Abstract
During the COVID-19 outbreak, the mobile cabin hospital has effectively isolated and treated patients diagnosed as mild-moderate disease. However, a detailed clinical course has not been well described. We included 483 patients who were isolated and treated from Feb 6, 2020, to Feb 15, 2020, including definite outcome (discharge or deterioration). Sixty-two patients were transferred to severe cases, of whom were trasfered to designated hospital for intensive care. By March 9, 2020, all patients were discharged without dead. The mobile cabin hospital provides feasible strategy of isolation of mild-moderate cases and timely intervention during the virus outbreak.
Electronic supplementary material
The online version of this article (10.1007/s10096-020-03927-3) contains supplementary material, which is available to authorized users.
Keywords: COVID-19, characteristics, outcomes, mobile cabin hospital
Introduction
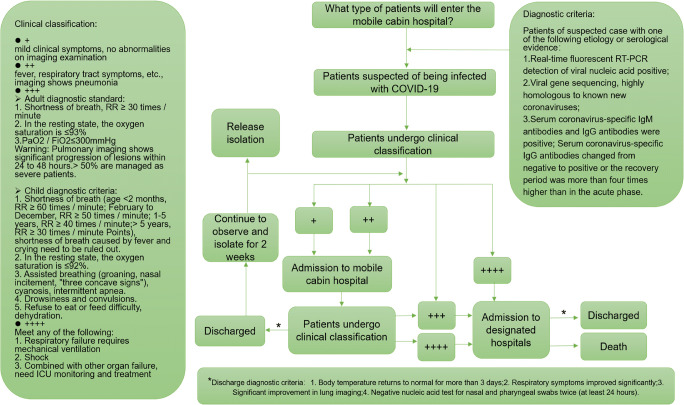
Since December 8, 2019, Wuhan, Hubei, China, has reported several cases of COVID-19. In addition to China, other countries including South Korea, Iran, and Italy also have reported cases of COVID-19 infection [1, 2]. According to the “New Coronavirus Infected Pneumonia Diagnosis and Treatment Plan (Trial Version 5),” during the study period [3], severe and critically ill patients are at risk for secondary systemic multiple organ failure, which in turn increases the risk of death. Therefore, it is necessary to treat critically ill patients and also prevent mild-moderate cases from developing into severe cases. The mobile cabin hospital has played an important role in stemming China’s outbreak of COVID-19 infection, especially in isolating and treating patients diagnosed as mild-moderate disease. However, information about these patient’s characteristics and the outcomes are scarce. Although previous studies reported the clinical characteristics of patients with COVID-19 pneumonia [4–7], limited research focused on the patients who developed from mild-moderate to severe disease, our study mainly analyzed the clinical characteristics of these cases admitted to the mobile cabin hospital (Fig. 1).
Fig. 1.
The flow chart of consultation for patients infected with COVID-19
Results
Among the cohort of 483 patients, 62 patients (12.8%) progressed to severe cases, and 421 patients (87.2%) were cured. The median age was 50 years, and 54.9% of cases were female. Besides, 61.7% of patients were exposed to the suspected/confirmed patients, and 45.5% of patients experienced family cluster infection. We found increasing odds of severe cases associated with comorbidities, including primary pulmonary disease (7.6% vs. 16.1%, p = 0.047), coronary heart disease (0.7% vs. 8.1%, p < 0.001), and abnormal laboratory test of renal (0.2%vs. 16.1%, p < 0.001), liver function (1.9%vs. 19.4%, p < 0.001), renal function (0.2% vs. 16.1%, p < 0.001), heart function (1.2% vs. 19.4%, p < 0.001), and abnormal lymphocyte (15.2% vs. 37.1%, p < 0.001) and leukocyte counts (23.0% vs. 37.1%, p = 0.025) (Table 1). By March 9, 2020, all patients were as follows: 62 patients who were diagnosed as severe cases were transferred to a designated hospital for intensive care, of whom, 23 refused (alive confirmed), 8 patients were cured and discharged, and 31 patients were still in the designated hospital to continue treatment and recovered.
Table 1.
Clinical characteristics of patients with coronavirus disease 2019
| Overall | Mild-moderate cases (+/++) | Severe cases (+++/++++) | pa value | |
|---|---|---|---|---|
| (n = 483) | (n = 421) | (n = 62) | ||
| Time from symptom to admission | 0.585 | |||
| Mean (SD, min, max) | 5.86 (5.23, 0.00, 30.0) | 5.90 (5.27, 0.00, 30.0) | 5.52 (4.91, 0.00, 18.0) | |
| Median (IQR) | 4.00 (2.00, 8.00) | 4.00 (2.00, 8.00) | 3.00 (2.00, 9.00) | |
| Time from admission to cure/severe illness | < 0.001 | |||
| Mean (SD, min, max) | 12.2 (4.71, 1.00, 23.0) | 12.5 (4.51, 1.00, 23.0) | 9.74 (5.32, 1.00, 23.0) | |
| Median (IQR) | 12.0 (9.00, 15.0) | 13.0 (9.00, 16.0) | 9.00 (5.25, 13.8) | |
| Time from symptom to cure/severe illness | 0.002 | |||
| Mean (SD, min, max) | 18.0 (7.41, 1.00, 50.0) | 18.4 (7.35, 1.00, 50.0) | 15.3 (7.32, 1.00, 50.0) | |
| Median (IQR) | 17.0 (13.0, 23.0) | 17.0 (13.0, 23.0) | 14.5 (9.25, 21.0) | |
| Demographic characteristics | 0.830 | |||
| Age | ||||
| Mean (SD, min, max) | 48.4 (12.4, 11.0, 83.0) | 48.5 (12.4, 11.0, 72.0) | 48.1 (12.9, 22.0, 83.0) | |
| Median (IQR) | 50.0 (39.0, 58.0) | 50.0 (39.0, 58.0) | 48.5 (37.3, 58.8) | |
| Age distribution | 0.360 | |||
| (~ 50] | 227 (47.0%) | 194 (46.1%) | 33 (53.2%) | |
| (50~ | 256 (53.0%) | 227 (53.9%) | 29 (46.8%) | |
| Sex | 0.341 | |||
| Female | 265 (54.9%) | 227 (53.9%) | 38 (61.3%) | |
| Male | 218 (45.1%) | 194 (46.1%) | 24 (38.7%) | |
| BMI | 0.738 | |||
| Mean (SD, min, max) | 23.3 (3.15, 15.0, 37.8) | 23.3 (3.15, 15.0, 37.8) | 23.4 (3.15, 16.5, 31.3) | |
| Median (IQR) | 22.9 (21.4, 25.4) | 22.9 (21.4, 25.4) | 23.1 (21.9, 25.5) | |
| BMI distribution | 0.485 | |||
| (~ 18.4] | 25 (5.2%) | 20 (4.8%) | 5 (8.1%) | |
| (18.5, 23.9] | 271 (56.1%) | 239 (56.8%) | 32 (51.6%) | |
| (24, 27.9] | 155 (32.1%) | 136 (32.3%) | 19 (30.6%) | |
| [28~) | 32 (6.6%) | 26 (6.2%) | 6 (9.7%) | |
| Huanan seafood wholesale market exposure | 0.574 | |||
| No | 475 (98.3%) | 413 (98.1%) | 62 (100%) | |
| Yes | 8 (1.7%) | 8 (1.9%) | 0 (0%) | |
| History suspected patient exposure | 0.080 | |||
| Uncertain | 185 (38.3%) | 168 (39.9%) | 17 (27.4%) | |
| Yes | 298 (61.7%) | 253 (60.1%) | 45 (72.6%) | |
| With other family member infected | 0.011 | |||
| No | 263 (54.5%) | 239 (56.8%) | 24 (38.7%) | |
| Yes | 220 (45.5%) | 182 (43.2%) | 38 (61.3%) | |
| Daily exercise | 0.730 | |||
| No | 189 (39.1%) | 163 (38.7%) | 26 (41.9%) | |
| Yes | 294 (60.9%) | 258 (61.3%) | 36 (58.1%) | |
| Daily self-care ability | < 0.001 | |||
| No | 22 (4.6%) | 8 (1.9%) | 14 (22.6%) | |
| Yes | 461 (95.4%) | 413 (98.1%) | 48 (77.4%) | |
| Smoking | 0.063 | |||
| Current smokers | 83 (17.2%) | 66 (15.7%) | 17 (27.4%) | |
| Give up smoking | 15 (3.1%) | 14 (3.3%) | 1 (1.6%) | |
| Never smokers | 385 (79.7%) | 341 (81.0%) | 44 (71.0%) | |
| Marital status | 0.782 | |||
| Divorce | 3 (0.6%) | 3 (0.7%) | 0 (0%) | |
| Married | 454 (94.0%) | 395 (93.8%) | 59 (95.2%) | |
| Unmarried | 26 (5.4%) | 23 (5.5%) | 3 (4.8%) | |
| Recent surgery history | < 0.001 | |||
| No | 101 (20.9%) | 99 (23.5%) | 2 (3.2%) | |
| Yes | 382 (79.1%) | 322 (76.5%) | 60 (96.8%) | |
| Antiviral drug treatment before admission | 0.874 | |||
| No | 164 (34.0%) | 144 (34.2%) | 20 (32.3%) | |
| Yes | 319 (66.0%) | 277 (65.8%) | 42 (67.7%) | |
| Antibiotic drug treatment before admission | 0.723 | |||
| No | 193 (40.0%) | 170 (40.4%) | 23 (37.1%) | |
| Yes | 290 (60.0%) | 251 (59.6%) | 39 (62.9%) | |
| Comorbidities at admission | ||||
| Primary pulmonary disease | 0.047 | |||
| No | 441 (91.3%) | 389 (92.4%) | 52 (83.9%) | |
| Yes | 42 (8.7%) | 32 (7.6%) | 10 (16.1%) | |
| Hypertension | 0.067 | |||
| No | 449 (93.0%) | 392 (93.1%) | 57 (91.9%) | |
| Yes | 34 (7.0%) | 29 (6.9%) | 5 (8.1%) | |
| Diabetes | 0.072 | |||
| No | 459 (95.0%) | 401 (95.2%) | 58 (93.5%) | |
| Yes | 24 (5.0%) | 20 (4.8%) | 4 (6.5%) | |
| Hyperlipidemia | 0.124 | |||
| No | 470 (97.3%) | 412 (97.9%) | 58 (93.5%) | |
| Yes | 13 (2.7%) | 9 (2.1%) | 4 (6.5%) | |
| Coronary heart disease | < 0.001 | |||
| No | 475 (98.3%) | 418 (99.3%) | 57 (91.9%) | |
| Yes | 8 (1.7%) | 3 (0.7%) | 5 (8.1%) | |
| History of myocardial infarction | 0.048 | |||
| No | 476 (98.6%) | 417 (99.0%) | 59 (95.2%) | |
| Yes | 7 (1.4%) | 4 (1.0%) | 3 (4.8%) | |
| Cerebral infarction | 0.849 | |||
| No | 478 (99.0%) | 416 (98.8%) | 62 (100%) | |
| Yes | 5 (1.0%) | 5 (1.2%) | 0 (0%) | |
| Cerebral hemorrhage | 0.266 | |||
| No | 482 (99.8%) | 420 (99.8%) | 62 (100%) | |
| Yes | 1 (0.2%) | 1 (0.2%) | 0 (0%) | |
| Malignant neoplasms | 0.849 | |||
| No | 478 (99.0%) | 416 (98.8%) | 62 (100%) | |
| Yes | 5 (1.0%) | 5 (1.2%) | 0 (0%) | |
| Other disease | 0.039 | |||
| No | 442 (91.5%) | 390 (92.6%) | 52 (83.9%) | |
| Yes | 41 (8.5%) | 31 (7.4%) | 10 (16.1%) | |
| Vital signs at admissionb | ||||
| Blood pressure | 0.943 | |||
| Hight blood pressure | 34 (7.0%) | 29 (6.9%) | 5 (8.1%) | |
| Normal blood pressure | 449 (93.0%) | 392 (93.1%) | 57 (91.9%) | |
| Breath | < 0.001 | |||
| Breathing faster | 32 (6.6%) | 20 (4.8%) | 12 (19.4%) | |
| Normal breathing | 451 (93.4%) | 401 (95.2%) | 50 (80.6%) | |
| Heart rate | < 0.001 | |||
| Increased heart rate | 32 (6.6%) | 21 (5.0%) | 11 (17.7%) | |
| Normal heart rate | 451 (93.4%) | 400 (95.0%) | 51 (82.3%) | |
| Symptom at admission | 0.897 | |||
| Mild | 45 (9.3%) | 39 (9.3%) | 6 (9.7%) | |
| Moderate | 438 (90.7%) | 382 (90.7%) | 56 (90.3%) | |
| Highest temperature | 0.029 | |||
| Mean (SD, min, max) | 37.7 (0.86, 36.0, 40.0) | 37.7 (0.86, 36.0, 40.0) | 37.9 (0.89, 36.5, 39.9) | |
| Median (IQR) | 37.8 (36.9, 38.4) | 37.7 (36.8, 38.3) | 37.9 (37.2, 38.6) | |
| Temperature distribution | 0.195 | |||
| < 37.5 °C | 176 (36.4%) | 158 (37.5%) | 18 (29.0%) | |
| 37.5–38.0 °C | 112 (23.2%) | 97 (23.0%) | 15 (24.2%) | |
| 38.1–39.0 °C | 137 (28.4%) | 113 (26.8%) | 24 (38.7%) | |
| > 39.0 °C | 58 (12.0%) | 53 (12.6%) | 5 (8.1%) | |
| Cough | 0.524 | |||
| No | 204 (42.2%) | 175 (41.6%) | 29 (46.8%) | |
| Yes | 279 (57.8%) | 246 (58.4%) | 33 (53.2%) | |
| Shortness of breath | 0.041 | |||
| No | 352 (72.9%) | 314 (74.6%) | 38 (61.3%) | |
| Yes | 131 (27.1%) | 107 (25.4%) | 24 (38.7%) | |
| Myalgia | 0.439 | |||
| No | 334 (69.2%) | 288 (68.4%) | 46 (74.2%) | |
| Yes | 149 (30.8%) | 133 (31.6%) | 16 (25.8%) | |
| Running nose | 0.240 | |||
| No | 408 (84.5%) | 352 (83.6%) | 56 (90.3%) | |
| Yes | 75 (15.5%) | 69 (16.4%) | 6 (9.7%) | |
| Arthralgia | 0.263 | |||
| No | 383 (79.3%) | 330 (78.4%) | 53 (85.5%) | |
| Yes | 100 (20.7%) | 91 (21.6%) | 9 (14.5%) | |
| Chest tightness | 0.288 | |||
| No | 365 (75.6%) | 322 (76.5%) | 43 (69.4%) | |
| Yes | 118 (24.4%) | 99 (23.5%) | 19 (30.6%) | |
| Nausea or vomiting | 0.015 | |||
| No | 415 (85.9%) | 355 (84.3%) | 60 (96.8%) | |
| Yes | 68 (14.1%) | 66 (15.7%) | 2 (3.2%) | |
| Headache | 0.892 | |||
| No | 381 (78.9%) | 333 (79.1%) | 48 (77.4%) | |
| Yes | 102 (21.1%) | 88 (20.9%) | 14 (22.6%) | |
| Fatigue | 0.833 | |||
| No | 461 (95.4%) | 401 (95.2%) | 60 (96.8%) | |
| Yes | 22 (4.6%) | 20 (4.8%) | 2 (3.2%) | |
| Pharyngalgia | 0.606 | |||
| No | 481 (99.6%) | 419 (99.5%) | 62 (100%) | |
| Yes | 2 (0.4%) | 2 (0.5%) | 0 (0%) | |
| Nasal congestion | 0.606 | |||
| No | 481 (99.6%) | 419 (99.5%) | 62 (100%) | |
| Yes | 2 (0.4%) | 2 (0.5%) | 0 (0%) | |
| Diarrhea | 0.012 | |||
| No | 447 (92.5%) | 395 (93.8%) | 52 (83.9%) | |
| Yes | 36 (7.5%) | 26 (6.2%) | 10 (16.1%) | |
| Chill | ||||
| No | 478 (99.0%) | 417 (99.0%) | 61 (98.4%) | |
| Yes | 5 (1.0%) | 4 (1.0%) | 1 (1.6%) | |
| Laboratory test results at admission c | ||||
| Leukocyte | 0.025 | |||
| Abnormal | 120 (24.8%) | 97 (23.0%) | 23 (37.1%) | |
| Normal | 363 (75.2%) | 324 (77.0%) | 39 (62.9%) | |
| Lymphocyte | < 0.001 | |||
| Abnormal | 87 (18.0%) | 64 (15.2%) | 23 (37.1%) | |
| Normal | 396 (82.0%) | 357 (84.8%) | 39 (62.9%) | |
| Blood glucose | 0.374 | |||
| Abnormal glucose | 24 (5.0%) | 19 (4.5%) | 5 (8.1%) | |
| Normal glucose | 459 (95.0%) | 402 (95.5%) | 57 (91.9%) | |
| Renal function | < 0.001 | |||
| Normal | 472 (97.7%) | 420 (99.8%) | 52 (83.9%) | |
| Abnormal | 11 (2.3%) | 1 (0.2%) | 10 (16.1%) | |
| Heart function | < 0.001 | |||
| Normal | 466 (96.5%) | 416 (98.8%) | 50 (80.6%) | |
| Abnormal | 17 (3.5%) | 5 (1.2%) | 12 (19.4%) | |
| Liver function | < 0.001 | |||
| Normal | 463 (95.9%) | 413 (98.1%) | 50 (80.6%) | |
| Abnormal | 20 (4.1%) | 8 (1.9%) | 12 (19.4%) | |
| Urine infection | 0.129 | |||
| No | 435 (90.1%) | 383 (91.0%) | 52 (83.9%) | |
| Yes | 48 (9.9%) | 38 (9.0%) | 10 (16.1%) | |
| Imaging of lung | < 0.001 | |||
| Normal | 458 (94.8%) | 415 (98.6%) | 43 (69.4%) | |
| Abnormal | 25 (5.2%) | 6 (1.4%) | 19 (30.6%) | |
| Mental state before admissiond | 0.076 | |||
| Nervous before admission | 166 (34.4%) | 138 (32.8%) | 28 (45.2%) | |
| Without nervous before admission | 317 (65.6%) | 283 (67.2%) | 34 (54.8%) | |
| Sleep quality since diagnosis | 0.005 | |||
| Bad | 123 (25.5%) | 97 (23.0%) | 26 (41.9%) | |
| Good | 20 (4.1%) | 19 (4.5%) | 1 (1.6%) | |
| Without influence | 340 (70.4%) | 305 (72.4%) | 35 (56.5%) |
aData are n (%) unless otherwise specified; p values demonstrate differences between No conversion to severe and conversion to severe patients. p < 0.05 was considered obviously significant
bHypertension, ≥ 140/90 mmHg; breath, 12–20 times/min; heart rate, 60–100 times/min
cNormal reference value [1]: leukocyte: adult, (4.0–10.0) × 10^9/L; child, (5.0–12.0) × 10^9/L [2]; lymphocyte percentage (Lymph%) 20–40%; lymphocyte absolute value (Lymph #) 1.1–3.2 × 10^9 [3]; fasting whole blood glucose 3.9~6.1 mmol/L, 1 h after meal 6.7~9.4 mmol/L, 2 h after meal ≤ 7.8 mmol/L
dHeart function: tachycardia (100 beats/min)
eLiver function: ALT 0–46 U/L; AST 0–46 U/L
fUrine infection: creatinine (30–110 umol/L)
Discussion
During the COVID-19 outbreak, the number of confirmed cases has exploded in China. The major challenge is to treat and isolate these patients, as well as reduce severe cases and mortality. The establishment of the mobile cabin hospital has witnessed the classification management effectively. In this study, all patients received a nucleic acid test before admission; after the patients were admitted to the mobile cabin hospital, the treatment was carried out according to the “New Coronavirus Infected Pneumonia Diagnosis and Treatment Plan” [3]. To our knowlegement, this is the largest retrospective cohort study among mild-moderate cases with COVID-19 infection; the clinical course with respect to mild-moderate and severe cases in Wuchang mobile cabin hospital were analyzed in this study.
Our results showed that there was no significant difference in fever between mild-moderate and severe cases, of whom 421 (87.2%) patients were not admitted to the ICU, and 263 (62.5%) patients were identified as having a fever but progressed to critically ill status, suggesting that there may be individual differences in body temperature monitoring and even in the early concealment of the virus [8]. Consistent with the transmission route, we also found that critically ill patients were characterized by familial cluster infections, which indirectly confirms that COVID-19 can be transmitted through contact [9–11]. If necessary, appropriate psychological intervention during the admission of a patient may contribute to elevating the patient’s condition.
So far, the COVID-19 infection has been managed by controlling the source of infection and cutting off the route of transmission dominates, but no effective treatment has been proposed. For critically ill patients, supportive treatments may continue for some time. According to our study, all the cases in the mobile cabin hospital were community-acquired viral infections; no cases of nosocomial infections were found. This also suggested that the safety isolation measures adopted by patients and medical workers in the mobile cabin hospital can significantly reduce the chance of cross-infection.
Several limitations should be highlighted. First, this was a retrospective study and inherent limitations existed; we tried our best to collect detailed information, but not all laboratory information were collected adequately. Second, 23 patients were lost to follow-up, including 10 of them who also refused our follow-up (for this part, the medical records suggested that they were alive), which enabled a lack of analyzing the outcome of patients after being transferred to designated hospital. These patients who lost to follow-up may have a certain impact on the results, especially deviations and existing biases, so exploring the potential risks associated with the deterioration of patients is infeasible. However, depending on this descriptive study, we found that severe cases were associated with comorbidities. We believe that our study population is representative of mild-moderate cases, especially those who transferred to severe cases, for which provided feasible tactics in management of COVID-19 infection.
Electronic supplementary material
(DOCX 786 kb)
(DOCX 40 kb)
Abbreviations
- BMI
Body mass index
- IQR
Interquartile range
- SD
Standard deviation
- COVID-19
Corona virus disease 2019
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bo Wang and Zhixian Wang contributed equally to this work.
Contributor Information
Shixuan Wang, Email: shixuanwang@126.com.
Tiejun Wang, Email: tiejunwanghp@163.com.
References
- 1.World Health Organization. Novel coronavirus(2019-nCoV): situation report—15. February 5, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200204-sitrep-15-ncov.pdf
- 2.Holshue ML, DeBolt C, Lindquist S et al First Case of 2019 Novel Coronavirus in the United States. N Engl J Med:2020 [DOI] [PMC free article] [PubMed]
- 3.National Health Committee of the People's Republic of China. New coronavirus pneumonia diagnosis and treatment plan. February 18, 2020. http://www.nhc.gov.cn/wjw/gfxwjj/list.shtml
- 4.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA [DOI] [PMC free article] [PubMed]
- 7.Yang X, Yu Y, Xu J et al (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med [DOI] [PMC free article] [PubMed]
- 8.Guan W-j, Ni Z-y, Hu Y et al (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med [DOI] [PMC free article] [PubMed]
- 9.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajema KL, Oster AM, McGovern OL, et al. Persons evaluated for 2019 novel coronavirus - United States, January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(6):166–170. doi: 10.15585/mmwr.mm6906e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 786 kb)
(DOCX 40 kb)