Abstract
Objectives:
Single institution studies have shown improved outcomes among patients with a pathologic complete response (pCR) following neoadjuvant therapy. We sought to evaluate the impact of pCR and near complete response (nCR) on overall survival (OS) using a large national database.
Methods:
The National Cancer Database was queried for patients diagnosed with pancreatic cancer from 2004–2014. A pCR was defined as no tumor identified in the pancreas following surgical resection. An nCR was defined as a primary tumor less than 1 cm without lymph node metastases. The primary outcome was OS.
Results:
A total of 5364 patients underwent neoadjuvant chemotherapy and/or radiation followed by pancreatectomy. Forty-one patients (0.8%) had a pCR, 54 (1%) had an nCR, and the remaining 5266 (98.2%) had an otherwise incomplete response. Patients with pCR had a median OS of 43 months compared with 24 months for nCR and 23 months for incomplete response (P < 0.0001). Only pCR was associated with improved OS on adjusted Cox regression.
Conclusions:
For patients diagnosed with pancreatic cancer who underwent neoadjuvant treatment and surgical resection, achieving a pCR was associated with improved OS compared to those with residual tumor. An association between nCR and improved survival was not observed.
Keywords: pathologic complete response, near complete response, overall survival, neoadjuvant therapy, pancreatic ductal adenocarcinoma
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) remains among the deadliest malignancies despite ongoing improvements in surgical and oncologic care.1,2 Surgical resection offers the only chance for cure. Advances in neoadjuvant therapy (NAT), including chemotherapy and radiation therapy, have provided means by which previously unresectable tumors could be rendered resectable. By reducing the chances of positive margin resections, previously unresectable tumors can now be safely explored with hopes of complete extirpation, while also reducing the incidence of nodal disease, and with potential impact on long-term oncologic outcomes.3–6 Based on early positive findings associated with the use of NAT, treatment of upfront resectable tumors has also expanded to incorporate use of neoadjuvant regimens.
With recent improvements in combination chemotherapy agents, including systemic agents such as 5-fluoruracil (5-FU), oxaliplatin, and irinotecan (FOLFIRINOX), or gemcitabine and nab-paclitaxel, response rates have substantially improved compared with historic regimens that most notably included single-agent gemcitabine regimens.7,8 With improved response rates, select patients have even been noted to develop a pathologic complete response (pCR) to therapy, particularly in combination with radiation regimens that have served to further sterilize the field.9 The prognostic benefit of pCR has been previously described in esophageal,10,11 gastric,12 and rectal cancer13,14 patients. By attaining a pCR after neoadjuvant treatment and resection, distinct overall survival (OS) advantages have been described, including in a recent 2019 meta-analysis of 21 studies of pCR in gastrointestinal cancers that demonstrated significantly improved OS among patients who achieved pCR.15 However, the incidence and effect of pCR in PDAC remains relatively unexplored. Single-institution studies have shown improved outcomes among those who have a pCR following NAT followed by surgical resection.16–20 A recent 2018 paper from Johns Hopkins by He and colleagues demonstrated that 10% of patients who underwent NAT had a pCR, with an OS of 60 months, compared with patients who did not undergo pCR, whose OS was 26 months.21
The principal objective of this study was to evaluate the incidence of both pCR, near complete responses (nCR), and incomplete responses (iCR) among patients with PDAC who underwent NAT through use of a large national database. Secondary objectives included identifying additional determinants of OS in patients with PDAC.
MATERIALS & METHODS
Study Design
The National Cancer Database (NCDB)22 was utilized to retrospectively evaluate patients diagnosed with PDAC from 2004–2014. The NCDB is a nationwide prospectively collected dataset created by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society that captures 70% of all new cancer diagnoses within the United States. The diagnosis of PDAC was confirmed through use of the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3).23 The following histology codes were used: 8000, 8010, 8020, 8035, 8140, 8144, 8154, 8160, 8211, 8255, 8260, 8310, 8480, 8490, 8500, 8503, 8521, 8560. Only patients who underwent surgical resection were included. The CoC site-specific surgical procedure codes were used to identify operations. Patients included had all undergone NAT, which consisted of either chemotherapy only, radiation only, or both chemotherapy and radiation. Patients were excluded if they had clinical stage 0 or IV PDAC, based on American Joint Commission on Cancer (AJCC) 7th edition,24 or if their clinical stage was unknown. Additional exclusion criteria included patients who died within 30 days of their operation as this was less likely related to disease progression. To account for variations in clinical practice trends that occurred within the study period, additional analysis of this cohort was performed based upon the year of diagnosis. The cohort was divided into the following two groups: 2004–2008 and 2009–2014. These groups were chosen due to the increased nationwide utilization of NAT in 2009 within the database. This study was exempt from Institutional Review Board review due to the de-identified nature of the database.
Variable Definitions
Patient demographics and clinical characteristics captured within NCDB include age, sex, race, and Charlson-Deyo Combined Comorbidity Score (CDCC).25 Additional demographic data consist of socioeconomic variables such as income level and insurance status. Income level data within NCDB is derived from the 2012 American Community Survey and compiles records on median household income for each patient’s zip code between 2008 and 2012. Income level is divided by quartile (quartile 1 less than $38,000, quartile 2 $38,000-$47,999, quartile 3 $48,000-$62,999, quartile 4 $63,000+). Facility-level data included within NCDB are comprised of facility type (academic, community hospital, integrated network) and hospital setting (metropolitan, urban, rural).
A pCR was defined as no tumor identified in the pancreas or associated lymph nodes by final pathology following surgical resection. A nCR was defined pathologically as a primary tumor less than 1 cm without lymph node metastases. An incomplete response (iCR) included all remaining patients. These definitions corresponded to the study by He and colleagues, which would allow for a more seamless comparison and provides a practical set of definitions by which the field can be reasonably defined.21 The primary outcome measured was OS for all groups and was defined in months from the date of diagnosis until death.
Statistical Analysis
All statistical analyses were performed using Stata software®, version SE 15.0 (StataCorp, College Station, Texas). Continuous variables were compared with the student 2-sample t-test. Categorical variables were analyzed with the Pearson’s chi-squared test. Median values are presented with interquartile range (IQR). All tests were 2-sided and statistical significance was accepted at the P < 0.05 level. Multivariable logistic regression was used to adjust for potential confounders. Cox proportional hazard analysis was used to analyze OS, which was modeled by use of a Kaplan Meier graph. Results of the multivariable adjusted cox analysis were reported as hazard ratios (HR) with corresponding 95% confidence intervals (95% CI) and P-values.
RESULTS
Patient Demographics
A total of 5364 patients were diagnosed with PDAC between 2004 and 2014 who met the inclusion criteria (Fig. 1). Of those patients, 41 (0.8%) had a pCR, 57 (1%) had a nCR, and the remaining 5266 (98.2%) had an iCR. Demographic variables, CDCC scores, operations, and AJCC clinical stages were comparable between the three groups (Table 1). The cohort was further categorized into two groups; 2004–2008 and 2009–2014. All but 1 patient with a pCR were diagnosed and received care between 2009–2014.
FIGURE 1.
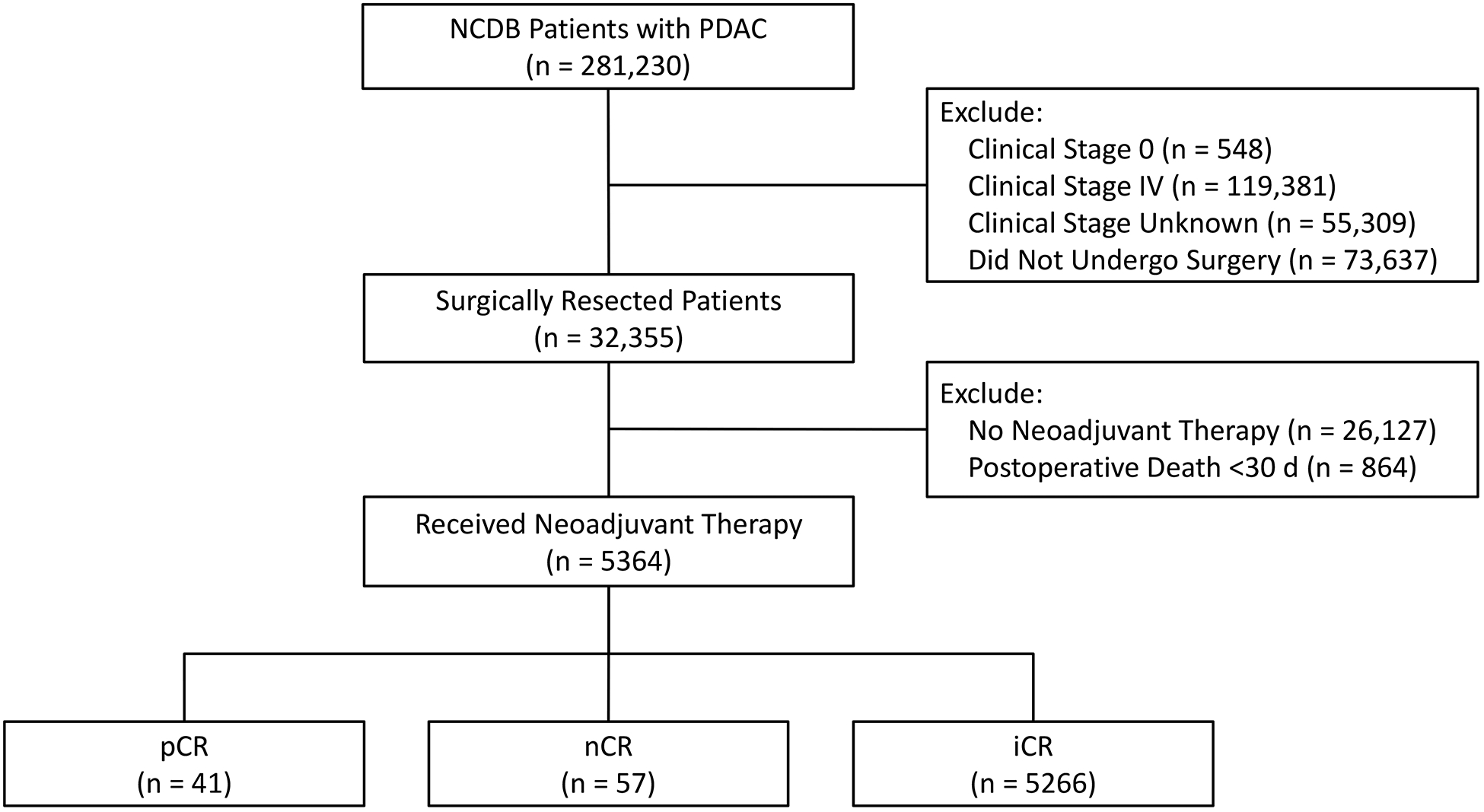
Patient allocation diagram. NCDB, National Cancer Database; PDAC, pancreatic ductal adenocarcinoma; d, days; pCR, pathologic complete response; nCR, near complete response; iCR, incomplete response.
Table 1.
Patient Demographics
| Total (n = 5364) |
pCR (n = 41) |
nCR (n = 57) |
iCR (n = 5266) |
P |
|---|---|---|---|---|
| Age, median (IQR), y | 63 (52–69) | 64 (57–68) | 64 (57–70) | 0.29 |
| Sex, male, n (%) | 20 (49) | 34 (60) | 2203 (51) | 0.43 |
| Race, n (%) | 0.68 | |||
| White | 34 (85) | 47 (85) | 4613 (89) | |
| Black | 4 (10) | 7 (13) | 467 (9) | |
| Asian | 2 (5) | 1 (2) | 120 (2) | |
| Hispanic, n (%) | 3 (8) | 3 (5) | 180 (4) | 0.29 |
| Insurance status, n (%) | 0.31 | |||
| None | 0 (0) | 2 (3) | 97 (2) | |
| Private | 22 (55) | 31 (55) | 2483 (48) | |
| Medicaid | 2 (5) | 1 (2) | 226 (5) | |
| Medicare | 14 (35) | 22 (40) | 2282 (44) | |
| Other | 2 (5) | 0 (0) | 67 (1) | |
| Median income quartile, n (%) | 0.41 | |||
| <$38,000 | 7 (17) | 5 (8.9) | 729 (14) | |
| $38,000–$47,999 | 8 (19.5) | 9 (16.1) | 1205 (23.2) | |
| $48,000–$62,999 | 9 (22) | 16 (28.6) | 1469 (28.3) | |
| >$63,000 | 17 (41.5) | 26 (46.4) | 1794 (34.5) | |
| Hospital type, n (%) | 0.42 | |||
| Academic center | 28 (68) | 44 (77) | 3391 (65) | |
| Community hospital | 9 (22) | 10 (18) | 1303 (25) | |
| Integrated network | 4 (10) | 3 (5) | 507 (10) | |
| Hospital setting, n (%) | 0.56 | |||
| Metropolitan | 37 (92) | 44 (83) | 4181 (82) | |
| Urban | 3 (8) | 8 (15) | 813 (16) | |
| Rural | 0 (0) | 1 (2) | 99 (2) | |
| Morbidity, n (%) | 0.38 | |||
| Charlson-Deyo 0 | 31 (75) | 43 (75) | 3599 (68) | |
| Charlson-Deyo 1 | 10 (25) | 12 (21) | 1358 (26) | |
| Charlson-Deyo 2+ | 0 (0) | 2 (4) | 309 (6) | |
| Clinical stage, n (%) | 0.06 | |||
| Stage I | 6 (15) | 17 (30) | 1251 (24) | |
| Stage II | 28 (68) | 24 (42) | 3091 (59) | |
| Stage III | 7 (17) | 16 (28) | 924 (17) | |
| Procedure, n (%) | 0.51 | |||
| Pancreaticoduodenectomy | 30 (73) | 43 (75) | 3873 (75) | |
| Distal pancreatectomy | 7 (17) | 4 (7) | 558 (11) | |
| Total pancreatectomy | 4 (10) | 10 (18) | 742 (14) | |
| Year of diagnosis | 0.05 | |||
| 2004–2008 | 1 (2) | 11 (19) | 778 (15) | |
| 2009–2014 | 40 (98) | 46 (81) | 4488 (85) |
Bold values are statistically significant.
Surgical Factors
Compared to patients who had an iCR, patients who had a pCR or nCR were more likely to have a chemotherapy regimen that consisted of a total neoadjuvant approach. Patients with an iCR were more likely to either receive no chemotherapy or to have undergone both neoadjuvant and adjuvant (perioperative) chemotherapy (P = 0.03). Greater than two-thirds of patients with a pCR had received neoadjuvant radiation (Table 2). While there were no significant differences in the median time from diagnosis to either chemotherapy or radiation among the groups, the pCR group had a significantly longer interval from diagnosis to surgery (195 days pCR vs 157 days nCR vs 139 days iCR; P < 0.0001).
Table 2.
Treatment Characteristics
| Total (n = 5364) |
pCR (n = 41) |
nCR (n = 57) |
iCR (n = 5266) |
P |
|---|---|---|---|---|
| Chemotherapy regimen, n (%) | 0.03 | |||
| No chemotherapy | 0 (0) | 0 (0) | 26 (1) | |
| Neoadjuvant | 34 (83) | 45 (85) | 3379 (66) | |
| Adjuvant | 0 (0) | 1 (1) | 71 (2) | |
| Both neoadjuvant & adjuvant | 7 (17) | 7 (13) | 1616 (31) | |
| Radiation regimen,* n (%) | 0.12 | |||
| No radiation | 10 (25) | 16 (28) | 1634 (31) | |
| Neoadjuvant | 30 (73) | 39 (68) | 2981 (57) | |
| Adjuvant | 1 (2) | 2 (4) | 575 (10) | |
| Both neoadjuvant & adjuvant | 0 (0) | 0 (0) | 34 (1) | |
| Diagnosis to chemotherapy, median (IQR), d | 28 (20–36) | 29 (20–41) | 27 (18–40) | 0.83 |
| Diagnosis to radiation, median (IQR), d | 51 (36–98) | 45 (26–95) | 68 (31–128) | 0.14 |
| Diagnosis to surgery, median (IQR), d | 195 (124–276) | 157 (127–212) | 139 (106–186) | 0.0001 |
Bold values are statistically significant
All patients included have undergone at least one component of neoadjuvant treatment, either chemotherapy and/or radiation. For example, if a patient received chemotherapy only in the adjuvant setting, then the patient would have received radiation in the neoadjuvant setting
With respect to negative margin resection, 100% of patients with a pCR had negative margins, compared to 95% of nCR patients, and 82% of iCR patients (P = 0.03, Table 3). There were no significant differences in hospital length of stay (LOS), 30-day readmission, or 90-day mortality between the three groups (Table 3).
Table 3.
Postoperative Outcomes
| Total (n = 5364) |
pCR (n = 41) |
nCR (n = 57) |
iCR (n = 5266) |
P |
|---|---|---|---|---|
| Resection margin, n (%) | 0.03 | |||
| Negative | 41 (100) | 54 (95) | 4152 (82) | |
| Microscopic | 0 (0) | 2 (3) | 528 (10) | |
| Macroscopic | 0 (0) | 0 (0) | 20 (1) | |
| Not otherwise specified | 0 (0) | 1 (2) | 328 (7) | |
| LOS, median (IQR), d | 8 (7–12) | 7 (6–10) | 8 (6–12) | 0.23 |
| Readmission, 30-d | 3 (8) | 6 (11) | 418 (8) | 0.80 |
| Mortality, 90-d | 0 (0) | 2 (4) | 141 (3) | 0.23 |
| Overall survival, median (IQR), mo | 43 (29–54) | 24 (16–37) | 23 (15–35) | 0.0001 |
Bold values are statistically significant
d, day; IQR, interquartile range; m, month
Analysis of Overall Survival
Patients with a pCR had a median OS of 43 months compared with 24 months for nCR and 23 months for iCR (P < 0.0001, Fig. 2). On multivariable analysis, pCR was associated with improved OS (HR, 0.38; 95% CI, 0.21–0.69; P = 0.002, Table 4). Conversely, age greater than 65 (HR, 1.15; 95% CI, 1.06–1.25; P < 0.001) and clinical stage II disease (HR, 1.13; 95% CI, 1.02–1.25; P = 0.015) were associated with worse OS. Microscopic positive margins (R1) were also associated with adverse survival on multivariable regression (HR, 1.57; 95% CI, 1.39–1.77; P < 0.001). As the majority of pCR and nCR patients were diagnosed between 2009–2014, an additional multivariable analysis was conducted to account for factors limited to the more recent study period. A pCR continued to be associated with improved OS (HR, 0.42; 95% CI, 0.23–0.76; P = 0.004, Table 5). Similarly, age greater than 65, (HR, 1.15; 95% CI, 1.06–1.26; P = 0.002), clinical stage II (HR, 1.16; 95% CI, 1.04–1.29; P = 0.01), and microscopically positive margin status (R1) (HR, 1.66; 95% CI, 1.44–1.90; P < 0.001) continued to be similarly associated with worse OS (Table 5).
FIGURE 2.

Kaplan-Meier survival analysis.
Table 4.
Multivariable Adjusted Cox Regression, All Years
| Variable | HR (95% CI) | P |
|---|---|---|
| Pathologic response | ||
| iCR | Reference | |
| nCR | 0.92 (0.63–1.33) | 0.654 |
| pCR | 0.38 (0.21–0.69) | 0.002 |
| Age, y | ||
| <65 | Reference | |
| ≥65 | 1.15 (1.06–1.25) | <0.001 |
| Sex | ||
| Male | Reference | |
| Female | 0.97 (0.90–1.05) | 0.452 |
| Race | ||
| White | Reference | |
| Black | 0.99 (0.87–1.14) | 0.959 |
| Asian | 0.80 (0.60–1.06) | 0.120 |
| Comorbidity | ||
| CDCC 0 | Reference | |
| CDCC 1 | 0.98 (0.89–1.07) | 0.589 |
| CDCC 2+ | 1.10 (0.94–1.30) | 0.244 |
| AJCC clinical stage | ||
| Clinical stage I | Reference | |
| Clinical stage II | 1.13 (1.02–1.25) | 0.015 |
| Clinical stage III | 1.13 (1.00–1.28) | 0.050 |
| Procedure | ||
| Pancreaticoduodenectomy | Reference | |
| Distal pancreatectomy | 0.90 (0.78–1.03) | 0.119 |
| Total pancreatectomy | 1.03 (0.92–1.15) | 0.619 |
| Margin status | ||
| Negative | Reference | |
| Microscopic | 1.57 (1.39–1.77) | <0.001 |
| Macroscopic | 1.70 (0.96–3.00) | 0.069 |
| Not otherwise specified | 1.55 (1.33–1.81) | <0.001 |
Bold values are statistically significant
CDCC indicates Charlson-Deyo Combined Comorbidity Score; AJCC, American Joint Committee on Cancer
Table 5.
Multivariable Adjusted Cox Regression, 2009–2014
| Variable | HR (95% CI) | P |
|---|---|---|
| Pathologic response | ||
| iCR | Reference | |
| nCR | 0.96 (0.60–1.53) | 0.863 |
| pCR | 0.42 (0.23–0.76) | 0.004 |
| Age, y | ||
| <65 | Reference | |
| ≥65 | 1.15 (1.06–1.26) | 0.002 |
| Sex | ||
| Male | Reference | |
| Female | 0.98 (0.90–1.08) | 0.728 |
| Race | ||
| White | Reference | |
| Black | 1.00 (0.86–1.17) | 0.990 |
| Asian | 0.80 (0.58–1.10) | 0.175 |
| Comorbidity | ||
| CDCC 0 | Reference | |
| CDCC 1 | 1.02 (0.92–1.13) | 0.734 |
| CDCC 2+ | 1.14 (0.95–1.38) | 0.160 |
| AJCC clinical stage | ||
| Clinical stage I | Reference | |
| Clinical stage II | 1.16 (1.04–1.29) | 0.010 |
| Clinical stage III | 1.12 (0.97–1.29) | 0.136 |
| Procedure | ||
| Pancreaticoduodenectomy | Reference | |
| Distal pancreatectomy | 0.90 (0.77–1.06) | 0.201 |
| Total pancreatectomy | 1.05 (0.93–1.18) | 0.472 |
| Margin status | ||
| Negative | Reference | |
| Microscopic | 1.66 (1.44–1.90) | <0.001 |
| Macroscopic | 1.90 (0.98–3.66) | 0.056 |
| Not otherwise specified | 1.49 (1.25–1.77) | <0.001 |
Bold values are statistically significant
CDCC indicates Charlson-Deyo Combined Comorbidity Score; AJCC, American Joint Committee on Cancer.
DISCUSSION
The results of this study indicate that for patients diagnosed with PDAC who undergo NAT followed by surgical resection, achieving a pCR is associated with improved OS (43 months) when compared to those with residual tumor within the specimen. Importantly, an association between nCR and improved survival was not observed (24 months for nCR vs. 23 months for iCR).
In an institutional series of 135 patients with PDAC, Chun and colleagues described a group of 68 “partial responders,” defined as having 50–94% fibrosis on pathology.17 While major responders (n = 21) with 95–100% fibrosis had a 66 month survival, patients who were partial responders had a median OS of 20 months, which was statistically similar to the 18 patients with a minor response (0–49% fibrosis) group whose median OS was 17 months. These findings were consistent with findings from this national database analysis. In a separate study, He and colleagues reported similar findings among their patients with borderline resectable and locally advanced disease. Patients with an nCR had a median OS of 27 months while patients with a limited response had a similar median OS of 26 months, which were both significantly lower than the pCR group whose median OS was 60 months.21 Both of these institutional reports were concordant with our nationwide findings that any pathological response short of a pCR appears to have no documented association with improved OS.
While the relationship between pCR and survival is well-established in other GI malignancies,10–14 the nationwide incidence of pCR among PDAC patients, by contrast, is just starting to emerge. There is distinct variation in pCR incidence rates at individual institutions compared to this nationwide database. Large cancer centers in the United States have thus far reported the following pCR rates: Fox Chase Cancer Center 7%, Moffitt Cancer Center 7%, and Johns Hopkins 10%.17,20,21 Similarly, a French collaborative multicenter study confirmed similar pCR rates of 10.8% with modern systemic neoadjuvant therapy regimens.26 Although the nationwide incidence of 0.8% reported above is substantially lower than those of individual high-volume cancer centers, it is important to note that the NCDB cohort is comprised of patients cared for at a variety of hospital types across the country and may more accurately represent outcomes across a variety of institutions across the United States. Importantly, however, this study, based on a large national database, still corroborates prior findings that pCR is associated with improved OS among patients diagnosed with PDAC and that nCR does not appear to be associated with improved performance. Additionally, the nationwide incidence of 0.8% reported in this study provides potential justification for centralization of care of patients with PDAC to high-volume hospitals that may offer enhanced multimodal and multidisciplinary care with strict processes that are more rigorously monitored and associated with improved oncologic outcomes, such as increased likelihood of obtaining a pCR and improved OS. Finally, the discrepancy in pCR rates may also be due to changes in trends of utilization of NAT. This study encompasses a timeframe that includes years prior to widespread implementation of NAT. Of the 41 patients within our cohort who had a pCR, 40 of them were diagnosed during or following 2009. For this reason, the data were additionally analyzed at separate time intervals.
While NAT constitutes the current standard of care for borderline resectable and locally advanced unresectable PDAC,4,5 the OS advantage conferred by pCR and overall improved outcomes has provided impetus for treating patients with upfront resectable tumors with NAT. Although no current prospective data have evaluated the use of NAT for resectable disease, several retrospective studies have demonstrated an association with improved survival in patients who undergo NAT. In a report by Mokdad and colleagues using the NCDB,6 the authors demonstrated that resectable patients who were treated with NAT were more likely to have a lower pathologic T stages, negative resection margins, and absence of lymph node metastases on final pathology. Similar findings were conferred in multiple prior studies.27–29 A positive-margin resection was a factor associated with worse OS on multivariable analysis in this study, and may have played some role in the worse outcomes associated with nCR and iCR. Interestingly, however, when outcomes were stratified according to margin positivity in the nCR group, there was no significant difference in OS between patients who had negative or positive margins (median OS 23 months vs 22 months, respectively). These data further confirm that a survival advantage was only associated with pCR as highlighted above.
Limitations of this study include the inability to accurately identify chemotherapy or radiation regimens. In addition, information regarding the extent of tumors with respect to local advancement at presentation could not be identified. The database additionally could not distinguish between tumor progression versus regression within the ultimately defined categories of nCR or iCR groups, given that initial corresponding tumor sizes not known. The data regarding margin is confounded by the outcome of complete response since every specimen with a complete response would have a negative margin. It is not surprising that age correlated with survival as this database only reports OS and not disease-specific survival. Finally, the majority of patients treated were in the top income bracket and had private insurance, which may not be representative of all patients with PDAC and is an important disparity to note.
CONCLUSION
For patients diagnosed with PDAC who underwent neoadjuvant treatment followed by surgical resection, achieving a pCR was associated with improved OS compared to those with residual tumor within the specimen. Interestingly, an association between nCR and improved survival was not observed.
Conflicts of Interest and Source of Funding:
NMS was supported by the NIH T32 Research Training in Aging grant 5T32AG023480-13. The NIH had no involvement in study design; collection, analysis or interpretation of data; writing of the report; or decision to submit the article for publication.
T.S.H. is on the Advisory Board for Merck and consults for Synthetic Biologics®. He additionally receives research support for clinical trials from Taiho®, Astra-Zeneca®, Tesaro®, BMS®, & IntraOp®.
Footnotes
This study was presented at the 2019 Annual Meeting of the Society for Surgical Oncology (SSO), on March 29, 2019, in San Diego, CA and at the 2019 Annual Meeting of the Pancreas Club, on May 18, 2019 in San Diego, CA.
The rest of the authors declare no conflict of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massucco P, Capussotti L, Magnino A, et al. Pancreatic resections after chemoradiotherapy for locally advanced ductal adenocarcinoma: analysis of perioperative outcome and survival. Ann Surg Oncol. 2006;13:1201–1208. [DOI] [PubMed] [Google Scholar]
- 4.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michelakos T, Pergolini I, Castillo CF, et al. Predictors of Resectability and Survival in Patients with Borderline and Locally Advanced Pancreatic Cancer who Underwent Neoadjuvant Treatment with FOLFIRINOX. Ann Surg. 2019;269:733–740 [DOI] [PubMed] [Google Scholar]
- 6.Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35:515–522. [DOI] [PubMed] [Google Scholar]
- 7.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2012;118:268–277. [DOI] [PubMed] [Google Scholar]
- 9.Katz MH, Shi Q, Ahmad SA, et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 2016;151:e161137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol. 2010;17:1159–1167. [DOI] [PubMed] [Google Scholar]
- 11.Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansour JC, Tang L, Shah M, et al. Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann Surg Oncol. 2007;14:3412–3418. [DOI] [PubMed] [Google Scholar]
- 13.de Campos-Lobato LF, Stocchi L, da Luz Moreira A, et al. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol. 2011;18:1590–1598. [DOI] [PubMed] [Google Scholar]
- 14.Dossa F, Acuna SA, Rickles AS, et al. Association between adjuvant chemotherapy and overall survival in patients with rectal cancer and pathological complete response after neoadjuvant chemotherapy and resection. JAMA. 2019;4:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan T, Zhang XF, Liang C, et al. The Prognostic Value of a Pathologic Complete Response After Neoadjuvant Therapy for Digestive Cancer: Systematic Review and Meta-Analysis of 21 Studies. Ann Surg Oncol. 2019;26:1412–1420. [DOI] [PubMed] [Google Scholar]
- 16.Moutardier V, Magnin V, Turrini O, et al. Assessment of pathologic response after preoperative chemoradiotherapy and surgery in pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2004;60:437–443. [DOI] [PubMed] [Google Scholar]
- 17.Chun YS, Cooper HS, Cohen SJ, et al. Significance of pathologic response to preoperative therapy in pancreatic cancer. Ann Surg Oncol. 2011;18:3601–3607. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118:3182–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Q, Rashid A, Gong Y, et al. Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Ann Diagn Pathol. 2012;16:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellon EA, Jin WH, Frakes JM, et al. Predictors and survival for pathologic tumor response grade in borderline resectable and locally advanced pancreatic cancer treated with induction chemotherapy and neoadjuvant stereotactic body radiotherapy. Acta Oncol. 2017;56:391–397. [DOI] [PubMed] [Google Scholar]
- 21.He J, Blair AB, Groot VP, et al. Is a Pathological Complete Response Following Neoadjuvant Chemoradiation Associated With Prolonged Survival in Patients With Pancreatic Cancer? Ann Surg. 2018;268:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Database. American College of Surgeons. https://www.facs.org/quality-programs/cancer/ncdb. Accessed 9 July 2018.
- 23.International Classification of Diseases for Oncology, Third Edition, First Revision. Geneva: World Health Organization, 2013. http://codes.iarc.fr/. Published 2013. Accessed 9 July 2018. [Google Scholar]
- 24.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 25.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 26.Pietrasz D, Turrini O, Vendrely V, et al. How Does Chemoradiotherapy Following Induction FOLFIRINOX Improve the Results in Resected Borderline or Locally Advanced Pancreatic Adenocarcinoma? An AGEO-FRENCH Multicentric Cohort. Ann Surg Oncol. 2019;26:109–117. [DOI] [PubMed] [Google Scholar]
- 27.Lee AJ, Simoneau E, Chiang YJ, et al. Is early-stage pancreatic adenocarcinoma truly early: stage migration on final pathology with surgery-first versus neoadjuvant therapy sequencing. HPB. 2019;21:1203–1210. [DOI] [PubMed] [Google Scholar]
- 28.Golcher H, Brunner TB, Witzigmann H, et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol. 2015;191:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzeng CW, Tran Cao HS, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18:16–24; [DOI] [PubMed] [Google Scholar]


