Abstract
Recent studies suggest that poly(A) polymerase I (PAP I) mediated polyadenylation in Escherichia coli is highly prevalent among mRNAs as well as tRNA precursors. Primary tRNA transcripts are initially processed endonucleolytically to generate pre-tRNA species, which undergo 5'-end maturation by the ribozyme RNase P. Subsequently, a group of 3' → 5' exonucleases mature the 3'-ends of the majority of tRNAs with few exceptions. PAP I competes with the 3' → 5' exonucleases for pre-tRNA substrates adding short poly(A) tails, which not only modulate the stability of the pre-tRNAs, but also regulate the availability of functional tRNAs. In this review, we discuss the recent discoveries of new tRNA processing pathways and the implications of polyadenylation in tRNA metabolism in E. coli.
Keywords: RNase E, RNase P, 3' → 5' exonucleases, poly(A) polymerase I, aminoacylation
Structure and Organization of tRNA genes
Transfer RNAs (tRNAs) are essential in all living organisms based on their role as adapter molecules that present the correct amino acids to the translation machinery so that the information contained in messenger RNAs (mRNAs) can be accurately decoded. To date over 6000 bacterial genomes have been sequenced and more than 363,000 tRNA genes have been annotated [1]. However, our understanding of tRNA processing is most advanced in the model organism Escherichia coli. It contains 86 tRNA genes that encode 40 mature tRNA isotypes associated with the standard 20 amino acids. The large majority of the tRNA genes (64) are expressed from polycistronic operons containing identical or unrelated tRNAs, rRNA or protein encoding genes (Table 1). The remainder of the tRNAs are expressed from monocistronic genes. While four tRNAs (tRNACys, tRNAHis, tRNASel and tRNATrp) are represented by only one gene each, the rest of the tRNAs are present in more than one copy, either in the same or different operons. There is a strong correlation between tRNA abundance and codon usage at a number of different growth rates [2, 3]. Unlike eukaryotes, all the tRNA genes in E. coli lack introns. However, all primary tRNA transcripts, irrespective of the organism, require extensive post-transcriptional processing (Box 1).
Table 1:
Organization of tRNA genes (bold) in the MG1655 E. coli genome.
| Monocistronic tRNA genes |
| argU, argW, aspV, asnV, asnU, asnT, glyU, ilex, ileY, leuX, metY, pheU, pheV, proK, proL, selC, serU, serW, serX, serT, thrW |
| Polycistronic tRNA operons |
| alaW alaX, argX hisR leuT proM, glyV glyX glyY, glyW cysT leuZ, leuQ leuP leuV, lysT valT lysW valZ lysY lysZ lysQ, metT leuW glnU glnW metU glnV glnX, metZ metW metV, serV argV argY argZ argQ, valU valX valY lysV, valV valW |
| Polycistronic operons containing tRNA and mRNA genes |
| secG leuU, thrU tyrU glyT thrT tufB, tyrT tyrV rttR |
| Polycistronic tRNA genes associated with rRNA operons |
| rrsA ileT alaT rrlA rrfA, rrsB gltT rrlB rrfB, rrsC gltU rrlC rrfC aspT trpT, rrsD ileU alaU rrlD rrfD thrV rrfF, rrsE gltV rrlE rrfE, rrsG gltW rrlG rrfG, rrsH ileV alaV rrlH rrfH aspU |
Box 1. Primary tRNA transcripts require extensive post-transcriptional processing.
There are ~375,000 functional tRNA molecules in an exponentially growing E. coli cell [59]. For a tRNA to be functional (i.e., to be able to be aminoacylated), it must have the nucleotides CCA at its immediate 3' terminus [60]. In many organisms, including eukaryotes and numerous bacterial species, the CCA determinant is added post-transcriptionally by the CCA-adding enzyme tRNA nucleotidyltransferase. The exact role of tRNA nucleotidyltransferase in the Gram-negative bacterium E. coli is not fully understood, since the CCA determinant is encoded in the DNA for every tRNA. It has been argued that the enzyme is needed for tRNA 3'-end repair [61, 62]. Surprisingly, the parasitic bacteria Mycoplasma and Ureaplasma (six genomes) also encodes the CCA triplet for all tRNA genes, but lack both tRNA nucleotidyltransferase and poly(A) polymerase (PAP) [63].
Even though all E. coli tRNAs have encoded CCA determinants, their primary transcripts must undergo extensive post-transcriptional processing in order to be functional. In fact, the processing of primary tRNA transcripts to produce mature tRNAs involves a significant number of ribonucleases with very distinct roles. In almost all cases, endoribonucleases (RNase E and/or RNase P) cleave the polycistonic tRNA transcripts to generate separate pre-tRNAs that contain extra nucleotides at both their 5'- and 3'-ends. Subsequently, additional enzymes (both endo- and exoribonucleases) are employed to generate the mature 3'- and 5'-termini.
To date, RNase P is the only known enzyme that is responsible for generating the mature 5'-ends of all tRNAs in all kingdoms of life. The sole exception is an obligatory parasitic archaeon Nanoarchaeum equitans where the primary tRNA transcripts start at the nucleotide that is the mature 5' terminus [64]. Inactivation of RNase P leads to accumulation of 5'-extended tRNAs and inviability in E. coli. Considering that many tRNAs with 5'-extensions can still be aminoacylated [10, 65–67], the exact reason for unviability in the absence of RNase P has yet to be determined.
In E. coli, the majority of tRNA 3'-end maturation involves one or more of the major 3' → 5' exonucleases (RNase T, RNase PH, RNase D, RNase BN, and RNase II) [4]. However, the 3'-ends of some tRNAs are matured by the endoribonuclease RNase E in a single step [19]. All these processing events are distinct from the many post-transcriptional modifications that generate the various altered nucleotides that are found in functional tRNAs.
tRNA processing pathways
For many years, it was thought that all E. coli tRNAs were matured via a common processing pathway following a ‘one shoe fits all’ model [4]. The observation of reduced RNase P activity on pre-tRNAs with long precursor sequences [5] and in the absence of RNase E led to the suggestion that an initial RNase E cleavage is required for RNase P to generate the mature 5' terminus [6–8]. Moreover, the involvement of RNase E in the initiation of tRNA processing has been proposed to be the basis of its essential function [6, 8, 9]. Thus, the consensus pathway that evolved over time was that the initial processing of all primary tRNA transcripts, both mono- and polycistronic, involved RNase E to generate pre-tRNAs containing a few extra nucleotides at both the 5'- and 3'-ends (Fig. 1A). Subsequently, the pre-tRNAs were matured at the 5'-end by RNase P and at the 3'-end by multiple 3' → 5' exoribonucleases, such as RNase T, RNase PH, RNase D and RNase BN (Box 2). Notable tRNA operons processed by this pathway are glyW cysT leuZ and argX hisR leuT proM (Fig. 1A)[6, 8].
Fig. 1.

tRNA processing pathways in E. coli (Figures are not drawn to scale). (A). RNase E initiates the processing of the argX hisR leuT proM polycistronic operon by removing the Rho-independent transcription terminator and separating the pre-tRNAs [8]. Subsequently, RNase P cleaves at the 5'-termini and RNase T and/or RNase PH removes the 3'-extension to the expose CCA determinant. (B) RNase P initiates the processing of leuQ leuP leuV polycistronic operon by separating pre-tRNAs with direct cleavages at the mature 5'-ends while the 3' Rho-dependent terminator is shortened by a combination of PNPase and RNase II [10]. Finally, RNase T and/or RNase PH matures the 3' ends. (C) In case of the secG leuU polycistronic transcript, once the Rho-independent transcription terminator is removed primarily by RNase E, RNase P cleaves at the leuU mature 5'-end and RNase T and/or RNase PH matures the 3'-end [12]. (D) Processing of the monocistronic leuX transcript [13] involves exonucleolytic removal of the Rho-independent transcription terminator by PNPase for ~90% of the transcripts (thick downward arrow). The Rho-independent transcription terminators in ~10% of the transcripts are removed endonucleolytically by RNase P followed by shortening of the 3' end by RNase II (thin downward arrow). Both pre-tRNAs are processed further by RNase P at the mature 5'-terminus and by RNase T and/or RNase PH at the 3'-terminus. (E) The processing of lysT operon [11] is initiated by RNase P by removing the Rho-independent transcription terminator (solid downward arrow). Subsequently, RNase P cleaves at the mature 5'-end of each of the upstream tRNAs sequentially from the 3'-end of the operon releasing pre-tRNAs with immature 3'-ends ranging from 2–150 nts. The longer 3'-extensions are shortened by a combination of PNPase and RNase II before RNase T and/or RNase PH mature the 3'-ends. RNase D and/or RNase BN (in small letters) also participate in the 3'-end maturation, albeit slowly, in all the above processing pathways only in the absence of the major exonucleases RNase T and RNase PH. In the absence of RNase P, RNase E can cleave at the indicated sites separating the pre-tRNAs (dashed downward arrow). (F) Processing of the proK, proL and proM transcripts [19] involves RNase E removing the Rho-independent transcription terminator in one step to generate the mature 3'-terminus without the need of any of the 3' → 5' exonucleases. RNase P cleaves at the mature 5' end.
Box 2. Processing the 3'-termini of pre-tRNAs.
Endonucleolytically generated pre-tRNAs very often contain nucleotides upstream of mature 5'-end and beyond CCA trinucleotide at the 3'-end. The removal of the extra nucleotides from the 3'-termini of most pre-tRNAs involves a hierarchy of at least five 3' → 5' exonucleases (RNase T, RNase PH, RNase D, RNase BN and RNase II) [55]. RNase T and RNase PH are considered to be the two primary exoribonucleases, which carry out the majority of tRNA 3'-end processing. RNase T is the most effective in tRNA 3'-end processing, but is inhibited by the presence of C residues in its active site [62]. For those tRNAs that have a C residue immediately downstream of the CCA determinant, RNase PH is primarily responsible for the final processing of its 3'-terminus [11, 62] (Table 2A). Several additional tRNAs where CCA determinant is followed immediately by C residue (Table 2B) are most likely also dependent on RNase PH and await experimental confirmation. If both RNase T and RNase PH are missing, cells grow more slowly but mature tRNAs are still generated through a combination of RNase D, RNase BN/Z and RNase II [18]. The loss of RNase D and RNase BN/Z, in the presence of RNase T and RNase PH, has no effect on either growth or tRNA 3'-end processing, but the absence of all five exonucleases is a synthetic lethal [68].
Many of the tRNA 3'-end processing exonucleases act on multiple RNA substrates [69]. While RNase PH and RNase D participate only in tRNA 3'-end processing RNase T participates in tRNA 3'-end processing, 23S and 5S rRNAs 3'-end maturation with equal preference [70, 71]. RNase BN, initially identified as a 3'→ 5' exonuclease involved in tRNA 3'-end maturation [72], has now been shown also to have endonuclease activity and is called RNase Z [73]. Unlike eukaryotic RNase Z which cleaves tRNA precursors endonucleolytically at the 3'-end to generate substrates for tRNA nucleotidyltransferase to add CCA determinant [74], E. coli RNase Z does not use pre-tRNA as substrate [75]. Instead, RNase Z functions primarily in mRNA decay [76]. RNase II is primarily involved in mRNA decay [77, 78].
However, it was noticed [8] that there were significant differences in the cleavage specificity of RNase E for many of the primary tRNA transcripts. In addition, it was also observed [10] that a number of tRNA operons lack predicted RNase E cleavage sites in the intergenic spacer regions, suggesting the involvement of ribonucleases other than RNase E in separation of these tRNAs. In fact, the processing of the transcripts of thevalV valW and leuQ leuP leuV operons was shown to be initiated exclusively by RNase P [10](Fig. 1B). It should be noted that these two operons are terminated by Rho-dependent transcription terminators unlike the glyW and argX operons, which are terminated by a Rho-independent transcription terminator where the processing is initiated by RNase E. Subsequently, RNase P also was shown to separate all the pre-tRNAs for some transcripts terminated with Rho-independent transcription terminators, such as the secG leuU and valU valX valY lysV operons, but only after the removal of the transcription terminator by RNase E (Fig. 1C) [11, 12].
However, not all Rho-independent transcription terminators are removed by RNase E. In the case of the leuX monocistronic transcript, PNPase, a 3' → 5' exonuclease, is primarily responsible for the removal of the terminator from ~90% of the transcripts, while RNase P removes the terminator from rest of the transcripts (Fig. 1D) [13]. Another type of processing has been described for the lysT valT lysW valZ lysY lysZ lysQ operon, which is also terminated with a Rho-independent transcription terminator [11]. In this case, RNase P processes the operon in the 3' → 5' direction starting with the removal of the Rho-independent transcription terminator (Fig. 1E) [11]. In the absence of RNase P, RNase E can cleave in the long intergenic regions (Fig. 1E) [11].
The cleavage specificity of RNase E on tRNA substrates is still poorly understood. RNase E is believed to cleave at an AU-rich sequence element (AUE) [14–17]. In fact, bacteria containing RNase E have been shown to have conserved AUE elements downstream of each tRNA [7]. Thus, most of the tRNA processing pathways described above generate pre-tRNAs that require one or more of the 3'→ 5' exoribonucleases for maturation of the 3'-ends, since RNase E endonucleolytic cleavages of primary tRNA transcripts normally occurs 1–3 nucleotides downstream of the CCA terminus at an AUE element [6, 8].
In contrast, the proK, proL, proM, metV metW, metY, and metZ tRNAs are matured exclusively by RNase E employing a one-step cleavage that exposes the CCA terminus without utilizing any of the 3' → 5' exonucleases [18] (Fig. 1F). This result was surprising, since RNase E removes the Rho-independent transcription terminators associated with proK, pheU and leuU primary transcripts very differently even though all these transcripts have similar AUE elements. Furthermore, while the downstream sequences of proL and leuX are similar, RNase E cleaves immediately downstream of the CCA of proL but leuX is not a substrate [19]. Instead, PNPase removes the Rho-independent transcription terminator of leuX [13]. With the exception of proK, proL, proM, metV metW, metY, and metZ tRNAs, all other tRNAs require 3'-end maturation by 3'→5' exonucleases (Box 2).
Discovering of the role of polyadenylation in tRNA processing
Polyadenylation in bacteria received very little or no attention for many years in spite of the fact that the first poly(A) polymerase (PAP) was discovered in E. coli in 1962 [20, 21]. However, since the identification of pcnB as the structural gene for PAP I thirty years later[22], significant progress has been made in identifying polyadenylation targets as well as its role in bacteria [13]. Genome-wide analysis in E. coli using oligo(dT)-dependent cDNAs derived from oligo(dT)-affinity purified transcripts suggested that the transcripts derived from the majority of the open reading frames (ORFs) are polyadenylated to some extent under exponentially growing conditions [23]. However, a recent RNAseq study using 5'-tag RACE approach has suggested that there are fewer polyadenylation targets in E. coli [24]. It should be noted that ligation-based techniques to determine the polyadenylation state of transcripts have been extremely inefficient in our hands.
Unlike in eukaryotes, only a limited fraction of bacterial mRNAs are polyadenylated at any given time. However, the actual extent of polyadenylation is known only for a few of the transcripts. These data suggest that the level of polyadenylation for any particular transcript is not dependent on either its size or abundance [25]. The level of polyadenylation can vary from as low as 0.4% to as high as 10%. Although these experiments have provided important insights into bacterial mRNA metabolism [25–27], its role in tRNA metabolism was still poorly understood.
The E. coli K12 strains MG1693 and W3110, which are widely used as the reference strains, are known to contain the rph-1 allele, which has a single base pair deletion in the C-terminal region of the rph gene resulting in a truncated RNase PH protein that has no 3' → 5' exonuclease activity [28, 29]. The significance of the rph-1 mutation was not realized until it was shown that many pre-tRNAs containing one or more C residues downstream of the CCA determinant were not substrates for RNase T, but were dependent on RNase PH for their final 3'-end processing (Table 2A)[30]. Thus, the pre-tRNAs that are not fully processed in rph-1 strains are prevented from being aminoacylated and become potential targets for polyadenylation. tRNATyr was one of the first tRNAs found to be polyadenylated in multiple exonuclease mutants [31]. Subsequently, using a temperature sensitive mutant of tRNATrp, it was proposed that defective tRNA precursors are polyadenylated and degraded as part of a general quality control pathway [32]. The demonstration of polyadenylation of pre-tRNAs (Table 3) in both a wild type control and a rph-1 single mutant [12, 13, 18, 29, 33] suggested that polyadenylation plays a more significant role in tRNA metabolism.
Table 2A:
tRNA 3'-ends processed by RNase PH
Table 3:
Polyadenylated tRNAs in E. coli.
| tRNAs* | No of tRNA genes | Genotypes | Reference |
|---|---|---|---|
| Arg2 | 4 | Δrnt rph-1 | [18] |
| Asn | 4 | Δrnt rph-1 | [18] |
| Cys | 1 | Wild type, rph-1, Δrnt rph-1 | [12, 18] |
| Glu2 | 4 | Δrnt rph-1 | [18] |
| His | 1 | Wild type, rph-1, Δrnt rph-1 | [12, 18] |
| Leu1 | 4 | Δrnt rph-1 | [18] |
| Leu2 | 1 | rph-1, Δrnt rph-1 | [12, 18] |
| Leu3 | 1 | Δrnt rph-1 | [18] |
| Leu4 | 1 | Δrnt rph-1 | [18] |
| Leu5 | 1 | rph-1, Δrnt rph-1 | [13, 18] |
| Lys | 6 | Δrnt rph-1 | [18] |
| Met m | 2 | Δrnt rph-1 | [18] |
| Phe | 2 | Δrnt rph-1 | [18, 29] |
| Ser1 | 1 | Δrnt rph-1 | [18] |
| Ser3 | 1 | Δrnt rph-1 | [18] |
| Ser5 | 2 | Δrnt rph-1 | [18] |
| Tyr1 | 1 | Δrnt Δrnd Δrbn rph-1 | [31] |
| Tyr2 | 2 | Δrnt Δrnd Δrbn rph-1 | [31] |
| Val1 | 5 | Δrnt rph-1 | [18] |
| Val2A | 1 | Δrnt rph-1 | [18] |
| Val2B | 1 | Δrnt rph-1 | [18] |
Length of poly(A) tails associated with these tRNAs was in the range of 1–5 nucleotides based on either Northern analysis or sequencing of cDNAs.
The prevalence of polyadenylation among specific tRNAs in E. coli at any given time is still unknown. However, based on cloning and sequencing data [18, 25], ~8–23% of hisR, cysT and leuX pre-tRNAs were reported to have poly(A) tails in a wild type strain. The percentage of poly(A) tails increased (~20–44%) in the rph-1 mutant, which was consistent with the role played by RNase PH as a 3'→5' tRNA 3'-end processing exonuclease. About 44% of pheU and pheV pre-tRNAs were reported to contain poly(A) tails in the rph-1 mutant where the pheV transcript is dependent on RNase PH for final 3'-end maturation [29]. In the absence of both RNase PH and RNase T, the percentage of transcripts with poly(A) tails increased to ~80% for hisR and ~53% for cysT. However, no poly(A) tails were reported at the 3'-mature termini of any of the above transcripts either in the rph-1 mutant or the wild type control. Interestingly, ~8–13% of the hisR and cysT tRNAs contained poly(A) tails at the mature 3'-termini in the rph-1 Δrnt double mutant [18].
Overall, 79/86 pre-tRNAs are extensively polyadenylated in the absence of both RNase T and RNase PH [18], indicating that these ribonucleases are major impediments to tRNA polyadenylation. This observation provided a possible explanation for why deregulation of PAP I synthesis in E. coli led to a significant increase in toxicity [34]. Specifically, there is an ongoing competition between the activities of tRNA 3'-end processing ribonucleases and polyadenylation by PAP I. Because of the significantly higher levels of 3'-end processing enzymes compared to PAP I in wild type cells, the majority of tRNA 3'-ends are matured faster, becoming substrates for aminoacylation rather than polyadenylation (Fig. 2A). Deregulation of PAP I synthesis changes the ratio of PAP I to the 3' → 5' exonucleases resulting in polyadenylation of mature tRNAs and a concomitant decrease in aminoacylation and protein synthesis resulting in cell death (Fig. 2B) [33].
Fig. 2:
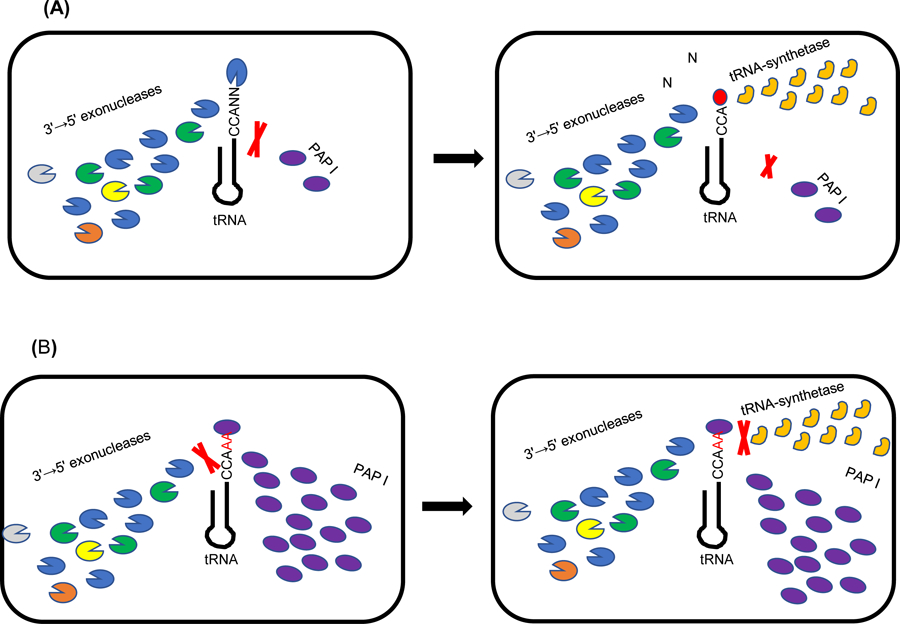
Graphical presentation (not drawn to scale) of competition between tRNA 3'-end processing 3'→5' exoribonucleases (RNase T, RNase PH, RNase D, RNase BN and RNase II), PAP I and aminoacyl tRNA-synthetases for the pre-tRNA/tRNA substrates. (A) pre-tRNAs are matured at the 3'-ends at a faster rate by higher level of exonucleases compared to PAP I. These tRNAs immediately become substrates for aminoacyl tRNA-synthetases, which add an amino acid (red circle) and are not available (red cross) for polyadenylation by PAP I. (B) Increased levels of PAP I outcompete the 3' → 5' exonucleases for the pre-tRNA/tRNA substrates and adds poly(A) tails making them unsuitable (red cross) for aminoacylation.
It should also be noted that E. coli contains a second enzyme, polynucleotide phosphorylase (PNPase) that can add polynucleotide tails onto the 3' ends of RNA species [35]. PNPase is a highly conserved protein and has been shown to synthesize polynucleotide tails in many other bacterial species [36–38]. However, there is no current evidence to suggest that either pre-tRNAs or mature tRNA species are substrates for the biosynthetic activity of PNPase. Furthermore, the generality of tRNA polyadenylation in prokaryotes may be limited by the number of organisms that contain a true poly(A) polymerase [39]. However, tRNA polyadenylation is not limited to prokaryotes, since polyadenylation of pre-tRNAs has now been observed in a eukaryotic organism [40].
Poly(A) tail lengths vary significantly between mRNAs and tRNAs
While E. coli PAP I primarily adds homopolymeric poly(A) tails, PNPase adds mostly poly(A) rich polynucleotide tails containing all four nucleotides [23, 34, 35, 41]. Transcripts with a Rho-independent transcription terminator are usually polyadenylated downstream of the terminator, which serves as a polyadenylation signal for mRNAs in E. coli [23, 41, 42]. Poly(A) tails found on mRNAs in wild-type cells are highly heterogeneous in length ranging from about 10–50 nts [25, 34, 43], but can be significantly increased in length to over 125 nt by increasing the intracellular levels of PAP I [34].
Transcripts with Rho-dependent transcription terminators are mostly targeted by PNPase for addition of polynucleotide tails [23, 41]. Polynucleotide tails tend to be very long, often over 200 nts (Mohanty and Kushner, unpublished results) [44]. The exact reason for why such a variation in tail length or what fraction of transcripts contains longer tails is not clear. Under normal physiological conditions, poly(A) tail length in exponentially growing cells is controlled through a competition between poly(A) synthesis by PAP I and degradation of the poly(A) tails by 3' → 5' exonucleases, specifically by PNPase and RNase II [35, 45, 46]. In addition, the SM-like protein Hfq, a small RNA binding protein, also affects the length of poly(A) tails associated with mRNAs in vivo [41, 47].
In contrast, the poly(A) tails found on pre-tRNA substrates are inherently short (≤10 nt) [18]. In fact, even under conditions where the synthesis of PAP I is significantly increased, the poly(A) tails found on tRNAs are still not longer than 10 nts [18, 33], suggesting a significantly reduced processivity of PAP I on tRNA substrates compared to mRNAs. Interestingly, PAP I can add long poly(A) tails to yeast tRNAs in vitro [48]. However, the basis for how PAP I discriminates among tRNAs and other RNA species in vivo is not clear. It has been reported that the RNA binding protein Hfq plays a significant role in increased processivity of PAP I during mRNA polyadenylation [41, 47, 49]. However, a comparison of poly(A) tail profiles in hfq mutants suggested no effect of Hfq on tRNA polyadenylation [18]. This is probably due to the fact that the poly(A) binding site of Hfq is distinct from its tRNA binding site, which is on its proximal surface [50].
PAP I belongs to the class II group of the nucleotidyltransferase superfamily, which includes bacterial tRNA nucleotidyltransfereases [51, 52]. Both enzymes are very similar in their amino acid sequences and are involved in template independent addition of nucleotides. However, while PAP I adds poly(A) tails to many different RNA substrates, tRNA nucleotidyltransferases add a maximum of three nucleotides (CCA) to tRNA 3'-ends. Mutational analysis suggests that a set of amino acids that determines the CCA-adding enzyme’s specificity for CTP and ATP are also conserved in PAP I, but in this case only confer ATP specificity to the enzyme [53]. Thus, it is possible that PAP I retains its intrinsic property to add short poly(A) tails to tRNA substrates in vivo. It is also possible that the complex secondary and tertiary structures associated with tRNAs play a role in limiting tRNA poly(A) tail lengths.
Unlike mRNA polyadenylation, the 3' → 5' exonucleases PNPase and RNase II, which are involved in limiting poly(A) tail lengths on mRNAs, have no effect on the level and the length of tRNA poly(A) tails [18]. This result is consistent with the fact that both these exoribonucleases require at least a 6–9 nt single-stranded region downstream from the base of a stable stem-loop structure for efficient binding activity [54]. Since the majority of the poly(A) tails associated with tRNAs are shorter than 10 nts, the binding of PNPase and/or RNase II is likely inhibited. Instead, RNase T and RNase PH directly regulate the level and length of tRNA polyadenylation [18]. The mechanism of regulation of tRNA polyadenylation by RNase T and RNase PH is not clear. It is most likely due to rapid processing of immature tRNA 3'-ends to mature 3'-ends, thereby promoting aminoacylation, rather than through the degradation of the poly(A) tails by these enzymes [33]. There is about 10-fold excess of RNase T compared to RNase PH under normal physiological conditions [33]. The number of molecules of both RNase T and RNase PH collectively is much higher than the 32–50 molecules of PAP I per cell [41]. Thus, the exoribonucleases have much higher probability of processing a tRNA substrate allowing it to enter the aminoacylation pathway rather than becoming a substrate for PAP I directed polyadenylation (Fig. 2). In fact, increased levels of RNase T and/or RNase PH eliminated the toxicity associated with overexpression of PAP I [33]. It should be noted that, the poly(A) tails reported on several eukaryotic pre-tRNAs are also 1–5 nt in length [40].
Role of polyadenylation in tRNA metabolism
The general consensus regarding polyadenylation in prokaryotes is that it serves as a targeting mechanism for the more rapid decay of defective mRNAs. A similar suggestion was made when it was shown that a mutated tRNATrp was degraded rapidly in a poly(A)-dependent manner [32]. In eukaryotes, the polyadenylated pre-tRNAs are also believed to be part of the surveillance pathway where defective pre-tRNAs are targeted to the TRAMP-exosome pathway [40].
With regard to prokaryotic tRNAs, a more detailed study in E. coli involving a variety of tRNAs suggested that under normal physiological conditions only pre-tRNAs are substrates for polyadenylation [18]. However, only a small fraction of the polyadenylated pre-tRNAs are actually degraded [18], possibly via PNPase-dependent pathway [32], but the bulk of the polyadenylated pre-tRNA species undergo a slow maturation process employing 3' → 5' exonucleases which compete with PAP I for the tRNA 3'-ends to generate mature 3'-ends with a CCA trinucleotide. Thus, polyadenylation of pre-tRNAs appears to slow down the processing by the 3' → 5' exoribonucleases resulting in a potential reduction in functional tRNA levels [18]. The aminoacylation of mature tRNAs most likely prevents their polyadenylation (Fig. 2A) (see outstanding questions).
Outstanding questions.
Is the abundance of polyadenylated pre-tRNAs/tRNAs and/or the length of their poly(A) tails regulated in response to growth conditions, growth phase, or stress?
Why are mature tRNA 3'-ends poorly polyadenylated compared to immature 3'-ends? Is it because of low PAP I levels or immediate aminoacylation?
The three proline and four initiator met tRNAs avoid polyadenylation at their 3'-end by employing an RNase E endonucleolytic cleavage immediately downstream of the CCA determinant. What distinguishes these tRNAs from the other 79 tRNAs found in E. coli?
Why is RNase P essential in E. coli? Is it because of its role in the 5'-end maturation of pre-tRNAs (see text), or the failure to separate the pre-tRNAs found in RNase P-dependent tRNA operons, or the rapid degradation of 5' immature tRNAs, which are subject to polyadenylation?
The maturation pathways of the 14 tRNAs embedded in ribosomal operons (Table 1) are still unknown. Do they follow one of the above described pathways (Fig. 1) or another independent pathway?
Consequently, the absence of 3' → 5' processing exonucleases significantly increases the extent of pre-tRNA polyadenylation [4, 18, 55, 56]. Since RNase T is both the most abundant and effective 3'-end processing enzyme, it effectively prevents polyadenylation of mature tRNAs (Fig. 2A) [33]. Increasing PAP I levels above RNase T levels leads to widespread polyadenylation of mature tRNAs resulting in the reduction of aminoacylation, inhibition of protein synthesis, and ultimately cell death (Fig. 2B) [33]. Accordingly, the intracellular level of PAP I plays a balancing role in maintaining normal levels of functional tRNAs in vivo.
While polyadenylation of mRNAs and defective RNAs is important for their rapid degradation as part of general quality control, PAP I levels are strictly regulated in multiple ways in order to avoid the polyadenylation of tRNAs and to maintain a normal level of functional tRNAs. PAP I expression has been shown to be limited by an inefficient start codon (AUU) and a poor ribosome binding site [57]. In addition, the presence of at least four internal Shine-Dalgarno-like elements within the coding sequence also limits protein synthesis by translational pausing [58].
Surprisingly, the three proline tRNAs are processed in a unique way and avoid polyadenylation at their 3'-ends. Previous studies have shown that the initial endonucleolytic processing of tRNA operons by RNase E leaves pre-tRNAs with 1–3 extra nucleotides downstream of the CCA determinant, which are subsequently removed by the 3' → 5' exoribonucleases[6, 8, 12]. The unprocessed nucleotides prevent aminoacylation, thereby providing substrates for polyadenylation by PAP I. However, a detailed analysis of the processing of the three proline tRNAs has clearly shown that RNase E cleaves immediately downstream of the CCA determinant for all three proline species, thereby avoiding polyadenylation [19]. A similar type of 3'-end endonucleolytic processing is also predicted for the four initiator methionine tRNAs, which are also not substrates for polyadenylation [18].
Concluding Remarks and Future Perspectives
The role of polyadenylation in tRNA metabolism is still emerging particularly since the full consequences of tRNA polyadenylation by PAP I and the various elements regulating this phenomenon are still not completely understood (See outstanding questions). Interestingly, the distribution of bacterial PAP is limited to β, γ and δ subdivisions of proteobacteria and in some Chlamydiales and Spirochaetales [52]. It is absent in α and ε proteobacterial subdivisions including all Gram-positive bacteria [52]. Thus, the limited occurrence of a true PAP I type enzyme in most bacteria raises the question as to why E. coli has the enzyme. Based on the demonstration that polyadenylation is involved in regulating functional tRNA levels in E. coli, it is possible that the absence of PAP I is related to avoiding the inherent toxicity associated with the polyadenylation of mature tRNAs. E. coli has dealt with this problem by significantly down regulating the expression of the PAP I, through the absence of a good ribosome binding site and the utilization of a nonoptimal translation start codon, such that there are only 32–50 molecules per cell [41]. Since increased expression of PAP I in wild type cells leads to significant toxicity [34], it is possible that the presence of the enzyme presents a selective disadvantage to organisms where expression has not been downregulated. Furthermore, it has been argued that many organisms lack a true PAP enzyme because they contain PNPase, which can carry out poly(A) additions [51]. However, it is not clear that this is a reasonable explanation, since PNPase adds long polynucleotide tails as opposed to the both short and long poly(A) tails that are added by PAP I to RNA substrates. More importantly, there is no current evidence that pre-tRNAs are substrates for the biosynthetic activity of PNPase.
Table 2B:
tRNA 3'-ends containing a ‘C’ nucleotide immediately downstream of CCA
| tRNAs | 3'-nucleotides following the CCA determinant |
|---|---|
| aspT, valT | CC |
| aspT, aspU, glnT, glnU, glnV, glnW, serU, thrT, thrV | CU |
| alaT, alaU, alaV, glnW, thrU, tyrV | UC |
| glnX | CA |
| leuT | CG |
| valU | AC |
Highlights.
The majority of transcripts in Escherichia coli undergo post-transcriptional addition of poly(A) tails by poly(A) polymerase I (PAP I). Recent studies suggest that in vivo 79/86 pre-tRNA species undergo PAP I-dependent polyadenylation to some extent prior to final 3' end maturation.
In wild type E. coli mature tRNAs are generally not polyadenylated due to higher levels of 3' → 5' tRNA-processing exonucleases compared to PAP I, which favors aminoacylation by aminoacyl-tRNA synthetases.
However, unregulated PAP I expression results in the polyadenylation of mature tRNAs hindering aminoacylation by aminoacyl-tRNA synthetases leading to inhibition of protein synthesis and cell death.
Glossary box
- Aminoacylation
Addition of an amino acid to the mature 3'-end of a tRNA
- Endoribonuclease
An enzyme that cleaves RNA molecules at internal sites
- Exoribonuclease
Enzymes that degrade RNA either from 3'- or 5'-end one nucleotide at a time
- Homopolymeric poly(A) tail
Poly(A) tail containing all A nucleotides
- Monocistronic gene
A single transcriptional unit (gene)
- PAP I
Poly(A) polymerase of Escherichia coli encoded by pcnB gene
- Polycistronic operon
A group of genes that are transcribed as a single mRNA
- Polynucleotide tail
Post-transcriptionally added tails containing all four nucleotides with mostly A nucleotides
- Rho-dependent terminators
The Rho factor is a prokaryotic protein that interacts with RNA polymerase to promote the termination of many prokaryotic transcripts
- Rho-independent transcription terminator
It is also known as intrinsic termination. Some prokaryotic transcripts do not require Rho factor for termination. Instead, these transcripts are terminated through a mechanism involving a terminal stem-loop structure followed by a U-rich sequences on the mRNA that interacts with RNA polymerase
References
- 1.Abe T, et al. , tRNADB-CE: tRNA gene database well-timed in the era of big sequence data. Front Genet, 2014. 5: p. 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg OG and Kurland CG, Growth rate optimised tRNA abundance and codon usage. J. Molecular Biology, 1997. 270: p. 544–550. [DOI] [PubMed] [Google Scholar]
- 3.Dong H, Nilsson L, and Kurland CG, Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J. Molecular Biology, 1996. 260: p. 649–663. [DOI] [PubMed] [Google Scholar]
- 4.Deutscher MP, Degradation of RNA in bacteria: comparison of mRNA and stable RNA. Nucleic Acids Research, 2006. 34: p. 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altman S, et al. , Molecular Biology of RNA, in Cleavage of RNA by RNase P, Inouye M and Dudock BS, Editors. 1987, Academic Press: Orlando: p. 3–15. [Google Scholar]
- 6.Li Z and Deutscher MP, RNase E plays an essential role in the maturation of Escherichia coli tRNA precursors. RNA, 2002. 8(1): p. 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, et al. , Co-evolution of tRNA 3' trailer sequences with 3' processing enzymes in bacteria. RNA, 2005. 11: p. 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ow MC and Kushner SR, Initiation of tRNA maturation by RNase E is essential for cell viability in E. coli. Genes Dev, 2002. 16(9): p. 1102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perwez T, et al. , Intragenic suppressors of temperature-sensitive rne mutations lead to the dissociation of RNase E activity on mRNA and tRNA substrates in Escherichia coli. Nucleic Acids Res, 2008. 36(16): p. 5306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohanty BK and Kushner SR, Ribonuclease P processes polycistronic tRNA transcripts in Escherichia coli independent of ribonuclease E. Nucleic Acids Res, 2007. 35(22): p. 7614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal A, Mohanty BK, and Kushner SR, Processing of the seven valine tRNAs in Escherichia coli involves novel features of RNase P. Nucleic Acids Res, 2014. 42(17): p. 11166–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohanty BK and Kushner SR, Rho-independent transcription terminators inhibit RNase P processing of the secG leuU and metT tRNA polycistronic transcripts in Escherichia coli. Nucleic Acids Res, 2008. 36(2): p. 364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohanty BK and Kushner SR, Processing of the Escherichia coli leuX tRNA transcript, encoding tRNAleu5, requires either the 3'–5' exoribonuclease polynucleotide phosphorylase or RNase P to remove the Rho-independent transcription terminator. Nucleic Acids Res, 2010. 38: p. 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaberdin VR, Probing the substrate specificity of Escherichia coli RNase E using a novel oligonucleotide-based assay. Nucleic Acids Research, 2003. 31: p. 4710–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDowall KJ, et al. , Site-specific RNase E cleavage of oligonucleotides and inhibition by stem-loops. Nature, 1995. 374: p. 287–290. [DOI] [PubMed] [Google Scholar]
- 16.Jourdan SS, Kime L, and McDowall KJ, The sequence of sites recognised by a member of the RNase E/G family can control the maximal rate of cleavage, while a 5'-monophosphorylated end appears to function cooperatively in mediating RNA binding. Biochem Biophys Res Commun, 2010. 391(1): p. 879–83. [DOI] [PubMed] [Google Scholar]
- 17.Chao Y, et al. , In vivo cleavage map illuminates the central role of RNase E in coding and non-coding RNA pathways. Mol Cell, 2017. 65(1): p. 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohanty BK, Maples VF, and Kushner SR, Polyadenylation helps regulate functional tRNA levels in Escherichia coli. Nucleic Acids Res, 2012. 40: p. 4589–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohanty BK, Petree JR, and Kushner SR, Endonucleolytic cleavages by RNase E generate the mature 3' termini of the three proline tRNAs in Escherichia coli. Nucleic Acids Res, 2016. 44: p. 6350–6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.August J, Ortiz PJ, and Hurwitz J, Ribonucleic acid-dependent ribonucleotide incorporation. I. Purification and properties of the enzyme. Journal of Biological Chemistry, 1962. 237: p. 3786–3793. [PubMed] [Google Scholar]
- 21.Hardy SJ and Kurland CG, The polynucleotide product of poly(A) polymerase from Escherichia coli. Biochemistry, 1966. 5: p. 3668–3676. [DOI] [PubMed] [Google Scholar]
- 22.Cao G-J and Sarkar N, Identification of the gene for an Escherichia coli poly(A) polymerase. Proceedings of the National Academy of Sciences of the United States of America, 1992. 89: p. 10380–10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohanty BK and Kushner SR, The majority of Escherichia coli mRNAs undergo post-transcriptional modification in exponentially growing cells. Nucleic Acids Res, 2006. 34(19): p. 5695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maes A, et al. , Landscape of RNA polyadenylation in E. coli. Nucleic Acids Res, 2017. 45(5): p. 2746–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohanty BK and Kushner SR, Bacterial/archaeal/organellar polyadenylation. WIREs RNA, 2010. 2: p.,256–276. [Google Scholar]
- 26.Bandyra KJ and Luisi BF, Licensing and due process in the turnover of bacterial RNA. RNA Biol, 2013. 10(4): p. 627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laalami S, Zig L, and Putzer H, Initiation of mRNA decay in bacteria. Cell Mol Life Sci, 2014. 71(10): p. 1799–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen KG, The Escherichia coli K-12 “wild types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J. Bacteriol, 1993. 175: p. 3401–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowden KE, et al. , The rph-1-Encoded Truncated RNase PH Protein Inhibits RNase P Maturation of Pre-tRNAs with Short Leader Sequences in the Absence of RppH. J Bacteriol, 2017. 199(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo Y and Deutscher MP, The physiological role of RNase T can be explained by its unusual substrate specificity. J Biol Chem, 2002. 277(33): p. 29654–61. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Pandit S, and Deutscher MP, Polyadenylation of stable RNA precursors in vivo. Proc. Natl. Acad. Sci. USA, 1998. 95: p. 12158–12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, et al. , RNA quality control: degradation of defective transfer RNA. EMBO J, 2002. 21: p. 1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohanty BK and Kushner SR, Deregulation of poly(A) polymerase I in Escherichia coli inhibits protein synthesis and leads to cell death. Nucleic Acids Res, 2013. 41: p. 1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohanty BK and Kushner SR, Analysis of the function of Escherichia coli poly(A) polymerase I in RNA metabolism. Molecular Microbiol, 1999. 34(5): p. 1094–108. [DOI] [PubMed] [Google Scholar]
- 35.Mohanty BK and Kushner SR, Polynucleotide phosphorylase functions both as a 3' - 5' exonuclease and a poly(A) polymerase in Escherichia coli. Proc Natl Acad Sci U S A, 2000. 97(22): p. 11966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campos-Guillen J, et al. , Addition of poly(A) and heteropolymeric 3' ends in B. subtilis wild-type and polynucleotide phosphorylase deficient strains. J. Bacteriology, 2005. 187: p. 4698–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rott R, et al. , RNA polyadenylation and degradation in cyanobacteria are similar to the chloroplast but different from Escherichia coli. J. Biological Chemistry, 2003. 278: p. 15771–15777. [DOI] [PubMed] [Google Scholar]
- 38.Bralley P, et al. , RNA 3'-tail synthesis in Streptomyces: in vitro and in vivo activities of RNase PH, the SCO3896 gene product and polynucleotide phosphorylase. Microbiol.-SGM, 2006. 152: p. 627–636. [DOI] [PubMed] [Google Scholar]
- 39.Raynal LC, Krisch HM, and Carpousis AJ, Bacterial poly(A) polymerase: an enzyme that modulates RNA stability. Biochimie, 1996. 78(6): p. 390–8. [DOI] [PubMed] [Google Scholar]
- 40.Wlotzka W, et al. , The nuclear RNA polymerase II surveillance system targets polymerase III transcripts. EMBO J, 2011. 30(9): p. 1790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohanty BK, Maples VF, and Kushner SR, The Sm-like protein Hfq regulates polyadenylation dependent mRNA decay in Escherichia coli. Molecular Microbiol, 2004. 54(4): p. 905–20. [DOI] [PubMed] [Google Scholar]
- 42.Mildenhall KB, et al. , RNase E-based degradosome modulates polyadenylation of mRNAs after Rho-independent transcription terminators in Escherichia coli. Mol Microbiol, 2016. 101: p. 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Hara EB, et al. , Polyadenylylation helps regulate mRNA decay in Escherichia coli. Proc Natl Acad Sci U S A, 1995. 92(6): p. 1807–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soreq H and Littauer UZ, Purification and characterization of polynucleotide phosphorylase from Escherichia coli. Journal of Biological Chemistry, 1977. 252: p. 6885–6888. [PubMed] [Google Scholar]
- 45.Marujo PE, et al. , RNase II removes the oligo(A) tails that destabilize the rpsO mRNA of Escherichia coli. RNA, 2000. 6(8): p. 1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Regnier P and Hajnsdorf E, The interplay of Hfq, poly(A) polymerase I and exoribonucleases at the 3' ends of RNAs resulting from Rho-independent termination: A tentative model. RNA Biol, 2013. 10(4): p. 602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hajnsdorf E and Régnier P, Host factor Hfq of Escherichia coli stimulates elongation of poly(A) tails by poly(A) polymerase I. Proc. Natl. Acad. Sci. USA, 2000. 97: p. 1501–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raynal LC, Krisch HM, and Carpousis AJ, The Bacillus subtilis nucleotidyltransferase is a tRNA CCA-adding enzyme. J Bacteriol, 1998. 180(23): p. 6276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Derout J, et al. , Hfq affects the length and the frequency of short oligo(A) tails at the 3' end of Escherichia coli rpsO mRNAs. Nucleic Acids Research, 2003. 31: p. 4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee T and Feig AL, The RNA binding protein Hfq interacts specifically with tRNAs. RNA, 2008. 14(3): p. 514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin G and Keller W, RNA-specific ribonucleotidyl transferases. RNA, 2007. 13(11): p. 1834–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin G and Keller W, Sequence motifs that distinguish ATP(CTP):tRNA nucleotidyl transferases from eubacterial poly(A) polymerases. RNA, 2004. 10(6): p. 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Just A, et al. , A comparative analysis of two conserved motifs in bacterial poly(A) polymerase and CCA-adding enzyme. Nucleic Acids Res, 2008. 36(16): p. 5212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spickler C and Mackie GA, Action of RNase II and polynucleotide phosphorylase against RNAs containing stem-loops of defined structure. J. Bacteriol, 2000. 182: p. 2422–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reuven NB and Deutscher MP, Multiple exoribonucleases are required for the 3' processing of Escherichia coli tRNA precursors in vivo. FASEB J, 1993. 7: p. 143–148. [DOI] [PubMed] [Google Scholar]
- 56.Li Z and Deutscher MP, Maturation pathways for E. coli tRNA precursors: a random multienzyme process in vivo. Cell, 1996. 86(3): p. 503–12. [DOI] [PubMed] [Google Scholar]
- 57.Binns N and Masters M, Expression of the Escherichia coli pcnB gene is translationally limited using an inefficient start codon: a second chromosomal example of translation initiated at AUU. Molecular Microbiol, 2002. 44: p. 1287–1297. [DOI] [PubMed] [Google Scholar]
- 58.Li GW, Oh E, and Weissman JS, The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature, 2012. 484(7395): p. 538–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mackie GA, RNase E: at the interface of bacterial RNA processing and decay. Nat Rev Microbiol, 2013. 11(1): p. 45–57. [DOI] [PubMed] [Google Scholar]
- 60.Reuven NB and Deutscher MP, Substitution of the 3' terminal adenosine residue of transfer RNA in vivo. Proc Natl Acad Sci U S A, 1993. 90(10): p. 4350–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu L and Deutscher MP, tRNA nucleotidyltransferase is not essential for Escherichia coli viability. The EMBO journal, 1987. 6(8): p. 2473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuo Y and Deutscher MP, Mechanism of action of RNase T. I. Identification of residues required for catalysis, substrate binding, and dimerization. J Biol Chem, 2002. 277(51): p. 50155–9. [DOI] [PubMed] [Google Scholar]
- 63.Mushegian AR and Koonin EV, A minimal gene set for cellular life derived by comparison of complete bacterial genomes. Proc Natl Acad Sci U S A, 1996. 93(19): p. 10268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Randau L, Schroder I, and Soll D, Life without RNase P. Nature, 2008. 453(7191): p. 120–3. [DOI] [PubMed] [Google Scholar]
- 65.Burkard U, Willis I, and Soll D, Processing of histidine transfer RNA precursors. Abnormal cleavage site for RNase P. J Biol Chem, 1988. 263(5): p. 2447–51. [PubMed] [Google Scholar]
- 66.Connolly SA, et al. , G-1:C73 recognition by an arginine cluster in the active site of Escherichia coli histidyl-tRNA synthetase. Biochemistry, 2004. 43(4): p. 962–9. [DOI] [PubMed] [Google Scholar]
- 67.Perret V, Florentz C, and Giege R, Efficient aminoacylation of a yeast transfer RNAAsp transcript with a 5' extension. FEBS Letters, 1990. 270: p. 4–8. [DOI] [PubMed] [Google Scholar]
- 68.Kelly KO and Deutscher MP, The presence of only one of five exoribonucleases is sufficient to support the growth of Escherichia coli. J. Bacteriology, 1992. 174: p. 6682–6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohanty BK and Kushner SR, Enzymes Involved in Post-transcriptional RNA Metabolism in Gram-negative bacteria. Microbiology Spectrum, 2018. 6: 10.1128/microbiolspec.RWR-0011-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Z and Deutscher MP, The tRNA processing enzyme RNase T is essential for maturation of 5S RNA. Proc. Natl. Acad. Sci. USA, 1995. 92: p. 6883–6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Z, Pandit S, and Deutscher MP, Maturation of 23S ribosomal RNA requires the exoribonuclease RNase T. RNA, 1999. 5: p. 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asha PK, et al. , Ribonuclease BN: Identification and partial characterization of a new tRNA processing enzyme. Proceedings of the National Academy of Sciences of the United States of America, 1983. 80: p. 3301–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ezraty B, Dahlgren B, and Deutscher MP, The RNase Z homologue encoded by Escherichia coli elaC gene is RNase BN. J. Biological Chemistry, 2005. 280: p. 16542–16545. [DOI] [PubMed] [Google Scholar]
- 74.Schierling K, et al. , tRNA 3' end maturation in archaea has eukaryotic features:the RNase Z from Haloferax volcanii. J. Molecular Biology, 2002. 316(895–902). [DOI] [PubMed] [Google Scholar]
- 75.Dutta T and Deutscher MP, Mode of action of RNase BN/RNase Z on tRNA precursors: RNase BN does not remove the CCA sequence from tRNA. J Biol Chem, 2010. 285(30): p. 22874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perwez T and Kushner SR, RNase Z in Escherichia coli plays a significant role in mRNA decay. Molecular Microbiol, 2006. 60(3): p. 723–37. [DOI] [PubMed] [Google Scholar]
- 77.Mohanty BK and Kushner SR, Genomic analysis in Escherichia coli demonstrates differential roles for polynucleotide phosphorylase and RNase II in mRNA abundance and decay. Molecular Microbiol, 2003. 50(2): p. 645–58. [DOI] [PubMed] [Google Scholar]
- 78.Pobre V and Arraiano CM, Next generation sequencing analysis reveals that the ribonucleases RNase II, RNase R and PNPase affect bacterial motility and biofilm formation in E. coli. BMC Genomics, 2015. 16: p. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]


