Abstract
Background
Some previous studies have reported inconsistent results on the association between alcohol intake and diabetic retinopathy (DR) risk. This study aimed to evaluate the potential effects of alcohol intake on subsequent DR risk using a meta-analytic approach.
Methods
Three electronic databases (PubMed, EmBase, and the Cochrane library) were systematically searched for observational studies from their inception till November 2019. The pooled odds ratio (OR) with 95% confidence interval (CI) were applied for the summary effect estimate using a random-effects model.
Results
A total of 15 studies (5 cohort studies, 4 case-control studies, and 6 cross-sectional studies) with 37,290 participants and 12,711 DR cases were selected for the final meta-analysis. The pooled OR indicated no significant association between alcohol intake and DR risk (OR: 0.91; 95%CI: 0.78–1.06; P = 0.225), irrespective of the studies being pooled cohort (OR: 0.95; 95%CI: 0.66–1.36; P = 0.761), case-control (OR: 0.97; 95%CI: 0.77–1.23; P = 0.818), or cross-sectional (OR: 0.86; 95%CI: 0.69–1.08; P = 0.190) ones. However, this association might have been affected by the type of diabetes mellitus and the adjusted status.
Conclusion
The results of this study showed that the potential impact of alcohol intake on DR risk may differ according to the type of diabetes mellitus and adjusted status. Further large-scale, prospective cohort studies should be conducted to verify the findings of this study and to evaluate DR risk in relation to the dose and type of alcohol intake.
Keywords: Alcohol, Diabetic retinopathy, Meta-analysis
Background
Globally, diabetes mellitus (DM) has been rapidly increasing and is estimated to have affected about 422 million people and caused 1.6 million deaths in 2014 [1]. Diabetic patients experience progressive changes in their metabolic and inflammatory indices and several inflammatory markers [2–4]. Microvascular abnormalities and eye-related complications are most common in DM patients [5, 6]. Diabetic retinopathy (DR) is one of the most severe complications of DM and accounts for nearly 40% of DM complications in patients aged ≥40 years. Patients with DR have an increased risk of permanent visual impairment, and their quality of life is adversely affected [7–9]. A study reported that the prevalence of DR exceeds 75% in patients with DM for more than 20 years [10]. DR is the leading cause of impaired vision and blindness in DM patients and accounts for 4.8% of blindness cases worldwide [11, 12]. Therefore, identifying potential risk factors for the progression of DR is important in DM patients.
Several studies have identified some of the potential risk factors for the progression of DR. A meta-analysis conducted by Song et al. contained 31 studies and found that insulin treatment, elevated fasting blood glucose levels, and high glycosylated hemoglobin concentrations are associated with an increased risk of DR in Chinese diabetic patients [13]. Moreover, several other risk factors, including hyperhomocysteinemia [14], vitamin D deficiency [15], obstructive sleep apnea [16], and obesity [17] have been demonstrated to be associated with an increased risk of DR. The investigating the potential role of alcohol intake on the risk of DR with an important public health implications owing to alcohol was the most widely consumed beverages. Therefore, to clarify the role of alcohol intake plays in DR is particularly important, as it not defined in general and DM populations. In this study, we attempted a large-scale examination of the available observational studies to determine the association between alcohol intake and DR risk. Stratified analyses were also conducted according to the study design.
Methods
Data sources, search strategy, and selection criteria
This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement issued in 2009 [18]. Observational studies that investigated the association of alcohol intake with DR were included in this study, without restrictions on language and published status. Three electronic databases PubMed, EmBase, and the Cochrane library were systematically searched throughout November 2019 using “alcohol” and “diabetic retinopathy” as the core search terms. Manual searches were also performed for the reference lists of the retrieved studies to identify any new eligible study.
A standardized approach was applied by two of the authors for the literature search and study selection, with any disagreement between them resolved by a group discussion until a consensus was reached. The inclusion criteria of this study were as follows: (1) Study design: observational studies, including cohort, case-control, and cross-sectional studies; (2) Participants: there were no restrictions, with the inclusion of general population as well type 1 DM, type 2 DM, or mixed patients; (3) Exposure: alcohol intake; and (4) Outcomes: studies reporting an effect estimate and 95% confidence intervals (CIs) for comparisons of high and low alcohol intake on the risk of DR. The maximally adjusted results were selected if the study reported several adjusted effect estimates.
Data collection and quality assessment
Data collection and quality assessment were performed by two authors, and any inconsistency was settled by an additional author by referring to the original article. The following data items were collected: first author’s surname, publication year, study design, country, sample size, male participant percentage, mean age, number of cases, DR diagnosis, DR definition, population status, exposure definition, effect estimate and its 95% CI, and covariates in the fully adjusted model. The quality of identified studies was assessed using Newcastle–Ottawa Scale (NOS), which has already been partially validated for assessing the quality of observational studies in meta-analyses [19]. NOS comprises a star system that includes selection (four items), comparability (one item), and outcome (three items) categories; the number of stars awarded ranges from 0 to 9.
Statistical analysis
The association between alcohol intake and DR risk on the basis of effect estimate and corresponding 95%CIs in each study as well as the pooled odds ratio (OR) with 95%CI was calculated using the random-effects model [20, 21]. I2 index and Q statistic was applied to assess heterogeneity among the studies, and I2 > 50.0% or P < 0.10 was considered as significant heterogeneity [22, 23]. The robustness of pooled conclusion was evaluated using a sensitivity analysis [24]. Subgroup analyses were also conducted based on countries, publication year, population status, adjusted status, and study quality according to the study design. The P value between the subgroups was assessed using an interaction test [25]. Publication bias was assessed using the funnel plot and Egger’s and Begg’s tests [26, 27]. All reported P values are two-sided, and P values < 0.05 were considered significant for all the included studies. Statistical analyses were performed using STATA software (version 12.0; Stata Corporation, College Station, TX, USA).
Results
Literature search
A total of 483 articles were identified in our initial electronic searches; 460 studies were excluded due to duplication and irrelevancy. A total of 23 potentially eligible studies were selected for further full-text evaluations, and 8 studies were excluded due to other disease status (n = 2), other exposure (n = 3), and the study being a review or meta-analysis (n = 3). Eventually, 15 observational studies were selected for the final quantitative analysis [28–42]. A manual search for the reference lists yielded two studies, and these two studies were included in the initial electronic searches. Figure 1 presents the study selection process; the baseline characteristics of the included studies and participants are summarized in Table 1.
Fig. 1.
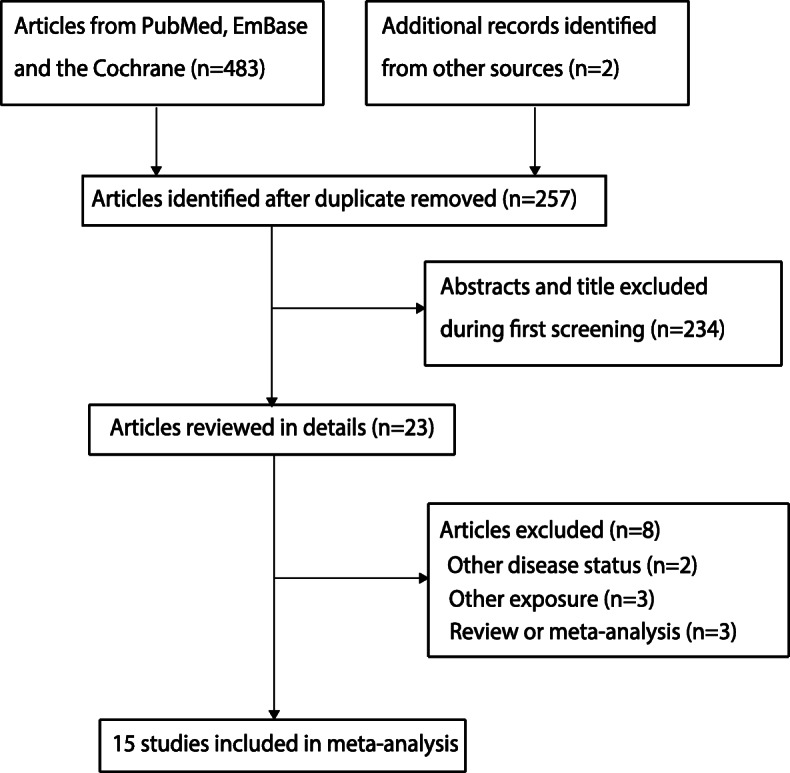
Flow diagram of the literature search and study selection process
Table 1.
Baseline characteristics of the selected studies
| Study | Publication year | Study design | Country | Sample size | Percent of male (%) | Mean age (years) | Number of cases | DR diagnosis | DR definition |
Diabetes | Exposure Definition | Adjustment/matched | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young [28] | 1984 | Cohort | UK | 296 | 100.0 | 20.0–59.0 | 66 | Fundoscopic | Four Grades | Mixed |
≤10 measures/ week, > 10 measures/week |
Crude | 6 |
| Moss [29] | 1994 | Cohort | USA | 916 | NA | ≥ 21.0 | 238 |
Fundus photographs |
ETDRS | Mixed | Average loz/ day increase | Age, sex, HbA1c, retinopathy | 8 |
| Kohner [30] | 1998 | Case control | UK | 2964 | 58.4 | 25.0–65.0 | 1102 |
Retinal photography |
ETDRS | T2DM | None, occasional, regular, heavy | Crude | 6 |
| Rasmidatta [31] | 1998 | Case control | Thailand | 198 | NA | 60.5 | 63 |
Fundoscopic examinations |
Three grades | T2DM | Nondrinker, drinker, not regular drinker | HbA1c, cholesterol, triglyceride, HDL, BP | 6 |
| Giuffrè [32] | 2004 | Case control | Italy | 132 | 38.6 | ≥ 40.0 | 45 |
Fundus examination |
ETDRS | Mixed | None, 1–19 years, 20 years or more | Crude | 6 |
| Hirai [33] | 2007 | Cross-sectional | USA | 537 | 50.1 | 45.3 | 309 |
Retinal photography |
ETDRS | T1DM | Alcohol/No alcohol | Crude | 4 |
| Beulens [34] | 2008 | Cross-sectional | Europe | 3250 | 29.7 | 15.0–60.0 | 304 |
Retinal photographs |
Three grades | T1DM | 0 g/week, 0.0–4.9 g/week, 5.0–29.9 g/ week, 30.0–69.9 g/week, 70.0–209.9 g/week, ≥210 g/week | Age, sex, centre, duration of illness, systolic BP, physical activity, smoking, BMI, presence of cardiovascular disease and HbA1c | 7 |
| Xu [35] | 2009 | Cohort | China | 4141 | 43.4 | ≥ 40.0 | 366 |
Fundus photographs |
NA | General | Consumers, non-consumers | BMI, HDL, LDL, arterial hypertension | 7 |
| Lee [36] | 2010 | Cohort | 14 countries | 1239 | 60.7 | 55.0–81.0 | 640 |
Retinal photography |
ETDRS | T2DM | 0, drinks /week 1–14, drinks/week > 14 drinks /week | Age, sex, HbA1c, systolic BP, duration of diabetes, BMI, cigarette smoking, ethnicity | 8 |
| Yang [37] | 2013 | Cross-sectional | Korea | 978 | 54.1 | ≥ 19.0 | 112 |
Fundus examination |
ETDRS | Mixed |
≥4 alcoholic drinks/week, < 3 drinks/week |
Age, gender, smoking status, regular exercise, BMI, serum total cholesterol, serum triglyceride, serum HDL cholesterol, anti-lipid drug use | 6 |
| Jongsareejit [38] | 2013 | Cross-sectional | Thailand | 933 | NA | 59.5 | 214 |
Indirect ophthalmoscope |
International scales |
T2DM | No, ever, current | Gender, age, diastolic BP, waist, total cholesterol, HDL, ccular perfusion pressure | 5 |
| Harjutsalo [39] | 2014 | Cross-sectional | Finland | 3608 | 52.6 | 28.9–46.8 | 1191 |
Retinal photography |
NA | T1DM | Heavy drinker light drinker | Crude | 3 |
| Fenwick [40] | 2015 | Cross-sectional | Australia | 395 | 64.1 | ≥ 18.0 | 235 |
Fundus photography |
ETDRS | T2DM | None, moderate, high | Education, income, language spoken at home, country of birth, lipid lowering drugs, hypertension drugs | 6 |
| Tseng [41] | 2015 | Cohort | China | 573 | 61.8 | 58.9 | 91 | Funduscopic | Three grades | T2DM | Drinker, no-drinker | Crude | 6 |
| Martín-Merino [42] | 2016 | Case control | UK | 17,130 | 55.9 | All stages | 7735 | Computerized records | NA | T2DM |
0–1 units/week 2–21 units/ week 22–34 units/week ≥35 units/week |
Sex, age at index date, diabetes duration, primary care practitioner visits, referrals and hospitalizations, smoking, first HbA1c; systolic BP, glaucoma; cataracts, or lens extraction, HDL and triglycerides, and hypoglycaemic agents, including oral hypoglycaemic drugs and insulin | 7 |
Study characteristics
Of the 15 included studies, 5 were cohort, 4 were case-control, and the remaining 6 were cross-sectional studies. The studies were published between 1984 and 2016, and the participants in the individual studies ranges from 132 to 17,130. A total of 11 studies were conducted in Western countries, and the remaining 4 studies were conducted in Eastern countries. Three studies included type 1 DM patients, seven included type 2 DM patients, four included both type 1 and type 2 DM patients, and the remaining study included the general population. Nine studies reported that effect estimates were adjusted for potential covariates, and the remaining six studies reported crude effect estimates. Studies were assessed using NOS: two studies were awarded 8 stars, three studies were awarded 7 stars, seven studies were awarded 6 stars, one study was awarded 5 stars, 1 study was awarded 4 stars, and the remaining study was awarded 3 stars.
Meta-analysis
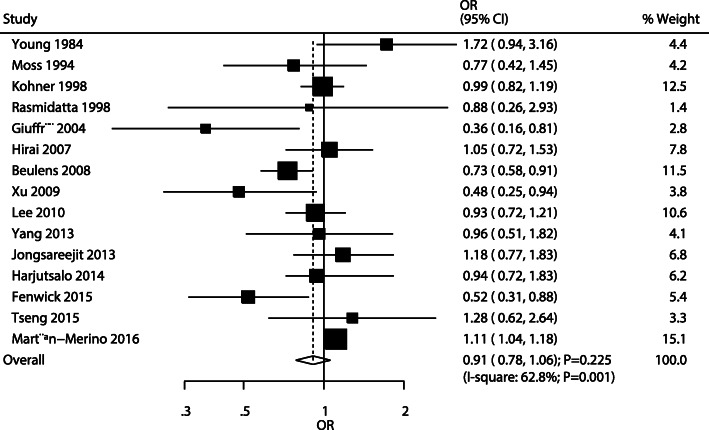
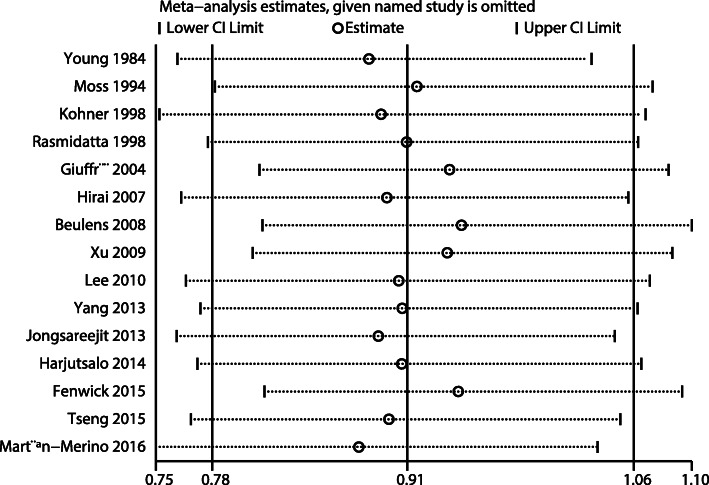
After pooling all the included studies, the pooled OR indicated no significant association between alcohol intake and DR risk (OR: 0.91; 95%CI: 0.78–1.06; P = 0.225; Fig. 2), and significant heterogeneity was observed across the studies (I2 = 62.8%; P = 0.001). The conclusion was not altered by sequentially excluding individual studies (Fig. 3). When stratified by study design, no significant associations were observed irrespective of the studies being pooled cohort (OR: 0.95; 95%CI: 0.66–1.36; P = 0.761), case-control (OR: 0.97; 95%CI: 0.77–1.23; P = 0.818), or cross-sectional (OR: 0.86; 95%CI: 0.69–1.08; P = 0.190) ones. Sensitivity analyses were also conducted according to the study design, showing that alcohol intake was not associated with DR risk in cohort and cross-sectional studies, whereas a potential significant association was observed in case-control studies (Additional file 1, Additional file 2 and Additional file 3).
Fig. 2.
The pooled odds ratio for the association of alcohol intake with diabetic retinopathy risk
Fig. 3.
Sensitivity analysis
Subgroup analysis
Subgroup analyses were conducted to evaluate the association between alcohol intake and DR risk according to the study design (Table 2). When stratified analyses were conducted for cohort studies, alcohol intake was found to be associated with a reduced DR risk if the study included general population; furthermore, the association between alcohol intake and DR risk could be affected by the adjusted status and study quality. When stratified analyses were conducted for case-control studies, alcohol intake was found to be associated with an increased DR risk if the analysis included pooled studies published in or after 2010, studies on type 2 DM patients, studies reporting adjusted effect estimates, and studies with high quality; however, alcohol intake was associated with a reduced DR risk if studies included both type 1 and type 2 DM patients. Moreover, the association of alcohol intake with DR risk could be affected by the population status. When stratified analyses were conducted for cross-sectional studies, alcohol intake was found to be associated with a reduced DR risk if the pooled studies were of high quality.
Table 2.
Subgroup analyses according to study design
| Study design | Factors | Group | OR and 95%CI | P value | Heterogeneity (%) | P value for Q test | P value between subgroups |
|---|---|---|---|---|---|---|---|
| Cohort studies | Countries | Western | 1.04 (0.70–1.53) | 0.855 | 50.5 | 0132 | 0.318 |
| Eastern | 0.77 (0.30–2.03) | 0.603 | 73.9 | 0.050 | |||
| Publication year | Before 2010 | 0.87 (0.42–1.80) | 0.703 | 75.3 | 0.018 | 0.722 | |
| 2010 or after | 0.96 (0.76–1.23) | 0.771 | 0.0 | 0.416 | |||
| Population | T2DM | 0.96 (0.76–1.23) | 0.771 | 0.0 | 0.416 | 0.086 | |
| Mixed | 1.15 (0.52–2.54) | 0.722 | 69.7 | 0.069 | |||
| General | 0.48 (0.25–0.93) | 0.030 | – | – | |||
| Adjusted status | Yes | 0.77 (0.53–1.11) | 0.162 | 41.3 | 0.182 | 0.024 | |
| No | 1.52 (0.96–2.42) | 0.076 | 0.0 | 0.540 | |||
| Study quality | High | 0.77 (0.53–1.11) | 0.162 | 41.3 | 0.182 | 0.024 | |
| Low | 1.52 (0.96–2.42) | 0.076 | 0.0 | 0.540 | |||
| Case control studies | Countries | Western | 0.97 (0.75–1.24) | 0.792 | 76.5 | 0.014 | 0.729 |
| Eastern | 0.88 (0.26–2.95) | 0.836 | – | – | |||
| Publication year | Before 2010 | 0.71 (0.36–1.41) | 0.332 | 64.9 | 0.058 | 0.086 | |
| 2010 or after | 1.11 (1.04–1.18) | 0.001 | – | – | |||
| Population | T2DM | 1.10 (1.03–1.16) | 0.003 | 0.0 | 0.490 | 0.007 | |
| Mixed | 0.36 (0.16–0.81) | 0.014 | – | – | |||
| Adjusted status | Yes | 1.11 (1.04–1.18) | 0.001 | 0.0 | 0.707 | 0.093 | |
| No | 0.65 (0.24–1.72) | 0.383 | 82.4 | 0.017 | |||
| Study quality | High | 1.11 (1.04–1.18) | 0.001 | – | – | 0.086 | |
| Low | 0.71 (0.36–1.41) | 0.332 | 64.9 | 0.058 | |||
| Cross-sectional studies | Countries | Western | 0.79 (0.61–1.03) | 0.080 | 46.9 | 0.130 | 0.088 |
| Eastern | 1.11 (0.77–1.58) | 0.583 | 0.0 | 0.599 | |||
| Publication year | Before 2010 | 0.85 (0.60–1.20) | 0.354 | 62.0 | 0.105 | 0.532 | |
| 2010 or after | 0.87 (0.61–1.24) | 0.451 | 48.4 | 0.121 | |||
| Population | T1DM | 0.85 (0.67–1.08) | 0.187 | 33.5 | 0.223 | 0.896 | |
| T2DM | 0.79 (0.36–1.77) | 0.573 | 82.2 | 0.018 | |||
| Mixed | 0.96 (0.51–1.81) | 0.900 | – | – | |||
| Adjusted status | Yes | 0.80 (0.59–1.10) | 0.170 | 54.4 | 0.087 | 0.144 | |
| No | 1.01 (0.75–1.35) | 0.973 | 00 | 0.718 | |||
| Study quality | High | 0.73 (0.58–0.91) | 0.006 | – | – | 0.114 | |
| Low | 0.92 (0.70–1.20) | 0.538 | 36.9 | 0.175 |
Publication bias
The publication bias could not be ruled out by reviewing the funnel plot for the association between alcohol intake and DR risk (Fig. 4). Although the Begg’s test indicated no significant publication bias (P = 0.692), the Egger’s test suggested significant publication bias (P = 0.044). The conclusions were unaltered after adjustments for publication bias through the trim and fill method [43].
Fig. 4.
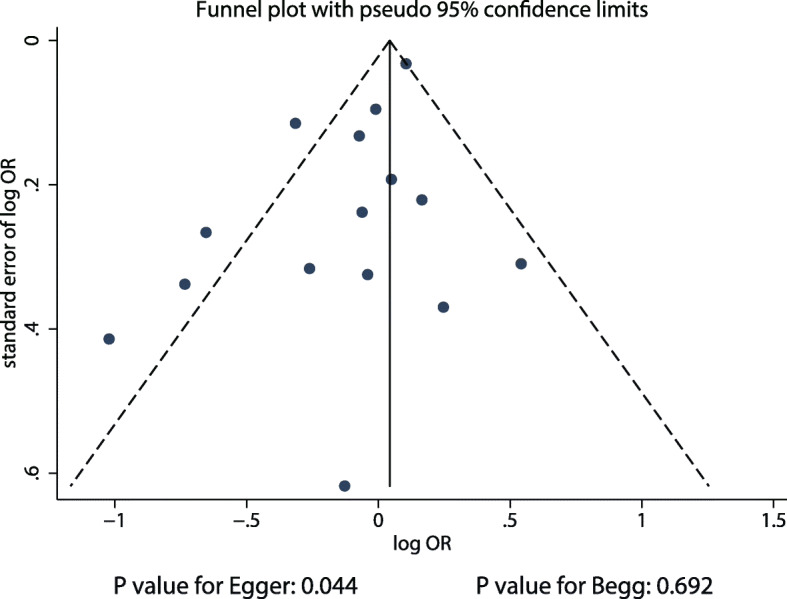
Publication bias
Discussion
This study was conducted on the basis of previously published observational studies, and it evaluated the association of alcohol intake with DR risk. This quantitative meta-analysis included 37,290 participants and 12,711 DR cases from 5 cohort studies, 4 case-control studies, and 6 cross-sectional studies across a wide range of participant characteristics. The findings of this study show no significant association between alcohol intake and DR risk, irrespective of the studies being pooled cohort, case-control, or cross-sectional ones. Sensitivity analysis suggested potential beneficial effects of alcohol intake on DR risk in case-control studies. Finally, the association of alcohol intake with DR risk according to study design varied when the studies were stratified by countries, publication year, population status, adjusted status, and quality.
A meta-analysis conducted by Zhu et al. included a total of 15 studies and found that alcohol intake was not associated with DR risk. Interestingly, wine or sherry intake was associated with a reduced DR risk [44]. They attributed the results to the potential protective effects of low to moderate alcohol intake on the risk of DM and cardiovascular disease [45, 46]. However, the inflammatory response and oxidative stress could be affected by alcohol and are significantly associated with DR risk [47, 48]. The stratified analyses from the previous meta-analysis were mixed owing to, studies with various designs, and the results of such stratified analyses are unreliable. Therefore, the present study may correct the inappropriate results reported by such stratified analyses.
Although no significant association between alcohol intake and DR risk was observed in most of the studies included in our meta-analysis, many of these studies reported inconsistent results. The Casteldaccia Eye Study found that the duration of alcohol intake between 1 and 19 years was not associated with DR risk, whereas alcohol intake for ≥20 years was associated with a reduced DR risk [32]. Beulens et al. reported that moderate alcohol intake was associated with a reduced risk of microvascular complications among type 1 DM patients [34]. The Beijing Eye Study suggested that alcohol intake was associated with a reduced DR risk in general population [35]. A study conducted by Fenwick et al. found that moderate white and fortified wine intake was correlated with DR risk among type 2 DM patients [40]. They pointed out the beneficial effects induced by alcohol intake due to increase in high-density lipoprotein levels, reduction in platelet aggregation, and decrease in fibrinogen levels [49]. However, a case-control study in a UK primary care setting indicated that alcohol intake was associated with an increased DR risk among type 2 DM patients [42]. A possible reason for this could be the moderate to heavy rate at which alcohol was consumed by the participants of that study, which has been associated with an increased DR risk.
The results of the subgroup analyses showed that the association of alcohol intake with DR risk is multifaceted when stratified by countries, publication year, population status, adjusted status, and study quality. First, we found that the association of alcohol intake with DR risk persisted even after stratification by countries, irrespective of the studies being cohort, case-control, or cross-sectional ones. However, the heterogeneity remained and was not fully explained. Second, we found that alcohol intake was associated with an increased DR risk in studies published in or after 2010 when stratified by case-control cohorts; this result was obtained from only one study and has been previously identified [42]. Third, we found that alcohol intake was associated with an increased DR risk if only type 2 DM patients were included, whereas the risk was significantly reduced if both type 1 and type 2 DM patients were included in stratified case-control cohorts. This could be due to the study conducted by Martín-Merino et al. contributing a large weight to the overall analysis [42]. A similar result was observed when the studies were stratified by adjusted status. Finally, when the studies were pooled by design as case-control or cross-sectional studies with high quality, conflicting results were observed. However, this observation was obtained from only one study, and the conclusions were not reliable.
There are several limitations to this study. First, most of the included studies (10/15) were designed as case-control or cross-sectional studies, making it difficult to distinguish cause-and-effect relationships. Second, the drinking habits and other lifestyle factors after the diagnosis of DM may have changed, altering the effects of alcohol intake on DR risk and biasing the results. Third, the adjusted status and included covariates were different across the included studies, which could affect the reliability of the pooled conclusion. Forth, stratified analyses according to sex, the dose and type of alcohol intake were not conducted owing to mostly included studies did not report these data. Finally, the inherent limitations of traditional meta-analysis, including publication bias and study level-based analysis, affect the reliability of conclusion and restrict the results of detailed analyses.
Conclusions
In conclusion, the findings of this study suggest no significant association between alcohol intake and DR risk. Moreover, this lack of association might have been affected by population status, adjusted status, and study quality. This association should be verified in further large-scale, prospective studies, and DR risk in relation to the dose and type of alcohol intake should also be explored.
Supplementary information
Additional file 1. Sensitivity for cohort studies.
Additional file 2. Sensitivity for case control studies.
Additional file 3. Sensitivity for cross-section studies.
Acknowledgements
Not applicable
Abbreviations
- DR
Diabetic retinopathy
- OR
Odds ratio
- CI
Confidence interval
- DM
Diabetes mellitus
- NOS
Newcastle–Ottawa Scale
Authors’ contributions
CC and DRP developed the concept of this study; WGX and ZJS did the literature research and data analysis; DL, JT, YTW and TZ contributed to the experimental technique; CC drafted the manuscript. All authors have read and approved the final manuscript.
Funding
The design of this study and statistics was funded by Experimental study on the management mode of berberine combined with family doctors to delay retinopathy in community type II diabetic patients (Community 2018SQ05), and Intervention study of berberine combined with comprehensive treatment for type II diabetic retinopathy in north Shanghai community (TYS2018M008).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12902-020-00588-3.
References
- 1.World Health Organization: Global report on diabetes. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf?ua=1. Accessed Nov 2019.
- 2.Dain A, Repossi G, Das UN, Eynard AR. Role of PUFAs, the precursors of endocannabinoids, in human obesity and type 2 diabetes. Front Biosci (Elite Ed) 2010;2:1432–1447. doi: 10.2741/e203. [DOI] [PubMed] [Google Scholar]
- 3.Dain A, Repossi G, Diaz-Gerevini GT, Vanamala J, Das UN, Eynard AR. Long chain polyunsaturated fatty acids (LCPUFAs) and nordihydroguaiaretic acid (NDGA) modulate metabolic and inflammatory markers in a spontaneous type 2 diabetes mellitus model (Stillman Salgado rats) Lipids Health Dis. 2016;15(1):205. doi: 10.1186/s12944-016-0363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosick R, Alvarado-Vazquez PA, Messersmith AR, Romero-Sandoval EA. High glucose induces a priming effect in macrophages and exacerbates the production of pro-inflammatory cytokines after a challenge. J Pain Res. 2018;11:1769–1778. doi: 10.2147/JPR.S164493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, Heller S, Marre M, Patel A, Poulter N, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57(12):2465–2474. doi: 10.1007/s00125-014-3369-7. [DOI] [PubMed] [Google Scholar]
- 6.Association AD Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusuhara S, Fukushima Y, Ogura S, Inoue N, Uemura A. Pathophysiology of diabetic retinopathy: the old and the new. Diabetes Metab J. 2018;42(5):364–376. doi: 10.4093/dmj.2018.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, Jonas JB, Lamoureux EL, Cheng CY, Klein BEK, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7(2):140–149. doi: 10.1016/S2213-8587(18)30128-1. [DOI] [PubMed] [Google Scholar]
- 9.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet (London, England) 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 10.Barcelo A, Aedo C, Rajpathak S, Robles S. The cost of diabetes in Latin America and the Caribbean. Bull World Health Organ. 2003;81(1):19–27. [PMC free article] [PubMed] [Google Scholar]
- 11.Kowluru RA. Mitochondria damage in the pathogenesis of diabetic retinopathy and in the metabolic memory associated with its continued progression. Curr Med Chem. 2013;20(26):3226–3233. doi: 10.2174/09298673113209990029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 13.Song P, Yu J, Chan KY, Theodoratou E, Rudan I. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J Glob Health. 2018;8(1):010803. doi: 10.7189/jogh.08.010803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu C, Wu Y, Liu G, Liu X, Wang F, Yu J. Relationship between homocysteine level and diabetic retinopathy: a systematic review and meta-analysis. Diagn Pathol. 2014;9:167. doi: 10.1186/s13000-014-0167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo BA, Gao F, Qin LL. The Association between Vitamin D Deficiency and Diabetic Retinopathy in Type 2 Diabetes: A Meta-Analysis of Observational Studies. Nutrients. 2017;9(3):307. [DOI] [PMC free article] [PubMed]
- 16.Zhu Z, Zhang F, Liu Y, Yang S, Li C, Niu Q, Niu J. Relationship of obstructive sleep Apnoea with diabetic retinopathy: a meta-analysis. Biomed Res Int. 2017;2017:4737064. doi: 10.1155/2017/4737064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu W, Wu Y, Meng YF, Xing Q, Tao JJ, Lu J. Association of obesity and risk of diabetic retinopathy in diabetes patients: a meta-analysis of prospective cohort studies. Medicine. 2018;97(32):e11807. doi: 10.1097/MD.0000000000011807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2009. Ottawa Hospital Research Institute web site. Ottawa: L’Hopital d’Ottawa Institut de Recherche; 2014. [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 22.Deeks JJ, Fellow JPHSSV, Altman DG. Analyzing data and undertaking meta-analyses. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 501. Oxford: The Cochrane Collaboration; 2008.
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;47:15–17. [Google Scholar]
- 25.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 28.Young RJ, McCulloch DK, Prescott RJ, Clarke BF. Alcohol: another risk factor for diabetic retinopathy? Br Med J (Clin Res Ed) 1984;288(6423):1035–1037. doi: 10.1136/bmj.288.6423.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moss SE, Klein R, Klein BE. The association of alcohol consumption with the incidence and progression of diabetic retinopathy. Ophthalmology. 1994;101(12):1962–1968. doi: 10.1016/s0161-6420(94)31076-1. [DOI] [PubMed] [Google Scholar]
- 30.Kohner EM, Aldington SJ, Stratton IM, Manley SE, Holman RR, Matthews DR, Turner RC. United Kingdom prospective diabetes study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol. 1998;116(3):297–303. doi: 10.1001/archopht.116.3.297. [DOI] [PubMed] [Google Scholar]
- 31.Rasmidatta S, Khunsuk-Mengrai K, Warunyuwong C. Risk factors of diabetic retinopathy in non-insulin dependent diabetes mellitus. J Med Assoc Thail. 1998;81(3):169–174. [PubMed] [Google Scholar]
- 32.Giuffre G, Lodato G, Dardanoni G. Prevalence and risk factors of diabetic retinopathy in adult and elderly subjects: the Casteldaccia eye study. Graefes Arch Clin Exp Ophthalmol. 2004;242(7):535–540. doi: 10.1007/s00417-004-0880-4. [DOI] [PubMed] [Google Scholar]
- 33.Hirai FE, Moss SE, Klein BE, Klein R. Severe hypoglycemia and smoking in a long-term type 1 diabetic population: Wisconsin epidemiologic study of diabetic retinopathy. Diabetes Care. 2007;30(6):1437–1441. doi: 10.2337/dc06-2264. [DOI] [PubMed] [Google Scholar]
- 34.Beulens JW, Kruidhof JS, Grobbee DE, Chaturvedi N, Fuller JH, Soedamah-Muthu SS. Alcohol consumption and risk of microvascular complications in type 1 diabetes patients: the EURODIAB prospective complications study. Diabetologia. 2008;51(9):1631–1638. doi: 10.1007/s00125-008-1091-z. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, You QS, Jonas JB. Prevalence of alcohol consumption and risk of ocular diseases in a general population: the Beijing eye study. Ophthalmology. 2009;116(10):1872–1879. doi: 10.1016/j.ophtha.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Lee CC, Stolk RP, Adler AI, Patel A, Chalmers J, Neal B, Poulter N, Harrap S, Woodward M, Marre M, et al. Association between alcohol consumption and diabetic retinopathy and visual acuity-the AdRem study. Diabet Med. 2010;27(10):1130–1137. doi: 10.1111/j.1464-5491.2010.03080.x. [DOI] [PubMed] [Google Scholar]
- 37.Yang JY, Kim NK, Lee YJ, Noh JH, Kim DJ, Ko KS, Rhee BD, Kim DJ. Prevalence and factors associated with diabetic retinopathy in a Korean adult population: the 2008-2009 Korea National Health and nutrition examination survey. Diabetes Res Clin Pract. 2013;102(3):218–224. doi: 10.1016/j.diabres.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Jongsareejit A, Potisat S, Krairittichai U, Sattaputh C, Arunratanachote W. The Thai DMS diabetes complications (DD.Comp.) project: prevalence and risk factors of diabetic retinopathy in Thai patients with type 2 diabetes mellitus. J Med Assoc Thail. 2013;96(11):1476–1482. [PubMed] [Google Scholar]
- 39.Harjutsalo V, Feodoroff M, Forsblom C, Groop PH. Patients with type 1 diabetes consuming alcoholic spirits have an increased risk of microvascular complications. Diabet Med. 2014;31(2):156–164. doi: 10.1111/dme.12307. [DOI] [PubMed] [Google Scholar]
- 40.Fenwick EK, Xie J, Man RE, Lim LL, Flood VM, Finger RP, Wong TY, Lamoureux EL. Moderate consumption of white and fortified wine is associated with reduced odds of diabetic retinopathy. J Diabetes Complicat. 2015;29(8):1009–1014. doi: 10.1016/j.jdiacomp.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Tseng ST, Chou ST, Low BH, Su FL. Risk factors associated with diabetic retinopathy onset and progression in diabetes patients: a Taiwanese cohort study. Int J Clin Exp Med. 2015;8(11):21507–21515. [PMC free article] [PubMed] [Google Scholar]
- 42.Martin-Merino E, Fortuny J, Rivero-Ferrer E, Lind M, Garcia-Rodriguez LA. Risk factors for diabetic retinopathy in people with type 2 diabetes: a case-control study in a UK primary care setting. Prim Care Diabetes. 2016;10(4):300–308. doi: 10.1016/j.pcd.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89–98. [Google Scholar]
- 44.Zhu W, Meng YF, Wu Y, Xu M, Lu J. Association of alcohol intake with risk of diabetic retinopathy: a meta-analysis of observational studies. Sci Rep. 2017;7(1):4. doi: 10.1038/s41598-017-00034-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raum P, Lamparter J, Ponto KA, Peto T, Hoehn R, Schulz A, Schneider A, Wild PS, Pfeiffer N, Mirshahi A. Prevalence and cardiovascular associations of diabetic retinopathy and Maculopathy: results from the Gutenberg health study. PLoS One. 2015;10(6):e0127188. doi: 10.1371/journal.pone.0127188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li XH, Yu FF, Zhou YH, He J. Association between alcohol consumption and the risk of incident type 2 diabetes: a systematic review and dose-response meta-analysis. Am J Clin Nutr. 2016;103(3):818–829. doi: 10.3945/ajcn.115.114389. [DOI] [PubMed] [Google Scholar]
- 47.Yu W, Fu YC, Wang W. Cellular and molecular effects of resveratrol in health and disease. J Cell Biochem. 2012;113(3):752–759. doi: 10.1002/jcb.23431. [DOI] [PubMed] [Google Scholar]
- 48.Srikanta AH, Kumar A, Sukhdeo SV, Peddha MS, Govindaswamy V. The antioxidant effect of mulberry and jamun fruit wines by ameliorating oxidative stress in streptozotocin-induced diabetic Wistar rats. Food Funct. 2016;7(10):4422–4431. doi: 10.1039/c6fo00372a. [DOI] [PubMed] [Google Scholar]
- 49.Moss SE, Klein R, Klein BE. Alcohol consumption and the prevalence of diabetic retinopathy. Ophthalmology. 1992;99(6):926–932. doi: 10.1016/s0161-6420(92)31872-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Sensitivity for cohort studies.
Additional file 2. Sensitivity for case control studies.
Additional file 3. Sensitivity for cross-section studies.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.