Abstract
Aim
This study aimed to explore the regulative mechanisms of miR-27a-3p in chemo-resistance of breast cancer cells.
Materials and Methods
qRT-PCR was employed to determine miR-27a-3p expression in two breast cancer cell lines, MCF-7 and MCF-7/adriamycin-resistant cell line (MCF-7/ADR). The two cell lines were treated with miR-27a-3p mimics or inhibitors or corresponding negative control (NC), respectively. The changes were investigated by qRT-PCR, CCK-8 assay, Western blot (WB), colony formation assay, and flow cytometry assay. Moreover, luciferase reporter assay was analyzed to verify the downstream target gene of miR-27a-3p. Further investigation in the correlation between miR-27a-3p and BTG2 was launched by WB, flow cytometry assay, and CCK-8 assay. The expression of Akt and p-Akt was detected by WB.
Key Findings
Significantly higher miR-27a-3p expression was confirmed in MCF-7/ADR as compared with sensitive cell line MCF-7 (P<0.05). The down-regulation of miR-27a-3p in MCF-7/ADR enhanced the sensitivity of cancer cells to adriamycin treatment, decreased multidrug resistance gene 1/P-glycoprotein (MDR1/P-gp) expression, enhanced the apoptosis-related proteins expression, increased adriamycin-induced apoptosis, and inhibited cell proliferation as compared to NC groups (P<0.05). The up-regulation of miR-27a-3p in MCF-7 showed the opposite results. BTG2 is identified as a direct target of miR-27a-3p and its down-regulation reversed ADR-resistance. BTG2 treatment exhibited inhibitory effect on PI3K/Akt pathway in MCF-7/ADR cells.
Significance
miR-27a-3p might be associated with resistance of breast cancer cells to adriamycin treatments, modulating cell proliferation and apoptosis by targeting BTG2 and promoting the PI3K/Akt pathway in breast cancer cells. miR-27a-3p/BTG2 axis might be a potential therapeutic target for clinical BC resistance.
Keywords: miR-27a-3p, chemoresistance, breast cancer, adriamycin, BTG2
Introduction
Breast cancer (BC) is one of the most common malignancies worldwide, accounting for 15% of cancer deaths in women.1 Chemotherapy plays an important role in reducing mortality and suppressing recurrence in many patients with BC.2 Adriamycin (ADR), an anthracycline chemotherapeutic compound, has been widely used in a variety of cancers for more than 30 years. At present, this agent is considered one of the most effective components of adjunctive and palliative therapy for BC.3 As an antineoplastic antibiotic, ADR damages DNA and induces reactive oxygen species (ROS) formation via intercalation into DNA and inhibition of topoisomerases I and II, thereby interfering with cell replication and activating apoptosis.4 Treatment with ADR has been found to contribute to prolonged survival time and improved overall survival in patients with various malignancies, including BC.5 However, chemoresistance to ADR can occur, presenting a major clinical obstacle to improved prognosis in patients with BC.6 To enhance the efficacy of ADR-based chemotherapy, researchers must elucidate the molecular basis of the resistance mechanism and then develop a new therapeutic strategy to reverse this resistance.
MicroRNAs (miRNAs) are small, noncoding RNAs (20–22 bp) that regulate gene expression via post-transcriptional repression or degradation.7 Recent reports have shown that miRNAs contribute to the tumor processes of cell proliferation, apoptosis, migration, and invasion.8–10 A miRNA of special interest is miR-27a-3p, which is located on chromosome 19.11 MiR-27a-3p is considered an oncogene because its overexpression is associated with a poor prognosis, as observed in patients with osteosarcoma or gastric cancer.12,13 Some reports have found that miR-27a-3p enhances colon cancer progression and differentiation.14 Besides, miR-27a-3p has been demonstrated to mediate chemoresistance of cancers such as leukemia, ovarian cancer, and hepatocellular carcinoma.15–17 In BC, miR-27a-3p has been found to regulate cancer cell growth, proliferation, migration, invasion, and angiogenesis.18,19 Studies have also found that miR-27a-3p is involved in the sensitivity of BC to radiotherapy,20 endocrine therapy,21 and cisplatin therapy.22 However, few studies have assessed the relationship between miR-27a-3p and ADR resistance in BC.
B-cell translocation gene 2 (BTG2), also known as NGF-inducible protein PC3/TIS21, belongs to the BTG/TOB gene family.23 The first identified member in the anti-proliferative gene family, BTG2 is believed to exhibit anticancer effects in many types of malignancies, including BC.24–26 Studies have demonstrated that BTG2 expression is down-regulated in BC, and there is evidence that BTG2 is involved in the incidence, progression, metastasis, and prognosis of BC.26,27 Recently, the potential relationship between miRNAs and BTG2 has attracted attention, since one study discovered that 3 miRNAs (miR-21, miR-23a, and miR-27a) acted as cooperative repressors of some tumor suppressor genes in pancreatic ductal adenocarcinoma.28 However, the specific relationship between BTG2 and miR-27a-3p in BC remains unknown.
According to the above researches, this research was designed to investigate the potential role of miR-27a-3p/BTG2 axis in the regulation of BC chemoresistance. This study will shed light on new strategies for clinical BC chemoresistance.
Materials and Methods
Cell Lines and Cell Cultures
The human chemosensitive BC cell line MCF-7 was obtained from the American Type Culture Collection, and the ADR-resistant cell line MCF-7/ADR was purchased from Xinyu Biotechnology (XY-h268, Shanghai, China). Cells were cultured in Roswell Park Memorial Institute-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco, Lofer, Austria) at 37°C in a humidified atmosphere of 5% CO2. To maintain the drug-resistant phenotype, ADR (0.5 μg/mL; Xinyu Biotechnology) was added to the culture medium of MCF-7/ADR. The medium was replaced every 1 to 2 days.
Information of the two cell lines is as follows: i) MCF-7 was estrogen receptor (ER)-positive breast cancer cells, the cells were derived from pleural effusion from a 69-year-old white women with breast cancer; ii) MCF-7/ADR was gradually induced as supplier from MCF-7 cells by using a certain concentration of adriamycin and STR data of cells were supplied in supplementary material (Figure S1 and 2). All cell lines were routinely tested for mycoplasma contamination and confirmed to be negative.
Chemicals and Antibodies
The polyclonal anti-Bcl-2, anti-Bcl-2 associated X (Bax), and anti-BTG2/PC3 antibodies were purchased from Abcam (Cambridge, MA, USA). The monoclonal anti-β-actin antibody was purchased from Paiwei Bio (Nanjing, China). The monoclonal anti-total Akt (Akt); anti-phospho-Akt (p-Akt); anti-Caspase3; anti-Cleaved-caspase3; anti-multidrug resistance protein 1 (MDR1)/ABCB1 antibody; and anti-rabbit IgG, HRP-linked antibody were purchased from Cell Signaling Technology (Beverly, MA, USA).
Cell Transfection
Specific concentrations of mimics or inhibitors of miR-27a-3p (RiboBio, Guangzhou, China) were transfected into cell cultures using the transfection reagent (RiboBio) to activate or inactivate miR-27a-3p expression, respectively. Negative controls (NC) were used for both reactions (see the RNA oligo sequences in Table 1). At 48 h after transfection, the transfection solution was replaced with complete culture medium. For RNA extraction and protein isolation, cells were treated for 48 h according to the manufacturer’s recommendations and were then harvested. The miRNA transfection efficiencies were determined by real-time polymerase chain reaction (PCR). The BTG2 DNA sequence and double-stranded siRNA against BTG2 (Genepharma, Shanghai, China) were then transfected into the MCF-7 and MCF-7/ADR cells using the transfection reagent, respectively.
Table 1.
RNA Oligo Sequences
| Name | Oligo Sequences |
|---|---|
| hsa-miR-27a-3p mimics | 5ʹ-UUCACAGUGGCUAAGUUCCGC-3ʹ 5ʹ-GGAACUUAGCCACUGUGAAUU-3’ |
| Mimic NC | 5ʹ-UUUGUACUACACAAAAGUACUG-3ʹ 5ʹ-CAGUACUUUUGUGUAGUACAAA-3’ |
| hsa-miR-27a-3p inhibitors | 5ʹ-GCGGAACUUAGCCACUGUGAA-3’ |
| Inhibitor NC | 5ʹ-CAGUACUUUUGUGUAGUACAAA-3’ |
Abbreviation: NC, negative control.
Quantitative RT-PCR (qRT-PCR) Analysis
Total RNA was extracted from MCF-7 and MCF-7/ADR cells using a Total RNA TriPure Isolation Reagent Kit (BioTeke, Beijing, China). The cDNA was obtained by reverse transcription using a PrimeScript RT Reagent Kit with gDNA Eraser (RR047A; Takara Bio Inc, Dalian, China). The bulge-loop miRNA qRT-PCR primer sets (one RT primer and a pair of qPCR primers for each set) specific for miR-27a-3p were designed by RiboBio. The qRT-PCR was performed with an AceQ qPCR SYBR Green Master Mix Kit (Q131-02; Vazyme Biotech, Nanjing, China) in an ABI 7500 RT-PCR system (Applied Biosystems, Foster City, CA, USA) using the following parameters: 1 cycle of predenaturation at 95°C for 10 min, followed by 40 cycles at 95°C for 2 sec, 60°C for 20 sec, and 70°C for 10 sec. At this point, miRNA expression was normalized to U6 and the relative fold changes were calculated using the 2−ΔΔCt method. Each sample was run independently in triplicate.
For mRNA expression analysis, total cellular RNA was isolated and reverse transcribed to cDNA. RT-PCR amplification was performed using the AceQ qPCR SYBR Green Master Mix Kit, and U6 was measured as an internal reference. The primers of mRNA and U6 were purchased from RiboBio (see the primer sequences in Table 2). The PCR reaction contained 2 µL RT product, 10 µL 2X SYBR Green Mix, 0.8 µL forward primer and 0.8 µL reverse primer (5 µM/l each), and nuclease-free water in a final volume of 20 µL. Standard PCR samples were analyzed using the ABI 7500 RT-PCR system, and all qRT-PCR reactions were performed in triplicate. The expression levels of the target genes were again normalized to U6 and calculated using the 2−ΔΔCt method. The qRT-PCR was carried out at least 3 times.
Table 2.
Primer Sequences for qRT-PCR
| Gene | Sequences |
|---|---|
| U6 | Forward 5ʹ-GCTTCGGCAGCACATATA-3ʹ Reverse 5ʹ-CGCTTCACGAATTTGCG-3’ |
| BTG2 | Forward 5ʹ-AACTGTTGCGTGCTTGAGTCTG-3ʹ Reverse 5ʹ-CAGTTGCTAACCTTGCTTGCTC-3’ |
Western Blot Assay
Cells were collected and lysed in RIPA lysis buffer on ice for 15 min. After the samples underwent centrifugation at 12,000 ×g for 10 min at 4°C, the protein concentrations were determined using a BCA reagent (Beyotime, Beijing, China). Equal amounts of protein were then subjected to 10% or 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore Corp., Bedford, MA, USA). After being blocked with 5% fat-free milk at room temperature (37°C) for 2 h, the membranes were incubated with primary antibodies against MDR1 (1:1250 dilution), Caspase3 (1:1000 dilution), Cleaved-caspase3 (1:1000 dilution), Bcl-2 (1:1000 dilution), Bax (1:1000 dilution), BTG2 (1:1000 dilution), Akt (1:1000 dilution), p-Akt (1:2000 dilution), and β-actin (1:1000 dilution) at 4°C overnight. The protein levels were normalized to β-actin. The blots were then labeled with anti-rabbit IgG secondary antibody (1:3000 dilution) for 1 h at 37°C. Protein bands were detected using an enhanced chemiluminescence reagent (Thermo Fisher Scientific, Waltham, MA, USA). All Western blot experiments were repeated at least 3 times.
Proliferation Assay
A cell counting kit-8 (CCK-8) assay (Dojindo Company, Shanghai, China) was performed to evaluate the cells’ sensitivity to the drug in triplication experiments. Briefly, cells (8 × 103) were plated in 96-well microplates and incubated overnight. To calculate the IC50 values for ADR, MCF-7 cells were transfected with 0, 0.22, 0.45, 0.90, 1.80, 3.60, 7.19, 14.38, 28.75, or 57.50 μM/l of ADR, and MCF-7/ADR cells were treated with 0, 3.60, 7.19, 14.38, 28.75, 57.50, 115.00, or 230.00 μM/l of ADR for 48 h. After treatment, the medium was replaced with 110 µL culture medium containing 10 µL CCK-8, and the cells were incubated for 3 h. Finally, the absorbance was measured at 450 nm (OD450) using an ELISA plate reader (Bio-Tek, Winooski, Vermont, USA). The IC50 values were calculated using Graphpad Prism 7.00 (GraphPad Software, San Diego, CA, USA).
Colony Formation Assay
Cell colony formation was measured with a plate colony formation assay. MCF-7 cells were treated with the miR-27a-3p mimics or with the mimic NC for 48 h and then seeded in 6-well plates at 1000 cells per well. MCF-7/ADR cells were treated with the miR-27a-3p inhibitors or with the inhibitor NC for 48 h and then seeded in 6-well plates at 800 cells per well. After 7 to 10 days, colonies were obviously formed with both cell types. The plates were then gently washed and stained with crystal violet. The number of colonies was counted by observing the proliferation of a single cell. Each assay was performed in triplicate on two separate occasions.
Flow Cytometry Assay
MCF-7 and MCF-7/ADR cells were seeded into 6-well plates and then treated with transfection for 48 h. After 48 h, the cells were washed twice with phosphate-buffered solution (PBS), stained for 15 min with the annexin V-FITC/PI Apoptosis Detection Kit (BD, Becton Dickinson Company, NJ, USA), and then collected. Samples were analyzed using FACSCanto TM II flow cytometry (Becton, Dickinson and Company). Triplicate experiments were performed for flow cytometry analysis.
Databases and Bioinformatics
To define the potential targets of miR-27a-3p, the miRNA targets predicted by publicly available computational algorithms were analyzed using miRWalk (http://mirwalk.umm.uni-heidelberg.de/human/mirna/46/), TargetScan (http://www.targetscan.org/cgi-bin/targetscan/vert_72/targetscan.cgi?species=Human&gid=&mir_sc=&mir_c=&mir_nc=&mir_vnc=&mirg=miR-27a-3p), PicTar (https://pictar.mdc-berlin.de/cgi-bin/PicTar_vertebrate.cgi), and StarBase v3.0 (http://starbase.sysu.edu.cn/).
Dual-Luciferase Reporter Assay
The wild type (WT) and mutant (MUT) 3ʹUTR of BTG2 predicted binding sequences with miR-27a-3p, were synthesized and inserted into the luciferase reporter plasmid (RiboBio). MCF-7 cells were seeded in 24-well plates, grown to 60% confluence, and co-transfected with plasmid plus miR-27a-3p mimics or miR-NC (RiboBio) using Lipo2000 (Invitrogen, Carlsbad, CA, USA). After 48 h of incubation, the luciferase activity was evaluated using the Dual-Luciferase Reporter Assay System (Promega, Madison city, WI, USA).
Statistical Analysis
Statistical analysis was performed with Graphpad Prism software 7.00. Data from triplicate experiments performed in a parallel manner were analyzed using Student’s t-test or one-way analysis of variance (ANOVA). All values are expressed as mean ± SD. P values < 0.05 were considered statistically significant.
Results
Differential Expressions of miR-27a-3p and MDR1 in BC
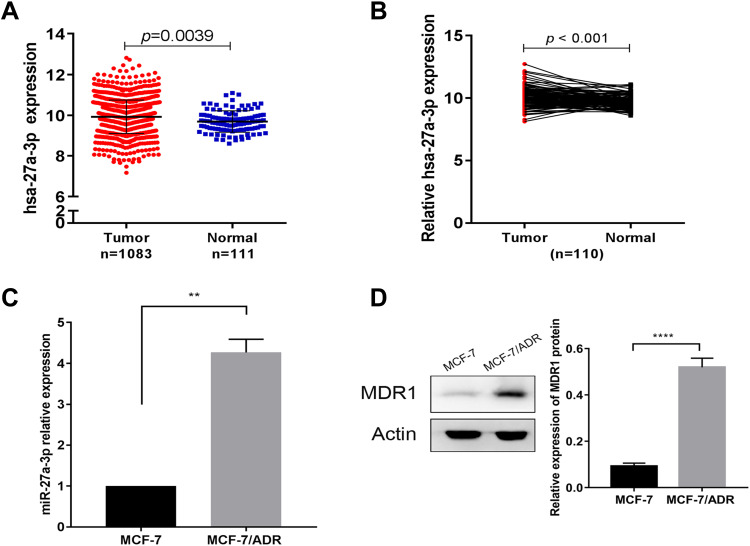
The miR-27a-3p expression data based on BC samples were screened from The Cancer Genome Atlas (TCGA). In 1083 tumors and 111 normal breast samples from TCGA database, the expression of miR-27a-3p was relatively higher in breast tumors than normal breast samples (Figure 1A). In 110 pairs of human BC tissues and adjacent normal tissues from TCGA database, relative miR-27a-3p expression was higher in BC samples than adjacent normal samples (Figure 1B). The expression levels of miR-27a-3p and MDR1 were measured in MCF-7 and MCF-7/ADR cell lines by qRT-PCR and Western blot, respectively. The relative expression of miR-27a-3p was significantly higher (approximately 4-fold) in MCF-7/ADR cells than in MCF-7 cells (Figure 1C). In addition, the expression level of MDR1 was obviously higher in the ADR-resistant MCF-7/ADR cells than in the parental MCF-7 cells (Figure 1D).
Figure 1.
miR-27a-3p was up-regulated in BC tissues and ADR-resistant cell lines. (A) miR-27a-3p expression in 1083 BC and 111 normal breast samples from TCGA database. (B) Expression levels of miR-27a-3p in 110 pairs of human BC tissues and adjacent normal tissues from TCGA database. (C) Expression levels of miR-27a-3p in MCF-7 cells and MCF-7/ADR cells based on qRT-PCR. (D) Expression levels of MDR1 in MCF-7 cells and MCF-7/ADR cells based on Western blot analysis. **P < 0.01; ****P < 0.0001. The data are expressed as mean ± SD.
miR-27a-3p Is Involved in ADR Resistance and Cell Proliferation
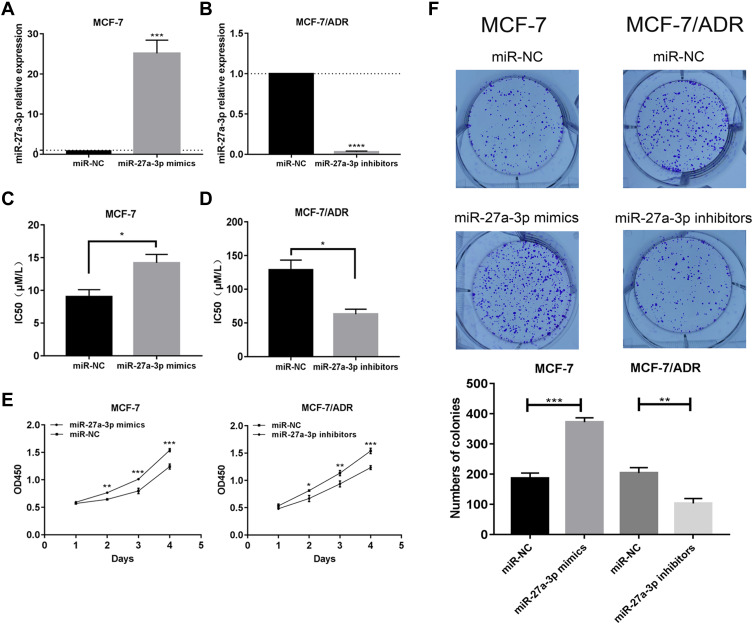
To investigate the function of miR-27a-3p in drug resistance, miR-27a-3p mimics, miR-27a-3p inhibitors, and respective miR-NC were successfully transferred into MCF-7 and MCF-7/ADR cells, respectively. The expression of miR-27a-3p was higher in mimics-transfected MCF-7 cells than in miR-NC-transfected MCF-7 cells (Figure 2A). The expression of miR-27a-3p was lower in inhibitors-transfected MCF-7/ADR cells than in miR-NC-transfected MCF-7/ADR cells (Figure 2B).
Figure 2.
miR-27a-3p promoted proliferation and resistance of BC cells in vitro. (A and B) qRT-PCR was performed to examine miR-27a-3p expression in MCF-7 and MCF-7/ADR cells transfected with either miR-27a-3p mimics or inhibitors. (C and D) IC50 was verified in MCF-7 and MCF-7/ADR cells transfected with either miR-27a-3p mimics or inhibitors. (E and F) Cell proliferation was determined with CCK-8 assays and colony formation assays in MCF-7 and MCF-7/ADR cells transfected with either miR-27a-3p mimics or inhibitors. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. The data are expressed as mean ± SD.
The sensitivity of MCF-7 and MCF-7/ADR cells to ADR was determined by CCK-8 assay. The IC50 values of ADR were 7.809 ± 0.29 μM/l in MCF-7 cells and 116.3 ± 0.98 μM/l in MCF-7/ADR cells. MCF-7 cells transfected with miR-27a-3p mimics had significantly decreased sensitivity to ADR when compared with cells transfected with miR-NC, as indicated by increased IC50 values (Figure 2C). Additionally, the IC50 values of ADR in MCF-7/ADR cells transfected with miR-27a-3p inhibitors were reduced significantly compared with the cells transfected with miR-NC (Figure 2D).
The CCK-8 assay also suggested that up-regulation of miR-27a-3p could induce MCF-7 cell growth, whereas down-regulation of miR-27a-3p in MCF-7/ADR cells suppressed proliferation (Figure 2E). The colony formation assay similarly demonstrated that promotion of miR-27a-3p could have a positive effect on proliferation (Figure 2F), whereas inhibition of miR-27a-3p attenuated the effect (Figure 2F).
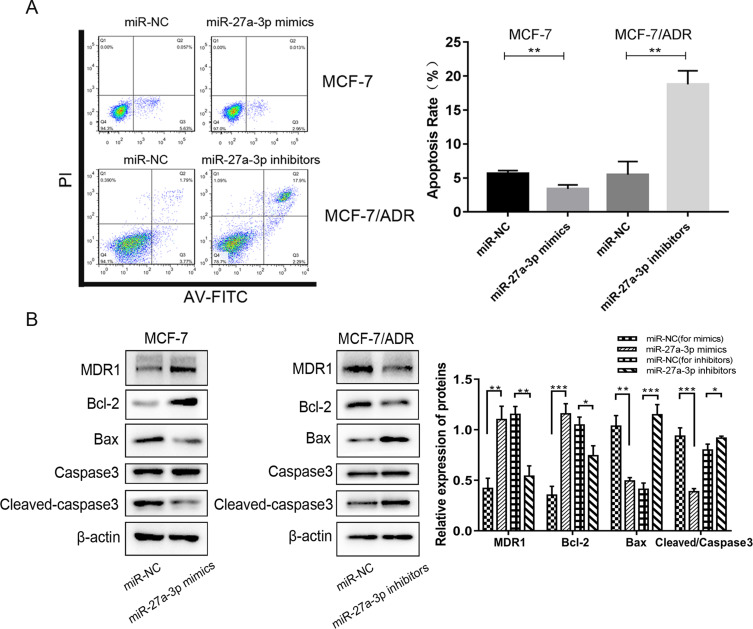
Dysregulation of miR-27a-3p Is Associated with ADR-Induced Apoptosis
To explore whether dysregulation of miR-27a-3p modulates ADR-induced apoptosis, we assessed the apoptosis rate of transfected cells at 48 h by flow cytometry assay. We found that overexpression of miR-27a-3p reduced apoptosis in MCF-7 cells compared to the corresponding miR-NC, and underexpression of miR-27a-3p in MCF-7/ADR cells induced the apoptosis rate (Figure 3A). Western blot demonstrated that miR-27a-3p suppression stimulated the expression of Bax and Cleaved-caspase3, and inhibited the expression of Bcl-2 and MDR1 (Figure 3B), whereas miR-27a-3p mimics decreased Bax and Cleaved-caspase3 protein levels, and increased Bcl-2 and MDR1 protein levels (Figure 3B). In brief, the results indicated that dysregulation of miR-27a-3p could negatively regulate apoptosis in MCF-7 and MCF-7/ADR cells.
Figure 3.
Dysregulation of miR-27a-3p is associated with ADR-induced apoptosis. (A) Flow cytometry analysis was analyzed in MCF-7 and MCF-7/ADR cells transfected with either miR-27a-3p mimics or inhibitors. (B) Western blot was performed to examine the effects of miR-27a-3p on apoptotic protein expression. Relative protein expression was normalized to β-actin. *P < 0.05; **P < 0.01; ***P < 0.001. The data are expressed as mean ± SD.
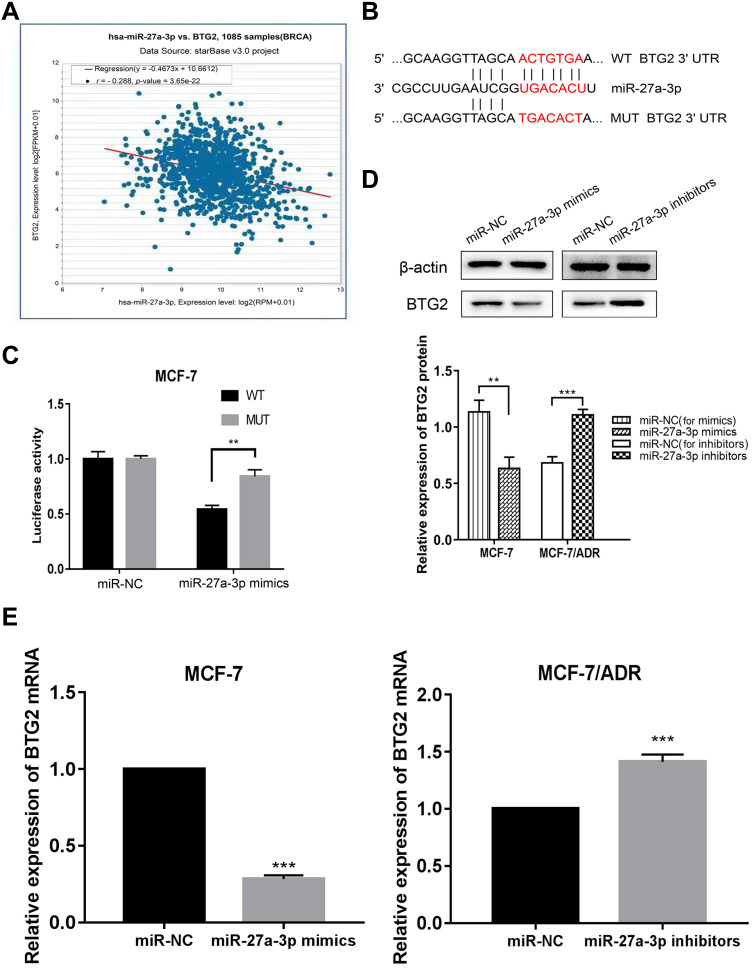
miR-27a-3p Directly Targets BTG2 and Mediates BTG2 Expression Post-Translationally
The practical experiments have demonstrated the essential role of miR-27a-3p in ADR-resistant BC, but the downstream targets of miR-27a-3p are still unknown. Through searching and filtering in miRNA bioinformatics prediction databases (miRWalk, TargetScan, PicTar, and StarBase v3.0), we identified BTG2 as a potential target gene of miR-27a-3p. In TCGA, BTG2 expression level was negatively associated with the expression level of miR-27a-3p (Pearson’s correlation, r = –0.288; Figure 4A). We also found that there is a highly complementary sequence in BTG2 mRNA 3ʹ-UTR with miR-27a-3p (Figure 4B). A dual-luciferase reporter assay showed that miR-27a-3p mimics decreased the luciferase activity of wild reporters but did not affect reporters carrying the mutation binding sites in MCF-7 cells (Figure 4C).
Figure 4.
miR-27a-3p directly targeted BTG2 and regulated its expression in BC cells. (A) A negative correlation was seen between the expression levels of miR-27a-3p and BTG2 in BC specimens from TCGA (r = −0.288; P < 0.05). (B) The seed sequence of miR-27a-3p is complementary to the 3ʹUTR of BTG2. (C) Luciferase activity was analyzed in MCF-7 cells transfected with miR-27a-3p mimics and miR-NC. (D) Western blot analysis demonstrated levels of BTG2 protein in MCF-7 and MCF-7/ADR cells transfected with miR-27a-3p mimics or inhibitors. (E) qRT-PCR was performed to examine the relative expression of BTG2 mRNA in MCF-7 and MCF-7/ADR cells transfected with miR-27a-3p mimics or inhibitors. **P < 0.01; ***P < 0.001. The data are expressed as mean ± SD.
To assess the connection between miR-27a-3p function and BTG2 expression, we performed Western blot and PCR analysis. Western blot demonstrated that the expression of BTG2 protein was lower in MCF-7 cells transfected with mimics than in those transfected with miR-NC, and was higher in MCF-7/ADR cells transfected with inhibitors than in those transfected with miR-NC (Figure 4D). Furthermore, qRT-PCR data demonstrated that the level of BTG2 mRNA decreased in MCF-7 cells transfected with miR-27a-3p mimics, and increased in MCF-7/ADR cells transfected with miR-27a-3p inhibitors (Figure 4E). These experiments indicated that BTG2 might be a direct target of miR-27a-3p and that its expression is inversely related to miR-27a-3p expression.
BTG2 Reverses Cell Proliferation and Apoptosis of BC
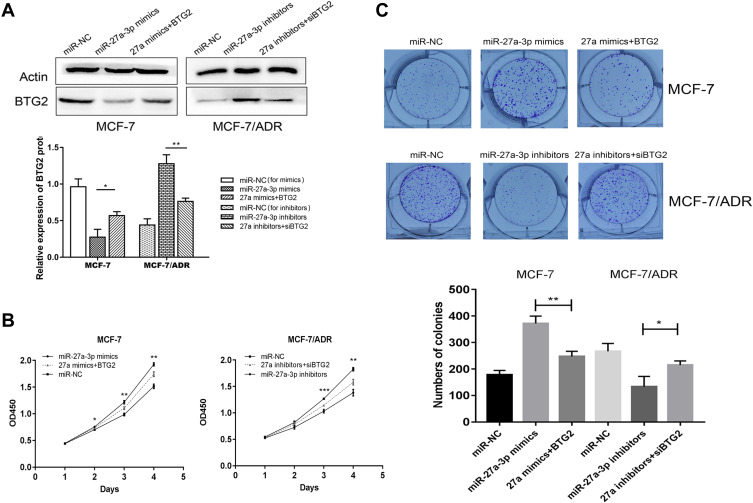
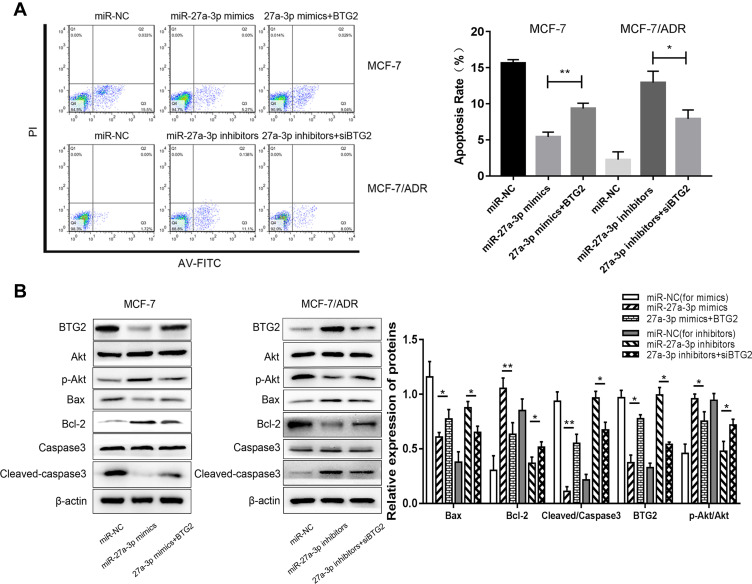
For further demonstration of the effect of BTG2 modulated by miR-27a-3p, BTG2 DNA sequence (BTG2) and siRNA (siBTG2) were transfected into MCF-7 and MCF-7/ADR cells at a stable concentration (50 nM/L), respectively. BTG2 in these transfected cell lines markedly reversed the protein expression levels (Figure 5A), indicating that the transfection of BTG2 is effective. CCK-8 assay and colony formation assay demonstrated that BTG2 partially reversed the effects on cell growth produced by miR-27a-3p mimics/inhibitors, and these effects were still present at 96 h after transfection (Figure 5B and C). Next, we analyzed the apoptosis of transfected cells after regulation of BTG2. We found that BTG2 could enhance ADR-induced apoptosis and expression of Bax and Cleaved-caspase-3 protein in MCF-7 cells transfected with miR-27a-3p mimics, while siBTG2 demonstrated the opposite effects in MCF-7/ADR cells transfected with miR-27a-3p inhibitors (Figure 6A and B). These results verified that BTG2 is involved in the regulation of cell apoptosis in BC.
Figure 5.
BTG2 reversed the effects of miR-27a-3p in BC cell proliferation. (A) The levels of BTG2 protein expression were measured by Western blot analysis. (B and C) CCK-8 and colony formation assays were used to assess cell proliferation in MCF-7 and MCF-7/ADR cells transfected with miR-27a-3p mimics, miR-27a-3p inhibitors, 27a-3p mimics+BTG2, 27a-3p inhibitors+siBTG2 and corresponding miR-NC. *P < 0.05; **P < 0.01; ***P < 0.001. The data are expressed as mean ± SD.
Figure 6.
BTG2 reversed the effects of miR-27a-3p in cell apoptosis of BC, and the PI3K/Akt signaling pathway was a potential downstream target of miR-27a-3p/BTG2. (A) Flow cytometry analysis was used to measure apoptosis of MCF-7 and MCF-7/ADR cells transfected with miR-27a-3p mimics, miR-27a-3p inhibitors, 27a-3p mimics+BTG2, 27a-3p inhibitors+siBTG2 and corresponding miR-NC. (B) Western blot analysis was used to assess BTG2, Akt, p-Akt, Bax, Bcl-2, Caspase3, and Cleaved-caspase3 in MCF-7 and MCF-7/ADR cells transfected with miR-27a-3p mimics, miR-27a-3p inhibitors, 27a-3p mimics+BTG2, 27a-3p inhibitors+siBTG2 and corresponding miR-NC. Relative protein expression was normalized to β-actin. *P < 0.05; **P < 0.01. The data are expressed as mean ± SD.
miR-27a-3p Increases the Activation of the PI3K/Akt Pathway by Targeting BTG2
Based on the above, we were able to clarify the effect of miR-27a-3p on BTG2 in ADR-resistant cells. To explore the mechanisms of miR-27a-3p and BTG2, we performed Western blot analysis to assess the expression of Akt and p-Akt protein in downstream pathways. In treatment with miR-27a-3p mimics or miR-NC in MCF-7 cells, the expression of p-Akt protein was strongly up-regulated (Figure 6B); in treatment with miR-27a-3p inhibitors or miR-NC, the level of p-Akt protein was down-regulated. Moreover, both BTG2 overexpression in the MCF-7 cells and BTG2 knockdown in the MCF-7/ADR cells reversed the p-Akt expression levels. In short, the experiments suggested that miR-27a-3p activated the PI3K/Akt pathway by targeting BTG2.
Discussion
ADR plays an important role in chemotherapy for patients with BC. However, the occurrence of drug resistance can block the efficacy of this treatment, resulting in severe restrictions on its clinical use. Previous research has established that miR-27a-3p is implicated in the biological behavior of tumor cells in numerous cancer types.29–31 Although research has also suggested that changes in miR-27a-3p expression are associated with the chemosensitivity of various malignancies,32–34 the specific role that miR-27a-3p plays in the regulation of ADR resistance in BC has not been well established. Further research regarding the mechanisms of ADR resistance are needed so that effective agents can be developed to reverse chemoresistance.
Several studies have shown that the up-regulation of miR-27a-3p promotes cancer chemoresistance in some malignancies such as gastric carcinoma35 and ovarian carcinoma.17 In contrast, other studies have found that miR-27a-3p serves as an inhibitor in drug resistance for certain tumors such as leukemia16 and hepatocellular carcinoma.15 In this study, we sought to clarify the potential correlation between miR-27a-3p and chemoresistance in BC. We found that miR-27a-3p had a positive correlation with ADR resistance in BC cells. More specifically, we observed that significantly higher expression of miR-27a-3p in the MCF-7/ADR cell line versus in the MCF-7 cell line was accompanied by increased expression of MDR1 protein. Furthermore, the sensitivity of MCF-7/ADR cells was elevated after transfection with inhibitors of miR-27a-3p, whereas the MCF-7 cell line demonstrated decreased sensitivity to ADR after treatment with mimics. Inhibitors of miR-27a-3p restrained MCF-7/ADR cell proliferation and promoted ADR-induced apoptosis in vitro, and the opposite effects were seen with mimics of miR-27a-3p. Taken together, these findings suggest the importance of miR-27a-3p in ADR resistance.
As a tumor suppressor, BTG2 is expressed at low levels in BC and is recognized as a regulator of tumor size, metastasis, recurrence, and overall survival.27,36 However, the association between BTG2 function and miR-27a-3p in ADR-resistant BC was previously poorly understood. In this study, we found that BTG2 was a direct target of miR-27a-3p. A dual-luciferase reporter assay demonstrated that miR-27a-3p down-regulated BTG2 expression by binding to the 3ʹUTR of BTG2 mRNA. Further research demonstrated that BTG2 reduced the effect of miR-27a-3p inhibitors/mimics. Our results also suggested the presence of an axis between miR-27a-3p and BTG2 that regulates the chemoresistance of BC cells through the PI3K/Akt signaling pathway. The PI3K/Akt signaling pathway has been found to be suppressed by BTG2 in many types of malignancies.37,38 Our data showed that miR-27a-3p mimics enhanced the expression of the signaling pathway, and BTG2 up-regulation partially overcame this promoted expression.
Overall, these results suggest that miR-27a-3p activates the PI3K/Akt signaling pathway by targeting BTG2 in MCF-7/ADR cells. These early findings may provide a starting point in developing a promising therapeutic strategy for overcoming chemoresistance. Recently, results from miRNA and siRNA studies have been applied clinically, with researchers applying these findings to targeted therapy for BC, gene editing, and early screening for cancer.39 The potential clinical value of miR-27a-3p in overcoming resistance to chemotherapy is also well worth pursuing. This study identified the role of miR-27a-3p and its target gene BTG2 in BC resistance, but insufficiency still exists.
The present work has several limitations. Expression of miR-27a-3p, is positively correlated with p-Akt protein level, but the detailed mechanism underlying this regulation remained unclear. Further investigations are still needed to clarify the mechanisms underlying this activation. Moreover, the effect of miR-27a-3p on chemoresistance of BC has not been verified in animal experiments, so it is not clear whether miR-27a-3p affects the volume of ADR-resistant tumor and the metastasis of the tumor in the lungs and the livers. We are also collecting clinical specimens to verify the expression of miR-27a-3p and BTG2.
Conclusion
In summary, our study demonstrates that miR-27a-3p participates in reversing drug resistance positively via regulating BTG2. The function of miR-27a-3p in breast cancer cell chemo-sensitivity and apoptosis was mediated by targeting BTG2 and subsequently promoting activation of PI3K/Akt signaling pathway.
Funding Statement
This present work was kindly supported by the Social Development Foundation of Science and Technology of Jiangsu (No. BE2016658), the Changzhou Sci & Tech Program (No.CE20165020), the High-Level Medical Talents Training Project of Changzhou (No.2016CZLJ007), the Project of Changzhou medical innovation team (No.CCX201807), grants from the Natural Science Foundation of China (81702591), and the Natural Science Foundation of Jiangsu Province (BK20170294).
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 2.Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(3):310–320. doi: 10.6004/jnccn.2018.0012 [DOI] [PubMed] [Google Scholar]
- 3.Cai F, Luis MAF, Lin X, et al. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: preventive strategies and treatment. Mol Clin Oncol. 2019;11(1):15–23. doi: 10.3892/mco.2019.1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabizon AA, Patil Y, La-Beck NM. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist Updat. 2016;29(1):90–106. doi: 10.1016/j.drup.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 5.Rivera E. Liposomal anthracyclines in metastatic breast cancer: clinical update. Oncologist. 2003;8(suppl 2):3–9. doi: 10.1634/theoncologist.8-suppl_2-3 [DOI] [PubMed] [Google Scholar]
- 6.Hassan MS, Ansari J, Spooner D, Hussain SA. Chemotherapy for breast cancer. Oncol Rep. 2010;24(5):1121–1131. doi: 10.3892/or_00000963 [DOI] [PubMed] [Google Scholar]
- 7.Pu M, Chen J, Tao Z, et al. Regulatory network of miRNA on its target: coordination between transcriptional and post-transcriptional regulation of gene expression. Cell Mol Life Sci. 2019;76(3):441–451. doi: 10.1007/s00018-018-2940-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Formosa A, Markert EK, Lena AM, et al. MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, mapped to the 14q32.31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene. 2014;33(44):5173–5182. doi: 10.1038/onc.2013.451 [DOI] [PubMed] [Google Scholar]
- 9.Pan BL, Tong ZW, Wu L, et al. Effects of microRNA-206 on osteosarcoma cell proliferation, apoptosis, migration and invasion by targeting ANXA2 through the AKT signaling pathway. Yonsei Med J. 2018;45(4):1410–1422. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, Li S, Li J, Wang D, Li Q. Effect of microRNA-135a on cell proliferation, migration, invasion, apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt signaling pathway in non-small cell lung cancer. Cell Physiol Biochem. 2017;42(4):1431–1446. doi: 10.1159/000479207 [DOI] [PubMed] [Google Scholar]
- 11.Kim SY, Kim AY, Lee HW, et al. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARγ expression. Biochem Biophys Res Commun. 2010;392(3):323–328. doi: 10.1016/j.bbrc.2010.01.012 [DOI] [PubMed] [Google Scholar]
- 12.Mu Y, Zhang L, Chen X, Chen S, Shi Y, Li J. Silencing microRNA-27a inhibits proliferation and invasion of human osteosarcoma cells through the SFRP1-dependent Wnt/β-catenin signaling pathway. Biosci Rep. 2019;39(6):BSR20182366. doi: 10.1042/BSR20182366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu CL, Cheng H, Li N, Zhou N, Tang XS. Relationship between microRNA-27a and efficacy of neoadjuvant chemotherapy in gastric cancer and its mechanism in gastric cancer cell growth and metastasis. Biosci Rep. 2019;39(5):BSR20181175. doi: 10.1042/BSR20181175 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Su C, Huang DP, Liu JW, Liu WY, Cao YO. miR-27a-3p regulates proliferation and apoptosis of colon cancer cells by potentially targeting BTG1. Oncol Lett. 2019;18(3):2825–2834. doi: 10.3892/ol.2019.10629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Ma T, Huang C, et al. MiR-27a modulates the MDR1/P-glycoprotein expression by inhibiting FZD7/β-catenin pathway in hepatocellular carcinoma cells. Cell Signal. 2013;25(12):2693–2701. doi: 10.1016/j.cellsig.2013.08.032 [DOI] [PubMed] [Google Scholar]
- 16.Feng DD, Zhang H, Zhang P, et al. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J Cell Mol Med. 2011;15(10):2164–2175. doi: 10.1111/j.1582-4934.2010.01213.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Hu S, Wang J, et al. MiR-27a modulates MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cells. Gynecol Oncol. 2010;119(1):125–130. doi: 10.1016/j.ygyno.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 18.Kong LY, Xue M, Zhang QC, Su CF. In vivo and in vitro effects of microRNA-27a on proliferation, migration and invasion of breast cancer cells through targeting of SFRP1 gene via Wnt/β-catenin signaling pathway. Oncotarget. 2017;8(9):15507–15519. doi: 10.18632/oncotarget.14662 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Tang W, Yu F, Yao H, et al. miR-27a regulates endothelial differentiation of breast cancer stem like cells. Oncogene. 2014;33(20):2629–2638. doi: 10.1038/onc.2013.214 [DOI] [PubMed] [Google Scholar]
- 20.Ren YQ, Fu F, Han J. MiR-27a modulates radiosensitivity of triple-negative breast cancer (TNBC) cells by targeting CDC27. Med Sci Monit. 2015;21:1297–1303. doi: 10.12659/MSM.893974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ljepoja B, García-Roman J, Sommer AK, Wagner E, Roidl A. MiRNA-27a sensitizes breast cancer cells to treatment with selective estrogen receptor modulators. Breast. 2019;43:31–38. doi: 10.1016/j.breast.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 22.Zhou S, Huang Q, Zheng S, Lin K, You J, Zhang X. miR-27a regulates the sensitivity of breast cancer cells to cisplatin treatment via BAK-SMAC/DIABLO-XIAP axis. Tumour Biol. 2016;37(5):6837–6845. doi: 10.1007/s13277-015-4500-1 [DOI] [PubMed] [Google Scholar]
- 23.Winkler GS. The mammalian anti-proliferative BTG/Tob protein family. J Cell Physiol. 2010;222(1):66–72. doi: 10.1002/jcp.21919 [DOI] [PubMed] [Google Scholar]
- 24.Tsui KH, Chiang KC, Lin YH, Chang KS, Feng TH, Juang HH. BTG2 is a tumor suppressor gene upregulated by p53 and PTEN in human bladder carcinoma cells. Cancer Med. 2018;7(1):184–195. doi: 10.1002/cam4.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei S, Hao C, Li X, Zhao H, Chen J, Zhou Q. Effects of BTG2 on proliferation inhibition and anti-invasion in human lung cancer cells. Tumor Biol. 2012;33(4):1223–1230. doi: 10.1007/s13277-012-0370-y [DOI] [PubMed] [Google Scholar]
- 26.Zhang YJ, Wei L, Liu M, et al. BTG2 inhibits the proliferation, invasion, and apoptosis of MDA-MB-231 triple-negative breast cancer cells. Tumor Biol. 2013;34(3):1605–1613. doi: 10.1007/s13277-013-0691-5 [DOI] [PubMed] [Google Scholar]
- 27.Möllerström E, Kovács A, Lövgren K, et al. Up-regulation of cell cycle arrest protein BTG2 correlates with increased overall survival in breast cancer, as detected by immunohistochemistry using tissue microarray. BMC Cancer. 2010;10(1):296–307. doi: 10.1186/1471-2407-10-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frampton AE, Castellano L, Colombo T, et al. Integrated molecular analysis to investigate the role of microRNAs in pancreatic tumour growth and progression. Lancet. 2015;385(4):S37. doi: 10.1016/S0140-6736(15)60352-X [DOI] [PubMed] [Google Scholar]
- 29.Chae DK, Ban E, Yoo YS, Kim EE, Baik JH, Song EJ. MIR-27a regulates the TGF-β signaling pathway by targeting SMAD2 and SMAD4 in lung cancer. Mol Carcinog. 2017;56(8):1992–1998. doi: 10.1002/mc.22655 [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Pan B, Sun L, et al. Circulating exosomal miR-27a and miR-130a act as novel diagnostic and prognostic biomarkers of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2018;27(7):746–754. doi: 10.1158/1055-9965.EPI-18-0067 [DOI] [PubMed] [Google Scholar]
- 31.Zhang LY, Chen Y, Jia J, Zhu X, He Y, Wu LM. MiR-27a promotes EMT in ovarian cancer through active Wnt/β-catenin signalling by targeting FOXO1. Cancer Biomark. 2019;24(1):31–42. doi: 10.3233/CBM-181229 [DOI] [PubMed] [Google Scholar]
- 32.Li J, Wang Y, Song Y, Fu Z, Yu W. miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Mol Cancer. 2014;13(1):193–202. doi: 10.1186/1476-4598-13-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danza K, Silvestris N, Simone G, et al. Role of miR-27a, miR-181a and miR-20b in gastric cancer hypoxia-induced chemoresistance. Cancer Biol Ther. 2016;17(4):400–406. doi: 10.1080/15384047.2016.1139244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Z, Xu L, Zhao S. Exosome-derived miR-27a produced by PSC-27 cells contributes to prostate cancer chemoresistance through p53. Biochem Biophys Res Commun. 2019;515(2):345–351. doi: 10.1016/j.bbrc.2019.05.120 [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Yang L, Hu J. Down-regulation of miR-27a might inhibit proliferation and drug resistance of gastric cancer cells. J Exp Clin Cancer Res. 2011;30(1):55–60. doi: 10.1186/1756-9966-30-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawakubo H, Carey JL, Brachtel E, et al. Expression of the NF-κB-responsive gene BTG2 is aberrantly regulated in breast cancer. Oncogene. 2004;23(50):8310–8319. doi: 10.1038/sj.onc.1208008 [DOI] [PubMed] [Google Scholar]
- 37.Li YJ, Dong BK, Fan M, Jiang WX. BTG2 inhibits the proliferation and metastasis of osteosarcoma cells by suppressing the PI3K/AKT pathway. Int J Clin Exp Pathol. 2015;8(10):12410–12418. [PMC free article] [PubMed] [Google Scholar]
- 38.Sundaramoorthy S, Ryu MS, Lim IK. B-cell translocation gene 2 mediates crosstalk between PI3K/Akt and NFκB pathways which enhances transcription of MnSOD by accelerating IκBα degradation in normal and cancer cells. Cell Commun Signal. 2013;11(1):69–83. doi: 10.1186/1478-811X-11-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catela Ivkovic T, Voss G, Cornella H, Ceder Y. microRNAs as cancer therapeutics: a step closer to clinical application. Cancer Lett. 2017;407(1):113–122. doi: 10.1016/j.canlet.2017.04.007 [DOI] [PubMed] [Google Scholar]