Abstract
Background
Circulatory abnormalities of retrobulbar vessels are increasingly being linked to the aetiopathogenesis of primary open angle glaucoma. These abnormalities can be assessed with a colour Doppler Imaging of retrobulbar vessels.
Aims and Objectives
To compare the Doppler ultrasound patterns of central retinal artery and ophthalmic artery in new patients with primary open angle glaucoma (POAG) diagnosis with those of non-glaucomatous group.
Design of the study
A hospital-based, comparative, cross-sectional study.
Setting
Department of Ophthalmology (Guinness Eye Centre) and Department of Radiology, Lagos University Teaching Hospital, Idi-Araba, Lagos, Nigeria.
Materials and Methods
End diastolic velocity (EDV), Peak systolic velocity (PSV) and resistivity index (RI) were measured in the central retinal artery (CRA) and ophthalmic artery (OA) of both eyes of newly diagnosed POAG patients. The CDI values of newly diagnosed POAG patients were compared with age-gender matched non-glaucomatous group. The Doppler values of the better and worse eye of patients with asymmetrical POAG were also compared.
Results
One hundred subjects (200 eyes) comprising of 50 POAG patients and 50 non-glaucomatous healthy subjects were recruited for this study. The male to female ratio was 1:1.1(24 males and 26 females) for POAG patients and 1:1 (25 males and 25 females) for non-glaucomatous subjects. The CRA and OA in both eyes of POAG patients had a significantly lower mean EDV and PSV compared with those of non-glaucomatous group (p< 0.001). The CRA and OA in both eyes of POAG patients had a significantly higher mean RI compared with those of non-glaucomatous group (p< 0.001). A significant positive Pearson correlation was seen between the IOP and the RI in the CRA and OA in both eyes. Also, statistically significant negative Pearson correlations were seen between the IOP and the PSV and EDV in the OA and CRA in both eyes.
Conclusion
This study demonstrated a significant reduction in EDV and PSV as well as an increase in RI of the CRA and OA in both eyes among POAG patients compared to the non-glaucomatous group.
Keywords: Central retinal artery, glaucoma, ophthalmic artery, Doppler, Lagos
Introduction
Glaucoma is a group of eye diseases with a distinctive optic neuropathy and neural tissue loss leading to the evolution of characteristic patterns of visual field defects.1 Worldwide, glaucoma is the second most common cause of blindness and the leading cause of irreversible loss of vision.2 It accounts for 12.3% of global blindness and 16.7% of blindness in adults 40 years and above in Nigeria.1,3 Glaucoma has been traditionally classified as primary or secondary (based on an identifiable underlying anatomical cause of aqueous outflow obstruction) as well as open angle or angle closure (based on the patency of the angle of anterior chamber).1 Primary open angle glaucoma (POAG) is the predominant type of glaucoma and runs a more aggressive course in black-skinned individuals as compared to Caucasians.4
Although the intraocular pressure (IOP) level is one of the primary risk factors for glaucoma development, susceptibility to glaucoma is also determined by the resilience of the optic nerve to the multiple pathogenic mechanisms involved in the neuropathy.1 Vascular theory is one of the major theories that have been proposed with respect to the aetiopathogenesis of glaucoma.5 In the vascular theory, glaucomatous optic neuropathy is thought to occur as a result of inadequate blood supply either due to elevated IOP or other causes of reduced ocular blood flow.6,7 Increasing evidence from clinical studies indicates that circulatory abnormalities are important in the aetiopathogenesis of glaucoma and that vascular factors leading to optic nerve ischemia may be contributory.6,8,9
There are various non-invasive methods of evaluating ocular blood flow. These include laser Doppler velocimetry (LDV), scanning laser flowmetry (SLF), laser Doppler flowmetry (LDF), colour Doppler imaging (CDI), Doppler Fourier-domain optical coherence tomography (Doppler FD-OCT) and optical coherence tomography-angiography (OCT-A).10–14 In 1989, Erickson et al11introduced orbital colour Doppler imaging (CDI).15 Orbital CDI is a cheap, readily available, non-invasive and relatively easy means of assessing the blood vessels of the orbit. It is useful for qualitative and quantitative assessment of the velocity of blood flow.15,16It employs the simultaneous use of Doppler imaging and B-scan to locate and assess blood flow velocity parameters in orbital blood vessels.
The vascular hypothesis in glaucomatous optic neuropathy has been suggested in many studies done outside Nigeria; however very few studies have been done in this country in this regard. Odunlami et al17, did a similar study with emphasis on blood flow velocity in the right eye alone using age-matched controls. This paucity of comprehensive Doppler information on retrobulbar arterial circulation among Nigerian glaucoma patients informed the need for this study.
This study sought to compare the ophthalmic artery and central retinal artery Doppler parameters in new patients with POAG diagnosis and age-sex matched non-glaucomatous group with a view to determining any association between Doppler changes and POAG. It also sought to determine if there are hemodynamic differences in each eye of asymmetric POAG patients since POAG is commonly a bilateral asymmetric disease.
Materials and Methods
This was a hospital-based, comparative, cross-sectional study comparing newly diagnosed primary open angle glaucoma (POAG) patients aged 40 years and above with age-matched non-glaucomatous group. It was conducted at the Lagos University Teaching Hospital (LUTH), Lagos, Nigeria over a period of nine months, from September 2016 to May 2017. The study was approved by the Health Research Ethics Committee of Lagos University Teaching Hospital (ADM/DCST/HREC/APP/1049) and the tenets of Helsinki declaration were adhered to strictly.
One hundred subjects were recruited for the study, comprising of 50 newly diagnosed POAG patients and 50 age-matched non-glaucomatous subjects. Consecutive consenting POAG patients who met the inclusion criteria were recruited from the Glaucoma clinic of the Department of Ophthalmology, LUTH while the age-matched non-glaucomatous subjects were recruited concurrently at the General Ophthalmology Clinic. Both eyes were used for the study which translated to a total of 200 eyes.
Patients younger than 40 years of age, those with other types of glaucoma apart from POAG, those with corneal abnormalities precluding accurate applanation tonometry, those with ocular surface inflammation or uveitis and those with any clinically/biochemically severe medical illness (diabetes mellitus, systemic hypertension, hypercholesterolaemia) or psychiatric condition were excluded from the study. Other exclusion criteria included being on antihypertensive therapy, having had intraocular surgery or laser surgery and having non-glaucomatous optic neuropathy or retinal defects.
POAG was defined by corrected lOP ≥ 21mmHg, open angle on gonioscopy, optic nerve head cup to disc ratio of at least 0.7 or a cup to disc ratio difference of more than 0.2 between the right and left eye which was consistent with visual field defects in two consecutive central 24-2 programme of Humphrey threshold perimetry test, according to the European Glaucoma Society (EGS) diagnostic criteria.20,21 For respondents with asymmetrical POAG (difference in glaucoma severity in each eye ), the severity of glaucoma was based on the cup to disc ratio (CDR) such that the eye with the lower CDR was classified as the better eye while the eye with the higher CDR was classified as the worse eye.
Both eyes of all the eligible POAG patients and non-glaucomatous group had blood flow velocity evaluation of their ophthalmic artery (OA) and central retinal artery (CRA) with the aid of a colour Doppler imaging (CDI) ultrasound machine (Toshiba Nemio XG diagnostic ultrasound system, 2000 model, probe 7.5MHz. linear phase array transducer). The transducer was placed over the ipsilateral common carotid artery to ascertain blood flow velocity and exclude any carotid artery disease.22 Only respondents with normal carotid waveform eventually had their blood flow velocity assessed for the study. To assess the blood flow velocity of CRA and OA, each participant lay supine and was asked to look straight up after which the eyes were closed. The ultrasound 7.5MHz probe was carefully applied to the closed eyelids using a coupling gel. Low wall filter settings, as well as an angle correction of ≤20°, were used occasionally requiring a colour box steering to accomplish this.16,17
Throughout the examination, the procedure was carefully done avoiding unnecessary manual pressure on the probe.23 The CDI window was localized over the retrobulbar region after which the ophthalmic and central retinal arterial blood flow were identified.23 Thereafter, a pulsed wave sample gate (1.5mm x 1.5mm) was then placed over the region of CDI flow to get a Doppler frequency shift trace.23 A trace was considered satisfactory if three consecutive waveforms were identified, allowing the mean values from the three cardiac cycles to be obtained. The OA was located superonasal to the optic nerve by orientating the sample volume about 10-15mm posterior to the eyeball.24 The optic nerve was identified as the visible hypoechoic stripe. The CRA was located at the anterior part of the optic nerve shadow by orientating the central part of the sample volume about 2-3mm posterior to the optic nerve head.24 The end diastolic velocity (EDV) and the peak systolic velocity (PSV) were measured with a cross-hair caliper. The resistivity index (RI) was automatically calculated by the machine using the Pourcelot equation. Both eyes were insonated, starting with the right. Intra-observer variability was reduced by taking an average of 3 measurements of velocimetric Doppler parameters (PSV, EDV, and RI) for each artery.
Data analysis was done with IBM Statistical Package for Social Science version 20 (Chicago, IL 2007.) The mean IOP, RI, PSV, and EDV of the POAG patients were compared with their age-matched non-glaucomatous patients using independent t-test after testing for normality with Kolmogorov-Smirnov test. Similarly, the Doppler values of the better and worse eye of patients with asymmetrical POAG were also compared using independent t-test. The linear relationships between intraocular pressure and OA and CRA Doppler parameters were determined with the Pearson correlation coefficient. A p-value of less than 0.05 was considered statistically significant.
Results
A total number of 100 subjects (200 eyes) comprising of 50 POAG patients and 50 non-glaucomatous healthy subjects were recruited for this study. There were 24 males and 26 females in the POAG group with a male to female ratio was 1:1.1 while the male to female ratio was 1:1 (25 males and 25 females) for non-glaucomatous subjects. The mean age for the POAG group was 51.62 ± 9.39 years (range, 40-69) while that of the non-glaucomatous group was 53.58 ± 9.44 years (range, 41-72). The age and gender distributions of the two groups were not significantly different (p>0.05).
The mean intraocular pressure of the POAG patients was significantly higher than those of the non-glaucomatous group. The mean right eye intraocular pressure (IOP) of the POAG group, 28.26 ± 3.42 mmHg, was significantly higher than the mean right eye IOP of the non-glaucomatous group 12.56 ± 1.93 mmHg, (p < 0.001; 95% Confidence Interval of difference between means, 14.60 – 16.80). Similarly, The mean left eye IOP of the POAG group, 27.98 ± 3.51 mmHg, was significantly higher than the mean left eye IOP of the non-glaucomatous group, 12.74 ± 1.90 mmHg, (p < 0.001; 95% Confidence Interval of difference between means, 14.12 – 16.36).
Table 1 compares the Doppler patterns of the central retinal artery (CRA) and the ophthalmic artery (OA) between the POAG group and the non-glaucomatous group. The CRA and OA in both eyes of POAG patients had a significantly lower mean end diastolic velocity (EDV) and peak systolic velocity (PSV) compared with those of non-glaucomatous group. Conversely, the CRA and OA in both eyes of POAG patients had a significantly higher mean RI compared with those of non-glaucomatous group.
Table 1. Comparison of Doppler parameters of the central retinal artery (CRA) and ophthalmic artery (OA) in both eyes of POAG and non-glaucomatous group.
| Arteries | Doppler Parameters | POAG Group (mean ± SD) | Non-glaucomatous Group (mean ± SD) | 95% CI of difference between means | p- value |
| Right EyeOA | PSV (cm/s) | 28.33± 3.25 | 42.31± 6.84 | 11.85 - 16.10 | <0.001 |
| EDV (cm/s) | 5.66 ± 2.06 | 16.08 ±3.62 | 9.26 – 11.60 | <0.001 | |
| RI | 0.80± 0.06 | 0.61± 0.08 | 0.17 – 0.22 | <0.001 | |
| CRA | PSV (cm/s) | 11.67± 1.02 | 16.92 ±2.73 | 4.43 – 6.06 | <0.001 |
| EDV (cm/s) | 1.88± 0.79 | 6.83± 1.60 | 4.44 – 5.45 | <0.001 | |
| RI | 0.84 ± 0.06 | 0.60 ± 0.04 | 0.22 – 0.26 | <0.001 | |
| Left eye | |||||
| OA | PSV (cm/s) | 28.25 ± 3.39 | 41.58 ± 6.11 | 11.37 –15.29 | <0.001 |
| EDV (cm/s) | 5.60 ± 2.06 | 16.08 ± 3.05 | 9.34 – 11.62 | <0.001 | |
| RI | 0.81 ± 0.05 | 0.62 ± 0.04 | 0.17 – 0.21 | <0.001 | |
| CRA | PSV (cm/s) | 11.72 ± 1.13 | 17.06 ± 2.96 | 4.46 – 6.23 | <0.001 |
| EDV (cm/s) | 1.86 ± 0.79 | 6.74 ± 1.60 | 4.38 – 5.38 | <0.001 | |
| RI | 0.85± 0.06 | 0.61 ± 0.04 | 0.22 – 0.25 | <0.001 | |
| SD = Standard Deviation CI= Confidence Interval | |||||
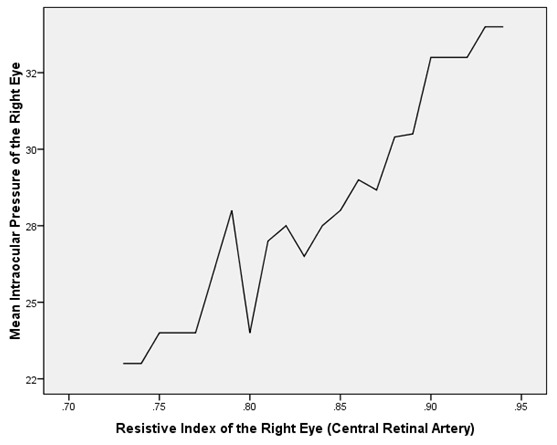
Table 2 depicts the correlation between the mean IOP and the Doppler patterns of the CRA and the OA in both eyes of the POAG group. The mean IOP was found to be negatively and significantly correlated with the PSV as well as EDV of the OA and CRA in both eyes of the POAG group (Table 2, Figure 1). On the contrary, the mean IOP was found to be positively and significantly correlated with the RI of the OA and CRA in both eyes of the POAG group (Table 2,Figure 2 and Figure 3).
Table 2. Relationship between mean IOP and the Doppler parameters of the central retinal artery (CRA) and the ophthalmic artery (OA) in both eyes of POAG group.
| Arteries | Doppler Parameters | Pearson Correlation Coefficient (r) | p-value |
| Right eye | |||
| OA | PSV (cm/s) | -0.906 | 0.02 |
| EDV (cm/s) | -0.908 | 0.03 | |
| RI | 0.863 | 0.02 | |
| CRA | PSV (cm/s) | -0.718 | 0.01 |
| EDV (cm/s) | -0.894 | 0.02 | |
| Left eye | |||
| OA | RI | 0.902 | 0.01 |
| PSV (cm/s) | -0.873 | 0.03 | |
| EDV (cm/s) | -0.858 | 0.02 | |
| CRA | RI | 0.812 | 0.02 |
| PSV (cm/s) | -0.681 | 0.01 | |
| EDV (cm/s) | -0.882 | 0.01 | |
| RI | 0.876 | 0.01 |
Figure 1: A graph showing an inverse relationship between the mean intraocular pressure (mmHg) and the peak systolic velocity (cm/s) in the right ophthalmic artery of POAG patients.
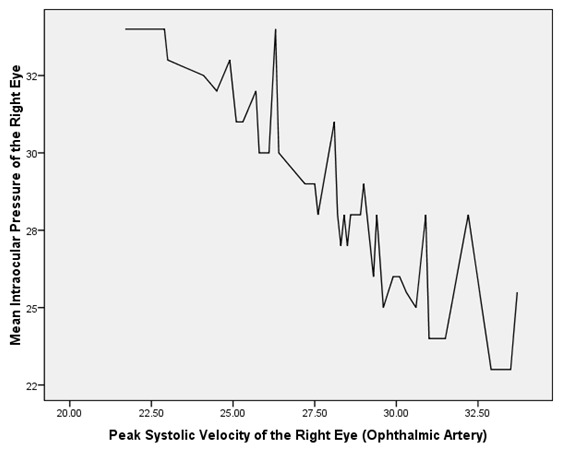
Figure 2: A graph showing a linear relationship between the mean intraocular pressure (mmHg) and the resistive index in the left central retinal artery of POAG patients.
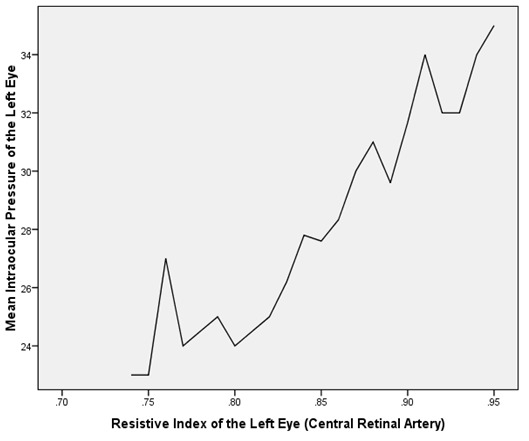
Figure 3: Graph showing a linear relationship between mean intraocular pressure (mmHg) and the resistive index (RI) in the right central retinal artery of POAG patients.
Thirty-five (70.0%) out of the 50 respondents with POAG had asymmetrical disease. The mean CDR in the better and worse eyes of respondents with asymmetrical POAG were 0.69±0.08 and 0.82±0.08 respectively (t=-6.60, p<0.001). There was no significant difference in the mean EDV, PSV and RI of the OA and CRA between the two eyes in POAG patients with asymmetric CDR. (Table 3).
Table 3. Comparison of the Doppler parameters of the central retinal artery (CRA) and ophthalmic artery (OA) of both eyes in asymmetrical POAG patients.
| Arteries | Doppler Parameters | Better eyeMean ± SD | Worse eyeMean ± SD | 95% CI of difference between means | p-value |
| OA | PSV (cm/s) | 27.62±3.46 | 28.44±3.45 | -2.00 – 0.62 | 0.30 |
| EDV (cm/s) | 5.34±2.06 | 5.97±2.20 | -1.22 – 0.40 | 0.32 | |
| RI | 0.81±0.05 | 0.80±0.05 | -0.01 – 0.03 | 0.42 | |
| CRA | PSV (cm/s) | 11.42±1.15 | 11.82±1.04 | -0.73 – 0.11 | 0.15 |
| EDV (cm/s) | 1.76±0.80 | 1.98±0.84 | -0.44 – 0.17 | 0.39 | |
| RI | 0.85±0.06 | 0.84±0.06 | -0.01 – 0.03 | 0.49 | |
| SD= Standard deviation CI=Confidence Interval | |||||
Discussion
This study recorded significantly lower mean values of end diastolic velocity (EDV) and peak systolic velocity (PSV) of the ophthalmic artery (OA) and central retinal artery (CRA) in both eyes of the POAG group compared to the non-glaucomatous group. This is similar to the findings of Odunlami et al17 who conducted a similar study among a Nigerian population in Ile-Ife. Similar observations have been reported from previous studies outside Nigeria. These studies suggested a possible relationship between decreased retrobulbar blood flow velocities and the aetiopathogenesis of glaucoma. Singh et al29 observed that the CRA and OA of the POAG patients they studied had significantly reduced EDV and PSV compared to normal subjects. This became normal after treatment once the target pressure was achieved. They postulated that reduced retrobulbar blood flow was probably a function of raised intraocular pressure (IOP) and not an independent factor in the aetiology of glaucoma.
Apart from the decreased EDV and PSV among POAG respondents in this study, the mean resistive indices (RI) of CRA and OA were significantly higher in both eyes (p<0.001) of the POAG group compared to the non-glaucomatous group. This aligns with the findings of previous studies from Nigeria17 Africa,30 Europe,25,27,28,31 Asia,22 and North America,26, The higher RI values suggest a higher resistance to blood flow in the ophthalmic and central retinal artery of POAG patients. The RI, being a ratio, is not dependent on the Doppler angle making its absolute value useful for the comparison of results among studies since values are constant irrespective of Doppler angle change.17,22 Elevated RI in the CRA and OA of untreated POAG patients has been attributed to elevated IOP which led to a direct impedance to blood flow in the retinal circulation.17,31 This is in agreement with the finding of a positive correlation between IOP and RI in OA and CRA of both eyes of POAG respondents in this study. Furthermore, the positive Pearson correlation values between the IOP and the RI were consistently higher in the CRA compared to the OA in both eyes of POAG patients. This may suggest that the CRA is more sensitive to detecting IOP changes than the OA. This may be due to the fact that the OA is a relatively large artery that has so many branches which include the CRA.
In this study, the differences between the mean values of the CDI parameters of the better eyes of respondents with asymmetrical POAG compared to their worse eyes were not statistically significant. Similarly, Singh et al29 observed no statistically significant difference in the CDI parameters of the OA, CRA and short posterior ciliary artery (SPCA) of asymmetrical POAG respondents with early, moderate and advanced glaucoma. This may suggest the contribution of other factors to asymmetric CDR other than differences in hemodynamic indices which can be explored in future studies. On the contrary, Hommer et al33 observed significantly lower mean velocities of blood flow in the CRA of the worse eyes of respondents with asymmetrical POAG compared to their better eyes. However, no significant difference was found with respect to the OA. This disparity in their findings may require further exploration; it may be mentioned, however, that Hommer et al33 studied only 15 asymmetrical POAG respondents.
This study is limited by the unavailability of information on the diameter of the vessels studied. Therefore, the velocity of blood flow determined with the CDI may not really translate to the volume of blood flow. Furthermore, the validity of RI as a measure of vascular resistance is uncertain as RI may be affected by several anatomical and physiological factors not related to resistance, such as conductance and blood pressure profile as pointed out by Stalmans et al.34 Finally, using the optic nerve head cup to disc ratio to classify asymmetric POAG into better and worse eye may not be as accurate as using visual field parameters such as mean deviation and pattern standard deviation.
Conclusions
In conclusion, this study has demonstrated a significant decrease in the PSV and EDV as well as a significant increase in the RI in the OA and CRA of respondents with POAG compared with the non-glaucomatous group. However, there was no significant difference in the EDV, PSV, and RI of the CRA and OA in better eyes compared with the worse eyes of respondents with asymmetrical POAG.
References
- 1.Girkin CA, Bhorade AM, Giaconi JA, Medeiros FA, Sit AJ, Tanna AP, et al. Introduction to Glaucoma. San Francisco: American Academy of Ophthalmology; 2016. Glaucoma, Basic and Clinical Science Course, 2016-2017. pp. 20–26. [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bulletin of the World Health Organization. 2004;32:848–851. [PMC free article] [PubMed] [Google Scholar]
- 3.Abdul MM, Sivasubramaniam S, Murthy GV, Gilbert C, Abubakar T, Ezelum C, et al. Causes of blindness and visual impairment in Nigeria, The Nigeria national blindness and visual impairment survey. . Invest Ophthalmol. & Vis. Sci. . 2009;50:4114–4120. doi: 10.1167/iovs.09-3507. [DOI] [PubMed] [Google Scholar]
- 4.Racette L, Wilson MR, Zangwill LM, Weinreb RN, Sample PA. Primary open-angle glaucoma in blacks: a review. . Surv Ophthalmol. 2003;48:295–313. doi: 10.1016/s0039-6257(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 5.Acar N, Berdeaux O, Juaneda P, Gregoire S, Cabaret S, Joffre C, et al. Red blood cells plasminogens and docosahexanoic acid are independently produced in primary open angle glaucoma. Exp. Eye Res. 2009;89:840–853. doi: 10.1016/j.exer.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Moore D, Harris A, Wudunn D, Kheradiya N, Siesky B. Dysfunctional regulation of ocular blood flow: A risk factor for glaucoma? Clin Ophthalmol. . 2008;2:849–861. doi: 10.2147/opth.s2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanagi M, Kawasaki R, Wang JJ, Wong TY, Crowston J, Kiuchi Y. Vascular risk factors in glaucoma: a review. Clin Exp Ophthalmol . 2011;39:252–258. doi: 10.1111/j.1442-9071.2010.02455.x. [DOI] [PubMed] [Google Scholar]
- 8.Grieshaber MC, Flammer J. Blood flow in glaucoma. Curr Opin Ophthalmol. . 2005;16:79–83. doi: 10.1097/01.icu.0000156134.38495.0b. [DOI] [PubMed] [Google Scholar]
- 9.Flammer J, Pache M, Resink T. Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retin Eye Res. 2001;20:319–349. doi: 10.1016/s1350-9462(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 10.Maram J, Srinivas S, Sadda SR. Evaluating ocular blood flow. Indian J Ophthalmol . 2017;65:337–346. doi: 10.4103/ijo.IJO_330_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson SJ, Hendrix LE, Massaro BM, Harris GJ, Lewandowski MF, Foley WD, et al. Colour Doppler flow imaging of the normal and abnormal orbit. Radiology. 1989;173:511–516. doi: 10.1148/radiology.173.2.2678264. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Bower BA, Izatt JA, Tan O, Huang D. Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2008;13(6) doi: 10.1117/1.2998480.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia JP, Jr Garcia PT, Rosen RB. Retinal blood flow in the normal human eye using the canon laser blood flowmeter. . Ophthalmic Res. 2002;34:295–299. doi: 10.1159/000065600. [DOI] [PubMed] [Google Scholar]
- 14.Gao SS, Jia Y, Zhang M, Su JP, Liu G, Hwang TS, et al. Optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:27–36. doi: 10.1167/iovs.15-19043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacKinnon JR, McKillop G, O’Brien C, Swa K, Butt Z, Nelson P. Colour Doppler imaging of the ocular circulation in diabetic retinopathy. Acta Ophthalmol Scand. 2000;78:386–389. doi: 10.1034/j.1600-0420.2000.078004386.x. [DOI] [PubMed] [Google Scholar]
- 16.Lieb WE, Cohen SM, Merton DA, Shields JA, Mitchell DG, Goldberg BB. Colour Doppler Imaging of the eye and orbit. Technique and normal vascular anatomy. Arch Ophthalmol . 1991;109:527–531. doi: 10.1001/archopht.1991.01080040095036. [DOI] [PubMed] [Google Scholar]
- 17.Odunlami OA, Ayoola O, Onakpoya OH, Adetiloye VA, Arogundade R. Ocular blood flow velocity in primary open angle glaucoma - A tropical African population study. Middle East Afr J Ophthalmol . 2013;20:174–178. doi: 10.4103/0974-9233.110617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araoye M.O. Research Methodology with Statistics for Health and Social Sciences. 1st ed. Ilorin: Nathadex Publishers.; 2004. pp. 115–129. [Google Scholar]
- 19.Ashaye A, Ashaolu O, Komolafe O, Ajayi BG, Olawoye O, Olusanya B, et al. Prevalence and types of glaucoma among an indigenous African population in southwestern Nigeria. Invest Ophthalmol Vis Sci. 2013;54:7410–7416. doi: 10.1167/iovs.13-12698. [DOI] [PubMed] [Google Scholar]
- 20.Terminology and Guidelines for Glaucoma. [2018 Aug 24];https://bjo.bmj.com/content/101/4/1. European Glaucoma Society. (4th ed.). part 1 doi: 10.1136/bjophthalmol-2021-egsguidelines. [DOI] [PubMed] [Google Scholar]
- 21.Crowston JG, Hopley CR, Healey PR, Lee A, Mitchell P. The effect of optic disc diameter on vertical cup to disc ratio percentiles in a population-based cohort: the Blue Mountains Eye Study. [2018 Dec 10]. https://bjo.bmj.com/content/88/6/766. https://bjo.bmj.com/content/88/6/766. [DOI] [PMC free article] [PubMed]
- 22.Sharma NC, Bangiya D. Comparative study of ocular blood flow parameters by colour Doppler imaging in healthy and glaucomatous eye. Indian J Radiol Imaging. . 2006;16:679–689. [Google Scholar]
- 23.Butt Z, McKillop G, O’Brien C, Allan P, Aspinall P. Measurement of ocular blood flow velocity using colour Doppler imaging in low tension glaucoma. . Eye. 1995;9(pt 1):29–33. doi: 10.1038/eye.1995.4. [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz OG, Ersoy B, Tuncyurek O, Urk V, Ozkol M, Ozhan B, et al. Doppler ultrasonography imaging of hemodynamic alteration of retrobulbar circulation in type 1 diabetic children and adolescents without retinopathy. Diabetes Res Clin Pract. 2008;79:243–248. doi: 10.1016/j.diabres.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Stalmanns I, Vandewalle E, Anderson DR, Costa VP, Frenkel RE, Garhofer G, et al. Use of Colour Doppler imaging in ocular blood flow research. Acta Ophthalmologica. 2011;89:609–630. doi: 10.1111/j.1755-3768.2011.02178.x. [DOI] [PubMed] [Google Scholar]
- 26.Rojanapongpun P, Drance SM, Morrison BJ. Ophthalmic artery flow velocity in glaucomatous and normal subjects. Br J Ophthalmol. 1993;77:25–27. doi: 10.1136/bjo.77.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akarsu C, Bilgili MY. Colour Doppler imaging in ocular hypertension and open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2004;242:125–129. doi: 10.1007/s00417-003-0809-3. [DOI] [PubMed] [Google Scholar]
- 28.Garhofer G, Fuchsjager-Maryl G, Vass C, Pemp B, Hommer A, Schmetterer W. Retrobulbar blood flow velocity in open angle glaucoma and their association with mean arterial blood pressure. Invest. Ophthalmol. 2010;51:6652–6657. doi: 10.1167/iovs.10-5490. [DOI] [PubMed] [Google Scholar]
- 29.Singh MD, Sharma C, Prasad A. A colour doppler study of retrobulbar blood flow parameters in patients of primary open angle glaucoma. Indian Journal of Clinical and Experimental Ophthalmology. 2015;1:84–90. [Google Scholar]
- 30.Mokbel TH, Shahin MM, El-Said EM, Abd EL-Ghaffar WM. Potential Diagnostic value of fluorescein angiography and color Doppler imaging in primary open angle glaucoma. Eur J Ophthalmol. 2009;19:957–962. doi: 10.1177/112067210901900610. [DOI] [PubMed] [Google Scholar]
- 31.Butt Z, O’ Brien C, Mckillop G, Aspinall P, Allan P. Colour Doppler imaging in untreated high and normal pressure open angle glaucoma. Invest Ophthalmol Vis.Sci, . 1997;38:690–696. [PubMed] [Google Scholar]
- 32.Rankin SJ, Walman BE, Buckley AR, Drance SM. Colour Doppler imaging and spectral analysis of the of the optic nerve vasculature in glaucoma. Am J Ophthalmol. 1995;119:683–693. doi: 10.1016/s0002-9394(14)72771-0. [DOI] [PubMed] [Google Scholar]
- 33.Hommer AB, Resch H, Garhofer G, Vass C, Schmetterer L. Ocular Blood Flow Parameters in Patients With Asymmetric Primary Open Angle Glaucoma. Invest Ophthalmol & Vis Sci . 2010;51:5008–5008. [Google Scholar]
- 34.Stalmans I, Vandewalle E, Anderson DR, Costa VP, Frenkel RE, et al. Use of Colour Doppler imaging in ocular blood flow research. Acta Ophthalmol. 2011;89(8):e609–e630. doi: 10.1111/j.1755-3768.2011.02178.x.. [DOI] [PubMed] [Google Scholar]


