Abstract
Interferons (IFNs) are widely used in treating coronavirus disease 2019 (COVID-19) patients. However, a recent report of ACE2, the host factor mediating SARS-Cov-2 infection, identifying it as interferon-stimulated raised considerable safety concern. To examine the association between the use and timing of IFN-α2b and clinical outcomes, we analyzed in a retrospective multicenter cohort study of 446 COVID-19 patients in Hubei, China. Regression models estimated that early administration (≤5 days after admission) of IFN-α2b was associated with reduced in-hospital mortality in comparison with no admission of IFN-α2b, whereas late administration of IFN-α2b was associated with increased mortality. Among survivors, early IFN-α2b was not associated with hospital discharge or computed tomography (CT) scan improvement, whereas late IFN-α2b was associated with delayed recovery. Additionally, early IFN-α2b and umifenovir alone or together were associated with reduced mortality and accelerated recovery in comparison with treatment with lopinavir/ritonavir (LPV/r) alone. We concluded that administration of IFN-α2b during the early stage of COVID-19 could induce favorable clinical responses.
Keywords: infectious disease, viral infection, anti-viral immunity, RNA virus, respiratory medicine, anti-retroviral agents, cytokine storm syndrome
Graphical Abstract

Highlights
-
•
242 of 446 analyzed COVID-19 patients received IFN-α2b, a type I IFN
-
•
Early initiation of IFN therapy was associated with reduced mortality
-
•
IFN therapy was not associated with recovery time for COVID-19
-
•
IFN-α2b was associated with better responses than were lopinavir/ritonavir
In a retrospective cohort study of 446 COVID-19 patients, Wang et al. determine that early administration of interferon-α2b was associated with reduced in-hospital mortality. In contrast, late interferon therapy increased mortality and delayed recovery, suggesting the timing of interferon therapy is crucial for favorable responses in COVID-19 patients.
Introduction
The ongoing pandemic of novel coronavirus disease 2019 (COVID-19) has resulted in the death of more than 383,000 persons worldwide as of June 4, 2020. There are currently no proven effective therapies for COVID-19 (Li and De Clercq, 2020; Sanders et al., 2020). Instead, a number of anti-viral medications are repurposed to treat COVID-19 patients based on their in vitro effectiveness against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes COVID-19 and other coronavirus strains (Mantlo et al., 2020; Wang et al., 2020).
Interferons (IFNs) have shown clinical efficacy in treating various viral infections and are widely repurposed to treat COVID-19 patients (Hung et al., 2020; Xie et al., 2020; Zuo et al., 2020). Mechanistically, severe COVID-19 patients exhibit IFN insufficiency similar with those seen in SARS-CoV infections, which could potentially be the immune evading mechanism of SARS-CoV-2 (Blanco-Melo et al., 2020; Channappanavar et al., 2016). Hence, it is speculated that early administration of IFNs could prevent rapid viral spreading and subsequent cytokine storm that causes the most damage (McKechnie and Blish, 2020; Nile et al., 2020; Park and Iwasaki, 2020). However, angiotensin-converting enzyme 2 (ACE2), the docking protein for SARS-CoV-2 (Zhou et al., 2020a), was recently demonstrated to be an interferon-stimulated gene (ISG) upregulated by IFN-α (Ziegler et al., 2020). Type I IFNs thus could pose potential risk for enhancing SARS-CoV-2 entry to ACE2-expressing target cells and delaying viral clearance (Acharya et al., 2020; Su and Jiang, 2020). Despite these vital experimental findings, current clinical evidence of the efficacy and safety profile of IFN-α in treating COVID-19 patients is scarce (Zhou et al., 2020b; Zuo et al., 2020).
Although randomized controlled trials are the optimal study design to evaluate the clinical efficacy of IFNs in COVID-19 patients, the urgent need to clarify the role of IFNs, especially type I IFNs, in SARS-CoV-2 infection warranted an observational study to evaluate the association between IFN-based therapies and clinical outcomes of COVID-19 patients. This retrospective multicenter cohort study analyzed 446 admitted patients with confirmed COVID-19 diagnosis who underwent anti-viral therapies in two regional medical centers of adjacent cities in Hubei, China. With input from first-line physicians, the aim of this study was to evaluate the association of the use of IFN-α2b with COVID-19 disease progress and uncover potential risk of IFN therapy for COVID-19 patients.
Results
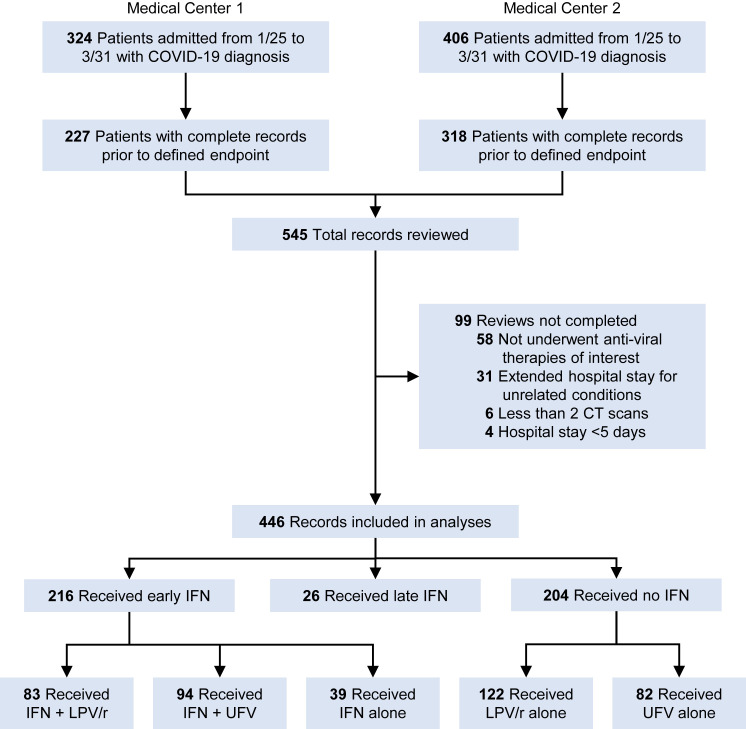
From the two participating medical centers, a total of 730 patients were admitted and diagnosed with COVID-19 during January 15 through March 31, representing 29.4% of the total confirmed COVID-19 cases in these two cities. Among them, a significant part of the diagnosis or treatment history was missing in 185 patient records, most of which were mild patients deemed to not require in-hospital care and those transferred to isolation stations before recovery. After reviewing the 545 complete records, a further 99 records were excluded due to not receiving any anti-viral therapies evaluated in this study, extended hospital stays for COVID-19-unrelated conditions, less than 2 computed tomography (CT) scans available for comparison, or hospital stays less than 5 days. The remaining 446 records were abstracted and included in the analyses (Figure 1 ). The date of final follow-up was May 22, 2020, and all patients included in this study were discharged or deceased prior to this date.
Figure 1.
Sampling Strategy of COVID-19 Patient Records
Among the analyzed patients, the age range was 8 to 96 with a median of 50, and 47.1% of the patients were female. For all patients, the median length from symptom onset to admission was 6 days, and the median length of hospital stay was 19 days. For survivors, the median length from admission to CT scan improvement is 10 days, and the median length from disease onset to hospital discharge was 26 days. Because patients were re-tested for SARS-CoV-2 nucleic acids only when symptoms and CT scans were improving, the length from admission to negative nucleic acids test, which represents viral shedding period, could not be precisely determined in this study. The most common preexisting confounders were hypertension (21.1%) and diabetes (7.4%). High-risk exposures to SARS-CoV-2 were reported in 62.6% of analyzed patients. Top symptoms at admission were fever (82.7%), cough (67.7%), fatigue, body or muscle aches (20.2%), and chest pain or shortness of breath (18.4%). Headache (5.2%) and diarrhea (2.5%) were not as relevant in this sample of patients. Laboratory tests showed 61.3% of eosinopenia, 46.2% of lymphopenia, and 36.7% of elevated lactic acid dehydrogenase (LDH) within 24 h of admission. During hospitalization, 6.1% patients required mechanical ventilation, and 1.1% were given extracorporeal membrane oxygenation (ECMO) treatment.
During January to March 2020, the Chinese Center for Disease Prevention and Control (CDC) published and kept updating a national guideline for treatment of COVID-19 patients (“Chinese management guideline for COVID-19 version 7,” 2020), which included antiviral agents such as IFNs, lopinavir/ritonavir (LPV/r), and umifenovir (UFV). During the pandemic, each hospital developed their own preferred drug regime based on both clinical impression and drug availability. The preferred regime was given to patients on a first-come first-serve basis before resorting to alternative drugs. Demographic characteristics and clinical features within 24 h of admission were comparable between Centers 1 and 2, except that Center 1 has higher prevalence of high-risk exposure, lower prevalence of eosinopenia at admission and less symptom counts based on fever, cough, fatigue, body or muscle aches, chest pain or shortness of breath, headache, and diarrhea reported at admission (Table S1). Among patients admitted to Center 1, only 25% received IFN-α2b, whereas 72.2% received LPV/r. In contrast, among patients admitted to Center 2, 73% received IFN-α2b and 36.3% received LPV/r. To assess the standard care at the two medical centers, we compared the cumulative event curves for hospital discharge and CT scan improvement. Among all survivors, patients admitted to Center 1 had longer hospitalization (Log-rank test, p < 0.001) (Figure S1A). However, after excluding patients that received LPV/r, which tended to prolong hospitalization due to various adverse effects, both centers showed comparable rate of hospital discharge (Log-rank test, p = 0.685) and CT scan improvement (Log-rank test, p = 0.749) (Figures S1B and S1C). Analysis of 58 patients that received no anti-viral therapy also showed no variation of hospital discharge (Log-rank test, p = 0.678) and CT scan improvement (Log-rank test, p = 0.561) between both centers (Figures S1D and S1E). The two cities where these two medical centers are located in are adjacent with approximately 50 miles of distance in between them. The population density, ethnic background, economy, job composition, and air pollution levels are comparable between the two cities. Together, we conclude that location of care was not associated with clinical outcomes in this study.
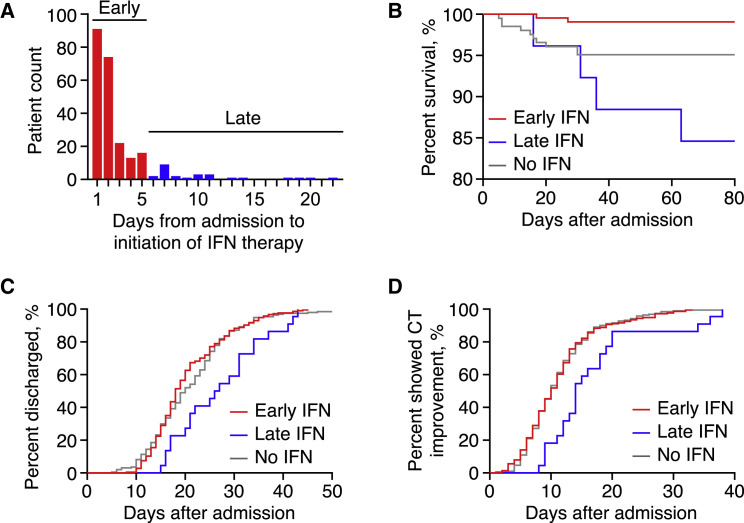
Because the timing of IFN therapy could be crucial to its efficacy against SARS-CoV-2 infection, we calculated the time from admission to initiation of IFN therapy and found that 68.2% of IFN users received the first dose within the first 2 days of hospitalization, and 89.3% received the first dose within the first 5 days. Together with the fact that the first CT re-evaluation and adjustment of treatment plans would also be carried out around the fifth day of hospitalization, we empirically determined that initiation of IFN therapy within the first 5 days of hospitalization was deemed early IFN therapy, and vice versa (Figure 2 A). By this criterion, the combined cohort from both medical centers was stratified to three groups: 216 (48.4%) received early IFN therapy, 26 (5.8%) received late IFN therapy, and 204 (45.7%) received no IFN therapy. No patient received IFN-α2b therapy prior to admission.
Figure 2.
Early IFN Therapy Associated with Reduced In-Hospital Mortality but not with Early Recovery of COVID-19
(A) Bar graph depicting initiation timing of IFN therapy among analyzed patients. Initiation of IFN therapy within first 5 days of hospitalization was empirically deemed early.
(B) Kaplan-Meier curve of in-hospital mortality in patients stratified by IFN therapy status (Log-rank test of all curves, p < 0.001).
(C and D) Cumulative event curves of hospital discharge (Log-rank test of all curves, p = 0.018) (C) and CT scan improvement (Log-rank test of all curves, p = 0.003) (D).
Demographic characteristics were not significantly different between early, late, and no IFN groups except location of care, and fewer female patients received early or late IFN (Table 1 ). Regarding clinical features at admission, the early and no IFN groups had comparable prevalence of high respiratory rate and low O2 saturation within 24 h of admission, whereas the late IFN group had higher prevalence than both early and no IFN groups. More symptoms were reported in the early IFN group, with 19.9% that reported 3 or more symptoms at admission, in comparison with 12.8% and 0% in the no and late IFN groups, respectively. The no IFN group had the lowest prevalence of lymphopenia and eosinopenia, whereas the late IFN group had the highest at admission. In general, patients in the late IFN group exhibited higher disease severity at admission, whereas severity in early and no IFN groups were comparable. For treatments, the median time from admission to first IFN dose was 2 and 8.5 days in the early and late IFN groups, respectively. Median duration of IFN therapy was 10 and 8.5 days in the early and late IFN groups, respectively. There was 60%–90% prevalence of the use of antibiotics and Lianhua Qingwen capsule, an herb-based medication with potential anti-viral efficacy, among all groups. For overall evaluation of hospitalization, patients in the late IFN group had significantly longer hospital stays, delayed CT scan improvement, and longer disease course from symptom onset to hospital discharge. The late IFN group also had more severe and critical patients retrospectively determined according to the Chinese CDC guideline, partly because they were more likely to initiate late IFN therapy after failing the first non-IFN regime, whereas disease severity in the early and no IFN groups was comparable. The no IFN group had less prevalence (80.9%) of positive SARS-CoV-2 nucleic acids tests than did other two groups (94.9% and 92.3%) despite having 97.5% of abnormal CT findings at admission. The prevalence of viral shedding after showing CT scan improvement was comparable between all 3 groups.
Table 1.
Patient Characteristics by IFN Administration
| No./Total No. (%) |
p Value∗ | |||
|---|---|---|---|---|
| Early IFN (n = 216) | No IFN(n = 204) | Late IFN (n = 26) | ||
| Demographic Characteristics | ||||
| Female sex | 93 (43.1) | 109 (53.4) | 8 (30.8) | .024 |
| Age, years, median (IQR) | 50 (39–57) | 49 (35–57) | 51.5 (45–63) | .560 |
| >60 | 39 (18.1) | 49 (24) | 6 (23.1) | .315 |
| Hypertension | 46 (21.3) | 40 (19.6) | 8 (30.8) | .419 |
| Diabetes | 16 (7.4) | 16 (7.8) | 1 (3.8) | .764 |
| High-risk exposure | 133 (61.6) | 126 (61.8) | 20 (76.9) | .296 |
| Admitted to Center 1 | 34 (15.7) | 132 (64.7) | 10 (38.5) | <.001 |
| Clinical FeaturesWithin 24 h of Admission | ||||
| Abnormal CT findings | 215 (99.5) | 199 (97.5) | 26 (100) | .174 |
| Respiratory rate >22/min | 35 (16.2) | 29 (14.2) | 7 (26.9) | .246 |
| O2 saturation, % | ||||
| <90 | 9 (4.2) | 14 (6.9) | 4 (15.4) | .108 |
| 90–93 | 41 (19) | 35 (17.2) | 7 (26.9) | |
| >93 | 166 (76.9) | 155 (76) | 15 (57.7) | |
| Symptom Count | ||||
| 0 | 2 (0.9) | 9 (4.4) | 0 (0) | .006 |
| 1 | 51 (23.6) | 71 (34.8) | 7 (26.9) | |
| 2 | 120 (55.6) | 98 (48) | 19 (73.1) | |
| 3 | 37 (17.1) | 23 (11.3) | 0 (0) | |
| 4 or more | 6 (2.8) | 3 (1.5) | 0 (0) | |
| Lymphopenia (<1.1 × 109/L) | 91/182 (50) | 57/153 (37.3) | 16/20 (80) | .001 |
| Eosinopenia (<0.02 × 109/L) | 125/179 (69.8) | 69/145 (47.6) | 17/20 (85) | <.001 |
| Treatments | ||||
| Time from admission to first IFN dose, days, median (IQR) | 2 (1–2) | N/A | 8.5 (7–11) | <.001 |
| Duration of IFN therapy, days, median (IQR) | 10 (7.5–13.5) | N/A | 8.5 (5–11) | .061 |
| Antibiotics | 183 (84.7) | 161 (78.9) | 25 (96.2) | .051 |
| Lianhua Qingwen capsule | 165 (76.4) | 132 (64.7) | 20 (76.9) | .024 |
| Overall | ||||
| Length of hospital stay, days, median (IQR) | 18 (15–25) | 19.5 (15–26) | 28 (20–34) | .001 |
| Time from admission to CT scan improvement, days, median (IQR) [no.] | 10 (7–13) [214] | 10 (7–14) [191] | 14 (12–19) [22] | .001 |
| Symptom onset to hospital admission >7 days | 63 (29.2) | 51 (25) | 9 (34.6) | .450 |
| Time from symptom onset to hospital discharge, days, median (IQR) [no.] | 25 (21–32) [209] | 26 (21–34) [193] | 32.5 (25–38) [22] | .025 |
| Severity Category | ||||
| Mild or asymptomatic | 1 (0.5) | 3 (1.5) | 0 (0) | .003 |
| Moderate | 159 (74) | 161 (80.1) | 11 (42.3) | |
| Severe | 33 (15.3) | 20 (10) | 8 (30.8) | |
| Critical | 23 (10.7) | 20 (10) | 7 (26.9) | |
| SARS-CoV-2 NA-positive | 205 (94.9) | 165 (80.9) | 24 (92.3) | <.001 |
| Viral shedding after CT scan improvement | 32/213 (15) | 24/195 (12.3) | 2/24 (8.3) | .545 |
| Outcome | ||||
| Recovered | 214 (99.1) | 194 (95.1) | 22 (84.6) | <.001 |
| Deceased | 2 (0.9) | 10 (4.9) | 4 (15.4) | |
Abbreviations: IFN, interferon α-2b; IQR, inter quartile range; NA, nucleic acids.
∗p values were calculated by Pearson chi-Square tests for categorical variables or Kruskal-Wallis 1-way ANOVA for continuous variables across all 3 groups.
Primary Outcome
In-hospital mortality was 3.6% among all patients and 14.4% among severe to critical patients (n = 111). No death was recorded in mild or moderate patients. By comparing Kaplan-Meier curves, significant difference in mortality was observed across the early (0.9%), late (15.4%), and no IFN (4.9%) groups (Log-rank test, p < 0.001) (Figure 2B). By using logistic regression, we determined that early IFN therapy was univariably associated with lower mortality (odds ratio [OR] = 0.18, p = 0.029), whereas late IFN therapy (OR = 3.53, p = 0.046), age >60 years (OR = 6.87, p < 0.001), hypertension (OR = 6.87, p < 0.001), diabetes (OR = 8.96, p < 0.001), respiratory rate >22/min at admission (OR = 10.1, p < 0.001), O2 saturation between 90%–93% (OR = 11.8, p < 0.001), or <93% (OR = 25.2, p < 0.001) were univariably associated with higher mortality (Figure S2).
In the primary analysis, both logistic regression and Cox proportional hazards models were tested and fitted to all patients. Factors associated with mortality were included in the model with the following exceptions. Gender and symptom count were included because of significant variation of prevalence between groups and potential association with mortality (female OR = 0.36, p = 0.083). High respiratory rate was excluded due to overlapping with low O2 saturation both statistically (Spearman’s ρ = 0.536, p < 0.001) and clinically. Symptom onset to admission >7 days was included as an indicator of pre-admission disease severity because severe cases typically were hospitalized within 7 days after symptom onset. After adjusting for gender, age, hypertension, diabetes, O2 saturation at admission, symptom count at admission, and symptom onset to admission >7 days, early IFN therapy was estimated to have an adjusted OR of 0.05 (95% confidence interval [CI], 0.01-0.37) and an adjusted hazard ratio (HR) of 0.10 (95% CI, 0.02-0.50) for in-hospital mortality in comparison with no IFN therapy, whereas late IFN therapy was estimated to have an adjusted OR of 6.82 (95% CI, 1.14–40.8) and an adjusted HR of 2.30 (95% CI, 0.64–8.27) for in-hospital mortality in comparison with no IFN therapy (Table 2 ). Proportional hazard assumption was not met during comparison of late IFN and no IFN groups.
Table 2.
Model-Adjusted Risks of In-Hospital Death, Hospital Discharge, and CT scan Improvement
| Outcome | Analyzed Categories | Model Type | Estimate (95% CI) |
|
|---|---|---|---|---|
| Early IFN versus No IFN | Late IFN versus No IFN | |||
| In-hospital mortality(odds ratio) | all | logistic regressiona | 0.05 (0.01–0.37)p = 0.004 | 6.82 (1.14–40.8)p = 0.035 |
| In-hospital mortality(hazard ratio) | all | Cox proportional hazardsb | 0.10 (0.02–0.50)p = 0.005 | 2.30 (0.64–8.27)cp = 0.203 |
| Hospital discharge (hazard ratio) | survivors | Cox proportional hazardsa | 1.14 (0.93–1.41)cp = 0.213 | 0.69 (0.44–1.08)p = 0.101 |
| CT scan improvement(hazard ratio) | survivors | Cox proportional hazardsa | 1.00 (0.81–1.22)cp = 0.975 | 0.50 (0.32–0.80)p = 0.004 |
Abbreviation: IFN, interferon α-2b.
Model adjusted for gender, age, hypertension, diabetes, oxygen saturation at admission, symptom onset to admission >7 days, plus symptom count at admission.
Model adjusted for gender, age, hypertension, diabetes, oxygen saturation at admission, and symptom onset to admission >7 days.
Proportional hazard assumption not met.
Secondary Outcomes
The length of hospital stays of patients who survived COVID-19 was significantly different between all 3 groups (Kruskal-Wallis test, p = 0.001). There is no significant difference between cumulative event curves of the early IFN and no IFN groups (Log-rank test, p = 0.335) for hospital discharge, whereas the late IFN group exhibited longer hospitalization than the no IFN group (crude HR[95%CI], 1.65[1.15–2.38]; Log-rank test, p = 0.016) (Figure 2C).
Among 430 survivors, all had CT scan improvement before hospital discharge, including those 6 admitted after positive SARS-CoV-2 nucleic acids tests with no abnormal CT findings at admission. There is no significant difference between cumulative event curves of the early IFN and no IFN groups (Log-rank test, p = 0.970) for CT scan improvement, whereas the late IFN group showed delayed CT scan improvement than the no IFN group (crude HR[95%CI], 0.53[0.37–0.75]; Log-rank test, p = 0.002) (Figure 2D).
By using generalized gamma with log link model, we determined that late IFN therapy, age >60 years, hypertension, respiratory rate >22/min at admission, O2 saturation between 90%–93% or <93%, lymphopenia, and eosinopenia were univariably associated with both longer hospitalization and delayed CT scan improvement, whereas diabetes and symptom onset to admission >7 days were univariably associated with delayed and early CT scan improvement, respectively (Figures S3B and S3C). After adjusting for gender, age, hypertension, diabetes, O2 saturation at admission, symptom count at admission, and symptom onset to admission >7 days, early IFN therapy was estimated to have adjusted HR of 1.14 (95% CI, 0.93–1.41) for hospital discharge and 1.00 (95% CI, 0.81–1.22) for CT scan improvement in comparison with no IFN therapy, whereas late IFN therapy was estimated to have adjusted HR of 0.69 (95% CI, 0.44–1.08) for hospital discharge and 0.50 (95% CI, 0.32–0.80) for CT scan improvement in comparison with no IFN therapy (Table 2).
Drug Combinations
Because IFNs are regularly used in conjunction with anti-retroviral agents to treat COVID-19 patients, we were interested in finding the combination associated with favorable clinical responses. After excluding patients that received late IFN therapy due to their complex treatment records, 83 patients were treated with the widely used IFN + LPV/r regime upon admission, and 94 were given the IFN + UFV regime instead. Among combined therapy users, 11 and 4 received LPV/r and UFV on the sixth day of admission or later, respectively. Other patients were put on single agent therapies (Table 3 ). Demographic characteristics were comparable across 5 groups, except the IFN + LPV/r group with significantly less females than other groups, and more elders among LPV/r users. Clinical features at admission were comparable between all groups except the UFV alone group with lower prevalence of eosinopenia. Overall, the IFN + LPV/r, IFN + UFV, and LPV/r alone groups shared similar patient characteristics, clinical features, and sample size.
Table 3.
Patient Characteristics by Treatments
| No./Total No. (%) |
p Value∗ | |||||
|---|---|---|---|---|---|---|
| IFN + LPV/r(n = 83) | LPV/r Alone(n = 122) | IFN + UFV(n = 94) | IFN Alone(n = 39) | UFV Alone(n = 82) | ||
| Demographic Characteristics | ||||||
| Female sex | 28 (33.7) | 66 (54.1) | 42 (44.7) | 23 (59) | 43 (52.4) | .021 |
| Age, years, median (IQR) | 53 (41.5–62) | 50 (39–61) | 48.5 (35–54) | 52 (41–56) | 48.5 (32–57) | .099 |
| >60 | 23 (27.7) | 34 (27.9) | 10 (10.6) | 6 (15.4) | 15 (18.3) | .012 |
| Hypertension | 19 (22.9) | 24 (19.7) | 21 (22.3) | 6 (15.4) | 16 (19.5) | .876 |
| Diabetes | 9 (10.8) | 13 (10.7) | 6 (6.4) | 1 (2.6) | 3 (3.7) | .180 |
| High-risk exposure | 46 (55.4) | 69 (56.6) | 64 (68.1) | 23 (59) | 57 (69.5) | .158 |
| Admitted to Center 1 | 24 (28.9) | 93 (76.2) | 6 (6.4) | 4 (10.3) | 39 (47.6) | <.001 |
| Clinical Features Within 24 h of Admission | ||||||
| Abnormal CT findings | 82 (98.8) | 118 (96.7) | 94 (100) | 39 (100) | 81 (98.8) | .293 |
| Respiratory rate >22/min | 16 (19.3) | 19 (15.6) | 12 (12.8) | 7 (17.9) | 10 (12.2) | .678 |
| O2 Saturation, % | ||||||
| <90 | 4 (4.8) | 9 (7.4) | 3 (3.2) | 2 (5.1) | 5 (6.1) | .638 |
| 90-93 | 15 (18.1) | 25 (20.5) | 16 (17) | 10(25.6) | 10 (12.2) | |
| >93 | 64 (77.1) | 88 (72.1) | 75 (79.8) | 27 (69.2) | 67 (81.7) | |
| Symptom Count | ||||||
| 0 | 1 (1.2) | 5 (4.1) | 1 (1.1) | 0 (0) | 4 (4.9) | .075 |
| 1 | 21 (25.3) | 39 (32) | 19 (20.2) | 11 (28.2) | 32 (39) | |
| 2 | 42 (50.6) | 62 (50.8) | 57 (60.6) | 21 (53.8) | 36 (43.9) | |
| 3 | 14 (16.9) | 15 (12.3) | 16 (17) | 7 (17.9) | 8 (9.8) | |
| 4 or more | 5 (6) | 1 (0.8) | 1 (1.1) | 0 (0) | 2 (2.4) | |
| Lymphopenia (<1.1 × 109/L) | 37/65 (56.9) | 34/82 (41.5) | 37/83 (44.6) | 17/34 (50) | 23/71 (32.4) | .061 |
| Eosinopenia (<0.02 × 109/L) | 44/64 (68.8) | 46/78 (59) | 57/82 (69.5) | 24/33 (72.7) | 23/67 (34.3) | <.001 |
| Treatment | ||||||
| Time from admission to first IFN dose, days, median (IQR) | 2 (1–3) | N/A | 1 (1–2) | 2 (1–2) | N/A | <.001 |
| Duration of IFN therapy, days, median (IQR) | 10 (7.5–14) | N/A | 10 (7–12) | 11 (9–13.5) | N/A | .259 |
| Antibiotics | 73 (88) | 95 (77.9) | 80 (85.1) | 30 (76.9) | 66 (80.5) | .308 |
| Lianhua Qingwen capsule | 65 (78.3) | 81 (66.4) | 76 (80.9) | 24 (61.5) | 51 (62.2) | .014 |
| Overall | ||||||
| Length of hospital stay, days, median (IQR) | 21 (18–28) | 23 (17–28) | 17 (13–21) | 16 (14–20.5) | 16.5 (13–22) | <.001 |
| Time from admission to CT scan improvement, days, median (IQR) [no.] | 11 (8–14) [82] | 11 (8–16) [111] | 9 (7–13) [93] | 10 (7–13.5) [39] | 8 (6–12) [80] | <.001 |
| Symptom onset to hospital admission >7 days | 19 (22.9) | 28 (23) | 35 (37.2) | 9 (23.1) | 23 (28) | .134 |
| Time from symptom onset to hospital discharge, days, median (IQR) [no.] | 28 (24–35) [79] | 29 (23–36) [113] | 24 (20–29) [91] | 23 (20–27) [39] | 24 (19–29) [80] | <.001 |
| Severity Category | ||||||
| Mild or asymptomatic | 1 (1.2) | 2 (1.6) | 0 (0) | 0 (0) | 1 (1.2) | .086 |
| Moderate | 58 (69.8) | 89 (73) | 72 (76.6) | 29 (74.4) | 72 (87.8) | |
| Severe | 12 (14.5) | 14 (11.5) | 13 (13.8) | 8 (20.5) | 6 (7.3) | |
| Critical | 12 (14.5) | 17 (13.9) | 9 (9.6) | 2 (5.1) | 3 (3.7) | |
| SARS-CoV-2 NA-positive | 83 (100) | 115 (94.3) | 89 (94.7) | 33 (84.6) | 50 (61) | <.001 |
| Viral shedding after CT scan improvement | 12 (14.6) | 20 (17.7) | 14 (15.2) | 6 (15.4) | 4 (4.9) | .123 |
| Outcome | ||||||
| Recovered | 82 (98.8) | 113 (92.6) | 93 (98.9) | 39 (100) | 81 (98.8) | .012 |
| Deceased | 1 (1.2) | 9 (7.4) | 1 (1.1) | 0 (0) | 1 (1.2) | |
Abbreviations: IFN, interferon α-2b; LPV/r, lopinavir and ritonavir; UFV, umifenovir; IQR, inter quartile range; NA, nucleic acids
∗p values were calculated by Pearson chi-Square tests for categorical variables or Kruskal-Wallis 1-way ANOVA for continuous variables across all 5 groups.
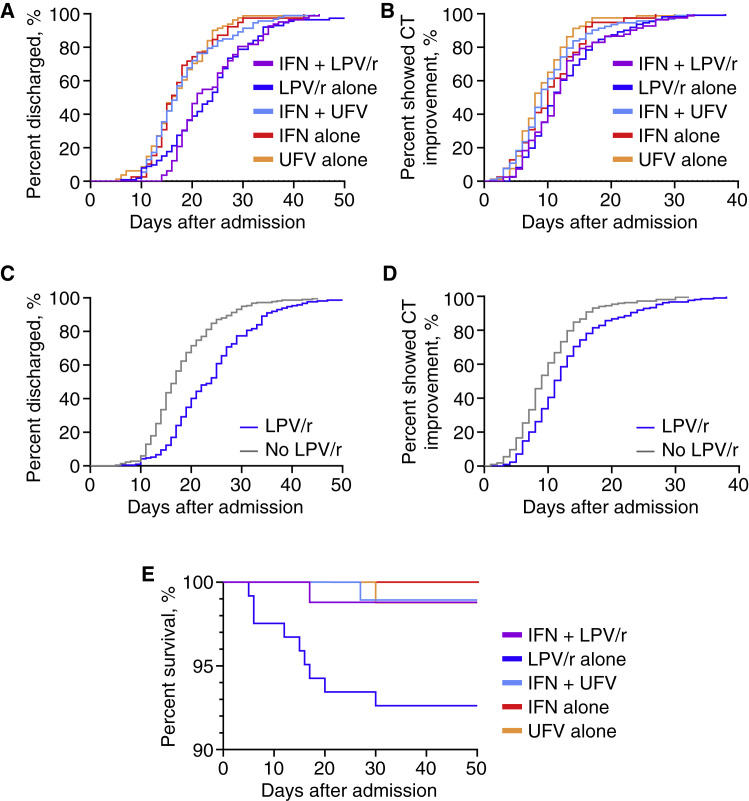
Cumulative event curves of survivors excluding those which received late IFN showed prolonged hospitalization of both the IFN + LPV/r and LPV/r alone groups in comparison with other non-LPV/r groups (Log-rank test for all curves, p < 0.001) (Figure 3 A). CT scan improvement in the IFN + LPV/r and LPV/r alone groups were also delayed to a lesser extent (Log-rank test for all curves, p < 0.001) (Figure 3B). Combining IFN with LPV/r or UFV was not associated with variations in hospital discharge or CT scan improvement in comparison with LPV/r or UFV alone (Log-rank tests, p > 0.100) (Figures 3A and 3B). After adjusting for gender, age, hypertension, diabetes, O2 saturation at admission, symptom count at admission, and symptom onset to admission >7 days, Cox proportional hazards model estimated that IFN alone, UFV alone, and IFN + UFV were associated with early hospital discharge in comparison with IFN + LPV/r, whereas UFV alone was associated with early CT scan improvement in comparison with IFN + LPV/r (Table 4 ). LPV/r alone exhibited no significant difference with IFN + LPV/r in hospital discharge and CT scan improvement, and proportional hazard assumption was not met in this pair of comparison. It is worth noting that LPV/r was associated with delayed hospital discharge (crude HR 0.51 [95% CI, 0.42–0.62]; Cox model adjusted HR 0.48 [95% CI, 0.3–-0.60]) and CT scan improvement (crude HR 0.66 [95% CI, 0.55–0.81]; Cox model adjusted HR 0.70 [95% CI, 0.57–0.86]) in comparison with no LPV/r (Figures 3C and 3D).
Figure 3.
IFN-Based Therapies Associated with More Favorable Clinical Response than LPV/r in COVID-19
(A and B) Cumulative event curves of hospital discharge (Log-rank test of all curves, p < 0.001) (A) and CT scan improvement (Log-rank test of all curves, p < 0.001) (B) in survivors, excluding those that received late IFN therapy stratified by treatments.
(C and D) Cumulative event curves of hospital discharge (Log-rank test, p < 0.001) (C) and CT scan improvement (Log-rank test, p < 0.001) (D) in survivors, excluding those that received late IFN therapy stratified by LPV/r use.
(E) Kaplan-Meier curves of in-hospital mortality in all patients, excluding those that received late IFN therapy stratified by treatments (Log-rank test of all curves, p = 0.011).
Table 4.
Model-Adjusted Risk of Hospital Discharge and CT scan Improvement
| Outcome | Analyzed Categories | Model Type | Estimate (95% CI) |
|||
|---|---|---|---|---|---|---|
| LPV/r Alone versusIFN + LPV/r | IFN Alone versusIFN + LPV/r | IFN + UFV versusIFN + LPV/r | UFV Alone versusIFN + LPV/r | |||
| Hospital discharge (hazard ratio) | survivors except late IFN | Cox proportional hazardsa | 0.89 (0.66–1.21)bp = 0.462 | 2.00 (1.34–2.97)p = 0.001 | 1.75 (1.28–2.38)p < 0.001 | 2.17 (1.56–3.00)p < 0.001 |
| CT scan improvement(hazard ratio) | survivors except late IFN | Cox proportional hazardsa | 0.98 (0.73–1.32)bp = 0.875 | 1.28 (0.87–1.90)p = 0.212 | 1.30 (0.96–1.77)p = 0.092 | 1.63 (1.17–2.25)p = 0.004 |
Abbreviations: IFN, interferon α-2b; LPV/r, lopinavir and ritonavir; UFV, umifenovir.
Model adjusted for gender, age, hypertension, diabetes, oxygen saturation at admission, symptom onset to admission >7 days, and symptom count at admission.
Proportional hazard assumption not met.
Lastly, we assessed the association of treatment groups with in-hospital mortality. Because the mortality rate is low, we only observed significant association of LPV/r alone with increased in-hospital mortality in comparison with other treatments (Figure 3E). Despite of showing comparably delayed hospital discharge and CT scan improvement, the IFN + LPV/r group showed significant lower mortality than the LPV/r alone group, which might be contributed by early use of IFNs.
Discussion
At the moment of writing, COVID-19 has been confirmed in more than 6.7 million people worldwide. The rapid spread of COVID-19 is in stark contrast with the lack of therapeutic tools, especially for patients in critical condition (Li and De Clercq, 2020). The WHO Solidarity trial for COVID-19, perhaps the largest-ever clinical trial, includes IFN plus LPV/r as one of the treatment options. In fact, this drug combination has been used in China to treat COVID-19 patients after the disease first broke out in Wuhan in January 2020 (Zeng et al., 2020). However, IFNs were given much less focus than were investigational antiviral agents such as Remdesivir and hydroxychloroquine (Sanders et al., 2020), and substantial evidence of its clinical efficacy in treating COVID-19 is still lacking. In this study, we took advantage of the drug stock disparity during the peak of COVID-19 outbreak between two medical centers in Hubei, China and conducted a retrospective cohort study comparing patients who underwent IFN-based therapies with those who received no IFNs. We found association of early IFN use with significantly reduced in-hospital mortality. However, no significant clinical benefit of IFNs was observed in moderately ill COVID-19 patients, and late administration of IFN could be associated with longer hospital stay and slower recovery of lung function. Additionally, we showed that using early IFNs with LPV/r is associated with more favorable clinical responses than by using LPV/r alone in COVID-19 patients.
Our findings resonate with recent findings about the role of IFNs in COVID-19 pathogenesis.
First, it has been reported that the acute inflammation often seen in critically ill COVID-19 patients is a result of repressed type I IFN expression and subsequent imbalanced IFN response with excessive cytokine production, which could be corrected by administration of therapeutic IFN (Blanco-Melo et al., 2020). Indeed, animal studies have already shown that early IFN treatment protects mice from lethal SARS-CoV or MERS-CoV infection (Channappanavar et al., 2016; Channappanavar et al., 2019). Together with our findings, it is plausible to speculate that early administration of IFNs in COVID-19 patients with high viral loads or compromised immune systems would slow down the viral replication and disease progression (Nile et al., 2020).
Second, ACE2 has recently been identified as a type I and III ISG in human airway epithelial cells (Ziegler et al., 2020), which implies that IFN signals could promote viral entry and replication (Su and Jiang, 2020). In this study, type I IFN was administered to the airway directly via an aerosol nebulizer, and thus should be highly potent to upregulate ACE2 expression in airway cells. In fact, our data showed no association of early IFN use with length of hospital stay and time to CT scan improvement in survived patients, and late IFN use was associated with slower CT scan improvement. These results point to the possibility that during the peak of viral replication in moderately ill patients, the beneficial and harmful effects of IFNs could cancel out each other. However, because critically ill patients often have a high viral load that could already saturate airway cells (Zheng et al., 2020), IFN-induced ACE2 expression thus might no longer pose significant risk as it does in moderately ill patients.
In summary, we conclude that among severe to critical COVID-19 patients, early treatment with IFN-α2b was associated with reduced in-hospital mortality, whereas no significant benefit was associated with IFN-α2b use in moderately ill patients. Randomized controlled trials are still needed to evaluate the clinical efficacy of IFN in treating COVID-19 patients. Nonetheless, our findings presented in this study should provide interest and motivation to scientists and physicians in future research of IFN therapy, and hopefully lead to effective therapies for COVID-19.
Limitation of Study
This study has several limitations. First, the retrospective design and the nonrandomized nature of assignment of therapies limit the interpretation of our results. Second, there is potential for survivorship bias due to the requirement of receiving IFNs for at least 3 consecutive days in the early IFN group, which is not required for the no IFN group. However, examination of 16 records of deceased patients found that the shortest duration from admission to in-hospital death was 5 days with a median value of 17 days, which could accommodate a 3-day course of IFNs or any other therapies. In fact, all deceased patients received at least one anti-viral therapy, which was among the entry criteria of this study. We also checked those records not included in this study and found no deceased patients among them. Nonetheless, we cannot rule out the possibility that some patients who received therapies of interest could have been deceased before confirmation of COVID-19 diagnosis and thus were excluded from the study. Third, detailed virologic data were not included in the study that precluded comparison with randomized controlled trials of IFNs (Hung et al., 2020). Forth, our regression models did not include location of care as a confounder because of multicollinearity with therapy choices between IFNs and LPV/r. Although we did investigate the potential variation of standard care and concluded no significant difference between the two medical centers, selection bias and confirmation bias could still affect the findings. Last, adjunctive and supportive therapies, such as corticosteroids, immunoglobulins, immunomodulatory agents, and herbal medicine, were not included in the analyses but could influence the length of hospital stay.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical Commercial Assays | ||
| Novel Coronavirus (2019-nCoV) Nucleic Acids Diagnostic Kit (PCR-Fluorescence Probing), Limit of Detection: 200 copies/mL | Sansure Biotech | China National Medical Products Administration Certificate #20203400064 |
| COVID-19 Coronavirus Real Time PCR Kit, Limit of Detection: 350 copies/mL | Bioperfectus Technologies | China National Medical Products Administration Certificate #20203400384 |
| Novel Coronavirus (2019-nCoV) Antibody Detection Reagent (Colloidal Gold Method), Sensitivity/Specificity: 86.43%/99.57% (IgM/IgG) | Wondfo Biotech | China National Medical Products Administration Certificate #20203400176 |
| Software and Algorithms | ||
| SPSS version 26 | IBM | https://www.ibm.com/analytics/spss-statistics-software |
| Prism version 8.0.1 | GraphPad | https://www.graphpad.com |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Peng Hong (peng.hong@downstate.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code availability
The manuscript includes all datasets generated and analyzed during this study.
Experimental Model and Subject Details
Human Sample
This study was approved by Medical Ethics Committees of Xiangyang Central Hospital and Suizhou Zengdu Hospital as a secondary analysis of identifiable data originally collected for non-research purposes. All patient identifications were replaced by anonymous codes during abstraction as stipulated by the Declaration of Helsinki. Hospital names were coded to prevent potential misinterpretation of findings.
We obtained all 730 inpatient records of confirmed COVID-19 patients admitted to the two COVID-19 designated hospitals during January 15 through March 31 directly from the two hospitals. COVID-19 diagnosis was confirmed by two consecutive positive results of quantitative PCR-based SARS-CoV-2 nucleic acids tests of throat or nasal swab samples, or in recovering patients by positive results of serological SARS-CoV-2 antibody tests (the most frequently used kits are listed in the Key Resource Table. Bioperfectus PCR was most often used in Center 1 and Sansure PCR kit was widely used at Center 2). Survivors were followed-up every two weeks according to local regulations and the date of last recorded follow-up was May 22, 2020.
Records were abstracted between May 8 and 22 by a trained team of 2 epidemiologists and 4 physicians into a standardized digital form based on the US CDC COVID-19 abstraction form with modifications to adapt local data and underwent daily quality control checks. Incomplete records such as those received unknown therapies at another hospital for at least 5 days before being admitted and those without key laboratory or radiological results due to transfers between departments are excluded. Patients requiring treatment for other conditions un-related to COVID-19 that extended hospital stay for at least 5 days were excluded. Patients with less than 2 CT scans available for comparison were excluded. Records with hospital stays less than 5 days were excluded. Patients not receiving any of IFN, LPV/r and UFV for more than 2 days because of lack of symptoms, late confirmation of diagnosis after recovery, severe adverse effects of anti-viral therapies, or successful treatment of disease using other therapies such as ribavirin, chloroquine, antibiotics and herbal medicine were excluded. A final sample of 446 was analyzed in this study (Figure 1). Patient information was collected on COVID-19 diagnosis, patient demographics, prior diagnosis of hypertension or diabetes, prior high-risk exposure such as close contact to COVID-19 patients or visiting high-risk locations, initial vital signs and laboratory test results within 24 h of admission, CT images and reports, and temporary and long-term prescriptions to describe the cohort and as potential confounders.
Method Details
Exposure
To evaluate the association of IFN with clinical outcomes, patients were categorized into 3 treatment groups based on the use and timing of IFN-based therapy during hospitalization: (1) early IFN, (2) late IFN, and (3) no IFN. Patients that received IFN aerosol within the first 5 days of hospitalization were assigned to early IFN group. To compare therapies based on IFN-α2b with or without anti-retroviral agents, patients were categorized into 5 treatment groups based on the first full-course anti-viral regime (continuous use of more than 7 days) they received during hospitalization: (1) IFN with LPV/r, (2) IFN with UFV, (3) IFN alone, (4) LPV/r alone, and (5) UFV alone.
Outcomes
The primary outcome was in-hospital mortality. Secondary outcomes included hospital discharge and CT scan improvement. The discharge criteria were defined as (1) normal body temperature for at least 3 days, (2) significant improvement of respiratory symptoms and function, (3) chest radiological imaging showing improvement of acute inflammation, (4) two consecutive negative SARS-CoV-2 nucleic acids tests of throat or nasal swab samples with at least 24 h interval between the two tests. CT scan improvement was defined as the first CT scan showing significant improvement of the lungs compared with the previous scan, or the second consecutive CT scan at least 5 days after the first CT scan both showing marginal or incremental improvements in the lungs over previous scans. In severe and critical cases with disease relapse, CT scan improvement occurred before relapse was not counted. For patients re-admitted because of positive SARS-CoV-2 nucleic acids tests during follow-up, their timing of CT scan improvement during the first hospital stay would still be valid if they showed no symptom and were without radiological disease activity during the second hospital stay.
Sample Size
An initial target sample size of 500 was determined based on the assumption of a 1:1 ratio of patients that received IFN or not, previously observed mortality rates (Guan et al., 2020) and α = 0.05. This sample size was calculated to have 90% power to detect a significant hazard ratio of 0.5 if the mortality rate is 19%, a previously observed mortality estimates in the US. For the actual mortality analysis in this study, post hoc power analysis estimates that the study achieved 90% power in logistic regression and 99% power in Cox proportional hazards regression at α = 0.05.
Quantification and Statistical Analysis
The distribution of treatment groups was summarized, and patient characteristics were assessed with Fisher’s exact test (between 2 groups) or Chi-square test (between >2 groups) for categorical variables and Mann-Whitney test (between 2 groups) or Kruskal-Wallis 1-way ANOVA (between >2 groups) for continuous variables. Survival and cumulative event curves were compared by Log-rank test. Proportional hazard assumption was tested by examination of the Kaplan-Meier curve. Odds ratios were estimated by logistic regression. Hazard ratios were estimated by Cox proportional hazards models fitted for time from admission to death or last follow-up, time from admission to discharge, and time from admission to CT scan improvement, after adjusting for comorbidities. Analyses were performed using SPSS 26 (IBM) or Prism 8 (GraphPad). Missing data were excluded pairwise from analyses. Significance was evaluated at α = 0.05 and all tests were 2-sided.
Acknowledgments
We thank Drs. Tianhao Su (Beijing Friendship Hospital), Kui Li, Zhengyan Wang, and Bo Zou (Suizhou Central Hospital), who did not receive any specific compensation for their contributions, for expert opinions on clinical findings. This study was supported by US Department of Veterans Affairs (5I01BX001353) and Sun Yat-sen University Seventh Hospital (393003).
Author Contributions
P.H. designed the study, abstracted records, analyzed data, and wrote the manuscript; N.W., Y.Z., L.Z., S.W., Y.G., and H.Y. abstracted records and analyzed data; Z.H., F.L., P.S., F.Q., X.W., X.Z., D.W., X.Q., G.T., Y.X., and Z.Z. conducted quality checks and interpreted clinical data; M.C. and Y.L. assisted statistical analysis and revised the manuscript. All authors read and approved the final manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: July 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.chom.2020.07.005.
Supplemental Information
References
- Acharya D., Liu G., Gack M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020;20:397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181:1036–1045.e9, e1039. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Zheng J., Wohlford-Lenane C., Abrahante J.E., Mack M., Sompallae R., McCray P.B., Jr., Meyerholz D.K., Perlman S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J. Clin. Invest. 2019;129:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of the People’s Republic of China; 2020. Chinese management guideline for COVID-19 (version 7)http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., China Medical Treatment Expert Group for Covid-19 Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y., Ng Y.Y., Lo J., Chan J., Tam A.R. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Mantlo E., Bukreyeva N., Maruyama J., Paessler S., Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020;179:104811. doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKechnie J.L., Blish C.A. The Innate Immune System: Fighting on the Front Lines or Fanning the Flames of COVID-19? Cell Host Microbe. 2020;27:863–869. doi: 10.1016/j.chom.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A., Iwasaki A. Type I and Type III Interferons - Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Su S., Jiang S. A suspicious role of interferon in the pathogenesis of SARS-CoV-2 by enhancing expression of ACE2. Signal Transduct. Target. Ther. 2020;5:71. doi: 10.1038/s41392-020-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Jiang Y., Zeng Y., Liu H. Combination antiviral therapy with lopinavir/ritonavir, arbidol and interferon-α1b for COVID-19. Antivir. Ther. (Lond.) 2020 doi: 10.3851/IMP3362. [DOI] [PubMed] [Google Scholar]
- Zeng Y.M., Xu X.L., He X.Q., Tang S.Q., Li Y., Huang Y.Q., Harypursat V., Chen Y.K. Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha, and ribavirin plus lopinavir/ritonavir plus interferon-alpha in patients with mild to moderate novel coronavirus disease 2019: study protocol. Chin. Med. J. (Engl.) 2020;133:1132–1134. doi: 10.1097/CM9.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Chen V., Shannon C.P., Wei X.S., Xiang X., Wang X., Wang Z.H., Tebbutt S.J., Kollmann T.R., Fish E.N. Interferon-α2b Treatment for COVID-19. Front. Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., HCA Lung Biological Network. Electronic address: lung-network@humancellatlas.org; HCA Lung Biological Network SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181:1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Liu Y., Zhong Q., Zhang K., Xu Y., Wang Z. Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID-19: A retrospective study in two designated hospitals in Anhui, China. J. Med. Virol. 2020 doi: 10.1002/jmv.26127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The manuscript includes all datasets generated and analyzed during this study.