Summary
While Hodgkin lymphoma (HL) is highly curable in younger patients, older patients have higher relapse and death rates, which may reflect age-related factors, distinct disease biology and/or treatment decisions. We described the association between patient, disease and geographic factors and first-line treatment in older patients (≥65 years) with incident HL using Surveillance, Epidemiology, and End Results (SEER)-Medicare data from 1999 to 2014 (n = 2825). First-line treatment initiated at ≤4 months after diagnosis was categorised as: full chemotherapy regimen (n = 699, 24.7%); partial chemotherapy regimen (n = 1016, 36.0%); single chemotherapy agent or radiotherapy (n = 382, 13.5%); and no treatment (n = 728, 25.8%). Among the fully treated, ABVD [doxorubicin (Adriamycin), bleomycin, vinblastine, dacarbazine]/AVD was most common (n = 635, 90.8%). Adjusted multinomial logistic regression identified factors associated with treatment. Older age, Medicaid dual eligibility, not married, frailty, cardiac comorbidity, prior cancer, earlier diagnosis date, histology, advanced disease Stage, B symptoms and South region were independently associated with increased odds of not receiving full chemotherapy regimens. In conclusion, we found variability in first-line HL treatment for older patients. Treatment differences by Medicaid and region may indicate disparities. Even after adjusting for frailty and cardiac comorbidity, age was associated with treatment, suggesting factors such as end-of-life care or shared decision-making may influence treatment in older patients.
Keywords: Hodgkin disease, aged, antineoplastic agents, SEER programme, healthcare disparities
Hodgkin lymphoma (HL) is a success story within haematological malignancies, with cure rates exceeding 85–90% in younger patients (National Cancer Institute; Evens, Sweetenham, & Horning, 2008; Appel et al., 2012; Meyer et al., 2012). First-line HL treatment typically includes multi-agent chemotherapy, such as ABVD [doxorubicin (Adriamycin), bleomycin, vinblastine, dacarbazine], with or without radiotherapy (RT) (Duggan et al., 2003; Hoppe et al., 2012; National Comprehensive Cancer Network, 2019). Despite treatment success in younger patients, older patients aged >60 or 65 years with HL have a 5-year overall survival of only 40–55% (Feltl, Vitek, & Zamecnik, 2006; Evens et al., 2008).
Various factors influence first-line treatment choice in older patients with HL, including inability to tolerate multi-agent chemotherapy regimens, risk of toxicity from aggressive therapy due to comorbidities and/or frailty, differences in HL biology, patient preference and clinicians’ reluctance to treat older patients as aggressively as younger patients (Evens et al., 2008; Bjorkholm, Svedmyr, & Sjoberg, 2011; Parikh, Grossbard, Green, Harrison, & Yahalom, 2015; Reagan, Magnuson, & Friedberg, 2016). Previous guidelines on HL treatment from the National Comprehensive Cancer Network (NCCN) stated that ‘individualized treatment may be necessary for older patients and patients with concomitant disease’, while more recent guidelines have specific recommendations for omitting certain drugs (e.g. bleomycin from ABVD) or using shorter courses or less toxic regimens (Hoppe et al., 2012; National Comprehensive Cancer Network, 2019). In clinical practice, drugs may be omitted from the regimen, or patients may receive fewer cycles, dose reductions or dose omissions (Engert et al., 2005; Evens et al., 2008). Additionally, some older patients are treated with palliative approaches, such as single chemotherapy agents or limited-field RT (Boll et al., 2013).
A challenge of studying older patients with HL is their underrepresentation in clinical trials (Engert et al., 2005; Abbasi, 2019). Most older patients are treated in community oncology practices, with less access to clinical trials. Further, available data about treatment of older patients with HL are limited by small sample size, short follow-up, decreased generalisability to community practices or retrospective design (Ballova et al., 2005; Evens et al., 2008; Evens et al., 2012; Evens et al., 2013; Forero-Torres et al., 2015; Friedberg et al., 2017). Therefore, we lack understanding of first-line treatment, second-line treatment after treatment failure and the associated outcomes in older HL patients.
The use of a large, longitudinal population-level database that combines clinical and claims data, such as Surveillance, Epidemiology, and End Results (SEER)-Medicare, provides data on older patients, including those treated at community practices and survival outcomes. The present study describes which HL treatments are used and identifies whether patient, disease and geographic factors are associated with treatment choice. We hypothesised that older age, frailty, comorbidity and lower socio-economic status would be associated with less treatment with full, multi-agent chemotherapy regimens.
Methods
SEER-Medicare
SEER-Medicare data link two population-based databases with detailed information on Medicare beneficiaries with cancer (National Cancer Institute; Warren, Klabunde, Schrag, Bach, & Riley, 2002). SEER contains information from cancer registries, while Medicare data contains information on claims for healthcare services, including treatment, from time of entry into Medicare until death. SEER data come from population-based tumour registries from 17 regions, which collect information (e.g. demographics, date of diagnosis, Stage, histology) on newly diagnosed patients residing in those regions. Medicare is the national health insurance programme for Americans aged ≥65 years and a small subset aged <65 years who qualify based on disability status or certain medical conditions. Nearly all Medicare beneficiaries have Part A to cover hospital, skilled-nursing facility, hospice and home healthcare. Most are also enrolled in Part B to cover physician and outpatient services. The majority of beneficiaries have fee-for-service coverage, rather than Part C (also known as Medicare Advantage). Claims for Part C services are not included in SEER-Medicare, so their healthcare service use cannot be determined. Part D is an optional outpatient prescription drug plan implemented in 2006 that covers approximately 70% of SEER-Medicare beneficiaries (National Cancer Institute). About 20% of Medicare beneficiaries are eligible for Medicaid based on low income (referred to as Medicaid dual eligibility) (Jacobson et al., 2012).
Sample
This retrospective cohort study utilised SEER-Medicare data from 1999 to 2014. Patients were aged ≥65 years at diagnosis with incident classical HL. The cohort was restricted to Medicare Part A and B fee-for-service coverage for 6 months prior to and 1 year after diagnosis (or until date of death) to fully capture claims for treatment. Exclusion criteria were: missing diagnosis month; unknown diagnostic confirmation; diagnosis reported from autopsy or death certificate; another cancer diagnosis <6 months before HL diagnosis; no claims within 6 months of diagnosis; and unknown Stage (Fig 1). Patients with only one to 10 claims within 6 months of diagnosis were reviewed for inclusion, and were required to have one or more HL-related claim(s). To understand potential differences by type of Medicare coverage, we compared available patient, disease and geographic factors by whether patients were enrolled in Medicare Part A and B (Table A1).
Fig 1.
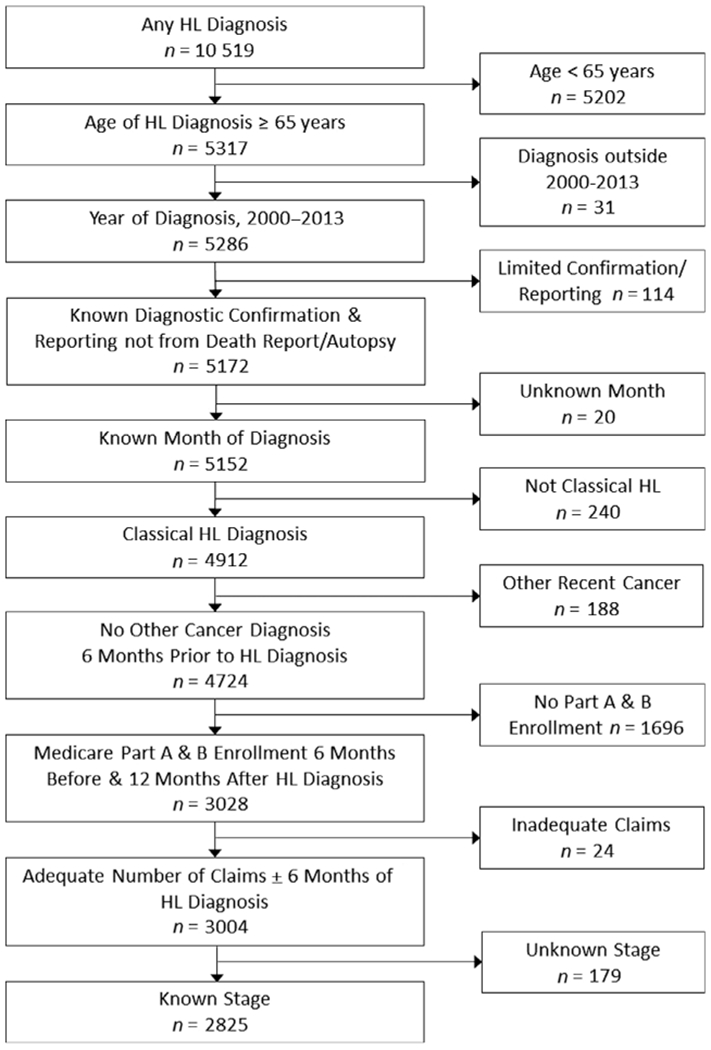
HL cohort development.
First-line treatment
First-line treatment at ≤4 months of diagnosis was determined from inpatient, outpatient and physician/supplier claims using chemotherapy J-codes, Healthcare Common Procedure Coding System (HCPCS) codes and Diagnosis Related Group (DRG) codes and was categorised as: (i) full chemotherapy regimens (hereafter ‘full regimen’); (ii) partial chemotherapy regimen (hereafter ‘partial regimen’); (iii) single chemotherapy agent or RT (hereafter ‘single agent/RT’) or (iv) no claims for chemotherapy or RT (hereafter ‘no treatment’). Full regimens were defined as the minimal number of cycles recommended for the patient’s Stage (early or advanced) based on NCCN guidelines and established chemotherapy regimens for HL, even though some patients were treated prior to the issuance of these recommendations (Table A2) (Canellos et al., 1992; Ballova et al., 2005; Boll et al., 2011; Hoppe et al., 2012; Gordon et al., 2013; National Comprehensive Cancer Network, 2019). To be classified as having received a full regimen, patients had to receive all drugs for the recommended number of cycles, but were allowed to miss one administration of one drug. Orally administered drugs (e.g. steroids, procarbazine) were not required for full regimens because they were only available in Part D pharmacy claims. Information on dose modifications was not available. Partial regimens included any multi-agent chemotherapy regimen that did not meet full regimen criteria. Single agent/RT included patients treated with one chemotherapy agent at a time and/or RT. No treatment was defined by lack of claims for chemotherapy or RT within 4 months of diagnosis.
Covariates
Patient and disease characteristics were defined based on SEER registry or Medicare data. Follow-up duration was defined as the number of months (from diagnosis) until the earliest of the following: death; end of continuous Medicare Part A and B fee-for-service enrolment; or the end of the available data (31/12/2014). Diagnosis date, age at diagnosis, gender, race/ethnicity, marital status, HL histology, Ann Arbor Stage and B symptoms were defined from SEER registry data. Medicaid dual eligibility was defined using the State ‘buy-in’ indicator. Frailty and comorbidity were defined using validated claims-based algorithms recorded in the 6 months prior to HL diagnosis (Quan et al, 2005; Segal et al., 2017). Based on published data, frailty was defined by a probability score of ≥0.12 (Segal et al., 2017). The current HL cancer was excluded from the comorbidity index. A separate cardiac comorbidity indicator was created for myocardial infarction or congestive heart failure, based on their hypothesised relationship with treatment choice. Prior cancer was defined as having a SEER-Medicare entry for any cancer >6 months prior to HL diagnosis. Missing data from the SEER registry or Medicare enrolment data are typically classified as unknown. These patients were included in analyses (except for unknown Stage), either as a separate category or collapsed with other small categories.
Geographic characteristics included region, population density and presence of a hospital providing chemotherapy within the health service area (HSA). Region and population density were determined from the Medicare enrolment file based on Zip Code. Population density was dichotomised into more populated (big metro, metro, urban) and less populated (less urban, rural). Regions included Northeast, Midwest, South and West based on SEER-registry data using Census Region Codes. The 2017–2018 Area Health Resources Files (AHRF) Access System was used to determine whether there was a hospital providing chemotherapy in the HSA during the year of HL diagnosis. Federal Information Processing Standards (FIPS) county and State codes provided the linkage between SEER-Medicare and AHRF data, using the SEER-Medicare HSA definition (National Cancer Institute).
Statistical analysis
Patient, disease and geographic characteristics were described for the whole sample and separately by first-line treatment. To further understand why patients did not receive treatment by 4 months, we explored the use of any treatment, use of hospice or death by 12 months after diagnosis (Table A3). We then described patient, disease and geographic characteristics for this group compared with treated patients (Table A4). Cell counts of <11 were suppressed and other cells were coarsened to avoid re-identification of patients in accordance with SEER-Medicare policy (Research Data Assistance Center, 2017).
Multinomial logistic regression estimated odds ratios (ORs) and 95% confidence intervals (CIs) to identify factors associated with first-line treatment. Univariate models between first-line treatment and all patient, disease and geographic characteristics were first estimated. A multivariable model that included all characteristics was then estimated to adjust for potential confounding between variables. Two-way interactions between age, Stage, frailty and cardiac comorbidity were added to a separate adjusted multivariable model based on our hypothesis that these factors would modify each other’s effect on treatment. Backwards elimination based on P < 0.05 was used to remove non-significant interaction terms. To understand the difference in the probability of each treatment category based on the non-interaction and interaction models, we used least square means to estimate the probability of each treatment category for specified levels of the interaction variables (e.g. early or advanced Stage; cardiac comorbidity or not; age 65, 75, 85 years); other variables were set to their reference or mean values. Model fit was assessed by examining potential influence points, linearity of continuous variables and collinearity (using variance inflation factors). Data were analysed using the Statistical Analysis System (sas) Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA).
Results
Sample
The cohort included 2825 patients diagnosed with HL, aged >65 years, who met eligibility criteria (Fig 1). The mean (SD, maximum) age was 76.0 (7.0, 98) years, 50.0% were female, 83.7% were White/non-Hispanic and 13.8% were Medicaid dual eligible (Table I). Over half (51.1%) met criteria for frailty, 78.7% had at least one comorbidity, 26.2% had a cardiac comorbidity and 15.9% had a prior cancer. The most common HL histology was nodular sclerosis (36.7%), patients were evenly distributed across Stage and 36.4% had B symptoms. Nearly all patients (95.5%) lived in an area with a hospital that provided chemotherapy. Patients had a median (interquartile range [IQR] Q1–Q3) of 21 (5–59) months of eligible follow-up and 1071 (37.9%) died by 12 months after diagnosis.
Table I.
Characteristics of patients at HL diagnosis by first-line treatment, n = 2825.
| Overall, n = 2825 | Full regimen, n = 699 | Partial regimen, n = 1016 | Single agent/RT, n = 382 | No treatment*, n = 728 | |
|---|---|---|---|---|---|
| Patient factors | |||||
| Age, years, mean (SD) | 76 (7.0) | 73.3 (5.9) | 74.9 (6.2) | 79.3 (7.2) | 78.2 (7.4) |
| Age categorical, n (%) | |||||
| 65–69 years | 619 (21.9) | 225 (32.2) | 242 (23.8) | 40 (10.5) | 112 (15.4) |
| 70–74 years | 671 (23.8) | 204 (29.2) | 265 (26.1) | 69 (18.1) | 133 (18.3) |
| 75–79 years | 654 (23.2) | 157 (22.5) | 262 (25.8) | 79 (20.7) | 156 (21.4) |
| ≥80 years | 881 (31.2) | 113 (16.2) | 247 (24.3) | 194 (50.8) | 327 (44.9) |
| Female, n (%) | 1413 (50.0) | 364 (52.1) | 489 (48.1) | 206 (53.9) | 354 (48.6) |
| Race/ethnicity, n (%) | |||||
| White/non-Hispanic | 2364 (83.7) | 596 (85.3) | 863 (84.9) | >320 (>83.8)† | 583 (80.1) |
| Black/non-Hispanic | 143 (5.1) | 34 (4.9) | 36 (3.5) | 21 (5.5) | 52 (7.1) |
| Hispanic | 233 (8.3) | 47 (6.7) | 91 (9) | 30 (7.9) | 65 (8.9) |
| Other race/non-Hispanic | 85 (3.0) | 22 (3.2) | 26 (2.6) | † | 28 (3.9) |
| Marital status, n (%) | |||||
| Married | 1601 (56.7) | 443 (63.4) | 621 (61.1) | 203 (53.1) | 334 (45.9) |
| Single/widowed/unknown | 1224 (43.3) | 256 (36.6) | 395 (38.9) | 179 (46.9) | 394 (54.1) |
| Medicaid dual enrolled, n (%) | 389 (13.8) | 71 (10.2) | 138 (13.6) | 45 (11.8) | 135 (18.5) |
| Frailty, n (%) | 1443 (51.1) | 205 (29.3) | 474 (46.7) | 260 (68.1) | 504 (69.2) |
| Comorbidity, n (%) | 2224 (78.7) | 497 (71.1) | 812 (79.9) | 293 (76.7) | 622 (85.4) |
| Cardiac comorbidity, n (%) | 740 (26.2) | 94 (13.5) | 247 (24.3) | 114 (29.8) | 285 (39.2) |
| Prior cancer, n (%) | 450 (15.9) | 98 (14) | 165 (16.2) | 80 (20.9) | 107 (14.7) |
| Disease factors | |||||
| Diagnosis year, n (%) | |||||
| 2000–2004 | 909 (32.2) | 190 (27.2) | 321 (31.6) | 163 (42.7) | 235 (32.3) |
| 2005–2009 | 1132 (40.1) | 318 (45.5) | 393 (38.7) | 138 (36.1) | 283 (38.9) |
| 2010–2013 | 784 (27.8) | 191 (27.3) | 302 (29.7) | 81 (21.2) | 210 (28.9) |
| Histology, n (%) | |||||
| Nodular Sclerosis | 1036 (36.7) | 310 (44.4) | 364 (35.8) | >143 (>37.4)† | 209 (28.7) |
| Mixed cellularity | 593 (21.0) | 150 (21.5) | 231 (22.7) | 70 (18.3) | 142 (19.5) |
| Lymphocyte rich | 122 (4.3) | 31 (4.4) | 33 (3.3) | 37 (9.7) | 21 (2.9) |
| Lymphocyte depleted | 92 (3.3) | 15 (2.2) | 38 (3.7) | † | 38 (5.2) |
| NOS | 982 (34.8) | 193 (27.6) | 350 (34.5) | 121 (31.7) | 318 (43.7) |
| Ann Arbor Stage, n (%) | |||||
| I | 663 (23.5) | 239 (34.2) | 114 (11.2) | 156 (40.8) | 154 (21.2) |
| II | 670 (23.7) | 282 (40.3) | 146 (14.4) | 96 (25.1) | 146 (20.1) |
| III | 767 (27.2) | 94 (13.5) | 413 (40.7) | 65 (17) | 195 (26.8) |
| IV | 725 (25.7) | 84 (12) | 343 (33.8) | 65 (17) | 233 (32) |
| B symptoms, n (%) | |||||
| No | 1167 (41.3) | 346 (49.5) | 362 (35.6) | 207 (54.2) | 252 (34.6) |
| Yes | 1027 (36.4) | 191 (27.3) | 451 (44.4) | 97 (25.4) | 288 (39.6) |
| Unknown | 631 (22.3) | 162 (23.2) | 203 (20) | 78 (20.4) | 188 (25.8) |
| Geographic factors | |||||
| Region, n (%) | |||||
| Northeast | 659 (23.3) | 151 (21.6) | 242 (23.8) | 87 (22.8) | 179 (24.6) |
| Midwest | 390 (13.8) | 114 (16.3) | 124 (12.2) | 59 (15.5) | 93 (12.8) |
| South | 685 (24.3) | 154 (22) | 256 (25.2) | 91 (23.8) | 184 (25.3) |
| West | 1091 (38.6) | 280 (40.1) | 394 (38.8) | 145 (38) | 272 (37.4) |
| Urban/rural, n (%) | |||||
| More populated | 2498 (88.4) | 618 (88.4) | 903 (88.9) | 334 (87.4) | 643 (88.3) |
| Less populated | 327 (11.6) | 81 (11.6) | 113 (11.1) | 48 (12.6) | 85 (11.7) |
| Hospital with chemotherapy, n (%) | 2698 (95.5) | 669 (95.7) | 971 (95.6) | 369 (96.6) | 689 (94.6) |
NOS, not otherwise specified.
No treatment refers to no claims for chemotherapy or RT.
Cell counts <11 were suppressed and other cells were coarsened to avoid re-identification of patients in accordance with SEER-Medicare policy
First-line treatment
Patients were classified into the following categories: 699 (24.7%) received full regimens, 1016 (36.0%) received partial regimens, 382 (13.5%) received a single agent/RT and 728 (25.8%) received no documented treatment. Among those receiving full regimens, 635 (90.8%) received ABVD/AVD (Table II). Among untreated patients (n = 728), 602 (82.7%) had an explanation for no treatment by 12 months (Table A3). For example, 90 (12.4%) died within the month of diagnosis, while 199 (27.3%) received hospice care by 6 months.
Table II.
Treatment details for patients receiving any first-line treatment, n = 2097.
| Treatment | n (%)* |
|---|---|
| Full chemotherapy regimen | 699 |
| Full ABVD/AVD | 635 (90.8) |
| Full BEACOPP | † |
| Full CHOP | † |
| Full COPP | 27 (3.9) |
| Full MOPP | † |
| Full PVAG | † |
| Full Stanford V | 22 (3.1) |
| Full VEPEMB | † |
| Partial chemotherapy regimen | 1016 |
| Partial chemotherapy regimen | 1016 (100) |
| Single chemotherapy agent or RT | 382 |
| Single chemotherapy agent only | 147 (38.5) |
| RT only | >244 (>58.6)† |
| Both | † |
Denominator for specific treatment is the number in the overall treatment category.
Cell counts < 11 were suppressed and other cells were coarsened to avoid re-identification of patients in accordance with SEER-Medicare policy.
Factors associated with first-line treatment
Univariate multinomial logistic regression indicated that the following factors were significantly associated with first-line treatment: age, marital status, Medicaid dual eligibility, frailty, cardiac comorbidity, prior cancer, diagnosis year, histology, Stage and B symptoms (Table III). Race/ethnicity, region, population density and hospitals providing chemotherapy were not associated with treatment.
Table III.
Multinomial logistic regression model of first-line treatment, n = 2825.
| Univariate, OR (95% CI) |
Multivariable, OR (95% CI) |
|||||
|---|---|---|---|---|---|---|
| Partial regimen vs. full regimen | Single agent/RT vs. full regimen | No treatmenta vs. full regimen | Partial regimen vs. full regimen | Single agent/RT vs. full regimen | No treatmenta vs. full regimen | |
| Patient factors | ||||||
| Age (per 5-year increase) | 1.23 (1.14–1.33) | 1.96 (1.78–2.16) | 1.75 (1.61–1.91) | 1.11 (1.00–1.25) | 1.75 (1.52–2.01) | 1.50 (1.33–1.69) |
| Race/ethnicity | ||||||
| White/non-Hispanic | Ref | Ref | Ref | Ref | Ref | Ref |
| Black/non-Hispanic | 0.73 (0.45–1.18) | 1.14 (0.65–2.00) | 1.56 (1.00–2.44) | 0.66 (0.38–1.14) | 1.30 (0.70–2.43) | 1.45 (0.86–2.46) |
| Hispanic | 1.34 (0.93–1.93) | 1.18 (0.73–1.91) | 1.41 (0.96–2.09) | 1.20 (0.76–1.89) | 1.46 (0.82–2.60) | 1.31 (0.81–2.13) |
| Other race/non-Hispanic | 0.82 (0.46–1.45) | 0.76 (0.35–1.66) | 1.30 (0.74–2.30) | 0.69 (0.35–1.34) | 0.91 (0.38–2.19) | 1.20 (0.61–2.35) |
| Marital status | ||||||
| Married | 0.91 (0.75–1.11) | 0.66 (0.51–0.84) | 0.49 (0.40–0.61) | 1.02 (0.81–1.29) | 0.96 (0.72–1.28) | 0.71 (0.56–0.91) |
| Single/widowed/unknown | Ref | Ref | Ref | Ref | Ref | Ref |
| Medicaid dual eligible | 1.39 (1.03–1.89) | 1.18 (0.80–1.76) | 2.01 (1.48–2.74) | 1.31 (0.90–1.92) | 1.09 (0.68–1.77) | 1.62 (1.10–2.39) |
| Frailty | 2.11 (1.72–2.59) | 5.14 (3.92–6.73) | 5.42 (4.32–6.80) | 1.53 (1.12–2.09) | 1.76 (1.19–2.60) | 1.82 (1.30–2.54) |
| Cardiac comorbidity | 2.07 (1.59–2.68) | 2.74 (2.01–3.73) | 4.14 (3.18–5.39) | 1.67 (1.23–2.25) | 2.16 (1.53–3.07) | 2.95 (2.18–4.00) |
| Prior cancer | 1.19 (0.91–1.56) | 1.63 (1.17–2.25) | 1.06 (0.79–1.42) | 1.35 (0.99–1.82) | 1.83 (1.28–2.62) | 1.17 (0.84–1.63) |
| Disease factors | ||||||
| Diagnosis year | 0.98 (0.95–1.00) | 0.91 (0.88–0.95) | 0.97 (0.94–1.00) | 0.93 (0.90–0.96) | 0.87 (0.83–0.90) | 0.93 (0.90–0.96) |
| Histology | ||||||
| Nodular sclerosis | Ref | Ref | Ref | Ref | Ref | Ref |
| Mixed cellularity | 1.31 (1.02–1.69) | 0.95 (0.67–1.33) | 1.40 (1.05–1.87) | 1.32 (0.99–1.76) | 0.86 (0.59–1.25) | 1.36 (0.99–1.88) |
| Lymphocyte depleted | 2.16 (1.17–4.00) | 0.14 (0.02–1.03) | 3.76 (2.02–7.01) | 1.61 (0.82–3.18) | 0.12 (0.02–0.93) | 2.64 (1.32–5.28) |
| Lymphocyte rich | 0.91 (0.54–1.51) | 2.42 (1.45–4.05) | 1.01 (0.56–1.80) | 1.51 (0.86–2.64) | 3.23 (1.84–5.69) | 1.51 (0.81–2.83) |
| NOS | 1.54 (1.23–1.95) | 1.27 (0.94–1.71) | 2.44 (1.90–3.14) | 1.35 (1.04–1.76) | 1.29 (0.93–1.78) | 2.22 (1.67–2.94) |
| Ann Arbor Stage | ||||||
| Early (I/II) | Ref | Ref | Ref | Ref | Ref | Ref |
| Advanced (III/IV) | 8.51 (6.82–10.62) | 1.51 (1.15–1.98) | 4.18 (3.33–5.23) | 8.60 (6.79–10.90) | 1.89 (1.40–2.55) | 4.12 (3.20–5.31) |
| B symptoms | ||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 2.26 (1.80–2.82) | 0.85 (0.63–1.15) | 2.07 (1.62–2.64) | 1.27 (0.98–1.63) | 0.70 (0.51–0.98) | 1.34 (1.01–1.77) |
| Unknown | 1.20 (0.93–1.54) | 0.81 (0.58–1.11) | 1.59 (1.22–2.08) | 0.99 (0.74–1.32) | 0.61 (0.43–0.88) | 1.32 (0.97–1.78) |
| Geographic factors | ||||||
| Region | ||||||
| Northeast | 1.14 (0.88–1.47) | 1.11 (0.80–1.55) | 1.22 (0.93–1.60) | 1.34 (1.00–1.79) | 1.15 (0.79–1.66) | 1.35 (0.98–1.85) |
| Midwest | 0.77 (0.57–1.04) | 1.00 (0.69–1.45) | 0.84 (0.61–1.16) | 0.76 (0.53–1.08) | 0.88 (0.57–1.35) | 0.78 (0.53–1.15) |
| South | 1.18 (0.92–1.52) | 1.14 (0.82–1.58) | 1.23 (0.94–1.61) | 1.45 (1.07–1.97) | 1.25 (0.85–1.85) | 1.41 (1.01–1.98) |
| West | Ref | Ref | Ref | Ref | Ref | Ref |
| Less populated | 0.96 (0.71–1.29) | 1.10 (0.75–1.61) | 1.01 (0.73–1.39) | 1.04 (0.72–1.50) | 1.37 (0.87–2.14) | 1.22 (0.82–1.81) |
| Hospital with chemotherapy | 1.03 (0.64–1.66) | 0.79 (0.41–1.53) | 1.26 (0.78–2.06) | 1.07 (0.62–1.84) | 1.37 (0.66–2.86) | 0.90 (0.51–1.59) |
NOS, not otherwise specified.
Bolding indicates P < 0.05.
No treatment refers to no claims for chemotherapy or RT.
In adjusted analysis comparing treatment with partial regimens to full regimens (Table III), frailty (OR 1.53, 95% CI 1.12–2.09), cardiac comorbidity (OR 1.67, 95% CI 1.23–1.25), not otherwise specified (NOS) histology (OR 1.35 vs. nodular sclerosis, 95% CI 1.04–1.76), advanced Stage (OR 8.60, 95% CI 6.79–10.90) and South region (OR 1.45 vs. West, 95% CI 1.07–1.97) were associated with higher odds of treatment with partial regimens compared with full regimens. More recent diagnostic year (OR 0.93, 95% CI 0.90–0.96) was associated with lower odds of treatment with partial regimens compared with full regimens.
In adjusted analysis comparing treatment with single agent/RT to full regimens (Table III), older age (OR 1.75 per 5-year increase, 95% CI 1.52–2.01), frailty (OR 1.76, 95% CI 1.19–2.60), cardiac comorbidity (OR 2.16, 95% CI 1.53–3.07), prior cancer (OR 1.83, 95% CI 1.28–2.62), lymphocyte-rich histology (OR 3.23 vs. nodular sclerosis, 95% CI 1.84–5.69) and advanced Stage (OR 1.89, 95% CI 1.40–2.55) were associated with higher odds of treatment with single agent/RT compared with full regimens. More recent diagnostic year (OR 0.87, 95% CI 0.83–0.90), lymphocyte-depleted histology (OR 0.12 vs. nodular sclerosis, 95% CI 0.02–0.93) and B symptoms (OR 0.70, 95% CI 0.51–0.98) were associated with lower odds of treatment with single agent/RT compared with full regimens.
In adjusted analysis comparing treatment with no treatment to full regimens (Table III), older age (OR 1.50 per 5-year increase, 95% CI 1.33–1.69), Medicaid dual eligibility (OR 1.62, 95% CI 1.10–2.39), frailty (OR 1.82, 95% CI 1.30–2.54), cardiac comorbidity (OR 2.95, 95% CI 2.18–4.00), lymphocyte-depleted histology (OR 2.64 vs. nodular sclerosis, 95% CI 1.32–5.28), NOS histology (OR 2.22 vs. nodular sclerosis, 95% CI 1.67–2.94), advanced Stage (OR 4.12, 95% CI 3.20–5.31), B symptoms (OR 1.34, 95% CI 1.01–1.77) and South region (OR 1.41 vs. West, 95% CI 1.01–1.98) were associated with higher odds of no treatment compared with full regimens. Being married (OR 0.71, 95% CI 0.56–0.91) and more recent diagnostic year (OR 0.93, 95% CI 0.90–0.96) were associated with lower odds of no treatment compared with full regimens.
We found significant two-way interactions between Stage and cardiac comorbidity (P = 0.03), as well as Stage and age (P = 0.003). A visual comparison of treatment probability based on least square means from the adjusted models with and without these interaction terms demonstrate few differences in the effect of Stage, age and cardiac comorbidity, when allowed to vary in the interaction model (Fig A1). That is, a comparison between the left (no interactions) and right (interactions) panels for a given age demonstrate similar probabilities of treatment for a given combination of Stage and cardiac comorbidity. Model assessment indicated no influence points, no violations of linearity and no collinearity.
Discussion
We present a large population-based analysis to evaluate first-line treatment amongst older adults with HL. One-quarter received full chemotherapy regimens, with ABVD/AVD being the most common, consistent with previously reported practices in the USA (Santoro et al., 1987; Evens et al., 2012). A substantial proportion of patients received AVD without bleomycin, probably due to existing comorbidities or fear of toxicity (Evens et al., 2013). In addition, we observed more aggressive treatment in recent years, perhaps reflecting less reluctance to treat older patients aggressively if they are able to tolerate treatment. Although only approved as first-line treatment after our study window, future research should study the use of brentuximab vedotin, which has shown preliminary tolerability and efficacy in older patients (Friedberg et al., 2017; Connors et al., 2018; Evens et al., 2018).
Wide variability in treatment of HL in older patients was observed. Based on our present analysis, this variability may not be fully explained by patient or disease factors, highlighting challenges in treating this patient population. Further, we found treatment differences by geographic region, which may reflect undesirable variation in care delivery and outcomes (Onega et al., 2008; Keating et al., 2018). The remaining variability in treatment selection, particularly palliative treatment (single chemotherapy agent or RT) or no documented treatment with chemotherapy or RT, may be explained by patient-provider shared decision-making and patient or family preferences, especially at the end-of-life (Zhang & Siminoff, 2003). We found that married patients were more likely to receive full regimens, possibly as a marker of social support or the influence of family in treatment choices (Aizer et al., 2013).
Similar to prior research in HL and other cancers, age influenced treatment choice (Evens et al., 2008; Given & Given, 2008; Bjorkholm et al., 2011). However, this was not fully explained by frailty or cardiac comorbidity given that the association between age and treatment remained significant after adjustment for these and other factors. This further highlights the role that shared decision-making and patient preference may play in treatment selection. In addition, frailty and cardiac comorbidity informed treatment. Frailty is caused by cumulative declines across multiple systems, which leads to decreased reserves, less resistance to stressors and ultimately, adverse outcomes (Fried et al., 2001). Typically, both frailty and presence of comorbidities increase with age, reducing patients’ ability to tolerate treatment with full chemotherapy regimens.
We found evidence of disparities in treatment of patients with Medicaid dual eligibility, who were more likely to receive no treatment. Given that Medicaid is the payer of last resort, we would not expect a lack of treatment claims if a patient with Medicaid dual eligibility actually received treatment (Social Security, 1965). As these results adjusted for other patient, disease and geographic factors, these suggest a possible disparity in care, similar to those observed in many health conditions (Institute of Medicine, 2003; Kawachi, Daniels, & Robinson, 2005; Warren et al., 2015; Popescu, Schrag, Ang, & Wong, 2016). Patients who are Medicaid dual eligibility may be sicker and have spent more money on healthcare (referred to as ‘spend down’), thereby qualifying for dual eligibility. Although Medicaid dual eligibility has reduced cost sharing, which may eliminate some cost-related barriers to care, there is evidence that patients who are dual eligible are sicker, have more comorbidities (including mental health issues) and face other socio-economic challenges (The Kaiser Commission on Medicaid & the Uninsured, 2010; Medicare Payment Advisory Commitee & Medicaid and CHIP Payment and Access Commission, 2018). Further, there is evidence of problems with care coordination for dual eligible beneficiaries and worse outcomes for Medicaid beneficiaries (Cassidy, 2012; Gold, Jacobson, & Garfield, 2012; Parikh et al., 2015). To improve care, disparities need to be addressed and care coordination needs to be improved for patients who are dual eligible.
We acknowledge the present study’s limitations. Although SEER-Medicare is the largest longitudinal population-based database of older adults with cancer, patients in the database are not necessarily representative of all older patients with cancer (e.g. elderly in SEER regions are less White, have lower poverty, live in urban areas, have lower cancer mortality) (Warren et al., 2002). In order to determine first-line treatment using claims data, patients were limited to those with Medicare Part A and B fee-for-service, therefore limiting generalisability by excluding patients without fee-for-service (i.e. Medicare Advantage). Similar to prior research, we found some differences in Part A and B enrolment by age, race/ethnicity, Medicaid dual eligibility, urban/rural and region (Neuman & Jacobson, 2018). However, there were minimal differences by disease factors (e.g. histology, Stage) and death by 12 months. The SEER registry provided information on some disease factors (e.g. Stage, B symptoms), but other factors, such as prognostic score or bulk, are not collected. Further, some patients have unknown status on available disease factors, which may be directly related to the treatment decisions (e.g. biopsy to determine histology not performed in patients receiving end-of-life care). Treatment misclassification is possible when using claims data, especially for no treatment, which was assumed based on the lack of any treatment-related claims. However, we were able to identify reasons for no treatment for nearly 82.7% of this group, therefore strengthening our confidence in treatment assignment. In addition, some patients classified as receiving partial regimens may have died before they were able to receive full regimens, but we estimate this as <18% (data not shown). Similarly, patients with advanced stage disease required more cycles for full chemotherapy regimens, which may partly explain why they were less likely to receive full regimens than patients with early stage disease.
In conclusion, one-quarter of older patients with HL received first-line treatment with full chemotherapy regimens. We found that factors such as age, frailty and cardiac comorbidity were associated with receipt of less aggressive or no treatment. Differences in treatment by insurance coverage and geography are disparities that need to be addressed, particularly as novel agents become available. Future research will link these treatments to clinical outcomes to better understand survival differences between older and younger patients and to identify opportunities to improve outcomes.
Acknowledgements
We thank Nicole Savidge for her assistance with manuscript preparation.
Funding
This project was supported by the National Center for Advancing Translational Sciences Award Number 1KL2TR002545 (Angie Mae Rodday) and National Heart, Lung, and Blood Institute Award Number K24HL132008 (Peter K. Lindenauer).
Appendix A
Fig A1.
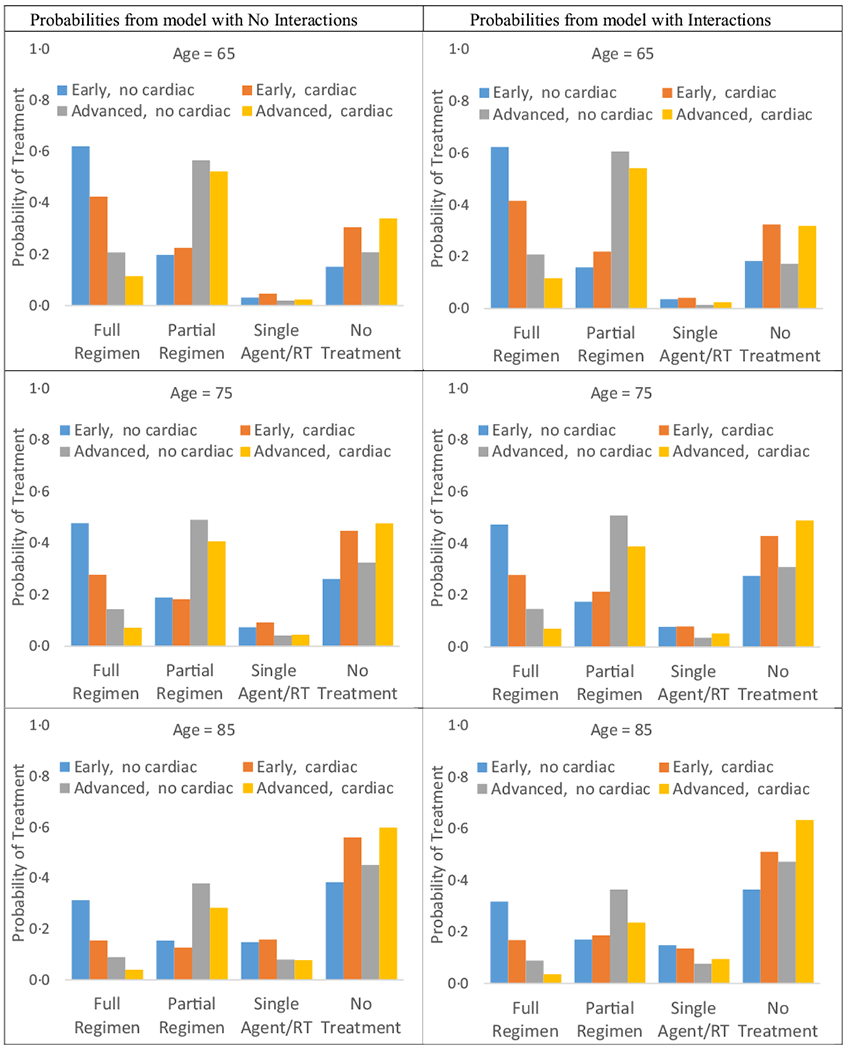
Probability of first-line treatment based on least square means from adjusted multinomial logistic regression models with and without interactionsa, n = 2825. aProbabilities estimated for the following levels of the interaction variables: early or advanced Stage; cardiac comorbidity or not; age 65, 75, 85 years. Other variables set to reference or mean values. No treatment refers to no claims for chemotherapy or RT.
Table A1.
Characteristics of patients at HL diagnosis by information enrolment in Medicare Part A and B, n = 4725
| Characteristic | Not enrolled in part A and B, n = 1696 | Enrolled in part A and B, n = 3028 | P |
|---|---|---|---|
| Patient factors | |||
| Age, years, mean (SD) | 74.2 (7.1) | 76.0 (7.0) | <0.001 |
| Female, n (%) | 758 (44.7) | 1507 (49.8) | <0.001 |
| Race/ethnicity, n (%) | |||
| White/non-Hispanic | 1232 (72.6) | 2516 (83.1) | <0.001 |
| Black/non-Hispanic | 276 (16.3) | 259 (8.6) | |
| Hispanic | 95 (5.6) | 152 (5.0) | |
| Other race/non-Hispanic | 93 (5.5) | 101 (3.3) | 0.02 |
| Marital status, n (%) | |||
| Married | 1002 (59.1) | 1685 (55.7) | |
| Single/widowed/unknown | 694 (40.9) | 1343 (44.4) | |
| Medicaid dual enrolled, n (%) | 180 (10.6) | 416 (13.7) | 0.002 |
| Prior cancer, n (%) | 228 (13.4) | 481 (15.9) | 0.02 |
| Disease factors | |||
| Year of diagnosis, n (%) | |||
| 2000–2004 | 596 (35.1) | 974 (32.2) | <0.001 |
| 2005–2009 | 585 (34.5) | 1221 (40.3) | |
| 2010–20013 | 515 (30.4) | 833 (27.5) | |
| Histology, n (%) | |||
| Nodular sclerosis | 607 (35.8) | 1109 (36.6) | 0.50 |
| Mixed cellularity | 363 (21.4) | 633 (20.9) | |
| Lymphocyte rich | 81 (4.8) | 127 (4.2) | |
| Lymphocyte depleted | 41 (2.4) | 96 (3.2) | |
| NOS | 604 (35.6) | 1063 (35.1) | |
| Ann Arbor Stage, n (%) | |||
| I | 349 (20.6) | 666 (22.0) | 0.73 |
| II | 375 (22.1) | 676 (22.3) | |
| III | 435 (25.7) | 778 (25.7) | |
| IV | 432 (25.5) | 728 (24.0) | |
| Unknown | 105 (6.2) | 180 (5.9) | |
| B symptoms, n (%) | |||
| No | 725 (42.8) | 1208 (39.9) | 0.04 |
| Yes | 596 (35.1) | 1060 (35.0) | |
| Unknown | 375 (22.1) | 760 (25.1) | |
| Died by 12 months | 605 (35.7) | 1137 (37.6) | 0.20 |
| Geographic factors | |||
| Region, n (%) | |||
| Northeast | 213 (12.6) | 714 (23.6) | <0.001 |
| Midwest | 113 (6.7) | 400 (13.2) | |
| South | 224 (13.2) | 715 (23.6) | |
| West | 1146 (67.6) | 1199 (39.6) | |
| Urban/rural, n (%) | |||
| More populated | 1636 (96.5) | 2684 (88.6) | <0.001 |
| Less populated | 59 (3.5) | 344 (11.4) |
NOS, not otherwise specified.
Table A2.
Definition of full chemotherapy regimens
| Cycles required |
||||
|---|---|---|---|---|
| Regimen | Drugs | Early Stage | Advanced Stage | Notes |
| ABVD/AVD | Doxorubicin (Adriamycin), bleomycin, vinblastine, dacarbazine | 2 | 6 | Bleomycin not required to be considered full |
| BEACOPP | Bleomycin, etoposide, doxorubicin (Adriamycin), cyclophosphamide, vincristine (Oncovin), procarbazine, prednisone | 4 | 6 | Procarbazine and prednisone not required to be considered full because orally administered |
| CHOP | Cyclophosphamide, hydroxydaunorubicin, vincristine (Oncovin), prednisone | 4 | 6 | Prednisone not required to be considered full because orally administered |
| COPP | Cyclophosphamide, vincristine (Oncovin), procarbazine, prednisone | 4 | 6 | Procarbazine and prednisone not required to be considered full because orally administered |
| MOPP | Mechlorethamine, vincristine (Oncovin), procarbazine, prednisone | 4 | 6 | Procarbazine and prednisone not required to be considered full because orally administered |
| PVAG | Prednisone, vinblastine, doxorubicin (Adriamycin), gemcitabine | 4 | 6 | Prednisone not required to be considered full because orally administered |
| Stanford V | Mechlorethamine, doxorubicin, vinblastine, vincristine, bleomycin, etoposide, prednisone | 2 | 3 | Prednisone not required to be considered full because orally administered |
| VEPEMB | Vinblastine, cyclophosphamide, prednisolone, procarbazine, etoposide, mitoxantrone and bleomycin | 4 | 6 | Procarbazine, prednisone, and etoposide not required to be considered full because orally administered |
Table A3.
Understanding patients with no treatment, n = 728
| Sequential classification*, n (%) | All classification†, n (%) | Cumulative total,‡ n | |
|---|---|---|---|
| Died within month of diagnosis | 90 (12.4) | 90 (12.4) | 90 |
| Received treatment by 6 months | 81 (11.1) | 81 (11.1) | 171 |
| Received hospice by 6 months | 172 (23.6) | 199 (27.3) | 343 |
| Died by 6 months | 183 (25.1) | 426 (58.5) | 526 |
| Received treatment by 12 months | 31 (4.3) | 112 (15.4) | 557 |
| Received hospice by 12 months | 21 (2.9) | 226 (31.0) | 578 |
| Died by 12 months | 24 (3.3) | 501 (68.8) | 602 |
| Unknown reason | 126 (17.3) | 126 (17.3) | 728 |
Patients are assigned to the group in which they first appear; patients may only be counted once.
Patients are assigned to any group to which they belong; patients may be counted more than once.
Patients from the sequential classification column are successively added to create the cumulative total.
Table A4.
Characteristics of patients at HL diagnosis by information about first-line treatment by 12 months, n = 2825
| Characteristic | Any treatment, n = 2097 | Some treatment explanation b 12 months, n = 602 | No treatment information by 12 months*, n = 126 |
|---|---|---|---|
| Patient factors | |||
| Age, years, mean (SD) | 75.2 (6.6) | 78.7 (7.3) | 76.1 (7.6) |
| Age categorical, n (%) | |||
| 65–69 years | 507 (24.2) | 81 (13.5) | 31 (24.6) |
| 70–74 years | 538 (25.7) | 102 (16.9) | 31 (24.6) |
| 75–79 years | 498 (23.8) | 135 (22.4) | 21 (16.7) |
| ≥80 years | 554 (26.4) | 284 (47.2) | 43 (34.1) |
| Female, n (%) | 1059 (50.5) | 300 (49.8) | 54 (42.9) |
| Race/ethnicity, n (%) | |||
| White/non-Hispanic | 1781 (84.9) | 480 (79.7) | 103 (81.8) |
| Black/non-Hispanic | 91 (4.3) | 41 (6.8) | 11 (8.7) |
| Hispanic | 168 (8.0) | 57 (9.5) | † |
| Other race/non-Hispanic | 57 (2.7) | 24 (4.0) | † |
| Marital status, n (%) | |||
| Married | 1267 (60.4) | 261 (43.4) | 73 (57.9) |
| Single/Widowed/Unknown | 830 (39.6) | 341 (56.6) | 53 (42.1) |
| Medicaid dual enrolled, n (%) | 254 (12.1) | 117 (19.4) | 18 (14.3) |
| Frailty, n (%) | 939 (44.8) | 448 (74.4) | 56 (44.4) |
| Comorbidity, n (%) | 1602 (76.4) | 533 (88.5) | 89 (70.6) |
| Cardiac comorbidity, n (%) | 455 (21.7) | 253 (42.0) | 32 (25.4) |
| Prior cancer, n (%) | 343 (16.4) | 83 (13.8) | 24 (19.1) |
| Disease factors | |||
| Year of diagnosis, n (%) | |||
| 2000–2004 | 674 (32.1) | 188 (31.2) | 47 (37.3) |
| 2005–2009 | 849 (40.5) | 242 (40.2) | 41 (32.5) |
| 2010–20013 | 574 (27.4) | 172 (28.6) | 38 (30.2) |
| Histology, n (%) | |||
| Nodular sclerosis | 827 (39.4) | 175 (29.1) | 34 (27.0) |
| Mixed cellularity | 451 (21.5) | 115 (19.1) | 27 (21.4) |
| Lymphocyte rich | 101 (4.8) | 12 (2.0) | † |
| Lymphocyte depleted | 54 (2.6) | 33 (5.5) | † |
| NOS | 664 (31.7) | 267 (44.4) | 51 (40.5) |
| Ann Arbor Stage, n (%) | |||
| I | 509 (24.3) | 105 (17.4) | 49 (38.9) |
| II | 524 (25.0) | 121 (20.1) | 25 (19.8) |
| III | 572 (27.3) | 161 (26.7) | 34 (27.0) |
| IV | 492 (23.5) | 215 (35.7) | 18 (14.3) |
| B symptoms, n (%) | |||
| No | 915 (43.6) | 207 (34.4) | 45 (35.7) |
| Yes | 739 (35.2) | 252 (41.9) | 36 (28.6) |
| Unknown | 443 (21.1) | 143 (23.8) | 45 (35.7) |
| Geographic factors | |||
| Region, n (%) | |||
| Northeast | 480 (22.9) | 151 (25.1) | 28 (22.2) |
| Midwest | 297 (14.2) | 79 (13.1) | 14 (11.1) |
| South | 501 (23.9) | 147 (24.4) | 37 (29.4) |
| West | 819 (39.1) | 225 (37.4) | 47 (37.3) |
| Urban/Rural, n (%) | |||
| More populated | 1855 (88.5) | 530 (88.0) | 113 (89.7) |
| Less populated | 242 (11.5) | 72 (12.0) | 13 (10.3) |
| Hospital with chemotherapy, n (%) | 2009 (95.8) | 568 (94.4) | 121 (96.0) |
NOS, not otherwise specified.
No treatment refers to no claims for chemotherapy or RT.
Cell counts < 11 were suppressed and other cells were coarsened to avoid re-identification of patients in accordance with SEER-Medicare policy.
Footnotes
Conflicts of interest
Angie Mae Rodday, Theresa Hahn, Anita J. Kumar, Peter K. Lindenauer and Susan K. Parsons: None. Jonathan W. Friedberg: Bayer (Honoraria; Data and Safety Monitoring Committee); Acerta (Data and Safety Monitoring Committee). Andrew M. Evens: Seattle Genetics (Scientific Advisory Board–Research Related); Epizyme (Scientific Advisory Board–Research Related); Novartis (Consultant—Data and Safety Monitoring Board); Pharmacyclics Novartis (Consultant—Data and Safety Monitoring Board).
References
- Abbasi J (2019) Older patients (still) left out of cancer clinical trials. JAMA, 322, 1751. [DOI] [PubMed] [Google Scholar]
- Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Hu JC & Nguyen PL (2013) Marital status and survival in patients with cancer. Journal of Clinical Oncology, 31, 3869–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel BE, Chen L, Buxton A, Wolden SL, Hodgson DC & Nachman JB (2012) Impact of low-dose involved-field radiation therapy on pediatric patients with lymphocyte-predominant Hodgkin lymphoma treated with chemotherapy: a report from the Children’s Oncology Group. Pediatric Blood & Cancer, 59, 1284–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballova V, Ruffer JU, Haverkamp H, Pfistner B , Muller-Hermelink HK, Duhmke E, Worst P, Wilhelmy M, Naumann R, Hentrich M & Engert A (2005) A prospectively randomized trial carried out by the German Hodgkin Study Group (GHSG) for elderly patients with advanced Hodgkin’s disease comparing BEACOPP baseline and COPP-ABVD (study HD9elderly). Annals of Oncology, 16, 124–131. [DOI] [PubMed] [Google Scholar]
- Bjorkholm M, Svedmyr E & Sjoberg J (2011) How we treat elderly patients with Hodgkin lymphoma. Current Opinion in Oncology, 23, 421–428. [DOI] [PubMed] [Google Scholar]
- Boll B, Bredenfeld H, Gorgen H, Halbsguth T, Eich HT, Soekler M, Markova J, Keller U, Graeven U, Kremers S, Geissler M, Trenn G, Fuchs M, von Tresckow B, Eichenauer DA, Borchmann P & Engert A (2011) Phase 2 study of PVAG (prednisone, vinblastine, dox orubicin, gemcitabine) in elderly patients with early unfavorable or advanced stage Hodgkin lymphoma. Blood, 118, 6292–6298. [DOI] [PubMed] [Google Scholar]
- Boll B, Goergen H, Arndt N, Meissner J, Krause SW, Schnell R, von Tresckow B, Eichenauer DA, Sasse S, Fuchs M, Behrin ger K, Klimm BC, Naumann R, Diehl V, Engert A & Borchmann P (2013) Relapsed hodgkin lymphoma in older patients: a compre hensive analysis from the German hodgkin study group. Journal of Clinical Oncology, 31, 4431–4437. [DOI] [PubMed] [Google Scholar]
- Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, Green MR, Gottlieb A & Peterson BA (1992) Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. New England Journal of Medicine, 327, 1478–1484. [DOI] [PubMed] [Google Scholar]
- Cassidy A (2012) Care for dual eligibles. Health Affairs, 10.1377/hpb20120613.634976. [DOI] [Google Scholar]
- Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, Younes A, Alekseev S, Hies A, Picardi M, Lech-Maranda E, Oki Y, Feldman T, Smolewski P, Savage KJ, Bartlett NL, Walewski J, Chen R, Ramchandren R, Zinzani PL, Cunningham D, Rosta A, Josephson NC, Song E, Sachs J, Liu R, Jolin HA, Huebner D & Radford J ECHELON-1 Study Group (2018) Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. New England Journal of Medicine, 378, 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan DB, Petroni GR, Johnson JL, Glick JH, Fisher RI, Connors JM, Canellos GP & Peterson BA (2003) Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: report of an intergroup trial. Journal of Clinical Oncology, 21, 607–614. [DOI] [PubMed] [Google Scholar]
- Engert A, Ballova V, Haverkamp H, Pfistner B , Josting A, Duhmke E, Muller-Hermelink K , Diehl V & German Hodgkin’s Study Group (2005) Hodgkin’s lymphoma in elderly patients: a comprehensive retrospective analysis from the German Hodgkin’s Study Group. Journal of Clinical Oncology, 23, 5052–5060. [DOI] [PubMed] [Google Scholar]
- Evens AM, Sweetenham JW & Horning SJ (2008) Hodgkin lymphoma in older patients: an uncommon disease in need of study. Oncology (Williston Park), 22, 1369–1379. [PubMed] [Google Scholar]
- Evens AM, Helenowski I, Ramsdale E, Nabhan C , Karmali R, Hanson B, Parsons B, Smith S, Larsen A, McKoy JM, Jovanovic B, Gregory S, Gordon LI & Smith SM (2012) A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood, 119, 692–695. [DOI] [PubMed] [Google Scholar]
- Evens AM, Hong F, Gordon LI, Fisher RI, Bartlett NL, Connors JM, Gascoyne RD, Wagner H, Gospodarowicz M, Cheson BD, Stiff PJ, Advani R, Miller TP, Hoppe RT, Kahl BS, Horning SJ & Horning SJ (2013) The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: a comprehensive analysis from the North American intergroup trial E2496. British Journal of Haematology, 161, 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evens AM, Advani RH, Helenowski IB, Fanale M, Smith SM, Jovanovic BD, Bociek GR, Klein AK, Winter JN, Gordon LI & Hamlin PA (2018) Multicenter Phase II study of sequential brentuximab vedotin and doxorubicin, vinblastine, and dacarbazine chemotherapy for older patients with untreated classical Hodgkin lymphoma. Journal of Clinical Oncology, 36, 3015–3022. [DOI] [PubMed] [Google Scholar]
- Feltl D, Vitek P & Zamecnik J (2006) Hodgkin’s lymphoma in the elderly: the results of 10 years of follow-up. Leukaemia & Lymphoma, 47, 1518–1522. [DOI] [PubMed] [Google Scholar]
- Forero-Torres A, Holkova B, Goldschmidt J, Chen R, Olsen G, Boccia RV, Bordoni RE, Friedberg JW, Sharman JP, Palanca-Wessels MC, Wang Y & Yasenchak CA (2015) Phase 2 study of frontline brentuximab vedotin monotherapy in Hodgkin lymphoma patients aged 60 years and older. Blood, 126, 2798–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA & Cardiovascular Health Study Collaborative Research Group (2001) Frailty in older adults: evidence for a phenotype. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 56, M146–156. [DOI] [PubMed] [Google Scholar]
- Friedberg JW, Forero-Torres A, Bordoni RE, Cline VJM, Patel Donnelly D, Flynn PJ, Olsen G, Chen R, Fong A, Wang Y & Yasenchak CA (2017) Frontline brentuximab vedotin in combination with dacarbazine or bendamustine in patients aged >/=60 years with HL. Blood, 130, 2829–2837. [DOI] [PubMed] [Google Scholar]
- Given B & Given CW (2008) Older adults and cancer treatment. Cancer, 113, 3505–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MR, Jacobson GA & Garfield RL (2012) There is little experience and limited data to support policy making on integrated care for dual eligibles. Health Aff (Millwood), 31, 1176–1185. [DOI] [PubMed] [Google Scholar]
- Gordon LI, Hong F, Fisher RI, Bartlett NL, Connors JM, Gascoyne RD, Wagner H, Stiff PJ, Cheson BD, Gospodarowicz M, Advani R, Kahl BS, Friedberg JW, Blum KA, Habermann TM, Tuscano JM, Hoppe RT & Horning SJ (2013) Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). Journal of Clinical Oncology, 31, 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Aoun P, Bello CM, Bierman PJ, Blum KA, Chen R, Dabaja B, Duron Y, Forero A, Gordon LI, Hernandez-Ilizaliturri FJ, Hochberg EP, Maloney DG, Mansur D, Mauch PM, Metzger M, Moore JO, Morgan D, Moskowitz CH, Poppe M, Pro B, Winter JN, Yahalom J, Sundar H &National Comprehensive Cancer Network (2012). Hodgkin lymphoma, version 2.2012 featured updates to the NCCN guidelines. Journal of National Comprehensive Cancer Network, 10, 589–597. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (2003). In Smedley BD, Stith AY & Nelson AR (Eds.), Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press (US). [PubMed] [Google Scholar]
- Jacobson G, Neuman T & Damico A (2012) Medicare’s role for dual eligible beneficiaries. Medicare Policy, https://www.kff.org/wp-content/uploads/2013/01/8138-02.pdf [Google Scholar]
- Kawachi I, Daniels N & Robinson DE (2005) Health disparities by race and class: why both matter. Health Aff (Millwood), 24, 343–352. [DOI] [PubMed] [Google Scholar]
- Keating NL, Huskamp HA, Kouri E, Schrag D , Hornbrook MC, Haggstrom DA & Landrum MB (2018) Factors contributing to geographic variation in end-of-life expenditures for cancer patients. Health Aff (Millwood), 37, 1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicare Payment Advisory Commitee & Medicaid and CHIP Payment and Access Commission (2018). Beneficiaries Dually Eligible for Medicare and Medicaid. [Google Scholar]
- Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Wells WA, Winter JN, Horning SJ, Dar AR, Shustik C, Stewart DA, Crump M, Djurfeldt MS, Chen BE, Shepherd LE&NCIC Clinical Trials Group, Eastern Cooperative Oncology Group (2012) ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. New England Journal of Medicine, 366, 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. SEER-medicare linked database. Retrieved 10/1/2019, from https://healthcaredelivery.cancer.gov/seermedicare/.
- National Cancer Institute. Surveillance Epidemiology and End Results Program. Health Service Areas (HSA). Retrieved 9/25/2019, from: http://seer.cancer.gov/seerstat/variables/countyattribs/hsa.htmlhttps://seer.cancer.gov/seerstat/variables/countyattribs/hsa.html.
- National Comprehensive Cancer Network (2019). Clinical Practice Guidelines in Oncology. Hodgkin Lymphoma. NCCN Evidence Blocks. Version 1.2019. Retrieved August 7, 2019, from https://www.nccn.org/professionals/physician_gls/pdf/hodgkin_blocks.pdf.
- Neuman P & Jacobson GA (2018) Medicare advantage checkup. New England Journal of Medicine, 379, 2163–2172. [DOI] [PubMed] [Google Scholar]
- Onega T, Duell EJ, Shi X, Wang D, Demidenko E & Goodman D (2008) Geographic access to cancer care in the U.S. Cancer, 112, 909–918. [DOI] [PubMed] [Google Scholar]
- Parikh RR, Grossbard ML, Green BL, Harrison LB & Yahalom J (2015) Disparities in survival by insurance status in patients with Hodgkin lymphoma. Cancer, 121, 3515–3524. [DOI] [PubMed] [Google Scholar]
- Popescu I, Schrag D, Ang A & Wong M (2016) Racial/ethnic and socioeconomic differences in colorectal and breast cancer treatment quality: the role of physician-level variations in care. Medical Care, 54, 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE & Ghali WA (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care, 43, 1130–1139. [DOI] [PubMed] [Google Scholar]
- Reagan PM, Magnuson A & Friedberg JW (2016) Hodgkin lymphoma in older patients. American Journal of Hematology/Oncology, 12, 13–19. [Google Scholar]
- Research Data Assistance Center (2017) CMS Cell Size Suppression Policy. Retrieved 1/2/2020, from https://www.resdac.org/articles/cms-cell-size-suppression-policy.
- Santoro A, Bonadonna G, Valagussa P, Zucali R, Viviani S, Villani F, Pagnoni AM, Bonfante V, Musumeci R, Crippa F & Tess JT (1987) Long-term results of combined chemotherapy-radiotherapy approach in Hodgkin’s disease: superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. Journal of Clinical Oncology, 5, 27–37. [DOI] [PubMed] [Google Scholar]
- Segal JB, Chang HY, Du Y, Walston JD, Carlson MC & Varadhan R (2017) Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Medical Care, 55, 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Social Security (1965) State plans for medical assistance. In: Sec. 1902. [42 U.S.C. 1396a]. [Google Scholar]
- The Kaiser Commission on Medicaid and the Uninsured (2010). Chronic disease and co-morbidity among dual eligibles: implications for patterns of medicaid and medicare service use and spending.
- Warren JL, Klabunde CN, Schrag D, Bach PB & Riley GF (2002) Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Medical Care, 40(Suppl. 8), IV-3-18 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- Warren JL, Butler EN, Stevens J, Lathan CS, Noone AM, Ward KC & Harlan LC (2015) Receipt of chemotherapy among medicare patients with cancer by type of supplemental insurance. Journal of Clinical Oncology, 33, 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AY & Siminoff LA (2003) The role of the family in treatment decision making by patients with cancer. Oncology Nursing Forum, 30, 1022–1028. [DOI] [PubMed] [Google Scholar]


