Abstract
Objective
To examine the accuracy of transcutaneous bilirubinometry (TCB) measurements during and after phototherapy (PT) in preterm infants.
Design
Prospective observational cohort study.
Setting
Level III neonatal centre.
Patients
Preterm infants (from 23+0 to 36+6 weeks of gestation) born between June 2017 and May 2018 requiring PT.
Interventions
TCB was measured from an exposed area of the skin (the sternum; TCBU) and the covered area of the skin under the nappy (the bony part of the upper outer quadrant of the buttock; TCBC) within an hour of obtaining total serum bilirubin (TSB).
Main outcome measures
Correlation and agreement between TCB (TCBU and TCBC) and TSB during and after PT.
Results
We have enrolled 196 preterm infants. There was a significant correlation between TSB and TCB during PT (r=0.72, 95% CI 0.66 to 0.77 in covered area; r=0.75, 95% CI 0.70 to 0.80 in uncovered area) and after PT (r=0.87, 95% CI 0.83 to 0.91). TCB underestimated TSB level during PT, with a mean TCBC–TSB difference of −25±43 (95% agreement limits of 62 to −112) and a mean TCBU–TSB difference of −48±46 (95% agreement limits of 45 to −140). The agreement between TCB and TSB after cessation of PT improved, with TCB underestimating TSB by a mean TCB–TSB difference of −10±31 (95% agreement limits of 52 to −72).
Conclusion
TCB measurements correlated strongly with TSB levels during and after PT. However, there was a wide and clinically relevant disagreement between TCB and TSB measurements during the PT phase, improving significantly after PT.
Keywords: jaundice, neonatology, monitoring
What is known about the subject?
Transcutaneous bilirubinometry (TCB) is a non-invasive test used as a validated screening tool for hyperbilirubinaemia in term infants.
The accuracy of TCB measurement during phototherapy (PT) is still controversial in term and preterm infants.
A few studies reported that TCB measurement from covered skin during PT could provide accurate approximations of total serum bilirubin (TSB) level in term infants.
What this study adds?
During and after PT, TCB measurements correlate significantly with TSB levels in preterm infants.
During PT, TCB underestimates TSB, with a significant wide disagreement between TCB and TSB measurements, making it unreliable.
After PT, there is a significant correlation and acceptable agreement between TCB and TSB measurements, with improving performance up to 12 hours after cessation of PT.
Introduction
Neonatal hyperbilirubinaemia is a very common condition, with approximately 50% of term and 80% of preterm infants developing jaundice in the first week of life.1 Hyperbilirubinaemia in most cases is a benign and self-limiting condition; however, severe hyperbilirubinaemia can occasionally occur and may be associated with irreversible brain damage, especially in preterm infants.2 3
Phototherapy (PT) is considered to be a safe and effective treatment for neonatal unconjugated hyperbilirubinaemia. The indication to commence treatment is based on the level of serum bilirubin, the age of the baby in hours and gestational age.4 Evidence is conflicting regarding the best therapeutic approach to hyperbilirubinaemia, especially in extremely low birthweight (ELBW) infants. A randomised clinical trial performed by the Neonatal Research Network found no significant difference in the rate of death or neurodevelopmental impairment at 18–22 months corrected age in ELBW infants who received aggressive PT versus those who received conservative PT. However, aggressive PT was associated with a reduction in the rate of neurodevelopmental impairment alone.5 However, post-hoc analysis showed that in the smallest and sickest subgroup (mechanically ventilated infants with birth weight less than 750 g), aggressive PT may increase mortality while reducing neurodevelopmental impairment.6
Measurement of total serum bilirubin (TSB) remains the gold standard for monitoring bilirubin levels during and after PT in term and preterm infants. However, obtaining heel stick or venous blood samples is painful, time-consuming, and increases the risk of local and systemic infection especially in preterm infants.7 A transcutaneous bilirubinometry (TCB) device works by directing light into the skin of the infant and measuring and analysing the intensity of the returned wavelengths to estimate TSB.8 TCB has been recommended as a non-invasive, painless and time-saving test for bilirubin estimation in term and late preterm infants prior to commencement of PT.9–11 However, TCB measurements are not recommended in the first 24 hours of life or in preterm infants below 35 weeks of gestation according to the National Institute for Health and Care Excellence (NICE) guidelines12 (https://www.nice.org.uk/guidance/cg98).
It seems that TCB has strong correlation and acceptable agreement in preterm infants before PT.13 14 The use of TCB for infants during and after PT is still controversial, as some studies reported that PT blanches the skin thereby affecting the correlation between TCB and TSB during and after PT,15 while others suggest that TCB readings from a covered skin area could be safely used to guide treatment during and after PT.16–18 We designed our study to examine the accuracy of TCB in estimating the TSB level in preterm infants undergoing PT and its reliability after PT.
Methods
This is a single-centre, prospective observational cohort study performed in the neonatal department of the Coombe Women and Infants University Hospital (CWIUH), Dublin, Ireland (level III neonatal centre). All preterm infants (from 23+0 to 36+6 weeks of gestation) born between June 2017 and May 2018 in CWIUH who developed significant jaundice requiring PT were eligible for enrolment to this study. During the study period, the protocols for screening, diagnosis and management of infants with jaundice were not changed. Infants with clinical or radiological evidence of major congenital anomalies (including those with gastrointestinal tract deformities and congenital heart diseases apart from patent ductus arteriosus, persistent foramen ovale and small (≤5 mm) muscular ventricular septal defect) were excluded from this study.
PT was commenced based on TSB levels according to hospital guidelines, taking into account the infant’s age in hours and gestation in weeks. The NICE treatment charts were used for preterm infants below 32 weeks of gestation (https://www.nice.org.uk/guidance/cg98/resources). In infants ≥32 weeks of gestation, a chart adapted from the National Health Service (Glasgow, UK) was used (online supplementary appendix 1). Standard PT units (Photo Therapy 4000, Draeger Medical, Germany) were used (overhead PT microlight units which can deliver ≥10 µW/cm2/nm and halogen spotlights which can deliver 20–25 µW/cm2/nm). Infants receiving PT were completely exposed, except for their eyes (covered with PT goggles for protection) and the nappy area (covered with a disposable nappy). PT was discontinued when the TSB fell below the relevant treatment threshold.
bmjpo-2020-000681supp001.pdf (707.8KB, pdf)
TCB was measured from uncovered/exposed (TCBU) and covered (TCBC) areas within an hour of obtaining TSB samples. The device was placed over an uncovered area (sternum) and pressed gently against the skin three times to obtain one reading (the average of the three measured values). The process was repeated over the covered area, the bony part of the upper outer quadrant of the buttock (covered by the nappy). After cessation of PT, TCBs were measured from the sternum. The measurements were obtained by experienced nurses trained and competent in the use of the Dräger Jaundice Meter (JM-105 or JM-103, Draeger Medical). The TCB devices were calibrated regularly according to the manufacturer’s instructions and hospital guidelines. Blood samples for TSB were obtained either by heel prick or venepuncture. The attending neonatologist directed the frequency of TSB measurements. TSB levels were measured in one clinical laboratory using direct spectrophotometry (Abbott Architect C8000, Abbott, USA).
Our primary outcome was the correlation and agreement between TCB (TCBU and TCBC) and TSB during and after PT.
Informed written consent was obtained from parents.
Data were entered into Microsoft Office Excel (MS Excel, Microsoft, USA) and analysed by the StatsDirect V.3.2.10 software (StatsDirect, UK). Descriptive statistics were used for all demographic variables of interest using frequency distribution and percentage for categorical variables. Mean and SD were used for normally distributed data, while non-normal distribution data were summarised using median and IQR. Paired-samples t-test was used to compare TCB and TSB paired measurements. When the differences between pairs were not normally distributed, we used the Wilcoxon signed-rank test for two sample comparisons. For independent variables we have used an unpaired t-test or Mann-Whitney U test as appropriate. Correlation between TCB (TCBU and TCBC) and TSB was calculated using Pearson’s correlation coefficient during and after PT. Bland-Altman (B-A) analysis was used to calculate and visualise the agreement between TSB and TCB. The agreement limits are demonstrated as 95% CI (95% CI=mean±1.96 SD), where the ideal agreement difference between measurements is zero. Our results were summarised using p values and 95% CIs. P values <0.05 were considered statistically significant. We have used a convenience sample for the study with planned 1-year enrolment.
Patient and public involvement
Patients or the public were not involved in the design, conduct or reporting plans of our research.
Results
One hundred and ninety-six preterm infants with jaundice who received PT were enrolled to the study. The mean (±SD) gestational age and birth weight of our cohort were 30.4 weeks of gestation (±3.2) and 1605 g (±638), respectively. The demographic description of our cohort is presented in table 1.
Table 1.
Baseline population characteristics
| Variable | N=196 |
| Male sex, n (%) | 105 (53.6) |
| Birth weight (g), mean±SD | 1605±638 |
| Gestational age (weeks), mean±SD | 30.4±3.2 |
| Mode of delivery, n (%) | |
| NVD | 56 (28.6) |
| Instrumental delivery | 3 (1.5) |
| Elective LSCS | 59 (30.1) |
| Emergency LSCS | 78 (39.8) |
| Apgar score at first minute, median (IQR) | 7 (5–9) |
| Apgar score at fifth minute, median (IQR) | 9 (8–10) |
| Blood group of infants (when done), n (%) | 175 (89.3) |
| A | 47 (26.9) |
| B | 18 (10.3) |
| AB | 2 (1.1) |
| O | 108 (61.7) |
| Rhesus+ | 150/175 (85.7) |
| Maternal group, n (%) | |
| A | 60 (30.6) |
| B | 24 (12.2) |
| AB | 5 (2.6) |
| O | 107 (54.6) |
| Rhesus+ | 176 (89.8) |
| Positive DCT, n (%) | 7 (3.6) |
| Maternal age (years), mean±SD | 32±6.2 |
DCT, direct Coombs test; LSCS, lower segment caesarean section; NVD, normal vaginal delivery.
There were 328 simultaneous measurements (TSB and TCB) during the PT phase and 142 pairs of readings after discontinuation of PT. The PT was commenced at a mean (±SD) of 32.5 (±20) hours of life and the median duration of PT exposure was 24 hours (IQR 24–32).
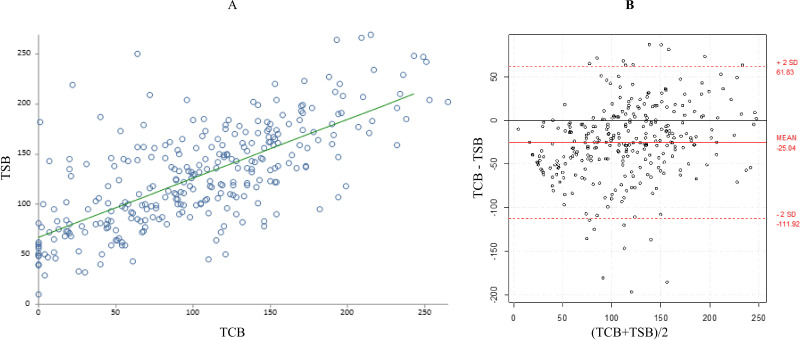
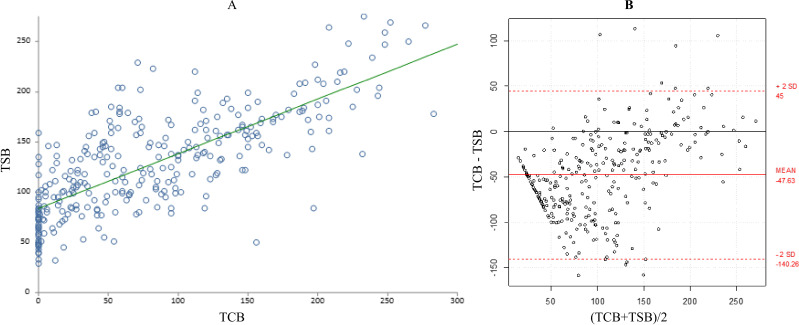
During the PT phase, the mean±SD TSB (127±51 μmol/L) and mean±SD TCBC (102±62) were statistically significantly different (p<0.0001) (table 2). Similarly, the difference between the mean±SD TSB (127±51 μmol/L) and mean±SD TCBU (79±70) was statistically significantly different during PT (p<0.0001) (table 2). Although there was a significant correlation between TSB and TCB measurements during PT (r=0.72, 95% CI 0.66 to 0.77 from covered area; r=0.75, 95% CI 0.70 to 0.80 from uncovered area; p<0.0001), B-A plots showed significant bias and imprecisions in the TCB readings. TCB underestimated TSB level with a mean TCB–TSB difference of −25±43 from the covered area (95% agreement limits of 62 to −112, p<0.0001) and of −48±46 from the uncovered area (95% agreement limits of 45 to −140, p<0.0001) (figures 1 and 2).
Table 2.
Paired samples of TSB and TCB from covered and uncovered skin during and after phototherapy
| Mean±SD (µmol/L) | Median (IQR) (µmol/L) | n | P value | |
| During PT | ||||
| TCBC | 102±62 | 102 (55–146) | 299 | <0.0001 |
| TSB | 127±51 | 124 (89–162) | ||
| TCBC–TSB difference | −25±43 | −25 (−49 to 1) | ||
| During PT | ||||
| TCBU | 79±70 | 61 (18–127) | 309 | <0.0001 |
| TSB | 127±51 | 122 (86–162) | ||
| TCBU–TSB difference | −48±46 | −48 (−79 to −18) | ||
| After PT | ||||
| TCB | 143±63 | 141 (100–188) | 142 | 0.0001 |
| TSB | 153±51 | 153 (115–187) | ||
| TCBC–TSB difference | −10±31 | −13 (−28 to 9) |
PT, phototherapy; TCB, transcutaneous bilirubinometry; TCBC, transcutaneous bilirubinometry readings from covered skin; TCBU, transcutaneous bilirubinometry readings from exposed skin; TSB, total serum bilirubin.
Figure 1.
(A) Correlation between total serum bilirubin (TSB) and transcutaneous bilirubinometry (TCB) from covered skin during phototherapy. (B) Bland–Altman plot showing the 95% limits of agreement between TCB from covered skin and TSB during phototherapy.
Figure 2.
(A) Correlation between total serum bilirubin (TSB) and transcutaneous bilirubinometry (TCB) from uncovered skin during phototherapy. (B) Bland-Altman plot showing the 95% limits of agreement between TCB from uncovered skin and TSB during phototherapy.
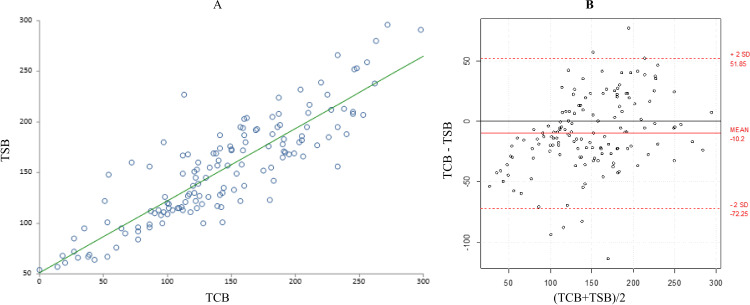
During the post-PT phase, TSB±SD (153±51 μmol/L) and TCB±SD (143±63) measurements were statistically significantly different (p=0.0001) (table 2). These measurements were taken at a median time of 12 hours (IQR 8–24) after PT. After cessation of PT, the correlation between TCB and TSB further improved (r=0.87, 95% CI 0.83 to 0.91, p<0.0001) (figure 3A). The B-A plot also showed an improvement in the agreement between TCB and TSB, but TCB continued to underestimate TSB level by −10±31 (95% agreement limits of 52 to −72, p=0.0001) (figure 3B). At 12 hours after cessation of PT, the correlation between TCB and TSB was improved compared with 8 hours after PT, with statistically significantly improving mean difference between TCB and TSB (p<0.0001) (table 3).
Figure 3.
(A) Correlation between total serum bilirubin (TSB) and transcutaneous bilirubinometry (TCB) after phototherapy. (B) Bland-Altman plot showing the 95% limits of agreement between TCB and TSB after phototherapy.
Table 3.
TCB and TSB pairs at 8 and 12 hours after phototherapy
| Hours after PT | n | TSB (µmol/L) Mean±SD Median (IQR) |
TCB (µmol/L) Mean±SD Median (IQR) |
Mean difference (TCB–TSB) (µmol/L) Mean±SD Median (IQR) |
Correlation (p value) |
| 8 hours | 40 | 133±51 | 95±54 | −37±28 | r=0.86 (<0.0001) |
| 124 (94–168) | 97 (53–138) | −32 (−49 to −22) | |||
| 12 hours | 36 | 147±52 | 131±51 | −16±19 | r=0.93 (<0.0001) |
| 135 (112–173) | 123 (95–154) | −17 (−23 to −7) |
PT, phototherapy; TCB, transcutaneous bilirubinometry; TSB, total serum bilirubin.
Discussion
Our study revealed a significant correlation between TCB and TSB during and after PT in preterm infants. However, our findings also showed a significant wide disagreement between TCB and TSB measurements during the PT phase. Although TCB readings from the covered skin had better agreement than those from the exposed skin, TCB measurements were associated with a large bias during PT. We noted that the TCB device could underestimate serum bilirubin level by up to 112 and 140 μmol/L from covered and uncovered areas, respectively, during PT.
In general, the significant correlation between TCB and TSB during PT is consistent with the findings in some previous studies in preterm infants.19 Cucuy et al20 conducted a study of 86 preterm infants with a mean gestational age of 32 weeks and a mean birth weight of 1637 g. Although they found good correlation between TSB and TCB during PT (r=0.8), it was not clear if their TCB readings were measured from the exposed or covered skin during PT. In addition to this, they did not provide information about the level of agreement between the TCB and TSB measurements.
There are only a few studies that examined 95% agreement limits between TCB and TSB in preterm infants during PT. Nagar and Kumar21 performed a smaller study on 90 preterm infants with a mean gestational age of 32.4 weeks and a mean birth weight of 1847 g. They found that TCB cannot be recommended for bilirubin measurement during PT in preterm infants due to the high risk of underestimation of TSB by up to 132 and 157 μmol/L from covered and uncovered skin, respectively. Although their sample was smaller and infants older than our cohort, their results were quite comparable with our findings.
Similarly, Hulzebos et al18 demonstrated that TCB underestimated TSB in very preterm infants during PT when measured on covered skin. The same research group proposed different cut-off rules to improve the prediction of PT thresholds when TCB was measured during PT on covered skin.18
Zecca et al22 conducted a study on 364 preterm and term infants requiring PT. The mean gestational age and the mean birth weight of their sample were 34.6 weeks of gestation and 2371 g, respectively, which were higher than the mean gestational age and the mean birth weight of our cohort. They reported a smaller bias between TCB readings from covered skin and TSB compared with our results. Their results demonstrated that TCB from exposed skin underestimated TSB by 54±51 μmol/L, while TCB from covered skin underestimated TSB by 3.1±53 μmol/L. However, B-A plots showed a wide TCB–TSB disagreement with a risk of underestimation of TSB by up to 106 μmol/L from covered skin and 153 μmol/L from exposed skin.
We have shown that TCB readings from both covered and uncovered areas were lower than TSB levels. We speculate that immaturity of the skin and the absence of subcutaneous fat in preterm infants may lead to rapid clearance of extravascular bilirubin levels from the skin following initiation of PT.9 23 This however would contravene the findings of De Luca and Dell’Orto24, who reported that TCB reading from covered skin and TSB correlated strongly (r=0.84, p<0.001) in their study of 60 extremely preterm infants undergoing PT and, unlike our findings, TCB overestimated TSB with a mean TCB–TSB difference of 47.8±41 μmol/L.
During the post-PT phase, our data revealed a better correlation between TCB and TSB reading as compared with that during the PT phase. More interestingly, the mean difference of TSB–TCB pairs was much lower than reported previously, even in paired measurements done in our study as early as 8 hours after PT.18 We observed improved correlation and decreasing mean difference of TSB–TCB pairs with increased time after PT, which would be different from the previous observation by Cucuy et al20 as they reported that time after PT did not have any significant effect on the correlation between TSB and TCB.
Moreover, our results showed a significant improvement in the agreement between TCB and TSB after cessation of PT. The level of underestimation of TSB in our study is similar to those observed in the study of Nagar and Kumar.21
The strength of our study is that it is a large prospective observational study with a substantial number of paired TCB–TSB measurements in comparison with previous studies. We have also provided recent data on the agreement between TCB and TSB which are more helpful in clinical practice than correlation coefficient. Thus, our study added significant findings to the literature on the use of the TCB device in preterm infants during and after PT.
The present study has some limitations. First, we did not examine the effect of the duration and recommencement of PT on the TSB–TCB correlation. Also, TCB was only measured from exposed skin (sternum) after PT was discontinued, and the TCB measurements from the covered area (nappy area) could have different correlation and agreement with TSB.
In conclusion, TCB measurements correlate strongly with TSB levels during and after PT. However, as a result of the wide and clinically relevant disagreement between TCB and TSB measurements during the PT phase, a TCB device cannot be recommended for monitoring bilirubin level during PT in our opinion. However, based on our results, we would advocate the use of TCB for TSB ‘rebound’ measurements at 12 hours after PT to avoid unnecessary serum sampling.
Supplementary Material
Acknowledgments
The authors would like to thank all the babies and families who participated in this study, along with the medical and midwifery staff at the CWIUH.
Footnotes
Contributors: AAR designed the study, contributed substantially to the data collection and analysis, and drafted the initial manuscript. AOS contributed substantially to the data collection and analysis, and reviewed and revised the manuscript. JM conceptualised and designed the study, supervised the conduct of the study, and coordinated the data analysis. He reviewed and revised the manuscript critically for important intellectual content. All the authors approved the final manuscript as submitted. They agree to be accountable for all aspects of the work.
Funding: AAR's work was supported by a grant from the Department of Cultural Affairs, Libya (ref: HG6-490-45693) (managed by University College Dublin).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The Research Ethics Committee of the Coombe Women and Infants University Hospital approved the study (study no 3-2017).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Deidentified participant data are available from the corresponding author upon reasonable request.
References
- 1.Woodgate P, Jardine LA. Neonatal jaundice. BMJ Clin Evid 2011;15. [PMC free article] [PubMed] [Google Scholar]
- 2.Watchko JF, Oski FA. Kernicterus in preterm newborns: past, present, and future. Pediatrics 1992;90:707–15. [PubMed] [Google Scholar]
- 3.Cashore WJ. The neurotoxicity of bilirubin. Clin Perinatol 1990;17:437–47. [PubMed] [Google Scholar]
- 4.Mitra S, Rennie J. Neonatal jaundice: aetiology, diagnosis and treatment. Br J Hosp Med 2017;78:699–704. 10.12968/hmed.2017.78.12.699 [DOI] [PubMed] [Google Scholar]
- 5.Morris BH, Oh W, Tyson JE, et al. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N Engl J Med 2008;359:1885–96. 10.1056/NEJMoa0803024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyson JE, Pedroza C, Langer J, et al. Does aggressive phototherapy increase mortality while decreasing profound impairment among the smallest and sickest newborns? J Perinatol 2012;32:677–84. 10.1038/jp.2012.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badiee Z, Mohammadizadeh M, Shamee M. Diagnostic usefulness of transcutaneous bilirubinometry in very preterm newborns. Int J Prev Med 2012;3:262–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed M, Mostafa S, Fisher G, et al. Comparison between transcutaneous bilirubinometry and total serum bilirubin measurements in preterm infants <35 weeks gestation. Ann Clin Biochem 2010;47:72–7. 10.1258/acb.2009.009072 [DOI] [PubMed] [Google Scholar]
- 9.Ozkan H, Oren H, Duman N, et al. Dermal bilirubin kinetics during phototherapy in term neonates. Acta Paediatr 2003;92:577–81. 10.1111/j.1651-2227.2003.tb02510.x [DOI] [PubMed] [Google Scholar]
- 10.Beck M, Kau N, Schlebusch H. Transcutaneous bilirubin measurement in newborn infants: evaluation of a new spectrophotometric method. Arch Dis Child Fetal Neonatal Ed 2003;88:F350–1. 10.1136/fn.88.4.f350-b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagar G, Vandermeer B, Campbell S, et al. Effect of phototherapy on the reliability of transcutaneous bilirubin devices in term and near-term infants: a systematic review and meta-analysis. Neonatology 2016;109:203–12. 10.1159/000442195 [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence Jaundice in newborn babies under 28 days Clinical guideline [CG98], 2016. [PubMed] [Google Scholar]
- 13.Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics 2013;132:871–81. 10.1542/peds.2013-1713 [DOI] [PubMed] [Google Scholar]
- 14.Hassan Shabuj M, Hossain J, Dey S. Accuracy of transcutaneous bilirubinometry in the preterm infants: a comprehensive meta-analysis. J Matern Fetal Neonatal Med 2019;32:734–41. 10.1080/14767058.2017.1390561 [DOI] [PubMed] [Google Scholar]
- 15.Juster-Reicher A, Flidel-Rimon O, Rozin I, et al. Correlation of transcutaneous bilirubinometry (TcB) and total serum bilirubin (TsB) levels after phototherapy. J Matern Fetal Neonatal Med 2015;28:1329–31. 10.3109/14767058.2014.953923 [DOI] [PubMed] [Google Scholar]
- 16.Tan KL, Dong F. Transcutaneous bilirubinometry during and after phototherapy. Acta Paediatr 2003;92:327–31. [PubMed] [Google Scholar]
- 17.Rylance S, Yan J, Molyneux E. Can transcutaneous bilirubinometry safely guide phototherapy treatment of neonatal jaundice in Malawi? Paediatr Int Child Health 2014;34:101–7. 10.1179/2046905513Y.0000000050 [DOI] [PubMed] [Google Scholar]
- 18.Hulzebos CV, Vader-van Imhoff DE, Bos AF, et al. Should transcutaneous bilirubin be measured in preterm infants receiving phototherapy? the relationship between transcutaneous and total serum bilirubin in preterm infants with and without phototherapy. PLoS One 2019;14:e0218131. 10.1371/journal.pone.0218131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arman D, Topcuoğlu S, Gürsoy T, et al. The accuracy of transcutaneous bilirubinometry in preterm infants. J Perinatol 2020;40:212–8. 10.1038/s41372-019-0445-3 [DOI] [PubMed] [Google Scholar]
- 20.Cucuy M, Juster-Reicher A, Flidel O, et al. Correlation between transcutaneous and serum bilirubin in preterm infants before, during, and after phototherapy. J Matern Fetal Neonatal Med 2018;31:1323–6. 10.1080/14767058.2017.1315662 [DOI] [PubMed] [Google Scholar]
- 21.Nagar G, Kumar M. Effect of phototherapy on the diagnostic accuracy of transcutaneous bilirubin in preterm infants. J Clin Neonatol 2017;6:148–53. [Google Scholar]
- 22.Zecca E, Barone G, De Luca D, et al. Skin bilirubin measurement during phototherapy in preterm and term newborn infants. Early Hum Dev 2009;85:537–40. 10.1016/j.earlhumdev.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 23.Kanti V, Bonzel A, Stroux A, et al. Postnatal maturation of skin barrier function in premature infants. Skin Pharmacol Physiol 2014;27:234–41. 10.1159/000354923 [DOI] [PubMed] [Google Scholar]
- 24.De Luca D, Dell'Orto V. Patched skin bilirubin assay to monitor neonates born extremely preterm undergoing phototherapy. J Pediatr 2017;188:122–7. 10.1016/j.jpeds.2017.05.080 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2020-000681supp001.pdf (707.8KB, pdf)