shown in the Results, 150K spectra had much lower intensity than RT ones. The baseline of the actual absorption spectra acquired by the first integration was very irregular and the intensity was so low compared to the baseline noise that the AUC generated by the second integration varied largely. Instead of AUC, the difference between the peak and the trough in the EPR spectra (Peak to Trough) has been extensively used for quantitation of signal intensity [20, 29, 31]. We found that the method of Peak to Trough provided much lesser variability (based on R2) for CP• standard curves compared to the quantitation using AUC. Therefore, the quantitation for 150K measurements was attained using the method of Peak to Trough (in AU). Another common method is to simulate the spectra then integrate.
As discussed below, the rate of CMH autoxidation was subtracted from the rate of CM• generation in samples. This value was then normalized to (divided by) the cell concentration to obtain the rate with the units of pmol / min / 106cells.
Data are expressed as mean ± standard deviation (SD).
Results
Reproducibility and stability of CP• standards
Fig. 1 shows our standard curve averaged from 6 independent preparations of 0–10μM CP•. R2=0.9843 demonstrates a good linear regression for standard curves. The slope and the intercept are 9.225±0.184 arbitrary units (AU) / μM and 0.2315±0.7950 AU, respectively. To increase the precision of our standard curves (Fig. 1), more than 10 mg CP• solid has been weighed, dissolved, and then diluted to 0–10μM for each independent preparation. In addition, the reproducibility of the standard curve and the potential variation from weighing the CP• solid suggest that generating a new standard curve on each experimental day is not necessary. Based on our standard curve, the equation that we used to quantitate CM• concentration is [CM•] (μM) = (AUC + 0.2315) / 9.225.
Figure 1. Reproducibility of CP• standards.
0–10 μM CP• in PBS containing 1mM DTPA was measured freshly (n=6). The standard curve is AUC = 9.225 × [CP•] - 0.2315, R2 = 0.9843. SD for the slope and the intercept are 0.1843 AU/μM and 0.7950 AU, respectively.
We have also tested the stability of the CP• solution. The same CP• stock solution was either diluted to 0–10μM then measured fresh (data included in Fig. 1) or stored at −20 or −80°C for up to 4 months, then thawed, diluted, and measured. The standard curves from CP• solutions after storage are not different from the standard curve shown in Fig. 1 (data not shown), indicating that CP• solution is stable for at least 4 months when stored at −20 or −80°C.
Reduction of CMH autoxidation and selection of experimental protocol
To increase the sensitivity of our method, we have attempted to reduce the baseline generation of CM• from CMH autoxidation. Typical concentrations of 0.2 [29, 31], 0.5 [15, 20, 31, 34], and 1.0 mM [20, 25, 27, 28, 33, 37] have been used for spin probes in previous reports. CMH concentration has been optimized for oxidant generation induced by PMA in human lymphoblasts, and ≤ 1mM has been suggested [21]. We have found that the higher the concentration, the faster the autoxidation (data partially shown in Fig. 9). On the other hand, the higher the concentration, the more competitive the spin probe is to endogenous antioxidants in samples that could interfere with oxidants detection. In this study, we use 0.5 mM that is in the middle of the range of commonly applied concentrations [20, 27–29, 31, 33, 34, 37].
Figure 9. CMH auto-oxidation measured at 150K.
0.2 and 0.5mM CMH in PBS containing 1mM DTPA were measured fresh or snap-frozen in quartz EPR tubes at different time points, frozen samples were stored at −80°C overnight, then measured by EPR at 150K. Representative time courses from two independent experiments.
Trace transition metals present in buffers catalyze redox reactions that can be inhibited by metal chelators. Here we have chosen metal chelators that have been applied in previous studies using spin probes [20, 21, 25–29, 31, 33–41] and compared their effects on CMH autoxidation. Starting with concentrations that have been applied in previous studies [20, 21, 25–29, 31, 33–41], we have also tested whether higher concentrations of these metal chelators would further reduce CMH autoxidation.
Fig. 2 shows representative results from two independent experiments. CM• generated from CMH autoxidation either in the presence or the absence of metal chelators increases linearly with time (R2 ≥ 0.998) up to 6 hrs. The slope of the linear regression is defined as the rate of CMH autoxidation. In Fig. 2A, the autoxidation rate of 0.5 mM CMH in PBS decreases from 42.3 to 16.1 nM/min in the presence of 2.5 μM DETC. However, higher concentrations of DETC (10 and 25 μM) are not able to further decrease the rate (19.8 and 18.3 nM/min, respectively). DTPA is more effective and can reduce the autoxidation to 8.22 nM/min at 0.1 mM, and further to 8.01 nM/min at 1.0 mM (Fig. 2B). However, no further decrease (8.48 nM/min) is observed at higher concentration 10.0mM (Fig. 2B). DFO reduces the autoxidation to 7.46 nM/min at 25 μM (Fig. 2C). Similar to DETC, higher concentrations of DFO (250 and 500 μM) do not further decrease the autoxidation rate (7.70 and 8.50 nM/min, respectively in Fig. 2C). Although DFO is the most effective inhibitor of CMH autoxidation compared to DETC and DTPA, it is not suitable for RNOS detection in cells. DFO can enter cells through endocytosis [63, 64] and can react with carbonate and nitric dioxide radicals [65], the major derivatives of peroxynitrite. Thus, we have chosen 1mM DTPA for our experiments with cells as follows.
Figure 2. Effects of metal chelation on CMH auto-oxidation.
CMH solid was dissolved in PBS directly for 0.5M, then the stock solution was diluted to 0.5mM with PBS containing various concentrations of (A) DETC; (B) DTPA or (C) DFO, CM• generation was followed by EPR. Shown are representative time courses from at least two independent experiments.
Chelex®, a resin-based polymer that binds polyvalent metal ions, has also been used to incubate with buffers prior to CMH addition to reduce CMH autoxidation [28, 37, 41].
Surprisingly, we have found that pre-treatment of PBS with Chelex® slightly increases the rate of CMH autoxidation (data not shown).
Temperature is a key determinant of reaction kinetics. We have further tested temperature effects on the rate of CMH autoxidation. Aliquots from the same 0.5 mM CMH solution have been maintained on ice, at room temperature (RT) or 37°C. As shown in Fig. 3, the rate of CMH autoxidation increases as the reaction temperature increases, more specifically, 1.08 nM/min on ice, 16.0 nM/min at RT and 54.2 nM/min at 37°C. These results suggest that within the range of experimental temperatures that are suitable for the aim of the study, the lower the temperature the sample is prepared at, the slower is the baseline generation of CM•, therefore, the more sensitive is the method. Here, we have chosen RT for our cell studies as follows.
Figure 3. Temperature effects on CMH auto-oxidation.
Solutions of 0.5mM CMH in 10 mM HEPES buffer containing 1mM DTPA were placed either on ice, at room temperature, or at 37°C, CM• generation was followed by EPR.
Some reports have diminished oxygen in CMH solution to reduce autoxidation [26–28, 31, 33, 34, 41]. As shown in Fig. 4, sparging nitrogen through 0.5 mM CMH can decrease the rate of CMH autoxidation from 7.63 nM/min to 1.69 nM/min in PBS containing 1 mM DTPA, and from 4.84 to 1.56 nM/min in HEPES containing 1 mM DTPA. It appears that the removal of oxygen did not completely inhibit CMH autoxiation, which may be due to residual oxygen left in the solution even after sparging for 20 min. Removing oxygen from CMH solution is only applicable to samples that would not be affected by hypoxia/anoxia. For instance, we prepare and maintain our CMH solution under room air for our cell studies (see below) since hypoxia/anoxia significantly alters RNOS generation in cells [66, 67].
Figure 4. Oxygen effects on CMH auto-oxidation.
CM• generation from 0.5mM CMH in PBS (PBSD) or 10mM HEPES(HEPESD) containing 1mM DTPA was followed by EPR. Oxygen was removed by sparging nitrogen gas through the solution for at least 20min. Shown are representative time courses from at least two independent experiments.
Reproducibility of CMH Autoxidation
As rationalized above, the protocol we would use for our cell studies is 0.5 mM CMH in PBS containing 1 mM DTPA under room air at RT. Using this protocol, CMH autoxidation from 31 independent experiments and followed within various time ranges are plotted in Fig. 5. Consistent with results above, all time courses are in good linear regression (R2 > 0.96) up to about 10 hrs. The rate of autoxidation (the slope of the linear regression from each time course) averages as 7.01 ± 1.60 nM/min excluding one outlier (16.0 nM/min) that is identified via the ROUT method with Q = 0.1%. This continuous generation of CM• by CMH itself, especially at a rate of about 7.01 nM/min, could be significant in samples with less oxidants generation. To increase the sensitivity and the accuracy of the detection, we suggest to measure CM• generation with time in samples, and that the rate of oxidants generation in samples should be calculated by subtracting the rate of baseline CMH autoxidation from the rate of CM• generation in samples. In addition, this baseline CMH autoxidation that is subtracted should be from the same CMH solution that is used to contain the sample on the same experimental day (which we call same pair in the following).
Figure 5. Reproducibility of CMH auto-oxidation.
CM• generation from 0.5mM CMH in PBS containing 1mM DTPA was followed by EPR. Shown are time courses from 31 independent experiments. Autoxidation rates were obtained by linear regression.
Detection of basal oxidant production from RAW264.7 cells
We first apply the above protocol/method to detect the basal production of oxidants from RAW264.7 cells. Following collection and washes, cells are resuspended (at time 0 in Fig. 6A) in CMH solution for cell concentrations ranging from 1–30 × 106 cells/ml. Fig. 6A shows time courses of CM• generation from cell suspensions at representative low (2.18 × 106 cells/ml), middle (16.8 × 106 cells/ml) and high (31.8 × 106 cells/ml) cell concentrations. The initial increase of CM• in cell suspension seems positively correlated with cell concentration. The increase slows down with time, which is more obvious with higher cell concentrations. We have found that there is no significant change (by paired t-test) in cell viability within at least an hour under our experimental condition (data not shown). This is consistent with previous report that 0.5 mM CMH is not toxic to cells [19]. Therefore, the slowdown is not due to cell death. It could be due to the potential changes with time of the oxygen content in cell suspension. RAW264.7 cells consume oxygen at a rate of 38 ± 4 pmol / s / 106 cells at 37°C [45]. In a closed system containing 107 cells/ml, oxygen can be depleted to below 100nM within 5–10 min [68]. Although the cell suspensions are open to the air and are mixed from time to time (see Methods), cells (especially at higher concentration) may consume oxygen fast enough to overcome the rate of external oxygen entering the cells. Therefore, we focus on the initial generation of CM• within the first 10–15min. Following subtraction of the rate of CMH autoxidation in the same pair, the initial rates of CM• generation at various cell concentrations are shown in Fig. 6B. A linear regression gives a slope of 4.0 ± 0.7 pmol / min / 106 cells (Fig. 6B). In addition to the known variation in cell counting, the scatter (R2 of 0.57) is likely an indication of the variability of the CMH method, especially after the CMH autoxiation is subtracted, which is variable as shown in Fig. 5.
Figure 6. Detection of CMH-detectable oxidants from various concentration of RAW264.7 cells.
CM• generation was followed once cells were resuspended in PBS containing 1mM DTPA and 0.5mM CMH. (A) representative time courses at low, middle and high cell concentrations, cells were resuspended at time 0. Initial rates within the first 10–15min (B) were obtained by linear regression and CMH auto-oxidation was subtracted, n = 11. The slope in (B) is 4.0 ± 0.7 pmol / min / 106 cells.
Detection of oxidant production from PMA-stimulated cells
We have further tested whether our current protocol/method is able to detect the oxidant generation from RAW264.7 cells stimulated by PMA. Although mole per cell has been highly recommended as dosing metrics for cell culture [69], it has not been frequently practiced. A search with “phorbol-12- myristate-13-acetate (or PMA) RAW264.7 cells reactive oxygen species” as keywords discovered 21 nonoverlapping publications from PubMed [15, 44–46, 50–61, 70–74]. Combined with 5 other publications in the same category that we have found separately [20, 21, 26, 47, 49], none reported PMA concentration in mole per cell metrics. Among these 26 reports, there are 10 where enough information is provided so that the concentration in mole per cell can be calculated [21, 26–28, 31, 33, 34, 41, 46, 49]. Applied PMA concentration ranges from 0.1–10 nmol / 106 cells [21, 26–28, 31, 33, 34, 41, 49] except that one report used 0.0016 nmol / 106 cells [46]. Here, we have chosen low PMA concentration of 0.1 nmol / 106 cells.
Once resuspended in CMH solution, cell suspensions of 1–4 × 106 cells/ml are simulated by 0.1 nmol / 106 cells of PMA (at time 0 in Fig. 7A & C). The same volume of dimethyl sulfoxide (DMSO) is used as control. Since DMSO can react with radicals and act as an antioxidant [75], less than 0.5‰ (in volume) of DMSO was applied. As shown by a representative group of time courses in Fig. 7A, CM• generation from cells stimulated with PMA or DMSO is also linear with time, R2 > 0.92 for all time courses that are used for rate calculation shown in Fig. 7B. The slopes of the linear regression have been used as the rate of oxidant generation following subtraction of CMH autoxidation and normalization to cell concentration. As shown in Fig. 7B (n = 6), CM• is generated at a rate of 2.5 ± 0.9 pmol / min / 106 cells in cells stimulated by PMA, although this rate is not different from that in control cells treated with DMSO (2.1 ± 1.5 pmol / min / 106 cells). The statistics are performed by both paired and unpaired t-test.
Figure 7. Generation of CMH- and DMPO-detectable oxidants in RAW264.7 cells stimulated by PMA.
PMA or DMSO (<0.5‰ of volume) was added to cell suspensions (1–4 × 106 cells/ml) in PBS containing 1mM DTPA and (A,B) 0.5mM CMH or (C,D) 50mM DMPO for final concentration of 0.1 nmol / 106 cells, CM• or DMPO-OH• generation was followed by EPR, the generation rate (B and D, initial rate for PMA stimulated cells in DMPO) was obtained by linear regression and normalized to cell concentration after CMH or DMPO auto-oxidation was subtracted. (A) and (C) are representative time courses, PMA and DMSO were added at time 0. n = 6 for (B) and 5–6 for (D). *, p = 0.001 by unpaired t-test.
Our result implies that the current CMH method is not sensitive enough to detect oxidant generation from RAW264.7 cells stimulated by PMA, at least at low concentration. To confirm that this is not due to fault in our experimental condition or design, we repeated the experiments with the classic spin trap DMPO. The experimental protocol/method and data analysis are exactly the same except that 0.5 mM CMH is replaced by 50 mM DMPO. Fig. 7C shows a representative group of time courses using DMPO. DMPO undergoes little autoxidation compared to CMH (0.075 ± 0.032 nM/min, n = 7). More importantly, compared to DMSO, PMA induces a burst generation of DMPO-OH•, which slows down with time. The slowdown is possibly due to cell adaptation and endogenous antioxidants arousal. There is no significant change in cell viability in the presence of PMA at this low concentration for at least an hour (by paired t-test, data not shown). This is in the presence of 50mM DMPO. Therefore, 50mM DMPO alone is not be toxic to cells under our experimental conditions, which is consistent to previous reports [16]. The rates of DMPO-OH• generation from DMSO-treated cells and from the burst for PMA-stimulated samples are summaries in Fig. 7D (n = 5–6). PMA stimulated DMPO-OH• generation at a rate of 0.26 ± 0.09 pmol / min / 106 cells that is greater than that from DMSO-treated cells (−0.06 ± 0.12 pmol / min / 106 cells), p = 0.001 by unpaired t-test. Our result indicates that PMA is able to stimulate RNOS generation under our experimental condition, which can be detected by DMPO.
Frozen samples for EPR measurement at 150K
The EPR technique is complicated and the instrument and supplies are expensive, which has limited its availability in laboratories. It is quite often that the samples are frozen and then shipped to the laboratory where the EPR measurements can be performed. Usually low temperature instead of RT measurements are employed to avoid changes in samples due to freeze-thaw processes. Indeed, low temperature EPR measurements of frozen samples have been frequently applied for tissues and in vivo studies [20, 26, 29, 31]. In the following study, we have snap-frozen CP• standards (Fig. 8) and the time course samples from CMH autoxidation (Fig.9), and stored them at −80°C. Their EPR spectra are measured at 150K using previous established instrumental settings [20], and the quantitation is compared to that from RT measurements using fresh samples from the same preparation.
Figure 8. CP• standard curves measured at RT or 150K.
Solutions of 0–10 μM CP• in PBS containing 1mM DTPA were measured fresh, or, snap-frozen in quartz EPR tubes and stored at −80°C for up to 12 months, then measurèd by EPR at 150K (n=4). The 150K results are enlarged in the insert.
Much lower intensity is obtained from frozen samples and 150K measurements (spectra not shown). For comparison, RT measurements for fresh samples from the same preparation are also analyzed using the same method (Peak to Trough, see Methods). As shown in Fig. 8, for the same preparation of CP• standards, RT measurement of fresh samples gives a peak to through value that is about 10 times more intense than that from 150K measurements of frozen samples. The same set of frozen CP• standards has been measured at 150K four times within a year. Although less intense, 150K measurements can still provide standard curves with good linear regression, R2 ≥ 0.994 (Fig. 8 insert). There are no outliers identified (by the ROUT method with Q = 1%) for either the slope or the Y-intercept of these standard curves (Fig. 8 insert), indicating the stability of CP• standards as discussed above. However, there is much more variation in 150K measurements of time course samples of CMH autoxidation (representative time courses from two independent experiments shown in Fig. 9). In addition to low intensity, 150K measurements of autoxidation time courses of 0.2 and 0.5 mM CMH have the linear regression with R2 = 0.619 and 0.735, respectively, much lesser than that from RT measurements of fresh samples from the same preparation (R2 = 0.998 and 0.999, respectively).
Discussion
Buffers including KHB [20, 21, 26–31], HEPES [32], and PBS [33–40] have been applied in studies using spin probes. As shown in Fig. 2, metal chelation significantly inhibits CMH autoxidation in PBS, indicating that metals, that are inevitable as contaminants in buffer salt, are the key catalysts of the autoxidation. The more salts included in the buffer, the more metal contamination is introduced. Hence, the component in the buffer used for spin probe studies should be simplified as long as it is still fulfilling experimental needs. It is also worth noting that HEPES may produce hydrogen peroxide either by itself [76] or by reacting with peroxynitrite [77]. Accounting for these factors, we have chosen PBS for our current studies using cell suspension.
To improve the sensitivity of the method using spin probes, metal chelator(s) should be added regardless of the buffer choice. In addition to efficiency, the biochemical properties of metal chelators should be considered for the choice of chelator(s). In addition to what has been described for DFO in Results, cell permeable DETC [78, 79] would interfere with intracellular redox chemistry, therefore, should not be used for samples containing cells.
As shown and discussed in Results, spin probe concentration, temperature, and oxygen content in samples in addition to buffer and metal chelators should also be considered for selection of experimental protocol. Overall, redox chemistry is complex, experimental details should be considered and tested rigorously for studies involving RNOS. The current study establishes an example for method development of RNOS detection. Although it focuses on the application in cell suspension, similar considerations and practices can be applied to studies, for instance, using tissues and by live imaging. Ideally, methods/protocols can be optimized and standardized in the society for reliable comparison of results amongst laboratories.
We have obtained the rate of CMH autoxidation as 7.01 ± 1.60 nM/min for 0.5 mM CMH in PBS containing 1 mM DTPA at RT under room air. To our knowledge, this is the first report on the autoxidation rate of spin probes under any condition. It is likely that superoxide can be generated from CMH autoxidation as oxygen acts as the one-electron oxidant. As described in the Introduction, the generated superoxide can further react with CMH generating hydrogen peroxide [23, 24] and subsequent intermediates in the presence of transition metals or heme via Fenton chemistry [24]. The generation of these intermediates can be tested by the addition of competitive species such as superoxide dismutase and catalase. Therefore, similar to what we emphasized in Introduction, it is the apparent rate of CMH autoxidation that we measured (the rate of CM• generation), which can be a result of CMH reaction with oxygen and downstream intermediates from that reaction. The high uncertainty of 22.8% (SD/mean) reveals low consistency of CMH autoxidation even in the presence of metal chelators. The SD value also demonstrates that the detection limit of this method is greater than 1.60 nM/min due to the CMH autoxidation. The significant CMH autoxidation and its low consistency suggest measuring and subtracting CMH autoxidation from samples in order to increase the sensitivity and the accuracy of the detection.
Also due to the continuous and significant CMH autoxidation, the rate of oxidant generation (in the form of CM• generation) instead of single point/time measurement [26, 30, 32] is suggested to be performed. Since current EPR technology is still limited to one measurement/sample at a time and each measurement takes minutes, single-point/time measurement at RT could be valuable only if the time frame from preparation of CMH solution till EPR measurement is exactly the same for each individual sample (including CMH alone). Otherwise, sensitivity and accuracy of 7.01 nM would be lost for every minute of difference in this time frame between samples. Achieving the same time frame requires that each CMH solution used for individual samples must be prepared directly from CMH solid, but not from the same stock solution with or without dilution. A typical example is shown in Fig. 4. The first measurements of sparged samples are actually greater than those of respective room air samples, which is simply because they were measured after the respective room air samples in real time. However, the rates over time demonstrated the effect of oxygen.
Reports with low temperature EPR tend to use single point/time measurement [20, 26, 29, 31]. Theoretically, low temperature EPR measurements use samples frozen at specific moments, consequently, eliminating the false increase of CM• due to continuous CMH autoxidation in the next sample while the current sample is measured, which takes place at RT measurements. However, single point/time measurements at low temperature can preserve the sensitivity and accuracy only if the addition of CMH solution into the samples is almost at the same time for each sample, and so is the freezing of samples. In addition, as discussed in the following, the reproducibility of low temperature EPR measurements of frozen samples containing spin probes is a concern.
RAW264.7 cells in 0.5mM CMH initially generate CM• at a rate of 4.0 ± 0.7 pmol / min / 106 cells post resuspension. One may deduce that this rate is the rate of basal oxidant generation from RAW264.7 cells. However, based on the low sensitivity of CMH in oxidants detection in stimulated cells by PMA as discussed in the following, this deduction should be questioned. This initial rate is comparable to CM• generation at 5.5 ± 0.5 pmol / min / 106 cells found in human lymphoblasts [21], while greater than CP• generation at 0.3–0.5 pmol / min / 106 cells (calculated based on 27–40 nM/min from 5 × 106 cells in 60 μl of solution) found in bovine aortic endothelial cells [41], although it is not clear whether spin probe autoxidation has been subtracted or not. Using spin traps, the generation of spin trap adducts (presented as superoxide production by the authors) at a rate of 3 ± 2 pmol / min / 106 cells from RAW264.7 cells has also been reported [47]. We question that all these rates are actually the basal generation rate of probe/trap-detectable oxidants from cells. On the other hand, we speculate that what CMH reacts with is the oxidants that are previously present in cells, and that the rate reflects the rate of transmembrane migration of CMH and/or oxidants previously present inside of cells. As clarified in Introduction, this assumes that the rate of CMH reaction with oxidants inside and outside of cells is much faster.
To our knowledge, most claims regarding the cell permeability of spin probes and spin traps are based upon speculation, while the actual investigations and data are very limited. There are studies using artificial cell membrane mimic systems [20, 80]. However, the results could be misleading if considered as authentic cell permeability since actual mammalian cells possess active transport that requires intact cell membranes [20]. We have found only one study on cell permeability of spin probes, where the accumulation of 1 mM spin probes in rat aortic smooth muscle cells for 20 min were measured [20]. Although the rate of CMH penetrating cells was not reported in that study, significant amount of CMH accumulated inside of cells within 20 min [20]. This time frame is consistent with what we used to obtain the initial rate of CM• generation once RAW264.7 cells were resuspended, indicating that this rate might be the rate of CMH migration across cell membrane.
RNOS generation from PMA-stimulated cells has been detected using spin traps [47, 49] and spin probes [20, 21, 26]. The PMA concentrations applied are calculated as much higher than 0.1 nmol / 106 cells given that the cell concentration and the volume of the incubation solution are provided (2.5 – 5 nmol / 106 cells) [21, 26, 49]. Using spin traps, Abbas K et al. detected superoxide generation at a rate of 80 ± 10 pmol/min/106 cells from RAW264.7 cells stimulated by 5 μM PMA, although the PMA concentration in mole per cell was not indicated [47]. Using CMH, oxidant generation of 48.6 ± 8.2 pmol/min/106 cells was found in human lymphoblasts stimulated by 10 μM PMA (based on 2.5 × 106 cells/ml in 0.1 ml buffer) [21]. However, oxidant generation was not detectable with lower PMA concentration at 0.2 nmol / 106 cells (0.5 μM and 2.5 × 106 cells/ml in 0.1 ml buffer) [21], which is consistent with our result in RAW264.7 cells with PMA at 0.1 nmol / 106 cells.
Spin traps 5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide (DEPMPO) and 5-(ethoxycarbonyl)-5-methyl-1-pyrroline-N-oxide (EMPO) are able to detect RNOS generation from human lymphoblasts stimulated by 4 nmol / 106 cells PMA, providing rates of 9.4 ± 0.9 and 16 ± 2.1 pmol / min / 106 cells, respectively [21]. Using the spin trap DMPO, we have detected RNOS generation at a rate of 0.26 ± 0.09 pmol / min / 106 cells from RAW264.7 cells that are stimulated by PMA as low as 0.1 nmol / 106 cells. However, the spin probe CMH has failed to detect this low oxidant generation. As shown in Fig. 7, although utilization of the CMH method provides greater rates of RNOS generation compared to the DMPO method, the variation (the error bar) is also greater, which could contribute to the lower sensitivity of the CMH method. The variability of the CMH method is also shown in Fig. 6B. Another possibility is that spin traps and spin probes detect different RNOS species as explained in the Introduction. Nevertheless, our results challenge previous conclusions that spin probes are more sensitive than spin traps [20, 21].
Usually, it is much easier to observe an EPR signal with spin probes rather than spin traps, which has been interpreted as spin probes being more sensitive (or having lower detection limit) than spin traps. However, the signal observed could be simply from the autoxidation of the probe, so could the difference between samples measured at different time points. Therefore, the rate of autoxidation must be considered and subtracted. In addition, the autoxidation could be a source of greater variability of the spin probe method. However, it is worth noting, this study is not designed to compare spin traps and spin probes in parallel, neither in vitro nor in vivo, rather, it exemplifies a detailed method development for RNOS detection and raises questions by presenting problems when spin probes are used. The pros and cons of using spin probes [19] or traps [10] have been reviewed previously.
The lower intensity of 150K measurements compared to that of RT measurements is likely due to the lower microwave power (2mW vs. 20mW) used in instrumental settings [31]. However, the power for 150K measurement may not be increased because CP• signal detected at liquid nitrogen temperature is saturated at less than 4mW [32]. Some reports have employed even lower power [26, 31, 32]. Berg et al. have claimed a precision and accuracy of ± 10 μM with a power of 0.2 mW for 150K measurements [31]. The variations of 150K measurements for CMH autoxidation could be explained by a lack of homogeneity of the frozen samples. As the solution crystallizes while freezing, there would be localized uneven distribution of concentrations of radicals. Another possibility is the instability of CMH (for instance, autoxidation) even under temperature as low as (and less than) −80°C. However, the samples have only been stored overnight, which is the least time span that long distance collaboration can conquer. All these aspects raise questions on the accuracy and the sensitivity of low temperature storage and measurement using spin probes.
Conclusions
Overall, this study establishes an example of the method development for RNOS detection, where experimental details are rigorously considered and tested. Spin probes are extremely reactive, and as a consequence, undergo rapid autoxidation and produce greater variability, which on the other hand harm their sensitivity as a method of RNOS detection. Autoxidation of spin probes should be counted for the accuracy of RNOS measurements. Although convenient, low temperature EPR measurements of frozen samples containing spin probes provide lower signal intensity and greater variability compared to RT measurements of fresh samples. More rigorous investigations on the methodology are in urgent demand for accurate and sensitive detection of RNOS, optimized and standardized methods/protocol can be developed and applied in the society, all of which could potentially advance invention of new spin traps or probes.
Method development for the detection of reactive oxygen species
Electron Paramagnetic Resonance coupled with spin probes and spin traps
Autoxidation of spin probes must be considered
Oxidant generation from RAW264.7 cells
Low temperature measurements provide less intensity and greater variability
Acknowledgements
This work was supported by NIH grant RO1HL119280 (T.E.T.), UAB institutional start-up funds (T.E.T.), and UAB Honors College Presidential Summer Fellowship (J.P.G.).
We thank Dr. Arlin B. Blood (Loma Linda University) for helpful discussion of the manuscript. This work is part of J.P.G.’s UAB undergraduate thesis, we thank Drs. Joe March, Rakesh Patel, Gayan Wijeratne from UAB for serving in the thesis committee.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Forman HJ, Augusto O, Brigelius-Flohe R, Dennery PA, Kalyanaraman B, Ischiropoulos H, Mann GE, Radi R, Roberts, LJ 2nd,, Vina J, and Davies KJ, Even free radicals should follow some rules: a guide to free radical research terminology and methodology. Free Radic Biol Med, 2015. 78: p. 233–5. [DOI] [PubMed] [Google Scholar]
- 2.Koppenol WH and Hider RH, Iron and redox cycling. Do’s and don’ts. Free Radic Biol Med, 2019. 133: p. 3–10. [DOI] [PubMed] [Google Scholar]
- 3.Alfadda AA and Sallam RM, Reactive oxygen species in health and disease. J Biomed Biotechnol, 2012. 2012: p. 936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schieber M and Chandel NS, ROS function in redox signaling and oxidative stress. Curr Biol, 2014. 24(10): p. R453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen YR, Harrison DG, Bhatnagar A, and American S Heart Association Council on Basic Cardiovascular, Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement From the American Heart Association. Circ Res, 2016. 119(5): p. e39–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khramtsov VV, In Vivo Electron Paramagnetic Resonance: Radical Concepts for Translation to the Clinical Setting. Antioxid Redox Signal, 2018. 28(15): p. 1341–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maulucci G, Bacic G, Bridal L, Schmidt HH, Tavitian B, Viel T, Utsumi H, Yalcin AS, and De Spirito M, Imaging Reactive Oxygen Species-Induced Modifications in Living Systems. Antioxid Redox Signal, 2016. 24(16): p. 939–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dikalov SI and Harrison DG, Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid Redox Signal, 2014. 20(2): p. 372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dikalov S, Griendling KK, and Harrison DG, Measurement of reactive oxygen species in cardiovascular studies. Hypertension, 2007. 49(4): p. 717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy M, Zielonka J, Karoui H, Sikora A, Michalski R, Podsiadly R, Lopez M, Vasquez-Vivar J, Kalyanaraman B, and Ouari O, Detection and Characterization of Reactive Oxygen and Nitrogen Species in Biological Systems by Monitoring Species-Specific Products. Antioxid Redox Signal, 2018. 28(15): p. 1416–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd, and H. Ischiropoulos, Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med, 2012. 52(1): p. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wardman P, Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med, 2007. 43(7): p. 995–1022. [DOI] [PubMed] [Google Scholar]
- 13.Xiao Y and Meierhofer D, Are Hydroethidine-Based Probes Reliable for Reactive Oxygen Species Detection? Antioxid Redox Signal, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A.A.-R. E, Mahmoud AM, Khalifa AM, and Ali SS, Physiological and pathophysiological reactive oxygen species as probed by EPR spectroscopy: the underutilized research window on muscle ageing. J Physiol, 2016. 594(16): p. 4591–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheinok S, Leveque P, Sonveaux P, Driesschaert B, and Gallez B, Comparison of different methods for measuring the superoxide radical by EPR spectroscopy in buffer, cell lysates and cells. Free Radic Res, 2018. 52(10): p. 1182–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbas K, Babic N, and Peyrot F, Use of spin traps to detect superoxide production in living cells by electron paramagnetic resonance (EPR) spectroscopy. Methods, 2016. 109: p. 31–43. [DOI] [PubMed] [Google Scholar]
- 17.Culcasi M, Rockenbauer A, Mercier A, Clement JL, and Pietri S, The line asymmetry of electron spin resonance spectra as a tool to determine the cis:trans ratio for spin-trapping adducts of chiral pyrrolines N-oxides: the mechanism of formation of hydroxyl radical adducts of EMPO, DEPMPO, and DIPPMPO in the ischemic-reperfused rat liver. Free Radic Biol Med, 2006. 40(9): p. 1524–38. [DOI] [PubMed] [Google Scholar]
- 18.Mason RP, Imaging free radicals in organelles, cells, tissue, and in vivo with immuno-spin trapping. Redox Biol, 2016. 8: p. 422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dikalov SI, Polienko YF, and Kirilyuk I, Electron Paramagnetic Resonance Measurements of Reactive Oxygen Species by Cyclic Hydroxylamine Spin Probes. Antioxid Redox Signal, 2018. 28(15): p. 1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dikalov SI, Kirilyuk IA, Voinov M, and Grigor’ev IA, EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic Res, 2011. 45(4): p. 417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dikalov SI, Li W, Mehranpour P, Wang SS, and Zafari AM, Production of extracellular superoxide by human lymphoblast cell lines: comparison of electron spin resonance techniques and cytochrome C reduction assay. Biochem Pharmacol, 2007. 73(7): p. 972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thierbach S, Bui N, Zapp J, Chhabra SR, Kappl R, and Fetzner S, Substrate-assisted O2 activation in a cofactor-independent dioxygenase. Chem Biol, 2014. 21(2): p. 217–25. [DOI] [PubMed] [Google Scholar]
- 23.Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, and Galis ZS, Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation, 2004. 109(4): p. 520–5. [DOI] [PubMed] [Google Scholar]
- 24.Dikalov SI, Vitek MP, and Mason RP, Cupric-amyloid beta peptide complex stimulates oxidation of ascorbate and generation of hydroxyl radical. Free Radic Biol Med, 2004. 36(3): p. 340–7. [DOI] [PubMed] [Google Scholar]
- 25.Weissmann N, Kuzkaya N, Fuchs B, Tiyerili V, Schafer RU, Schutte H, Ghofrani HA, Schermuly RT, Schudt C, Sydykov A, Egemnazarow B, Seeger W, and Grimminger F, Detection of reactive oxygen species in isolated, perfused lungs by electron spin resonance spectroscopy. Respir Res, 2005. 6: p. 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elajaili HB, Hernandez-Lagunas L, Ranguelova K, Dikalov S, and Nozik-Grayck E, Use of Electron Paramagnetic Resonance in Biological Samples at Ambient Temperature and 77 K. J Vis Exp, 2019(143). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai H, Dikalov S, Griendling KK, and Harrison DG, Detection of reactive oxygen species and nitric oxide in vascular cells and tissues: comparison of sensitivity and specificity. Methods Mol Med, 2007. 139: p. 293–311. [DOI] [PubMed] [Google Scholar]
- 28.Kuzkaya N, Weissmann N, Harrison DG, and Dikalov S, Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem, 2003. 278(25): p. 22546–54. [DOI] [PubMed] [Google Scholar]
- 29.Gomes BRB, Firmino M, Jorge JS, Ferreira MLO, Rodovalho TM, Weis SN, Souza GEP, Morais PC, Sousa MV, Souza PEN, and Veiga-Souza FH, Increase of reactive oxygen species in different tissues during lipopolysaccharide-induced fever and antipyresis: an electron paramagnetic resonance study. Free Radic Res, 2018. 52(3): p. 351–361. [DOI] [PubMed] [Google Scholar]
- 30.Gioscia-Ryan RA, Battson ML, Cuevas LM, Zigler MC, Sindler AL, and Seals DR, Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. Aging (Albany NY), 2016. 8(11): p. 2897–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berg K, Ericsson M, Lindgren M, and Gustafsson H, A high precision method for quantitative measurements of reactive oxygen species in frozen biopsies. PLoS One, 2014. 9(3): p. e90964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozlov AV, Szalay L, Umar F, Fink B, Kropik K, Nohl H, Redl H, and Bahrami S, Epr analysis reveals three tissues responding to endotoxin by increased formation of reactive oxygen and nitrogen species. Free Radic Biol Med, 2003. 34(12): p. 1555–62. [DOI] [PubMed] [Google Scholar]
- 33.Dikalov S, Skatchkov M, Fink B, and Bassenge E, Quantification of superoxide radicals and peroxynitrite in vascular cells using oxidation of sterically hindered hydroxylamines and electron spin resonance. Nitric Oxide, 1997. 1(5): p. 423–31. [DOI] [PubMed] [Google Scholar]
- 34.Dikalov S, Grigor’ev IA, Voinov M, and Bassenge E, Detection of superoxide radicals and peroxynitrite by 1-hydroxy-4-phosphonooxy-2,2,6,6-tetramethylpiperidine: quantification of extracellular superoxide radicals formation. Biochem Biophys Res Commun, 1998. 248(2): p. 211–5. [DOI] [PubMed] [Google Scholar]
- 35.Dikalov S, Skatchkov M, and Bassenge E, Spin trapping of superoxide radicals and peroxynitrite by 1-hydroxy-3-carboxy-pyrrolidine and 1-hydroxy-2,2,6, 6-tetramethyl-4-oxo-piperidine and the stability of corresponding nitroxyl radicals towards biological reductants. Biochem Biophys Res Commun, 1997. 231(3): p. 701–4. [DOI] [PubMed] [Google Scholar]
- 36.Dikalov S, Skatchkov M, and Bassenge E, Quantification of peroxynitrite, superoxide, and peroxyl radicals by a new spin trap hydroxylamine 1-hydroxy-2,2,6,6-tetramethyl-4-oxopiperidine. Biochem Biophys Res Commun, 1997. 230(1): p. 54–7. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Garrido A, Brown DI, Lyle AN, Dikalova A, Seidel-Rogol B, Lassegue B, San Martin A, and Griendling KK, NADPH oxidase 4 mediates TGF-beta-induced smooth muscle alpha-actin via p38MAPK and serum response factor. Free Radic Biol Med, 2011. 50(2): p. 354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fink B, Dikalov S, and Bassenge E, A new approach for extracellular spin trapping of nitroglycerin-induced superoxide radicals both in vitro and in vivo. Free Radic Biol Med, 2000. 28(1): p. 121–8. [DOI] [PubMed] [Google Scholar]
- 39.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, and Harrison DG, Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension, 2002. 40(4): p. 511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschlager N, Hornig B, Drexler H, and Harrison DG, Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation, 2003. 107(10): p. 1383–9. [DOI] [PubMed] [Google Scholar]
- 41.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, and Harrison DG, Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol, 2003. 285(6): p. H2290–7. [DOI] [PubMed] [Google Scholar]
- 42.Marchandot B, Kibler M, Charles AL, Trinh A, Petit Eisenmann H, Zeyons F, Von Hunolstein JJ, Reydel A, Matsushita K, Kindo M, Hoang Minh T, Leddet P, De Poli F, Messas N, Jesel L, Ohlmann P, Geny B, and Morel O, Does Transcatheter Aortic Valve Replacement Modulate the Kinetic of Superoxide Anion Generation? Antioxid Redox Signal, 2019. 31(5): p. 420–426. [DOI] [PubMed] [Google Scholar]
- 43.Thomas VC, Chaudhari SS, Jones J, Zimmerman MC, and Bayles KW, Electron Paramagnetic Resonance (EPR) Spectroscopy to Detect Reactive Oxygen Species in Staphylococcus aureus. Bio Protoc, 2015. 5(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu Y, Li X, Fang Y, Shi W, Li X, Chen W, Xian M, and Ma H, Reactive oxygen species-triggered off-on fluorescence donor for imaging hydrogen sulfide delivery in living cells. Chem Sci, 2019. 10(33): p. 7690–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okoye FB, Nworu CS, Akah PA, Esimone CO, Debbab A, and Proksch P, Inhibition of inflammatory mediators and reactive oxygen and nitrogen species by some depsidones and diaryl ether derivatives isolated from Corynespora cassiicola, an endophytic fungus of Gongronema latifolium leaves. Immunopharmacol Immunotoxicol, 2013. 35(6): p. 662–8. [DOI] [PubMed] [Google Scholar]
- 46.Zhao K, Huang Z, Lu H, Zhou J, and Wei T, Induction of inducible nitric oxide synthase increases the production of reactive oxygen species in RAW264.7 macrophages. Biosci Rep, 2010. 30(4): p. 233–41. [DOI] [PubMed] [Google Scholar]
- 47.Abbas K, Hardy M, Poulhes F, Karoui H, Tordo P, Ouari O, and Peyrot F, Detection of superoxide production in stimulated and unstimulated living cells using new cyclic nitrone spin traps. Free Radic Biol Med, 2014. 71: p. 281–90. [DOI] [PubMed] [Google Scholar]
- 48.Chin MP, Schauer DB, and Deen WM, Nitric oxide, oxygen, and superoxide formation and consumption in macrophages and colonic epithelial cells. Chem Res Toxicol, 2010. 23(4): p. 778–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbas K, Hardy M, Poulhes F, Karoui H, Tordo P, Ouari O, and Peyrot F, Medium-throughput ESR detection of superoxide production in undetached adherent cells using cyclic nitrone spin traps. Free Radic Res, 2015. 49(9): p. 1122–8. [DOI] [PubMed] [Google Scholar]
- 50.Chung TH, Wu YP, Chew CY, Lam CH, and Tan KT, Imaging and Quantification of Secreted Peroxynitrite at the Cell Surface by a Streptavidin-Biotin-Controlled Binding Probe. Chembiochem, 2018. 19(24): p. 2584–2590. [DOI] [PubMed] [Google Scholar]
- 51.Gieche J, Mehlhase J, Licht A, Zacke T, Sitte N, and Grune T, Protein oxidation and proteolysis in RAW264.7 macrophages: effects of PMA activation. Biochim Biophys Acta, 2001. 1538(2–3): p. 321–8. [DOI] [PubMed] [Google Scholar]
- 52.Johann AM, von Knethen A, Lindemann D, and Brune B, Recognition of apoptotic cells by macrophages activates the peroxisome proliferator-activated receptor-gamma and attenuates the oxidative burst. Cell Death Differ, 2006. 13(9): p. 1533–40. [DOI] [PubMed] [Google Scholar]
- 53.Lee SY and Cho JY, Inhibitory effects of honokiol on LPS and PMA-induced cellular responses of macrophages and monocytes. BMB Rep, 2009. 42(9): p. 574–9. [DOI] [PubMed] [Google Scholar]
- 54.Liu W, Wu H, Chen L, Wen Y, Kong X, and Gao WQ, Park7 interacts with p47(phox) to direct NADPH oxidase-dependent ROS production and protect against sepsis. Cell Res, 2015. 25(6): p. 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Li Q, Gong X, Li H, Chen Z, Tong L, and Tang B, Rapid determination of superoxide free radical in hepatocellular carcinoma cells by MCE with LIF. Electrophoresis, 2009. 30(6): p. 1077–83. [DOI] [PubMed] [Google Scholar]
- 56.Lu X, Mestres G, Singh VP, Effati P, Poon JF, Engman L, and Ott MK, Selenium- and Tellurium-Based Antioxidants for Modulating Inflammation and Effects on Osteoblastic Activity. Antioxidants (Basel), 2017. 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsebatlela TM, Anderson AL, Gallicchio VS, Elford H, and Rice CD, 3,4-Dihydroxy-benzohydroxamic acid (Didox) suppresses pro-inflammatory profiles and oxidative stress in TLR4-activated RAW264.7 murine macrophages. Chem Biol Interact, 2015. 233: p. 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiao S, Li W, Tsubouchi R, Haneda M, Murakami K, Takeuchi F, Nisimoto Y, and Yoshino M, Rosmarinic acid inhibits the formation of reactive oxygen and nitrogen species in RAW264.7 macrophages. Free Radic Res, 2005. 39(9): p. 995–1003. [DOI] [PubMed] [Google Scholar]
- 59.Szliszka E, Skaba D, Czuba ZP, and Krol W, Inhibition of inflammatory mediators by neobavaisoflavone in activated RAW264.7 macrophages. Molecules, 2011. 16(5): p. 3701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Knethen A and Brune B, PKC alpha depletion in RAW264.7 macrophages following microbial/IFNgamma stimulation is PC-PLC-mediated. Antioxid Redox Signal, 2005. 7(9–10): p. 1217–22. [DOI] [PubMed] [Google Scholar]
- 61.Yeh SL, Wang HM, Chen PY, and Wu TC, Interactions of beta-carotene and flavonoids on the secretion of pro-inflammatory mediators in an in vitro system. Chem Biol Interact, 2009. 179(2–3): p. 386–93. [DOI] [PubMed] [Google Scholar]
- 62.DeChatelet LR, Shirley PS, and Johnston RB Jr., Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood, 1976. 47(4): p. 545–54. [PubMed] [Google Scholar]
- 63.Cable H and Lloyd JB, Cellular uptake and release of two contrasting iron chelators. J Pharm Pharmacol, 1999. 51(2): p. 131–4. [DOI] [PubMed] [Google Scholar]
- 64.Doulias PT, Christoforidis S, Brunk UT, and Galaris D, Endosomal and lysosomal effects of desferrioxamine: protection of HeLa cells from hydrogen peroxide-induced DNA damage and induction of cell-cycle arrest. Free Radic Biol Med, 2003. 35(7): p. 719–28. [DOI] [PubMed] [Google Scholar]
- 65.Bartesaghi S, Trujillo M, Denicola A, Folkes L, Wardman P, and Radi R, Reactions of desferrioxamine with peroxynitrite-derived carbonate and nitrogen dioxide radicals. Free Radic Biol Med, 2004. 36(4): p. 471–83. [DOI] [PubMed] [Google Scholar]
- 66.Fuhrmann DC and Brune B, Mitochondrial composition and function under the control of hypoxia. Redox Biol, 2017. 12: p. 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, and Chouaib S, Hypoxia: a key player in antitumor immune response. A Review in the Theme: Cellular Responses to Hypoxia. Am J Physiol Cell Physiol, 2015. 309(9): p. C569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bosworth CA, Toledo JC Jr., Zmijewski JW, Li Q, and Lancaster JR Jr., Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc Natl Acad Sci U S A, 2009. 106(12): p. 4671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doskey CM, van ‘t Erve TJ, Wagner BA, and Buettner GR, Moles of a Substance per Cell Is a Highly Informative Dosing Metric in Cell Culture. PLoS One, 2015. 10(7): p. e0132572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pae HO, Jun CD, Yoo JC, Kwak HJ, Lee SJ, Kook YA, Park RK, and Chung HT, Enhancing and priming of macrophages for superoxide anion production by taxol. Immunopharmacol Immunotoxicol, 1998. 20(1): p. 27–37. [DOI] [PubMed] [Google Scholar]
- 71.Xie W, Li M, Xu N, Lv Q, Huang N, He J, and Zhang Y, MiR-181a regulates inflammation responses in monocytes and macrophages. PLoS One, 2013. 8(3): p. e58639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inoue T, Takeshita K, and Fukushima K, Effects of KE-758; an active metabolite of the new anti-rheumatic drug KE-298, D-penicillamine, bucillamine and auranofin on the proliferation of murine lymphocytes, and the production of nitric oxide by murine macrophages. Int Immunopharmacol, 2001. 1(5): p. 833–42. [DOI] [PubMed] [Google Scholar]
- 73.Roychowdhury S, Wolf G, Keilhoff G, Bagchi D, and Horn T, Protection of primary glial cells by grape seed proanthocyanidin extract against nitrosative/oxidative stress. Nitric Oxide, 2001. 5(2): p. 137–49. [DOI] [PubMed] [Google Scholar]
- 74.Curtis TD, Gram L, and Knudsen GM, The Small Colony Variant of Listeria monocytogenes Is More Tolerant to Antibiotics and Has Altered Survival in RAW 264.7 Murine Macrophages. Front Microbiol, 2016. 7: p. 1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brigham KL, Meyrick B, Berry LC Jr., and Repine JE, Antioxidants protect cultured bovine lung endothelial cells from injury by endotoxin. J Appl Physiol (1985), 1987. 63(2): p. 840–50. [DOI] [PubMed] [Google Scholar]
- 76.Grady JK, Chasteen ND, and Harris DC, Radicals from “Good’s” buffers. Anal Biochem, 1988. 173(1): p. 111–5. [DOI] [PubMed] [Google Scholar]
- 77.Kirsch M, Lomonosova EE, Korth HG, Sustmann R, and de Groot H, Hydrogen peroxide formation by reaction of peroxynitrite with HEPES and related tertiary amines. Implications for a general mechanism. J Biol Chem, 1998. 273(21): p. 12716–24. [DOI] [PubMed] [Google Scholar]
- 78.Kober T, Konig I, Weber M, and Kojda G, Diethyldithiocarbamate inhibits the catalytic activity of xanthine oxidase. FEBS Lett, 2003. 551(1–3): p. 99–103. [DOI] [PubMed] [Google Scholar]
- 79.Mulsch A, Schray-Utz B, Mordvintcev PI, Hauschildt S, and Busse R, Diethyldithiocarbamate inhibits induction of macrophage NO synthase. FEBS Lett, 1993. 321(2–3): p. 215–8. [DOI] [PubMed] [Google Scholar]
- 80.Anzai K, Aikawa T, Furukawa Y, Matsushima Y, Urano S, and Ozawa T, ESR measurement of rapid penetration of DMPO and DEPMPO spin traps through lipid bilayer membranes. Arch Biochem Biophys, 2003. 415(2): p. 251–6. [DOI] [PubMed] [Google Scholar]