Abstract
GSK3 are involved in different physical and pathological conditions and inflammatory regulated by macrophages contribute to significant mechanism. Infection stimuli may modulate GSK3 activity and influence host cell adaption, immune cells infiltration or cytokine expressions. To further address the role of GSK3 modulation in macrophages, the signal transduction of three major organs challenged by endotoxin, virus and genetic inherited factors are briefly introduced (lung injury, myocarditis and autosomal dominant polycystic kidney disease). As a result of pro-inflammatory and anti-inflammatory functions of GSK3 in different microenvironments and stages of macrophages (M1/M2), the rational resolution should be considered by adequately GSK3.
Keywords: PI3K/Akt/GSK3β/MCP-1 axis, M1/M2 macrophages, Pulmonary edema, HMGB1/IL-17A/autophagy, Podocyte, Mitotic catastrophe
1. Introduction
It has been a critical field for GSK3 as a major regulator of peripheral inflammatory responses. GSK3 also participates in stimulus-induced production of several cytokines that are related to inflammatory cell migration and disease symptoms. GSK3, a serine/threonine protein kinase signaling molecule, is widely expressed in versatile cell types. GSK3 is responsible for glycogen metabolism and involved in downstream of insulin signaling. Insulin-mediated signaling could be an activator of PI3K and, subsequently phosphorylation of both GSK3α (Ser21) and GSK3β (Ser9) [1]. GSK3 is rapidly phosphorylated and inhibited by the activation of the phosphoinositide 3-kinase (PI3K) pathway, contributing to deposition of glycogen [2]. Two isoforms in mammalian cells are GSK3α (51 kDa) and GSK3β (47 kDa) with 85% overall structural similarity and the kinase domains shares 97% identity. However, only 36% identity found on the C-terminus [3]. In general the functions of GSK3 are dependent on tyrosine residues phosphorylation (Tyr279 for GSK3α and Tyr216 for GSK3β) as active forms. In contrast, the serine residues phosphorylation (Ser21 for GSK3α and Ser9 for GSK3β) are a suppressive status [4]. More than 80 proposed substrates has been reported to interact with GSK3β [5]. GSK3β can be negatively regulated through phosphorylation on Ser9 by different kinases, for example MAPK, ERK or Akt [6]. In monocytes, PI3K/Akt-dependent inhibition of GSK3β activity can regulate Toll-like receptor (TLR)-dependent activation [7]. Further mechanism reveals the insulin-mediated signaling pathway as an activator of PI3K and subsequently a phospho-inactivator of both GSK3α (Ser21) and GSK3β (Ser9) [8]. Even GSK3α and β share highly structural similarity, GSK3β null mice die during late embryogenesis with cardiac abnormalities [9,10], however, GSK3α null mice are survival with elevated insulin sensitivity [11].
2. GSK3 role on inflammation
GSK3 activity is necessary for full stimulation of the production of several pro-inflammatory cytokines, such as IL-6, IL-1β, and tumor necrosis factor (TNF) in the peripheral blood mononuclear cells [7]. Moreover it has been found that Toll-like receptor activation in monocytes may induce interleukin 10 production and accompany with reduced level of pro-inflammatory cytokines after GSK3 and NF-κB inhibitions [7]. Even studies demonstrate that GSK3 is necessary for the full transcriptional activity of NF-κB [9], GSK3 may support NF-κB transcriptional activity in a promoter-specific manner, indicating that GSK3 selectively modulates subset NF-κB mediated genes [12]. For example, NF-κB-mediated expression of IL-6 and monocyte chemoattractant protein-1 (MCP-1) require GSK3β for efficient expression, but not IκBα and macrophage inflammatory protein-2 [13] [14]. This selective action of GSK3β on NF-κB-induced gene expression is of significance to explore the anti-inflammatory utility of GSK3 inhibitors with differently NF-κB responses.
Decreased phosphorylation of NF-κB, JNK, ERK and P38MAPK in LPS-stimulated macrophages is related to IL-10-dependent manner by GSK3β phosphorylation [15]. In addition, TLR signaling can induce the phosphorylation of GSK3β through the PI3K/Akt signaling pathway [16]. By means of increasing the production of IL-10, it results in reduction of phosphorylation of NF-κB, JNK, ERK and P38 MAPK in LPS-stimulated macrophages. The enhancement of GSK3β phosphorylation (inactivation) via PI3K/Akt is involved in the methane-rich saline-induced production of IL-10. However, another studies indicate that 6-bromoindirubin-3-oxime (BIO) inhibits GSK3 activity and then triggers a feedback regulatory loop aiming to restore physiological GSK3 activity. This loop results in gsk-3β gene and protein upregulation and it also reduces the levels of the inhibitory GSK3α (Ser21) and GSK3β (Ser9) [15].
3. GSK3 in lung inflammation
Recently the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is leading to the global outbreak of coronavirus disease 2019 (COVID-19). The clinical manifestation is involved in acute respiratory distress syndrome (ARDS). Currently many trials are exploring the clinical feasible means to manage it [17]. Particularly chloroquine is an existing medicine with approved indication for anti-malarial use. It reveals inhibitory effect on SARS-CoV-2 [18]. In view of the intracellular versatile functions of GSK3, viruses could likely leverage this important kinase to multiplicate in host cells. It has been shown that multiplication of the SARS coronavirus depends on GSK3-mediated phosphorylation of the nucleocapsid (N) protein which is a necessary process for viral replication [19]. In addition, it was shown that GSK3β promoted the life cycle of influenza virus through facilitating the viral entry step [20]. GSK3β also participates in other viral multiplication, such as HCV, HIV, coxsackievirus group B3 (CVB3) and venezuelan equine encephalitis virus [17] [20,21] [21]. As for bacterial infection, inhibition of GSK3β-related regulations by chloroquine-modulated PI3K/Akt signaling may increase survivability in B. pseudomallei-infected mice. The underling mechanism appears high level of phosphorylated Akt (Ser473) and GSK3β (Ser9) in liver samples of mice administered with chloroquine and decreased phosphorylation of NF-κB p65 (Ser536). Further analysis showed that chloroquine may reduce pro-inflammatory cytokines (TNF-α, IFN-γ, IL-1β and IL-18) and increase levels of anti-inflammatory cytokines (IL-4 and IL-10) in B. pseudomallei-infected mice [22].
3.1. GSK3 in acute respiratory distress syndrome
Alveolar neutrophils and macrophages are of significance in lung immune regulations. Neutrophils exhibit the acute phase effector and migrate into the inflammatory sites by mediation of versatile cytokines and chemokines [23]. Neutrophils represent over 50% of all circulating leukocytes and, mostly in the pulmonary pool [24]. As injury occurred in lung, the activated neutrophils exhibit the process of degranulation and to release intracellular enzyme, such as neutrophil extracellular traps (NETs), which is composed of chromatin fibers mixed with anti-microbial proteins [25]. Particularly, increasing studies have been working on NETs induced by different stimuli-triggered neutrophil activations (such as LPS, calcium ionophore A23187 and phorbol 12-myristate 13-acetate) [26]. Inhibition of GSK3β diminishes LPS-induced neutrophil activation, lung injury as well as reduced level of pulmonary edema, TNF-α, MIP-2 and HMGB1. The underlying mechanism revealed that GSK3β-dependent phosphorylation of threonine 479 on AMPK is associated with pT172 dephosphorylation and inactivation of AMPK. Moreover, GSK3β-dependent inhibition of AMPK in LPS-treated neutrophils activation is dependent on IKK1/2 [27]. Given that AMPK has anti-inflammatory functions [28], it appears that GSK3β inhibitors (BIO or SB216763) prevent Thr172-AMPK dephosphorylation then attenuates proinflammatory cytokine production [29] and protection from bleomycin-induced lung injury [30] (Fig. 1 ).
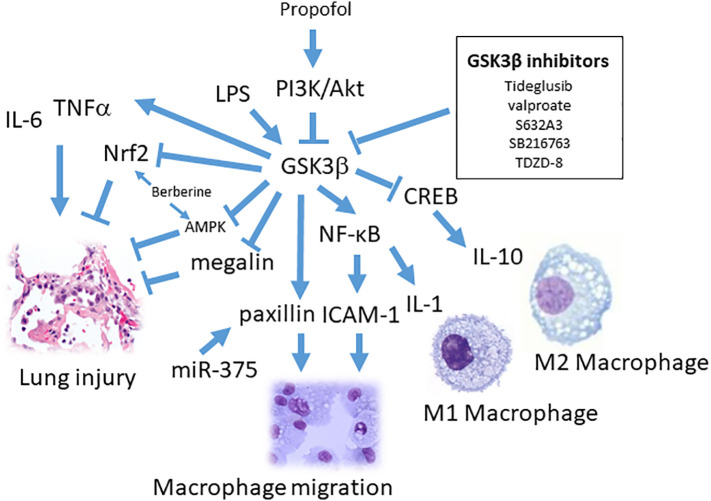
Fig. 1.
The role of GSK3β on macrophages regulation and lung injury. LPS could stimulate GSK3β-mediated IL-6 and TNFα production. GSK3β may inhibit AMPK, Nrf2 and megalin during lung injury and propofol, berberine and GSK3β inhibitors could reverse it. GSK3β and miR-375 enhance macrophage migration, respectively, by activating paxillin (PXN) expression. GSK3β differently regulate transcription factor NFκB or CREB-mediated M1 macrophage pro-inflammatory (IL-1β) or M2 macrophage anti-inflammatory (IL-10) responses, respectively.
Protein-rich edema is frequently manifested in patient with ARDS. Impairment of edema removal is associate with alveolar hypoxia and systemic hypoxemia [31]. Protein clearance from the distal air spaces is facilitated by an the regulation of megalin (LRP2), belonging to the low-density lipoprotein (LDL)-receptor superfamily [32]. Studies have shown a detrimental role of GSK3β during ARDS via inhibition of alveolar epithelial protein transport. Thus megalin is negatively regulated by GSK3β. Tideglusib and valproate (GSK3β inhibitors) may reduce alveolar protein concentrations and suppress acute lung injury [33]. Similarly. the observation of utilizing ventilator-induced ARDS in mice, it appears that increased mRNA and protein expression of GSK3β, implying the GSK3β role in edema removal [34] (Fig. 1). To further analyze alveolar fluid composition, albumin is disclosed to be the abundant protein and clathrin-mediated endocytosis and transcytosis is responsible albumin transferring [32]. Other study shows that activation of GSK3β by its dephosphorylation at Ser9 inhibits albumin uptake and the phosphatase PP1 is responsible in alveolar epithelial cells. In vitro studies show that TGF-β treated alveolar epithelial cells inhibiting uptake of albumin and decreases megalin abundance on the plasma membrane. It shows that GSK3β phosphorylates megalin leading to its subsequent downregulation [35]. Moreover Acyl-CoA:lysophosphatidylcholine acyltransferase 1 (LPCAT1) is a key enzyme in the synthesis of dipalmitoylphosphatidylcholine, an important surfactant. In LPS-induced ARDS experimental model, LPCAT1 is phosphorylated by GSK3β, then ubiquinated and targeted for degradation [36].
In order to relief ARDS, it appears a positive outcome by inhibition strategy of GSK3β through inhaling aerosolized insulin, [37]. In acute necrotizing pancreatitis (ANP)-elicited lung injury, protective effect of p-GSK3β (Ser9) exhibits by treatment of GSK3β inhibitors (TDZD-8 or SB216763) [38]. Since NF-κB could be phosphorylated by GSK3β and lead to transcriptional responses, inhibitors of GSK3β may decrease the activation of NF-κB and then reduce downstream gene expression (IL-1β, IL-6, and TNF-α) [29]. In addition, cell adhesion related signals such as ICAM-1 modulated leukocyte adhesion and migration in acute pancreatitis and higher level of ICAM-1 were indicated in lung tissues [[39], [40], [41]]. Converging all evidences reveal the axis of GSK3β/NF-κB/ICAM-1 pathway of leukocyte infiltration in lung injury (Fig. 1). Meanwhile, by applying GSK3β inhibitors (TDZD-8 and SB216763 pretreatment,1 mg/kg) to treat ANP rats, it is found that the increased expression of p-GSK3β (Ser9) and IL-10 levels in lung [38].
3.2. GSK3 modulation by cholinergic signaling
As ARDS patients rescued by ventilated treatment, it frequently exhibits strong respiratory efforts. These respiratory efforts may result in increased mechanical lung injury due to high transpulmonary pressures [42]. To prevent it, a routine medication with neuromuscular blockade effect is employed. Cisatracurium acts on cholinergic receptors, blocking neuromuscular transmission. This action is antagonized by acetylcholinesterase inhibitors. Cisatracurium is used during medication of ARDS patients. It has been reported that cisatracurium may decrease mechanical lung injury in terms of clinical trial evaluation [43]. Further analyze the possible mechanism reveals that cisatracurium could decrease cell migration and up-regulated E-Cadherin [44].In line with other studies indicate that GSK3 kinase activity negatively regulates the expression of the E-cadherin and also inhibits epithelial-mesenchymal transition (EMT) [45,46]. Co-treatment of cisatracurium and ventilation reveals reduction of the inflammatory injury by decreased HMGB1 expression in lung tissue [47]. It has been indicated that HMGB1 could trigger inflammatory cytokines via macrophages and result in onset of acute lung injury [48]. HMGB1 participates in lung inflammation and could modulate AKT/GSK3β/β-catenin signaling pathways in human bronchial epithelial cells [49]. In parallel, by increasing endogenous cholinergic activity through acetylcholinesterase inhibitor (physostigmine) treatment at a low dose (0.1 mg/kg), it shows a rapid increases of both phospho-Ser21-GSK3α and phospho-Ser9-GSK3β in mice [50].
3.3. GSK3 on cell migration
GSK-3 regulation is known to be involved in cell migration. Elevated PI3K and p-Akt expression in the lung tissue by propofol treatment of LPS-challenged rats resulted in the reduction of acute lung injury. This protective effect is accompanied with prolonged survival, decreased the concentrations of protein, TNF-α, and IL-6. The activated PI3/Akt induced by propofol may attenuate GSK3β activity [51] (Fig. 1). Propofol is a potent intravenous anesthetic and sedative agent widely used to facilitate intubation and ventilation [52]. Propofol suppresses acute lung injury (ALI) by influencing migration, phagocytosis, and oxidative ability of macrophages [53]. Cell migration is considered to participate in inflammatory responses. GSK-3 has also been demonstrated to regulate microtubule stability through phosphorylation of three microtubule/tubulin-associated proteins, Tau, microtubule-associated protein 1B, and collapsin response mediator protein 2 [54,55]. Paxillin (PXN) is a 68-kDa focal adhesion-associated protein that plays an important role in controlling cell spreading and migration. PXN has been described as regulators of macrophage migration [56]. GSK-3 phosphorylates PXN (S126/S130) during the process of macrophage migration after LPS treatment and reversely regulated by the mutation of PXN with S126A/S130A substitutions [57]. Additional studies reveal miR-375 derived from cancer cells influences macrophage migration by enhanced PXN and tensin 3 (TNS3) mRNA levels [58]. LPS-stimulated macrophages results in PXN-mediated cytoskeleton changes through ERK/GSK3 pathway [57]. Other molecular mechanisms involved in the protective function of propofol on brain and liver are PI3K/Akt pathway activation [59] and sustaining the mitochondrial function by GSK3β regulation, respectively [60].
3.4. GSK3 alternation by antibiotics with immune modulation effects
Group B streptococci are Gram-positive bacteria for pneumonia, sepsis and particularly in newborns meningitis. S632A3 is a glutarimide antibiotic with immune inhibitory effect on LPS-stimulated macrophages. This inflammatory suppression occurs via inactivation of GSK3β and the differential promotion of CREB–CBP interactions over NF-κB signaling and leads to increased IL-10 production [61] (Fig. 1). It has been indicated that phosphorylated CREB (Ser133) interacted with CBP could reduce NF-κB activity [[62], [63], [64]] [65]. Since GSK3β can inhibit DNA-binding activity of CREB [12], GSK3-β inhibition augments the binding of CREB (Ser133) and suppresses the binding of NF-κB p65 (Ser276) to the nuclear coactivator CBP. This mechanism accounts for the inactive form of GSK3 to be involved in IL-10 production by means of the transcription ability of CREB within LPS-stimulated monocytes [7]. Of particular the M2 macrophage produced IL-10 is indicated to mediate via CREB transcriptional activity [66] (Fig. 1). Macrolides (erythromycin and azithromycin) have improved ARDS mortality and shortened usage of mechanical ventilation [67] and anti-inflammatory M2 macrophage production [68]. Several other antibiotics, minocycline, may influence GSK3β activity [69]. Furthermore, it has been indicated that PI3K/Akt and GSK3β pathway is responsible for protective effect of minocycline in ketamine-induced neural stem cell injury. The phosphorylated GSK3β level restored after minocycline treatment in ketamine-induced injury. In contrast, PI3K inhibitor (LY294002) reduces the protective effect of minocycline [70].
Berberine is a natural alkaloid isolated from plants and has long been used in botanical medicines. Berberine effectively alleviated lung injury by reducing pulmonary edema and neutrophil infiltration via activation of Nrf2 signaling [71] (Fig. 1). Furthermore, berberine reduces LPS-induced lung injury in a Nrf2 dependent manner [72]. GSK3β may phosphorylate Nrf2 and trigger ubiquitination-mediated degradation. As a result, attenuating GSK3β activity provides further feasibility for Nrf2 responsive transcription [73]. Inhibiting GSK3β by BIO treatment increases the expression of MDR genes and the drug efflux activity [74]. Berberine reduces production of pro-inflammatory cytokines in various cell lines via inhibiting NF-κB pathway [75,76]. In addition, reduced phosphorylation level of PI3K/Akt, ERK, and GSK3β also involves in berberine-regulated effects [77]. Moreover the ATP-binding cassette (ABC) transporters express in lung (particularly alveolar macrophages, airway smooth muscle cells, epithelial cells) participate in inflammatory responses [78]. Wnt signaling appears critical and increase p-glycoprotein (p-gp) transporter (ABCB1) by Wnt agonist (1.5–20 uM AMBMP) [79]. Other GSK-3β inhibitors such as 1-azakenpaullone (0.05–0.5 uM), BIO (0.1–1 uM) and LiCl (5000–10,000 uM) may increase MDR protein expression [79] [80]. Comparable effect of berberine-associated upregulated MDR expression were found in oral, gastric, colon and liver cancer cell lines [81] [82], suggesting the axis of PI3K/Akt/ERK/GSK3β/Nrf2/MDR signaling involved in berberine-mediated alleviation of pulmonary edema and injury [71] [72].
4. GSK3β-mediated inflammation in heart
Base on the observation of GSK3β null mice embryogenesis, it implies that GSK3β modulation participates important role of heart physical functions and related diseases [83]. Myocarditis remains a leading cause of heart failure (HF) in children and young adults [84]. From the manifestation of coxsackievirus B3 (CVB3)-positive myocarditis, endomyocardial biopsy results demonstrate higher proteins expression of S100A8 and S100A9. A decrease in myocardial expression of S100A8 and S100A9 is associated with an improved clinical outcome in CVB3-positive patient [85]. This higher level of S100A8 and S100A9 has been involved in the activation of NLRP3 inflammasome [86]. NLRP3 can be activated by infectious triggers known as pathogen-associated molecular patterns, including CVB3 RNA [87]. By activation of the NLRP3 inflammasome, procaspase-1 is converted to active caspase-1 and subsequently activates pro–IL-1 into mature IL-1β. IL-1β is a major pro-inflammatory cytokine secreted primarily from monocytes and macrophages and then to amplify the innate immune responses [88]. As a result, NLRP3 inflammasome has been considered as the detrimental function in myocarditis (Fig. 2 ). By using GSK-3β inhibitor (TDZD-8), the role of NLRP3 inflammasome is attenuated and following by a decrease in IL-1β-associated inflammatory responses [89]. Studies indicate that critical NLRP3 phosphorylation site 198 replaced with alanine mutant (NLRP3-S198A corresponding to mouse NLRP3-S194A) showed an obvious abrogation in inflammasome activation. In contrast, the constitutive phosphorylated S198 mutants (S198D and S198E) manifested much higher activity in IL-1β processing. Comparable results are found from knock-in mice harboring the Nlrp3 S194A allele (Nlrp3S194A/S194A), accompanying much reduced peritoneal inflammation. Further elucidating the kinase responsible for the S194 phosphorylation of NLRP3, showing JNK inhibitor (SP600125) exhibited a robust inhibition of inflammasome activation. Since JNK1 can phosphorylate mouse NLRP3 at S194 and human NLRP3 at S198 [90]. JNK1 is demonstrated to cooperate with GSK3 for efficient down streaming proteins phosphorylation [91] (Fig. 2). These evidences indicate S100A8, S100A9/GSK3/JNK1/NLRP3 signaling in IL-1β regulation.
Fig. 2.
The role of GSK3β on myocarditis and dilated cardiomyopathy. Coxsackievirus B3 (CVB3) infection induces myocardial expression of S100A8/S100A9 and leads to activation of NLRP3 inflammasom/caspase-1/IL-1β signal pathway. GSK3β cooperates with JNK1 to trigger NLRP3 inflammasom-related myocarditis. Activated M1 macrophages secreting HMGB1 may involve in dilated cardiomyopathy. Propofol inhibits GSK3β by phosphorylation and prevents M1 macrophage polarization. Berberine protects myocytes via AMPK activation or Nrf2 mediated inhibition of M1 macrophages. Berebrine triggers M2 macrophage polarization, MDR1 expression and anti-inflammatory response.
4.1. GSK3β modulation in dilated cardiomyopathy
After acute phase myocarditis-induced inflammatory effects in heart, the following persisted and altered immune cells are critical for other complications. There is estimated around 20% of patients with myocarditis developing dilated cardiomyopathy (DCM) [92]. Studies indicate that different regulations of monocyte differentiation involve in the cardiac microenvironment of myocarditis patients [93]. Given the autoimmune myocarditis animal model and clinical observations, monocytes and macrophages represent around 75% of migrating cells surrounding the injured myocardium [94]. From endomyocardial biopsy with virus-negative patients, altered immune activation with chronic DCM treated with prednisone and azathioprine are beneficial to cardiac function [92]. Given the chronic inflammation related cardiovascular diseases, M1 and M2 macrophages derived from circulating monocytes and local tissue-resident macrophages function an important role [95]. M1 macrophages are originated from the inflammatory Ly6Chi blood monocyte and M2 macrophages mostly from Ly6Clo subsets [96]. Normally M1 macrophages produce MCP-1, IL-12, IL-23, and TNF, which are all crucial for defense effect. M2 macrophages stimulated by IL-4 and IL-13, are responsible for anti-inflammatory, wound healing and tissue remodeling responses through IL-10,arginase I and chemokines secretion [97] [96] [98]. M2 macrophages are accumulated on collagen rich fields and to be an independent determinant of cardiac fibrosis [99].
Activated M1 macrophages secrete large amounts of pro-inflammatory mediators such as high mobility group protein (HMGB1). HMGB1 interacts with three receptors (advanced glycation end products, RAGE, TLR2/TLR4) and induces NFκB and ERK1/2 responsive cytokine production [100]. Soluble MD-2 is a risk factor for survival in patients with DCM. HMGB1 could interact with MD-2 during dilated cardiomyopathy [101]. In the parallel evidences reveal IL-17A is responsible for myocarditis progression to dilated cardiomyopathy but is dispensable for myocarditis [102]. IL-17A signaling enhances Ly6Chi monocyte chemotaxis and neutrophil infiltration, worsening dilated cardiomyopathy outcomes [103] [104] (Fig. 2). A recent study indicated that IL-17 and HMGB1 could aggravate the cardiomyocyte apoptosis and autophagy. Extracellular HMGB1 was reported to cause cardiac remodeling, hypertrophy, reperfusion injury and myocardial fibrosis [92,93]. Since autophagy is regulated by autophagy-related proteins, such as, LC3-II/LC3-I and Beclin-1 [105], the increased ratio of LC3-II to LC3-I and the Beclin-1 expression are of significance in H/R group. Thus, it reveals that HMGB1/IL-17A/autophagy signaling is involved in cardiomyocytes injury [106]. Many studies have focused canonical wnt/β-catenin pathway on myocardial infarction and cardiac fibrosis [107]. The dilated cardiomyopathy is reported to associate with atrial fibrosis [108]. Canonical wnt/β-catenin pathway could induce several types of MMPs via modulation of the Axin, APC, and GSK3β protein complex after cardiac injury [109] [110]. The activation of the GSK3β/β-catenin pathway was ever reported to contribute to HMGB1-induced chondrocyte expression of matrix metalloproteinases (MMPs) [111]. The increased phosphorylated inactive GSK-3 has participated in the cytoplasmic accumulation and translocation of β-catenin into the nucleus, resulting in the expressions of downstream target genes [112]. In murine macrophage RAW264.7 cells, GSK3 participates in LPS-induced MMP-9, IL-1β and IL-6 gene expression [113]. Extracellular HMGB1 also induces EMT involved Akt phosphorylation, GSK3β inactivation, nuclear translocation of β-catenin and expression of receptor for RAGE [49]. These studies reveal a mechanism of extracellular HMGB1 in EMT through Akt/GSK3β/β-catenin/MMP/RAGE signaling pathway in cardiac remodeling. However, emerging evidences report that intracellular HMGB1 has an additional functions since attenuation of nuclear HMGB1 result in the detrimental conditions [[114], [115], [116]] (Fig. 2). Intriguing, studies found cardiac nuclear HMGB1 inhibited phosphorylation of ATM and subsequent activation of ERK1/2 and NF-κB signaling [117]. Excessive DNA damage causes phosphorylation of ATM and patients by using left ventricular assist device decreased the expression of p-ATM in cardiomyocytes [118], indicating a different aspect of protective axis by nuclear HMGB1/ATM/ERK/NF-κB signaling.
Neutrophils play a critical role for cardiac inflammation in the experimental autoimmune myocarditis (EAM), indicating relevant features of post-viral heart disease [119,120]. The sustained cardiac inflammation results in tissue remodeling and eventually DCM. Neutrophils slow rolling on venule of inflamed sites require lymphocyte function-associated antigen 1 (LFA-1) and the macrophage 1-antigen (Mac-1) [121]. After neutrophils adhesion, NETs formation participates in myocarditis and targeting NETs may considered as rational treatment [122]. However, recent results appear that blocking LFA-1 by its neutralized antibody enhances infiltration of CD11b + monocytes, F4/80+ macrophages, CD4+ T cells, Ly6G+ neutrophils, and CD133+ progenitor cells on the model of EAM, accompanied with an increased heart weight/body weight ratio [123]. It has been found that more specific monoclonal antibody is critical for integrin-based anti-inflammatory therapy [124] since LFA-1 and Mac-1 have common β2-subunit (CD18) and its deficiency causes recurrent infection and leukocytosis [125]. This compensatory elevated leukocytes could in part explain the phenomena associated with LFA-1 neutralized antibody. In addition, activation of LFA-1 by chemokines allows neutrophils and other leukocytes to undergo arrest, resulting in firm adhesion on endothelia [126]. Previous studies showed that endothelial cells GSK3β activation participated in leukocytes recruitment via reduction in Ser-21/9 and increase in Tyr-279/216 GSK3α/β. The activated GSK3 in endothelial cells led to NF-κB activation and then modulated the expression of endothelial adhesion molecules P- and E-selectins and ICAM-1 [127].
4.2. GSK3β role on cholinergic signaling in heart
The role of the parasympathetic nervous system in modulating inflammation has been recently established and considered as cholinergic antiinflammatory pathway. Stimulation of vagal efferent in the spleen results in the local release of acetylcholine that inhibits the release of proinflammatory cytokines by macrophages [128,129]. In general, there are two major sources of cholinergic signaling in heart: neuronal cholinergic signaling mediated by the release of acetylcholine from the vagus nerve, and the nonneuronal cholinergic system. The later one has been described recently and it is mediated by the release of acetylcholine by cardiomyocytes [130,131]. Physostigmine is a rapid and short-acting inhibitor of acetylcholinesterase. It appears that the low dose (0.1 mg/kg) of physostigmine administration results in rapid increases of both phospho-Ser21-GSK3α and phospho-Ser9-GSK3β in the hippocampus, cerebral cortex, and striatum, with a maximal effect occurring after 15 min [50]. Acetylcholine is released by cardiomyocytes attenuates the detrimental effects, such as oxidative stress, and modulates energy metabolism by enhancing glucose utilization in the heart [132]. Moreover, the immunomodulatory role of cholinergic signaling resulted in increased recruitment of anti-inflammatory cells to the heart and that the increased influx of FOXP3+ Tregs resulted in macrophage polarization toward M2 cells [133].
4.3. GSK3β modulated in cardioprotective effect
Since activated M1/HMGB1/IL17 axis involves in cardiomyocyte injury, the modulation of M1 macrophage is conceived as a rational target for treatment. Propofol is a clinical approved medicine for anesthetic indication. In addition to its anesthetic properties, propofol has been found to suppress the production of IL-6, IL-1β, and TNF-α by several types of cells. These effects indicate that propofol prevents inflammatory responses during polarization of human M1 macrophages by suppressing the expression of IL-6 and IL-1β through the GABAA receptor and the Nrf2-mediated signal transduction pathway [134]. Propofol (25 and 50 μM) could increase the levels of GSK-3β phosphorylation at the serine residue, Ser9 and reduce infarct size [135] (Fig. 2). Converging these results indicate that GABAA receptor/GSK-3β/Nrf2/IL-6/IL-1β signaling pathway functions as an active role in propofol cardioprotective effects.
From a clinical study with chronic congestive heart failure patients, berberine (1.2 to 2.0 g/day for 8 weeks) improved quality of life and decreased ventricular premature complexes of electrocardiogram [136]. Similar cardiac protection effects by berberine could be found on autoimmune myocarditis model [137], myocardial infarction [138] and doxorubicin-induced cardiac injury [139]. Moreover, AMPK and Nrf2 pathways is considered to be required for anti-inflammatory effect of berberine in LPS-stimulated macrophages [140] (Fig. 1). Nrf2 inhibits the transcriptional induction of a subset of M1-induced genes (such as IL6 and IL1β) without going through ARE modulation [141]. As a result, the in vivo underlying mechanism of inhibitory response of berberine could attribute to inhibit M1 polarization by Akt1/AMPK/Nrf2/NF-κB signaling pathway [142]. Interestingly, it has found that M2 macrophages appear higher level expression of MDR1 than M1 macrophages [143]. Nrf2 is involved in upregulation of MDR1 and anti-inflammatory after berberine treated DSS-Induced Colitis [144]. Polarized M2 macrophages were appeared after a regiment of berberine administration (100 mg/kg/d for 16 weeks) in adipose tissue [145]. Thus, the Nrf2-mediated transcriptional inhibition is controlling a suppressive role for M1 macrophages polarization. In contrast, GSK3/Nrf2/MDR signaling facilitates M2 macrophages skewing [141] (Fig. 2).
Many studies appear GSK-3 signaling in cardiac myocyte proliferation since GSK-3 phosphorylates four glycogen synthase regulatory serine residues (Ser641, Ser645, Ser649, and Ser653) and results in inhibiting glycogen synthase activity [146]. Glycogen synthesis in cardiac muscle is critical for normal heart development and its impairment leads to congenital heart defects and death [147]. Inhibition of GSK-3 by BIO promotes proliferation in mammalian cardiac myocytes. Employing sustained delivery hydrogel of BIO and IGF-1, it enhanced the proliferation of cardiac myocytes, promoted revascularization at the injury site and improved cardiac function.
4.4. GSK3β, MIF and MCP-1 in autosomal dominant polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic disease, characterized by renal cysts and numerous extra-renal manifestations [148]. Modulation of GSK3β has been considered as a rational mechanism to reduce renal cyst expansion. The abrogation of GSK3β activity could be accompanied with decreased c-Myc/p-ERK/Cyclin D1 and elevated β-catenin expression [149] (Fig. 3 ). In cyst fluid and kidney epithelial cells of ADPKD patients, high level accumulation of macrophage migration inhibitory factor (MIF) was founded [150]. MIF may activate PI3K/Akt pathway and subsequently inhibit GSK3β [151]. In a parallel observation, MIF stimulates cyst initiation and expansion via p-ERK/mTOR mediated cell proliferation pathway [150]. Thus, PI3K/Akt/p-ERK/mTOR axis participates a crucial role for polycystic kidney formation [152]. To view another aspect, young ADPKD patients with normal blood pressure and renal function exhibit early vascular changes and bi-ventricular diastolic dysfunction [153,154]. From clinical studies reveal that ADPKD has higher association with hypertension, left ventricular hypertrophy and cerebral aneurysms [155,156]. Mutations in PKD1 and PKD2 genes are known to account for ADPKD. PKD1 mutations account for ~85% and PKD2 mutations for ~15% of cases [157]. PKD1 and PKD2 encode the polycystin-1 (PC1) and polycystin-2 (PC2), respectively. PC1 interacts with PC2 and function as non-selective calcium-regulated cation channel [158]. Polycystic kidneys contain higher level of macrophages. Given the potential signal for increased macrophage numbers in cystic kidneys, animal studies show that MCP-1 is highly upregulated as cyst expansion in PKD1 and PKD2 knockout mice [159]. Moreover, tubular cell is reported to be the major source of MCP1 [160] and converted M2 macrophages could initiate renal cysts and tubule epithelial cells proliferation. However, M1 macrophages reveals limited proliferation promotion effects [161]. Moreover macrophages also participate MCP-1 production in an activated Akt and phosphorylated GSK3β-dependent manner [14] (Fig. 3).
Fig. 3.
The role of phosphorylated GSK3β on polycystin-1/2 mutation of polycystic kidney disease. MIF stimulates cyst initiation and expansion via p-ERK/mTOR mediated cell proliferation pathway. GSK3β inhibitor (TDZD-8) or berberine may decrease renal cell proliferation and cyst expansion via p-ERK/p70-S6/c-Myc/Cyclin-D1/β-catenin modulation. From the macrophage aspect, high level of MIF in cyst modulate PI3K/Akt/GSK3β/MCP-1 axis and promotes renal tubule cell proliferation and cyst expansion. Phosphorylated GSK3β (inactive form) results in MCP-1 production and β-catenin activation during the process of vascular remodeling and aneurysm. M1/M2 macrophages participates in different stages of aneurysm development. Long-term lithium treatment may influence podocyte cell cycle and renal function by GSK3β suppression (phosphorylated form). GSK3β degradation by microRNA-92a triggers podocyte re-entry cell cycle and renal failure via Ajuba and YAP/TAZ signal pathway.
4.5. GSK3β in aneurysm
Polycystin 1 and polycystin 2, two essential protein products of PKD1 and PKD2 respectively, are expressed in vascular smooth muscle and endothelium. Thus, interactions of these proteins with a hereditary pathological pathway might be the underlying mechanism involved in the early development of vascular remodeling and aneurysms in ADPKD [162,163]. This could be explained for why many extrarenal manifestations, especially in those of cardiovascular and cerebrovascular abnormalities, are found in associated with ADPKD. Intracranial aneurysm, a cerebrovascular disorder with weakness cerebral vessel, leads to a localized dilation or ballooning of this vessel. Because macrophages are universal finding in human intracranial aneurysm samples, the infiltration of macrophages secrete proinflammatory cytokines and MMPs. This aneurysm localized effect could recruit additional inflammatory cells and destroy extracellular matrix of arterial vessel wall [98]. In addition, abdominal aortic aneurysm tissue is also indicated accumulating macrophages has been considered as an important source of matrix-degrading proteases and to degrade the extracellular matrix. The role of MMPs in preclinical models of abdominal aortic aneurysm is well established [164] and in human abdominal aortic aneurysm as well [165]. In general there are two primarily sources of endogenous elastases (neutrophils and macrophages). The neutrophil elastase is an intracellular, granule-associated enzyme and macrophage elastase appears to be a secretory enzyme. Unstimulated macrophages secrete very little elastase activity but can be triggered to secrete higher levels of this enzyme by phagocytosis [166] [167]. By utility of elastase-induced abdominal aortic aneurysm, it appears that M1 macrophages predominate in early stages of aneurysm development while M2 macrophages accumulate at late stage [168] [169] (Fig. 3). Further analysis, IL-23 blockade inhibits the expansion of macrophages and profoundly reduced the expression of macrophage-associated inflammatory mediators. The primarily source of IL-23 is derived from macrophages and dendritic cells [170]. The IL-12/IL-23 axis participate in human abdominal aortic aneurysm and this signal transduction is conceived as a potential therapeutic pathway [171]. In angiotensin II–induced aortic aneurysm, the suprarenal aortas samples appear higher phosphorylation of GSK3β and activated Wnt signaling (Fig. 3). Moreover sclerostin is an inhibitory molecule of the Wnt/β-catenin signaling pathway [172] and sclerostin reduction is observed in aortic aneurysm. Sclerostin may protect AngII-infused mice from macrophage infiltration [173]. Of particular higher phosphorylated GSK3β leading to β-catenin activation is found on aneurysm [173]. Thus, inhibition of GSK3β by lithium has been shown to activate Wnt pathway and promote endothelial cell senescence [174]. It is also the evidence of arterial aging that Wnt/β-catenin activation is involved in vascular smooth muscle cells resting transformation [175]. From the anti-sclerostin antibody (Romosozumab) clinical trial for osteoporosis, it indicates high risk of cardiovascular events and remodeling, implying Wnt/β-catenin activation [176]. This could explain the role of sclerostin reduction in aortic aneurysm, where Wnt/GSK3β/β-catenin might promote the senescent phenotypes of arterial cells.
4.6. GSK3β in renal cells
Specifically deletion of GSK3 in podocytes of mice could result into a spectrum of kidney disease, ranging from albuminuric mesangial hyper-cellularity to glomerulosclerosis, severe hypertension and renal failure. The rationale of crescentic glomerulopathy is the mature podocyte to re-enter the cell cycle and proliferate. Some evidences indicate that terminally differentiated podocytes can re-enter the cell cycle in response of inflammatory glomerulonephritis [177] and HIV activated pathway of Wnt signaling in nephropathy [178]. Since podocytes may prevent albuminuria and preserve renal function, its well differentiated phenotypes are of significance [179,180]. On the disease setting, podocytes initiate mitosis with incompletely division by mitotic catastrophic mechanism. This insult may lead to crescentic rapidly progressive glomerulonephritis (RPGN) [181]. In addition, much higher level expression of GSK3β and phosphorylated form have linked to progression of albuminuria and diabetic kidney injury. Further analysis, renal glomeruli and tubules manifest higher phosphorylated GSK3β(Tyr216) [182] Since there are many comorbidities associated with diabetes, the function of GSK3β in kidney could be conceived with more attention during disease or therapy-related renal function interference [183,184]. Particularly, recent approach using detection of GSK3β activity in urinary exfoliated cells for early evaluation renal function in diabetic patients [182].
4.7. GSK3β in podocyte cell re-entry
Among the patients diagnosed with crescentic RPGN, elevated miR-92a is observed in kidney biopsies, particularly the field with podocytes. As a results, by means of specific abrogating miR-92a in podocyte, it reduces albuminuria and kidney failure [185]. (Fig. 3). As a result, podocyte exhibits cell cycle arrest mediated by p57kip2 regulation after inhibiting miR-92a. It is noticed that GSK3β appears to act as a direct target of miR-92a and triggers downstream β-catenin activation [186,187]. Furthermore, attenuated GSK3 results in the expression of Ajuba and accordingly nucleus YAP/TAZ accumulation contributes cell cycle re-entry and mitotic catastrophe [188] (Fig. 3). It is believed that inhibiting podocyte GSK3β activity exhibits beneficial renal diseases treatment [189]. Lithium is a prescribed medicine for bipolar disorder and is found to inhibit estimated 25% of GSK3 activity at the bio-available dose [190]. For long term administration of lithium on patients or animal models, renal side effects could be observed, such as diabetes insipidus, and an increased principal cell proliferation [191] [192,193].
4.8. GSK3β in renal protection
Given certain therapy-induced kidney adverse effects, podocyte dysfunction has been regarded as a critical role. Adriamycin elicits prominent oxidative stress and alters the activity of redox-sensitive kinases in target organs, such as kidneys. It reports that glomerular staining of podocyte marker (podocin and WT-1) decreased following adriamycin injury. Adriamycin exposure consistently diminished protein expression levels of podocin and WT-1, suggesting podocyte influence. The adriamycin-induced podocyte injury was followed by overactivity of GSK3β in the glomerulus [189]. By using GSK3βinhibitor, TDZD-8 reduces the adriamycin-elicited GSK3β overactivity and increased expression of podocin and WT-1 in glomerulus [161,194]. Attenuation of GSK3 has been shown to reduce proteinuria, podocyte injury and glomerulosclerosis in the experimental model of diabetic nephropathy [195], podocytopathy elicited by adriamycin and LPS treatment [189,196]. GSK3β was found to phosphorylate serine 467 of NFκB and then involves in transcription of podocyte cytoskeleton rearranged genes (MCP-1, B7-1, and cathepsin L) [196,197].
Berberine reduces cell growth in autosomal dominant polycystic kidney disease (ADPKD) cystic cells by cell cycle G0/G1 arrest and p-ERK/p70-S6 reduction (a downstream kinase of mTOR) [198]. Furthermore, berberine attenuates macrophages infiltration in intracranial aneurysms [199]. Previous reports have demonstrated the berberine anti-inflammatory effects are mediated by inhibiting several key signaling pathways involved in macrophages infiltration, such as Nrf2 and MAPK/JNK/p38/ERK pathway [72,200] and SRC-FAK pathway [201]. FAK/PYK2 may phosphorylate GSK3β(Tyr216) and to stabilize β-catenin [202]. Phosphorylation of GSK3β(Tyr216) is required for GSK3β's full kinase activity [203]. Berberine may inhibit FAK phosphorylation in macrophages [199,204]. Converging all these evidences, it reveals that berberine inhibits cyst and macrophage infiltration via FAK/GSK3β(Tyr216)/β-catenin modulation (Fig. 3).
5. Concluding remarks
GSK3 activity is required in physiological settings and interfering its function is associated in many diseases. Here we describe macrophages participating on acute respiratory distress syndrome by GSK3β activation and differently regulated transcription factor NFκB or CREB-mediated M1 macrophage pro-inflammatory or M2 macrophage anti-inflammatory responses, respectively. In the setting of myocarditis, GSK3β cooperates with JNK1 to trigger NLRP3/caspase-1/IL-1-related inflammation. As for the chronic inflammation in heart, secreted HMGB1 induces MMP9-related cell remodeling and highly MDR1 expression on M2 macrophages involve in dilated cardiomyopathy. For inherited genetic disturbance of polycystin-1/2 on polycystic kidney disease, Akt/GSK3β axis modulation results in MCP-1 production in macrophages. M2 macrophage enhances renal tubule cell proliferation. The polycystin-1/2 mutations manifested vascular remodeling and aneurysm could involve high phosphorylated GSK3β (inactive form) and β-catenin activation. Particularly for an excessive GSK3β suppression, a reciprocal modulation of GSK3β should be considered and of significance to prevent podocyte re-entering cell cycle and glomerular function reduction.
CRediT authorship contribution statement
Wei-Lun Liu:Writing - original draft, Resources.Fu-Tien Chiang:Supervision, Resources.Juliana Tze-Wah Kao:Resources, Supervision.Shih-Hwa Chiou:Resources, Supervision.Heng-Liang Lin:Conceptualization, Project administration, Writing - review & editing, Visualization.
Declaration of competing interest
All authors declare no conflicts of interest and no financial support for this publication.
Footnotes
This article is part of a Special Issue entitled: GSK-3 and related kinases in cancer, neurological and other disorders edited by James McCubrey, Agnieszka Gizak and Dariusz Rakus
References
- 1.Moore S.F., et al. Dual regulation of Glycogen Synthase Kinase 3 (GSK3)α/β by Protein Kinase C (PKC)α and Akt promotes thrombin-mediated integrin αIIbβ3 activation and granule secretion in platelets. J. Biol. Chem. 2013 Feb;288(6):3918–3928. doi: 10.1074/jbc.M112.429936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roach P. Glycogen and its metabolism. Curr. Mol. Med. Mar.2002;2(2):101–120. doi: 10.2174/1566524024605761. [DOI] [PubMed] [Google Scholar]
- 3.Woodgett J.R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. Aug.1990;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beurel E. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol. Ther. 2015 Apr.;0:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland C. What are the bona fide GSK3 substrates? Int. J. Alzheimers Dis. 2011;2011:505607. doi: 10.4061/2011/505607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doble B.W. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. Apr.2003;116(7):1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin M., Rehani K., Jope R.S., Michalek S.M. Toll-like receptor–mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. Aug.2005;6(8):777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross D.A.E., et al. Insulin activates protein kinase B, inhibits glycogen synthase kinase-3 and activates glycogen synthase by rapamycin-insensitive pathways in skeletal muscle and adipose tissue. FEBS Lett. Apr.1997;406(1–2):211–215. doi: 10.1016/S0014-5793(97)00240-8. [DOI] [PubMed] [Google Scholar]
- 9.Hoeflich K.P., Luo J., Rubie E.A., Tsao M.-S., Jin O., Woodgett J.R. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature. Jul.2000;406(6791):86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 10.Kerkela R., et al. Deletion of GSK-3β in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J. Clin. Invest. Nov.2008;118(11):3609–3618. doi: 10.1172/JCI36245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacAulay K., et al. Glycogen synthase kinase 3α-specific regulation of murine hepatic glycogen metabolism. Cell Metab. Oct.2007;6(4):329–337. doi: 10.1016/j.cmet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Grimes C.A., Jope R.S. CREB DNA binding activity is inhibited by glycogen synthase kinase-3β and facilitated by lithium. J. Neurochem. Dec.2001;78(6):1219–1232. doi: 10.1046/j.1471-4159.2001.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinbrecher K.A., Wilson W., Cogswell P.C., Baldwin A.S. Glycogen synthase kinase 3 functions to specify gene-specific, NF-B-dependent transcription. Mol. Cell. Biol. Oct.2005;25(19):8444–8455. doi: 10.1128/MCB.25.19.8444-8455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park D.-W., et al. The JAK2-Akt-glycogen synthase kinase-3β signaling pathway is involved in toll-like receptor 2-induced monocyte chemoattractant protein-1 regulation. Mol. Med. Rep. Apr.2012;5(4):1063–1067. doi: 10.3892/mmr.2012.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X., et al. Methane limit LPS-induced NF-κB/MAPKs signal in macrophages and suppress immune response in mice by enhancing PI3K/AKT/GSK-3β-mediated IL-10 expression. Sci. Rep. Jul. 2016;6(1):29359. doi: 10.1038/srep29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehani K., et al. Cotinine-induced convergence of the cholinergic and PI3 kinase-dependent anti-inflammatory pathways in innate immune cells. Biochim. Biophys. Acta, Mol. Cell Res. Mar.2008;1783(3):375–382. doi: 10.1016/j.bbamcr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Fisher D., Heymann D. Q&A: the novel coronavirus outbreak causing COVID-19. BMC Med. Feb. 2020;18(1):57. doi: 10.1186/s12916-020-01533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. Feb.2020 doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 19.Wu C.-H., et al. Glycogen synthase kinase-3 regulates the phosphorylation of severe acute respiratory syndrome coronavirus nucleocapsid protein and viral replication. J. Biol. Chem. Feb.2009;284(8):5229–5239. doi: 10.1074/jbc.M805747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.König R., et al. Human host factors required for influenza virus replication. Nature. Feb.2010;463(7282):813–817. doi: 10.1038/nature08699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saleh M., et al. Glycogen synthase kinase 3β enhances hepatitis C virus replication by supporting miR-122. Front. Microbiol. 2018;9:2949. doi: 10.3389/fmicb.2018.02949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganesan H., Embi N., N M., Sidek Mohd. The anti-malarial chloroquine modulated cytokine levels and increased animal survivability via Akt-mediated inhibition of GSK3β in burkholderia pseudomallei-infected mice. Trop. Biomed. 2018;35(3):709–723. [PubMed] [Google Scholar]
- 23.Mócsai A., Walzog B., Lowell C.A. Intracellular signalling during neutrophil recruitment. Cardiovasc. Res. Aug.2015;107(3):373–385. doi: 10.1093/cvr/cvv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ussov W.Y., et al. Relationship between granulocyte activation, pulmonary granulocyte kinetics and alveolar permeability in extrapulmonary inflammatory disease. Clin. Sci. (Lond.) Sep.1996;91(3):329–335. doi: 10.1042/cs0910329. [DOI] [PubMed] [Google Scholar]
- 25.Matthay M.A., Ware L.B., Zimmerman G.A. The acute respiratory distress syndrome. J. Clin. Invest. Aug.2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petretto A., et al. Neutrophil extracellular traps (NET) induced by different stimuli: a comparative proteomic analysis. PLoS One. Jul.2019;14(7):e0218946. doi: 10.1371/journal.pone.0218946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park D.W., et al. GSK3β-dependent inhibition of AMPK potentiates activation of neutrophils and macrophages and enhances severity of acute lung injury. Am. J. Phys. Lung Cell. Mol. Phys. Nov.2014;307(10):L735–L745. doi: 10.1152/ajplung.00165.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao X., et al. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am. J. Phys. Lung Cell. Mol. Phys. Sep.2008;295(3):L497–L504. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki T., et al. Inhibition of AMPK catabolic action by GSK3. Mol. Cell. 2013;50:407–419. doi: 10.1016/j.molcel.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurrieri C., et al. 3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione (SB216763), a glycogen synthase kinase-3 inhibitor, displays therapeutic properties in a mouse model of pulmonary inflammation and fibrosis. J. Pharmacol. Exp. Ther. Mar.2010;332(3):785–794. doi: 10.1124/jpet.109.153049. [DOI] [PubMed] [Google Scholar]
- 31.Hastings R.H., Folkesson H.G., Matthay M.A. Mechanisms of alveolar protein clearance in the intact lung. Am. J. Phys. Lung Cell. Mol. Phys. Apr.2004;286(4):L679–L689. doi: 10.1152/ajplung.00205.2003. [DOI] [PubMed] [Google Scholar]
- 32.Buchäckert Y., et al. Megalin mediates transepithelial albumin clearance from the alveolar space of intact rabbit lungs. J. Physiol. Oct.2012;590(20):5167–5181. doi: 10.1113/jphysiol.2012.233403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vohwinkel C.U., et al. Restoration of megalin-mediated clearance of alveolar protein as a novel therapeutic approach for acute lung injury. Am. J. Respir. Cell Mol. Biol. 2017;57(5):589–602. doi: 10.1165/rcmb.2016-0358OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffman O., Burns N., Vadász I., Eltzschig H.K., Edwards M.G., Vohwinkel C.U. Detrimental ELAVL-1/HuR-dependent GSK3β mRNA stabilization impairs resolution in acute respiratory distress syndrome. PLoS One. 2017;12(2):e0172116. doi: 10.1371/journal.pone.0172116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuseff M.I., Farfan P., Bu G., Marzolo M.-P. A cytoplasmic PPPSP motif determines megalin’s phosphorylation and regulates receptor’s recycling and surface expression. Traffic. Sep.2007;8(9):1215–1230. doi: 10.1111/j.1600-0854.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 36.Zou C., et al. LPS impairs phospholipid synthesis by triggering beta-transducin repeat-containing protein (beta-TrCP)-mediated polyubiquitination and degradation of the surfactant enzyme acyl-CoA:lysophosphatidylcholine acyltransferase I (LPCAT1) J. Biol. Chem. Jan.2011;286(4):2719–2727. doi: 10.1074/jbc.M110.192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shapiro H., Kagan I., Shalita-Chesner M., Singer J., Singer P. Inhaled aerosolized insulin: a ‘topical’ anti-inflammatory treatment for acute lung injury and respiratory distress syndrome? Inflammation. Oct.2010;33(5):315–319. doi: 10.1007/s10753-010-9187-2. [DOI] [PubMed] [Google Scholar]
- 38.Jin H., Yang X., Zhao K., Zhao L., Chen C., Yu J. Glycogen synthase kinase-3 beta inhibitors protectagainst the acute lung injuries resulting from acute necrotizing pancreatitis. Acta Cir. Bras. Aug.2019;34(6):e201900609. doi: 10.1590/s0102-865020190060000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werner J., Z’graggen K., Castillo C. Fernández-del, Lewandrowski K.B., Compton C.C., Warshaw A.L. Specific therapy for local and systemic complications of acute pancreatitis with monoclonal antibodies against ICAM-1. Ann. Surg. Jun.1999;229(6):834–840. doi: 10.1097/00000658-199906000-00010. (discussion 841-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundberg A.H., et al. Blocking pulmonary ICAM-1 expression ameliorates lung injury in established diet-induced pancreatitis. Ann. Surg. Feb.2001;233(2):213–220. doi: 10.1097/00000658-200102000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han X., et al. Enhancement of ICAM-1 via the JAK2/STAT3 signaling pathway in a rat model of severe acute pancreatitis-associated lung injury. Exp. Ther. Med. Mar.2016;11(3):788–796. doi: 10.3892/etm.2016.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beitler J.R., et al. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: the BREATHE criteria. Intensive Care Med. Sep.2016;42(9):1427–1436. doi: 10.1007/s00134-016-4423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papazian L., et al. Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. Sep.2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 44.Yabasin I.B., et al. Cisatracurium retards cell migration and invasion upon upregulation of p53 and inhibits the aggressiveness of colorectal cancer. Front. Physiol. 2018;9:941. doi: 10.3389/fphys.2018.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bachelder R.E., Yoon S.-O., Franci C., de Herreros A.G., Mercurio A.M. Glycogen synthase kinase-3 is an endogenous inhibitor of snail transcription: implications for the epithelial-mesenchymal transition. J. Cell Biol. Jan.2005;168(1):29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou B.P., et al. Dual regulation of snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. Oct.2004;6(10):931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 47.He T., Tao J., Wang X., Wang X. Effects of cisatracurium in combination with ventilation on inflammatory factors and immune variations in sepsis rats. Exp. Ther. Med. Mar. 2018 doi: 10.3892/etm.2018.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng Y., et al. Toll-like receptor 4 mediates acute lung injury induced by high mobility group box-1. PLoS One. May 2013;8(5):e64375. doi: 10.1371/journal.pone.0064375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y.-C., et al. High mobility group box 1-induced epithelial mesenchymal transition in human airway epithelial cells. Sci. Rep. Jan.2016;6:18815. doi: 10.1038/srep18815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Sarno P., Bijur G.N., Zmijewska A.A., Li X., Jope R.S. In vivo regulation of GSK3 phosphorylation by cholinergic and NMDA receptors. Neurobiol. Aging. Mar. 2006;27(3):413–422. doi: 10.1016/j.neurobiolaging.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsing C.-H., Chen C.-L., Lin W.-C., Lin C.-F. Propofol treatment inhibits constitutive apoptosis in human primary neutrophils and granulocyte-differentiated human HL60 cells. PLoS One. Jun. 2015;10(6):e0129693. doi: 10.1371/journal.pone.0129693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marik P.E. Propofol: therapeutic indications and side-effects. Curr. Pharm. Des. 2004;10(29):3639–3649. doi: 10.2174/1381612043382846. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka T., et al. The intravenous anesthetic propofol inhibits lipopolysaccharide-induced hypoxia-inducible factor 1 activation and suppresses the glucose metabolism in macrophages. J. Anesth. Feb.2010;24(1):54–60. doi: 10.1007/s00540-009-0829-1. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura T., Kawano Y., Arimura N., Kawabata S., Kikuchi A., Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. Jan. 2005;120(1):137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Leibinger M., et al. Boosting CNS axon regeneration by harnessing antagonistic effects of GSK3 activity. Proc. Natl. Acad. Sci. U. S. A. 2017;114(27):E5454–E5463. doi: 10.1073/pnas.1621225114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abshire M.Y., Thomas K.S., Owen K.A., Bouton A.H. Macrophage motility requires distinct α5β1/FAK and α4β1/paxillin signaling events. J. Leukoc. Biol. Feb. 2011;89(2):251–257. doi: 10.1189/jlb.0710395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai X., Li M., Vrana J., Schaller M.D. Glycogen synthase kinase 3- and extracellular signal-regulated kinase-dependent phosphorylation of paxillin regulates cytoskeletal rearrangement. Mol. Cell. Biol. Apr. 2006;26(7):2857–2868. doi: 10.1128/MCB.26.7.2857-2868.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frank A.-C., et al. Apoptotic tumor cell-derived microRNA-375 uses CD36 to alter the tumor-associated macrophage phenotype. Nat. Commun. 2019;10(1):1135. doi: 10.1038/s41467-019-08989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H., Wang G., Yu Y., Wang Y. The role of phosphoinositide-3-kinase/Akt pathway in propofol-induced postconditioning against focal cerebral ischemia-reperfusion injury in rats. Brain Res. Nov. 2009;1297:177–184. doi: 10.1016/j.brainres.2009.08.054. [DOI] [PubMed] [Google Scholar]
- 60.Zhao G., et al. Role of glycogen synthase kinase 3β in protective effect of propofol against hepatic ischemia-reperfusion injury. J. Surg. Res. Nov.2013;185(1):388–398. doi: 10.1016/j.jss.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Deng H., et al. S632A3, a new glutarimide antibiotic, suppresses lipopolysaccharide-induced pro-inflammatory responses via inhibiting the activation of glycogen synthase kinase 3β. Exp. Cell Res. Dec.2012;318(20):2592–2603. doi: 10.1016/j.yexcr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 62.Sheppard K.-A., et al. Transcriptional activation by NF-κB requires multiple coactivators. Mol. Cell. Biol. Sep.1999;19(9):6367–6378. doi: 10.1128/MCB.19.9.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhong H., Voll R.E., Ghosh S. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell. Apr.1998;1(5):661–671. doi: 10.1016/S1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 64.Parker D., et al. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol. Cell. Biol. Feb.1996;16(2):694–703. doi: 10.1128/MCB.16.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parry G.C., Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J. Immunol. Dec.1997;159(11):5450–5456. [PubMed] [Google Scholar]
- 66.Ruffell D., et al. A CREB-C/EBP cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. Oct.2009;106(41):17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.A. J. Walkey and R. S. Wiener, “Macrolide antibiotics and survival in patients with acute lung injury.,” Chest, vol. 141, no. 5, pp. 1153–1159, May2012, doi: 10.1378/chest.11-1908. [DOI] [PMC free article] [PubMed]
- 68.D.Amantea et al., “Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards M2 phenotype.,” Exp. Neurol., vol. 275 Pt 1, pp. 116–25, Jan. 2016, doi: 10.1016/j.expneurol.2015.10.012. [DOI] [PubMed]
- 69.Lu Y., et al. Protective effect of minocycline against ketamine-induced injury in neural stem cell: involvement of PI3K/Akt and Gsk-3 Beta pathway. Front. Mol. Neurosci. 2016;9:135. doi: 10.3389/fnmol.2016.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szeto G.L., Pomerantz J.L., Graham D.R.M., Clements J.E. Minocycline suppresses activation of nuclear factor of activated T cells 1 (NFAT1) in human CD4+ T cells. J. Biol. Chem. Apr.2011;286(13):11275–11282. doi: 10.1074/jbc.M110.210518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang Y., et al. Berberine ameliorates lipopolysaccharide-induced acute lung injury via the PERK-mediated Nrf2/HO-1 signaling axis. Phytother. Res. Jan.2019;33(1):130–148. doi: 10.1002/ptr.6206. [DOI] [PubMed] [Google Scholar]
- 72.Mo C., et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. Feb.2014;20(4):574–588. doi: 10.1089/ars.2012.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahmed S.M.U., Luo L., Namani A., Wang X.J., Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim. Biophys. Acta Mol. basis Dis. 2017;1863(2):585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 74.Das S., Samant R.S., Shevde L.A. Nonclassical activation of Hedgehog signaling enhances multidrug resistance and makes cancer cells refractory to smoothened-targeting Hedgehog inhibition. J. Biol. Chem. Apr.2013;288(17):11824–11833. doi: 10.1074/jbc.M112.432302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shang W., Liu J., Yu X., Zhao J. Effects of berberine on serum levels of inflammatory factors and inflammatory signaling pathway in obese mice induced by high fat diet. Zhongguo Zhong Yao Za Zhi. Jun.2010;35(11):1474–1477. [PubMed] [Google Scholar]
- 76.Cui G., Qin X., Zhang Y., Gong Z., Ge B., Zang Y.Q. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J. Biol. Chem. Oct.2009;284(41):28420–28429. doi: 10.1074/jbc.M109.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song Y.C., et al. Berberine regulates melanin synthesis by activating PI3K/AKT, ERK and GSK3β in B16F10 melanoma cells. Int. J. Mol. Med. Apr.2015;35(4):1011–1016. doi: 10.3892/ijmm.2015.2113. [DOI] [PubMed] [Google Scholar]
- 78.Chai A.B., Ammit A.J., Gelissen I.C. Examining the role of ABC lipid transporters in pulmonary lipid homeostasis and inflammation. Respir. Res. 2017;18(1):41. doi: 10.1186/s12931-017-0526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim J.C., et al. Activation of beta-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J. Neurochem. Aug.2008;106(4):1855–1865. doi: 10.1111/j.1471-4159.2008.05537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leost M., et al. Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur. J. Biochem. Oct.2000;267(19):5983–5994. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- 81.H. L.Lin, T. Y.Liu, W. Y.Lui, and C. W. Chi, “Up-regulation of multidrug resistance transporter expression by berberine in human and murine hepatoma cells.,” Cancer, vol. 85, no. 9, pp. 1937–42, May1999. [PubMed]
- 82.Lin H.L., Liu T.Y., Wu C.W., Chi C.W. Berberine modulates expression of mdr1 gene product and the responses of digestive track cancer cells to paclitaxel. Br. J. Cancer. Oct.1999;81(3):416–422. doi: 10.1038/sj.bjc.6690710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kerkela R., et al. Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J. Clin. Invest. Nov.2008;118(11):3609–3618. doi: 10.1172/JCI36245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caforio A.L.P., Marcolongo R., Jahns R., Fu M., Felix S.B., Iliceto S. Immune-mediated and autoimmune myocarditis: clinical presentation, diagnosis and management. Heart Fail. Rev. Nov.2013;18(6):715–732. doi: 10.1007/s10741-012-9364-5. [DOI] [PubMed] [Google Scholar]
- 85.Müller I., et al. Pathogenic role of the damage-associated molecular patterns S100A8 and S100A9 in Coxsackievirus B3-Induced Myocarditis. Circ. Heart Fail. Nov. 2017;10(11) doi: 10.1161/CIRCHEARTFAILURE.117.004125. [DOI] [PubMed] [Google Scholar]
- 86.Simard J.-C., et al. S100A8 and S100A9 induce cytokine expression and regulate the NLRP3 inflammasome via ROS-dependent activation of NF-κB(1.) PLoS One. 2013;8(8):e72138. doi: 10.1371/journal.pone.0072138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tschöpe C., et al. NOD2 (nucleotide-binding oligomerization domain 2) is a major pathogenic mediator of coxsackievirus B3-induced myocarditis. Circ. Heart Fail. Sep.2017;10(9) doi: 10.1161/CIRCHEARTFAILURE.117.003870. [DOI] [PubMed] [Google Scholar]
- 88.Tschopp J., Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010;10(3):210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 89.Zhao J., et al. Lupus nephritis: glycogen synthase kinase 3β promotion of renal damage through activation of the NLRP3 inflammasome in lupus-prone mice. Arthritis Rheumatol. (Hoboken, N.J.) Apr.2015;67(4):1036–1044. doi: 10.1002/art.38993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song N., et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol. Cell. Oct. 2017;68(1):185–197.e6. doi: 10.1016/j.molcel.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 91.Wei W., Jin J., Schlisio S., Harper J.W., Kaelin W.G. The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. Jul.2005;8(1):25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 92.C.Tschöpe, L. T.Cooper, G.Torre-Amione, and S. Van Linthout, “Management of myocarditis-related cardiomyopathy in adults,” Circ. Res., vol. 124, no. 11, pp. 1568–1583, May2019, doi: 10.1161/CIRCRESAHA.118.313578. [DOI] [PubMed]
- 93.Hou X., et al. The cardiac microenvironment instructs divergent monocyte fates and functions in myocarditis. Cell Rep. Jul.2019;28(1):172–189.e7. doi: 10.1016/j.celrep.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barin J.G., Rose N.R., Ciháková D. Macrophage diversity in cardiac inflammation: a review. Immunobiology. May 2012;217(5):468–475. doi: 10.1016/j.imbio.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tabas I., Bornfeldt K.E. Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. Feb.2016;118(4):653–667. doi: 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fisher E.A. Regression of atherosclerosis: the journey from the liver to the plaque and back. Arterioscler. Thromb. Vasc. Biol. Feb.2016;36(2):226–235. doi: 10.1161/ATVBAHA.115.301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. Apr.2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanematsu Y., et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. Jan.2011;42(1):173–178. doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cassetta L., Cassol E., Poli G. Macrophage polarization in health and disease. Sci. World J. 2011;11:2391–2402. doi: 10.1100/2011/213962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takahashi M. High-mobility group box 1 protein (HMGB1) in ischaemic heart disease: beneficial or deleterious? Cardiovasc. Res. Oct. 2008;80(1):5–6. doi: 10.1093/cvr/cvn212. [DOI] [PubMed] [Google Scholar]
- 101.Kuemmel A., Feldtmann R., Stohbach A., Riad A., Chamling B., Felix S.B. The involvement and interplay of HMGB1 with soluble MD-2 in dilated cardiomyopathy and its impact in immune cell recruitment. Eur. Heart J. Oct. 2019;40(Supplement_1) doi: 10.1093/eurheartj/ehz746.0102. [DOI] [Google Scholar]
- 102.G. C.Baldeviano et al., “Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy.,” Circ. Res., vol. 106, no. 10, pp. 1646–55, May2010, doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed]
- 103.Chen G., et al. Sca-1+ cardiac fibroblasts promote development of heart failure. Eur. J. Immunol. 2018;48(9):1522–1538. doi: 10.1002/eji.201847583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liao Y.-H., et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J. Am. Coll. Cardiol. Jan.2012;59(4):420–429. doi: 10.1016/j.jacc.2011.10.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gurusamy N., et al. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc. Res. Apr.2010;86(1):103–112. doi: 10.1093/cvr/cvp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu X., Zhang K., Chen Z., Jiang H., Xu W. The HMGB1-IL-17A axis contributes to hypoxia/reoxygenation injury via regulation of cardiomyocyte apoptosis and autophagy. Mol. Med. Rep. Jan.2018;17(1):336–341. doi: 10.3892/mmr.2017.7839. [DOI] [PubMed] [Google Scholar]
- 107.Lorenzon A., Calore M., Poloni G., DeWindt L.J., Braghetta P., Rampazzo A. Wnt/β-catenin pathway in arrhythmogenic cardiomyopathy. Oncotarget. Sep. 2017;8(36):60640–60655. doi: 10.18632/oncotarget.17457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ohtani K., Yutani C., Nagata S., Koretsune Y., Hori M., Kamada T. High prevalence of atrial fibrosis in patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. Apr.1995;25(5):1162–1169. doi: 10.1016/0735-1097(94)00529-Y. [DOI] [PubMed] [Google Scholar]
- 109.Wu B., Crampton S.P., Hughes C.C.W. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. Feb.2007;26(2):227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.A.Deb, “Cell-cell interaction in the heart via Wnt/-catenin pathway after cardiac injury,” Cardiovasc. Res., vol. 102, no. 2, pp. 214–223, May2014, doi: 10.1093/cvr/cvu054. [DOI] [PMC free article] [PubMed]
- 111.Shu Z., Miao X., Tang T., Zhan P., Zeng L., Jiang Y. The GSK-3β/β-catenin signaling pathway is involved in HMGB1-induced chondrocyte apoptosis and cartilage matrix degradation. Int. J. Mol. Med. Mar.2020;45(3):769–778. doi: 10.3892/ijmm.2020.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.M. P. Yavropoulou and J. G. Yovos, “The role of the Wnt signaling pathway in osteoblast commitment and differentiation.,” Hormones (Athens)., vol. 6, no. 4, pp. 279–94, doi:10.14310/horm.2002.1111024. [DOI] [PubMed]
- 113.Kim S., Bong N., Kim O.S., Jin J., Kim D.-E., Lee D.K. Lithium chloride suppresses LPS-mediated matrix metalloproteinase-9 expression in macrophages through phosphorylation of GSK-3β. Cell Biol. Int. Feb.2015;39(2):177–184. doi: 10.1002/cbin.10352. [DOI] [PubMed] [Google Scholar]
- 114.Kang R., et al. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology. Apr.2014;146(4):1097–1107. doi: 10.1053/j.gastro.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.H.Huang et al., “Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: a role for intracellular high-mobility group box 1 in cellular protection.,” Hepatology, vol. 59, no. 5, pp. 1984–1997, May2014, doi: 10.1002/hep.26976. [DOI] [PMC free article] [PubMed]
- 116.Messer J., Chang E. Intracellular HMGB1: defender of client proteins and cell fate. Oncotarget. Apr. 2015;6(11):8432–8433. doi: 10.18632/oncotarget.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takahashi T., et al. Cardiac nuclear high-mobility group box 1 ameliorates pathological cardiac hypertrophy by inhibiting DNA damage response. JACC. Basic Transl. Sci. Apr.2019;4(2):234–247. doi: 10.1016/j.jacbts.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Canseco D.C., et al. Human ventricular unloading induces cardiomyocyte proliferation. J. Am. Coll. Cardiol. Mar.2015;65(9):892–900. doi: 10.1016/j.jacc.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neu N., Klieber R., Frühwirth M., Berger P. Cardiac myosin-induced myocarditis as a model of postinfectious autoimmunity. Eur. Heart J. Aug. 1991;12(Suppl D):117–120. doi: 10.1093/eurheartj/12.suppl_d.117. [DOI] [PubMed] [Google Scholar]
- 120.Weckbach L.T., et al. Midkine drives cardiac inflammation by promoting neutrophil trafficking and NETosis in myocarditis. J. Exp. Med. 2019;216(2):350–368. doi: 10.1084/jem.20181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dunne J.L., Ballantyne C.M., Beaudet A.L., Ley K. Control of leukocyte rolling velocity in TNF-α–induced inflammation by LFA-1 and Mac-1. Blood. Jan.2002;99(1):336–341. doi: 10.1182/blood.V99.1.336. [DOI] [PubMed] [Google Scholar]
- 122.Jorch S.K., et al. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017;23:279–287. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 123.Weckbach L.T., et al. Blocking LFA-1 aggravates cardiac inflammation in experimental autoimmune myocarditis. Cells. 2019;8(10) doi: 10.3390/cells8101267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wolf D., et al. A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Nat. Commun. Dec. 2018;9(1):525. doi: 10.1038/s41467-018-02896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kishimoto T.K., Hollander N., Roberts T.M., Anderson D.C., Springer T.A. Heterogeneous mutations in the β subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. Jul.1987;50(2):193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- 126.Lefort C.T., Ley K. Neutrophil arrest by LFA-1 activation. Front. Immunol. 2012;3:157. doi: 10.3389/fimmu.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Su Y., Qadri S.M., Cayabyab F.S., Wu L., Liu L.E. Regulation of methylglyoxal-elicited leukocyte recruitment by endothelial SGK1/GSK3 signaling. Biochim. Biophys. Acta, Mol. Cell Res. Nov.2014;1843(11):2481–2491. doi: 10.1016/j.bbamcr.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 128.Olofsson P.S., Rosas-Ballina M., Levine Y.A., Tracey K.J. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol. Rev. Jul.2012;248(1):188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang H., et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. Jan.2003;421(6921):384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 130.Rocha-Resende C., et al. Non-neuronal cholinergic machinery present in cardiomyocytes offsets hypertrophic signals. J. Mol. Cell. Cardiol. Aug.2012;53(2):206–216. doi: 10.1016/j.yjmcc.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roy A., et al. Cardiac acetylcholine inhibits ventricular remodeling and dysfunction under pathologic conditions. FASEB J. Feb.2016;30(2):688–701. doi: 10.1096/fj.15-277046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kakinuma Y., Akiyama T., Sato T. Cholinoceptive and cholinergic properties of cardiomyocytes involving an amplification mechanism for vagal efferent effects in sparsely innervated ventricular myocardium. FEBS J. Sep.2009;276(18):5111–5125. doi: 10.1111/j.1742-4658.2009.07208.x. [DOI] [PubMed] [Google Scholar]
- 133.Rocha J.A., et al. Increase in cholinergic modulation with pyridostigmine induces anti-inflammatory cell recruitment soon after acute myocardial infarction in rats. Am. J. Physiol. Integr. Comp. Physiol. Apr.2016;310(8):R697–R706. doi: 10.1152/ajpregu.00328.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kochiyama T., et al. Effect of propofol on the production of inflammatory cytokines by human polarized macrophages. Mediat. Inflamm. 2019;2019:1919538. doi: 10.1155/2019/1919538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kamada N., Kanaya N., Hirata N., Kimura S., Namiki A. Cardioprotective effects of propofol in isolated ischemia-reperfused Guinea pig hearts: role of KATP channels and GSK-3beta. Can. J. Anaesth. Sep.2008;55(9):595–605. doi: 10.1007/BF03021433. [DOI] [PubMed] [Google Scholar]
- 136.Zeng X.-H., Zeng X.-J., Li Y.-Y. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. Jul.2003;92(2):173–176. doi: 10.1016/s0002-9149(03)00533-2. [DOI] [PubMed] [Google Scholar]
- 137.Liu X., Zhang X., Ye L., Yuan H. Protective mechanisms of berberine against experimental autoimmune myocarditis in a rat model. Biomed. Pharmacother. Apr. 2016;79:222–230. doi: 10.1016/j.biopha.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 138.Zhang Y.-J., et al. Berberine attenuates adverse left ventricular remodeling and cardiac dysfunction after acute myocardial infarction in rats: role of autophagy. Clin. Exp. Pharmacol. Physiol. Dec.2014;41(12):995–1002. doi: 10.1111/1440-1681.12309. [DOI] [PubMed] [Google Scholar]
- 139.Xiong C., et al. Protective effect of berberine on acute cardiomyopathy associated with doxorubicin treatment. Oncol. Lett. Feb. 2018 doi: 10.3892/ol.2018.8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Z., et al. Antioxidant and Anti-Inflammatory Activities of Berberine in the Treatment of Diabetes Mellitus. Evid Based Complement Alternat Med. 2014; 2014: 289264. 2014 Feb.;2014:1–12. doi: 10.1155/2014/289264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kobayashi E.H., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu Y., et al. Berberine inhibits macrophage M1 polarization via AKT1/SOCS1/NF-κB signaling pathway to protect against DSS-induced colitis. Int. Immunopharmacol. Apr.2018;57:121–131. doi: 10.1016/j.intimp.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 143.Cory T.J., He H., Winchester L.C., Kumar S., Fletcher C.V. Alterations in P-glycoprotein expression and function between macrophage subsets. Pharm. Res. 2016;33(11):2713–2721. doi: 10.1007/s11095-016-1998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jing W., et al. Berberine upregulates P-glycoprotein in human Caco-2 cells and in an experimental model of colitis in the rat via activation of Nrf2-dependent mechanisms. J. Pharmacol. Exp. Ther. Aug.2018;366(2):332–340. doi: 10.1124/jpet.118.249615. [DOI] [PubMed] [Google Scholar]
- 145.Lin J., et al. Berberine, a traditional chinese medicine, reduces inflammation in adipose tissue, polarizes M2 macrophages, and increases energy expenditure in mice fed a high-fat diet. Med. Sci. Monit. Jan. 2019;25:87–97. doi: 10.12659/MSM.911849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.MacAulay K., Woodgett J.R. Targeting glycogen synthase kinase-3 (GSK-3) in the treatment of type 2 diabetes. Expert Opin. Ther. Targets. Oct.2008;12(10):1265–1274. doi: 10.1517/14728222.12.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pederson B.A., Chen H., Schroeder J.M., Shou W., DePaoli-Roach A.A., Roach P.J. Abnormal cardiac development in the absence of heart glycogen. Mol. Cell. Biol. Aug.2004;24(16):7179–7187. doi: 10.1128/MCB.24.16.7179-7187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Torres V.E., Harris P.C., Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. Apr.2007;369(9569):1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 149.Tao S., et al. Glycogen synthase kinase-3β promotes cyst expansion in polycystic kidney disease. Kidney Int. Jun.2015;87(6):1164–1175. doi: 10.1038/ki.2014.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen L., et al. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J. Clin. Invest. Jun.2015;125(6):2399–2412. doi: 10.1172/JCI80467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lue H., et al. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. Aug.2007;26(35):5046–5059. doi: 10.1038/sj.onc.1210318. [DOI] [PubMed] [Google Scholar]
- 152.Margaria J.P., Campa C.C., DeSantis M.C., Hirsch E., Franco I. The PI3K/Akt/mTOR pathway in polycystic kidney disease: A complex interaction with polycystins and primary cilium. Cell. Signal. Feb. 2020;66:109468. doi: 10.1016/j.cellsig.2019.109468. [DOI] [PubMed] [Google Scholar]
- 153.Bardaji A., Vea A., Gutierrez C., Ridao C., Richart C., Oliver J. Left ventricular mass and diastolic function in normotensive young adults with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. Dec.1998;32(6):970–975. doi: 10.1016/S0272-6386(98)70071-X. [DOI] [PubMed] [Google Scholar]
- 154.Oflaz H., et al. Biventricular diastolic dysfunction in patients with autosomal-dominant polycystic kidney disease. Kidney Int. Nov.2005;68(5):2244–2249. doi: 10.1111/j.1523-1755.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 155.Chapman A.B., Johnson A.M., Rainguet S., Hossack K., Gabow P., Schrier R.W. Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. Aug.1997;8(8):1292–1297. doi: 10.1681/ASN.V881292. [DOI] [PubMed] [Google Scholar]
- 156.Chapman A.B., Stepniakowski K., Rahbari-Oskoui F. Hypertension in autosomal dominant polycystic kidney disease. Adv. Chronic Kidney Dis. Mar.2010;17(2):153–163. doi: 10.1053/j.ackd.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rossetti S., et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. Jul.2007;18(7):2143–2160. doi: 10.1681/ASN.2006121387. [DOI] [PubMed] [Google Scholar]
- 158.Casuscelli J., et al. Analysis of the cytoplasmic interaction between polycystin-1 and polycystin-2. Am. J. Physiol. Physiol. Nov.2009;297(5):F1310–F1315. doi: 10.1152/ajprenal.00412.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Karihaloo A., et al. Macrophages promote cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. Oct. 2011;22(10):1809–1814. doi: 10.1681/ASN.2011010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tesch G.H., Schwarting A., Kinoshita K., Lan H.Y., Rollins B.J., Kelley V.R. Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J. Clin. Invest. Jan.1999;103(1):73–80. doi: 10.1172/JCI4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.K. I.Swenson-Fields et al., “Macrophages promote polycystic kidney disease progression,” Kidney Int., vol. 83, no. 5, pp. 855–864, May2013, doi: 10.1038/ki.2012.446. [DOI] [PMC free article] [PubMed]
- 162.Ecder T., Schrier R.W. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat. Rev. Nephrol. Apr.2009;5(4):221–228. doi: 10.1038/nrneph.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Perrone R.D., Malek A.M., Watnick T. Vascular complications in autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. Oct.2015;11(10):589–598. doi: 10.1038/nrneph.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pyo R., et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Invest. Jun.2000;105(11):1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thompson R.W., et al. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J. Clin. Invest. Jul.1995;96(1):318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J. Exp. Med. Aug.1975;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kuhn C., Senior R.M. The role of elastases in the development of emphysema. Lung. Dec.1978;155(1):185–197. doi: 10.1007/BF02730693. [DOI] [PubMed] [Google Scholar]
- 168.Raffort J., Lareyre F., Clément M., Hassen-Khodja R., Chinetti G., Mallat Z. Monocytes and macrophages in abdominal aortic aneurysm. Nat. Rev. Cardiol. Aug.2017;14(8):457–471. doi: 10.1038/nrcardio.2017.52. [DOI] [PubMed] [Google Scholar]
- 169.Cheng Z., et al. Diverse roles of macrophage polarization in aortic aneurysm: destruction and repair. J. Transl. Med. Dec. 2018;16(1):354. doi: 10.1186/s12967-018-1731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Volpe E., et al. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat. Immunol. Jun.2008;9(6):650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 171.Yan H., Hu Y., Akk A., Ye K., Bacon J., Pham C.T.N. Interleukin-12 and -23 blockade mitigates elastase-induced abdominal aortic aneurysm. Sci. Rep. Dec. 2019;9(1):10447. doi: 10.1038/s41598-019-46909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Moester M.J.C., Papapoulos S.E., Löwik C.W.G.M., van Bezooijen R.L. Sclerostin: current knowledge and future perspectives. Calcif. Tissue Int. Aug.2010;87(2):99–107. doi: 10.1007/s00223-010-9372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Krishna S.M., et al. Wnt signaling pathway inhibitor sclerostin inhibits angiotensin II–induced aortic aneurysm and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. Mar.2017;37(3):553–566. doi: 10.1161/ATVBAHA.116.308723. [DOI] [PubMed] [Google Scholar]
- 174.Mao C.D., Hoang P., Di Corleto P.E. Lithium inhibits cell cycle progression and induces stabilization of p53 in bovine aortic endothelial cells. J. Biol. Chem. Jul.2001;276(28):26180–26188. doi: 10.1074/jbc.M101188200. [DOI] [PubMed] [Google Scholar]
- 175.Marchand A., et al. The Wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell. Apr.2011;10(2):220–232. doi: 10.1111/j.1474-9726.2010.00661.x. [DOI] [PubMed] [Google Scholar]
- 176.Asadipooya K., Weinstock A. Cardiovascular outcomes of romosozumab and protective role of alendronate. Arterioscler. Thromb. Vasc. Biol. Jul.2019;39(7):1343–1350. doi: 10.1161/ATVBAHA.119.312371. [DOI] [PubMed] [Google Scholar]
- 177.Moeller M.J. Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J. Am. Soc. Nephrol. Jan.2004;15(1):61–67. doi: 10.1097/01.ASN.0000102468.37809.C6. [DOI] [PubMed] [Google Scholar]
- 178.Shkreli M., et al. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat. Med. Jan.2012;18(1):111–119. doi: 10.1038/nm.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hiromura K., et al. Podocyte expression of the CDK-inhibitor p57 during development and disease. Kidney Int. Dec.2001;60(6):2235–2246. doi: 10.1046/j.1523-1755.2001.00057.x. [DOI] [PubMed] [Google Scholar]
- 180.Barisoni L., Mokrzycki M., Sablay L., Nagata M., Yamase H., Mundel P. Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int. Jul.2000;58(1):137–143. doi: 10.1046/j.1523-1755.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- 181.Nitta K., et al. Glomerular expression of cell-cycle-regulatory proteins in human crescentic glomerulonephritis. Virchows Arch. Oct.1999;435(4):422–427. doi: 10.1007/s004280050420. [DOI] [PubMed] [Google Scholar]
- 182.Liang X., et al. Glycogen synthase kinase 3β hyperactivity in urinary exfoliated cells predicts progression of diabetic kidney disease. Kidney Int. Jan.2020;97(1):175–192. doi: 10.1016/j.kint.2019.08.036. [DOI] [PubMed] [Google Scholar]
- 183.Eckardt K.-U., et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. Jul.2013;382(9887):158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 184.Tonelli M., et al. Chronic kidney disease and mortality risk: a systematic review. J. Am. Soc. Nephrol. Jul.2006;17(7):2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 185.Henique C., et al. Genetic and pharmacological inhibition of microRNA-92a maintains podocyte cell cycle quiescence and limits crescentic glomerulonephritis. Nat. Commun. Dec. 2017;8(1):1829. doi: 10.1038/s41467-017-01885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang H., et al. MicroRNA-92 promotes invasion and chemoresistance by targeting GSK3β and activating Wnt signaling in bladder cancer cells. Tumor Biol. Dec.2016;37(12):16295–16304. doi: 10.1007/s13277-016-5460-9. [DOI] [PubMed] [Google Scholar]
- 187.Zhang G.-J., et al. MiR-92a promotes stem cell-like properties by activating Wnt/B2-catenin signaling in colorectal cancer. Oncotarget. Nov. 2017;8(60) doi: 10.18632/oncotarget.21667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hurcombe J.A., et al. Podocyte GSK3 is an evolutionarily conserved critical regulator of kidney function. Nat. Commun. Dec. 2019;10(1):403. doi: 10.1038/s41467-018-08235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang Z., Bao H., Ge Y., Zhuang S., Peng A., Gong R. Pharmacological targeting of GSK3β confers protection against podocytopathy and proteinuria by desensitizing mitochondrial permeability transition. Br. J. Pharmacol. Feb.2015;172(3):895–909. doi: 10.1111/bph.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kaidanovich-Beilin O., Woodgett J.R. GSK-3: functional insights from cell biology and animal models. Front. Mol. Neurosci. 2011;4 doi: 10.3389/fnmol.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Aiff H., et al. Effects of 10 to 30 years of lithium treatment on kidney function. J. Psychopharmacol. May 2015;29(5):608–614. doi: 10.1177/0269881115573808. [DOI] [PubMed] [Google Scholar]
- 192.Bendz H., Schön S., Attman P.-O., Aurell M. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. Feb.2010;77(3):219–224. doi: 10.1038/ki.2009.433. [DOI] [PubMed] [Google Scholar]
- 193.Christensen B.M., Kim Y.-H., Kwon T.-H., Nielsen S. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am. J. Physiol. Physiol. Jul.2006;291(1):F39–F48. doi: 10.1152/ajprenal.00383.2005. [DOI] [PubMed] [Google Scholar]
- 194.Cassini M.F., et al. Mcp1 promotes macrophage-dependent cyst expansion in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. Oct.2018;29(10):2471–2481. doi: 10.1681/ASN.2018050518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lin C.-L., Wang J.-Y., Huang Y.-T., Kuo Y.-H., Surendran K., Wang F.-S. Wnt/β-catenin signaling modulates survival of high glucose–stressed mesangial cells. J. Am. Soc. Nephrol. Oct.2006;17(10):2812–2820. doi: 10.1681/ASN.2005121355. [DOI] [PubMed] [Google Scholar]
- 196.Bao H., Ge Y., Peng A., Gong R. Fine-tuning of NFκB by glycogen synthase kinase 3β directs the fate of glomerular podocytes upon injury. Kidney Int. Jun.2015;87(6):1176–1190. doi: 10.1038/ki.2014.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.C.Li, Y.Ge, L.Dworkin, A.Peng, and R. Gong, “The β isoform of GSK3 mediates podocyte autonomous injury in proteinuric glomerulopathy,” J. Pathol., vol. 239, no. 1, pp. 23–35, May2016, doi: 10.1002/path.4692. [DOI] [PMC free article] [PubMed]
- 198.Bonon A., Mangolini A., Pinton P., del Senno L., Aguiari G. Berberine slows cell growth in autosomal dominant polycystic kidney disease cells. Biochem. Biophys. Res. Commun. Nov.2013;441(3):668–674. doi: 10.1016/j.bbrc.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 199.Quan K., et al. Berberine attenuates macrophages infiltration in intracranial aneurysms potentially through FAK/Grp78/UPR axis. Front. Pharmacol. May 2018;9 doi: 10.3389/fphar.2018.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gao M., Chen L., Yang L., Yu X., Kou J., Yu B. Berberine inhibits LPS-induced TF procoagulant activity and expression through NF-κB/p65, Akt and MAPK pathway in THP-1 cells. Pharmacol. Rep. Jun.2014;66(3):480–484. doi: 10.1016/j.pharep.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 201.Cheng W.-E., Ying Chang M., Wei J.-Y., Chen Y.-J., Maa M.-C., Leu T.-H. Berberine reduces Toll-like receptor-mediated macrophage migration by suppression of Src enhancement. Eur. J. Pharmacol. Jun.2015;757:1–10. doi: 10.1016/j.ejphar.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 202.Gao C., Chen G., Kuan S.-F., Zhang D.H., Schlaepfer D.D., Hu J. FAK/PYK2 promotes the Wnt/β-catenin pathway and intestinal tumorigenesis by phosphorylating GSK3β. Elife. Sep. 2015;4 doi: 10.7554/eLife.10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hughes K., Nikolakaki E., Plyte S.E., Totty N.F., Woodgett J.R. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. Feb.1993;12(2):803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tang J., et al. CD147 induces UPR to inhibit apoptosis and chemosensitivity by increasing the transcription of Bip in hepatocellular carcinoma. Cell Death Differ. Nov.2012;19(11):1779–1790. doi: 10.1038/cdd.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]