Abstract:
Skin manifestations of COVID-19 infections are diverse and are new to the dermatology community. We had the opportunity to examine the clinical and histopathological features of several patients who were divided into 3 groups. The first group included 8 COVID-19–positive patients who were hospitalized and quarantined at home. The second group included children and young adults who presented with chilblain erythema, erythema multiforme, and urticaria-like lesions. This group of patients was negative for the COVID-19 gene sequences by polymerase chain reaction but had a high risk of COVID-19 infection. The third group included clinically heterogeneous and challenging lesions. These patients were not subject to either polymerase chain reaction tests or serological analyses because they sought dermatological attention only for a dermatosis. The histopathological analysis of these cases showed a wide spectrum of histopathological patterns. What appears to be constant in all skin biopsies was the presence of prominent dilated blood vessels with a swollen endothelial layer, vessels engulfed with red blood cells, and perivascular infiltrates, consisting mainly of cytotoxic CD8+ lymphocytes and eosinophils. In 2 cases, there was diffuse coagulopathy in the cutaneous vascular plexus. In the early phases of the disease, there were numerous collections of Langerhans cells in the epidermis after being activated by the virus. The presence of urticarial lesions, chilblains, targetoid lesions (erythema multiforme–like lesions), exanthema, maculohemorrhagic rash, or chickenpox-like lesions associated with the histopathological features mentioned previously should cause clinical dermatologists to suspect the possibility of COVID-19 infection, especially in patients with fever and cough.
Key Words: COVID-19 skin disease, COVID-19 skin histopathology, COVID-19 dermatopathology, COVID-19 skin pathology
INTRODUCTION
In the initial and dramatic phases of the COVID-19 emergency in Northern Italy, the intensive care units were at the verge of collapse. Infectious disease specialists and anesthesiologists had no time to deal with the cutaneous manifestations caused by this disease. It was a month or so after the beginning of the outbreak when dermatologists became aware of the cutaneous manifestations of this disease. The virus can affect the skin producing different lesions, including urticarial rashes, livedoid vasculopathy, and papulovesicular exanthems. During the acute phase, the skin lesions were overlooked, leading to a delay in the description of the clinical and histopathological manifestations of the coronavirus. As a matter of fact, up to this point, there are only a few histopathological descriptions of the cutaneous manifestations of the disease.
MATERIAL AND METHODS
For this study, we divided the patients into 3 groups. The first group was composed of 8 patients who tested positive for COVID-19 with active infection; some of these patients were hospitalized, others were quarantined at home, and a few were treated for the disease. We recently published the data of these patients.1,2 Clinically, some of these patients did not have serious clinical symptomatology and presented with a maculopapular rash and were treated with oxygen support, hydroxychloroquine, and antibiotics (Fig. 1A).
FIGURE 1.
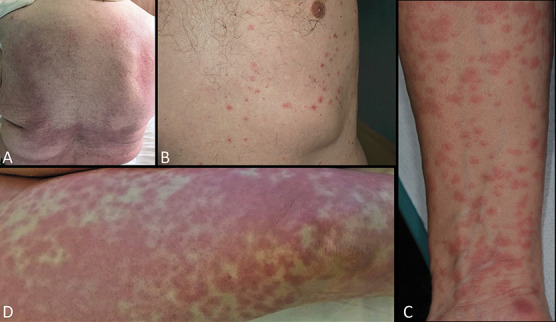
First group: COVID-19 active infection with PCR positive test: A, Papulomacular exanthema. B, Chickenpox appearance of crusted papular lesions. C, Papulomacular lesions coalescing into small plaques. D, Diffuse livedoid exanthema in an intubated patient.
The second group included patients from the Lecco hospital, located at 50 km from Milan (Lombardy region), and their clinical findings have been already reported.3 This group comprised children and young adults (13–39 years of age), with lesions clinically similar to chilblain erythema, erythema multiforme, and urticaria. These patients tested negative for COVID-19 gene sequencing by polymerase chain reaction (PCR) but had most probably a COVID-19 infection, either being in the same household of relatives affected by the disease or having similar skin lesions as those presented in affected siblings. Also, the close timing of the appearance of the skin lesions with the COVID-19 pandemic, rapid outbreak, and the reporting of several cases with similar features from other areas affected by the pandemic strongly support a concomitant COVID-19 infection. In all cases, the clinical picture was reproducible with chilblain erythema at the limbs (Figs. 2A–C, F) and urticarial lesions, some of which showed a target-like appearance similar to erythema multiforme. Generally, few days after the onset, the urticarial stage evolves toward the chilblain feature (Figs. 2D, E).
FIGURE 2.
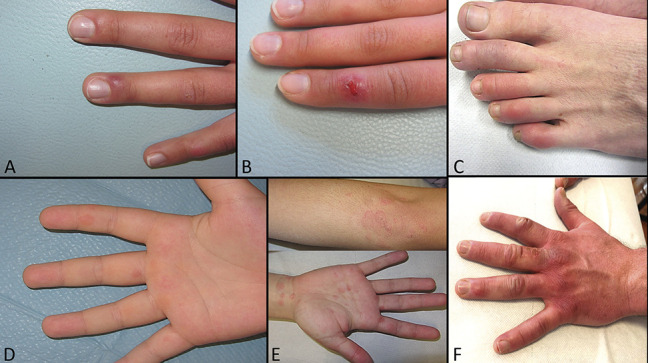
Second group: most probably COVID-19 infection with a negative PCR test. A and B, Chilblain erythema, one in the early ulcerated phase. C, Erythematous indurated plaques on the digits of a 28-year-old man. D, Early targetoid phase of chilblains. E, Erythema multiforme–like lesions. F, Fully developed chilblain erythema in a 36-year-old man.
Finally, we identified the third group that was composed of patients who were at high suspicious for active SARS-CoV-2 infection. This clinically heterogeneous and not previously reported group was the most challenging and tricky to diagnose. These patients did not have the possibility to be tested by PCR and/or serology because they were seeking attention at the dermatological clinic only for a dermatosis. At the time of their visit in Milan (the capital of the Lombardy region), these tests were reserved only for medical staff, paramedical staff, and patients with systemic symptoms strongly suspicious for an SARS-CoV-2 infection. They presented with fever, cough, and ageusia, but they were also immunocompromised by either a previous malignant condition or HIV positivity (Figs. 3A–J).
FIGURE 3.
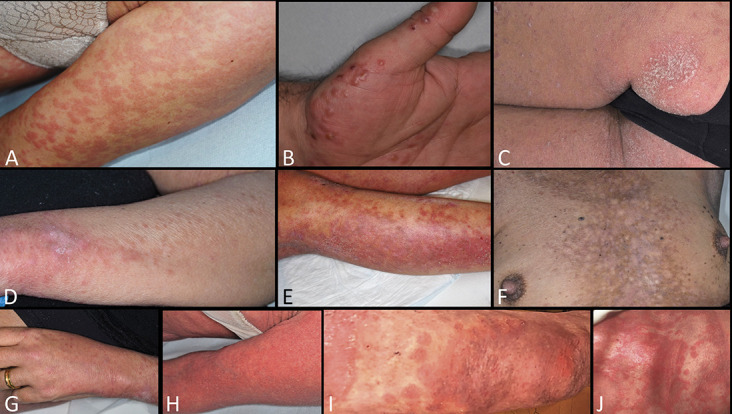
Third group: highly suspicious for COVID-19 infection—PCR test not performed. A, An neoplastic immunodeficient patient with exanthematous lesions. B, Papulovesicular lesions in an HIV+ patient. C, Psoriasiform plaques on the buttock. D, Papules coalescing into plaques. E, Maculohemorrhagic rash. F, Livedoid reticulated lesions. G, Papules on the hand. H–J, Erythrodermic psoriasis with maculohemorrhagic rash.
HISTOPATHOLOGICAL FINDINGS
First Group: COVID-19 With Active Infection—Positive PCR Test
In the purely exanthematic phase, the histopathological examination showed only mild spongiosis. The dermis was edematous with highly dilated capillaries. There were also a few extravasated red blood cells and rare eosinophils among the collagen bundles (Fig. 4A).
FIGURE 4.
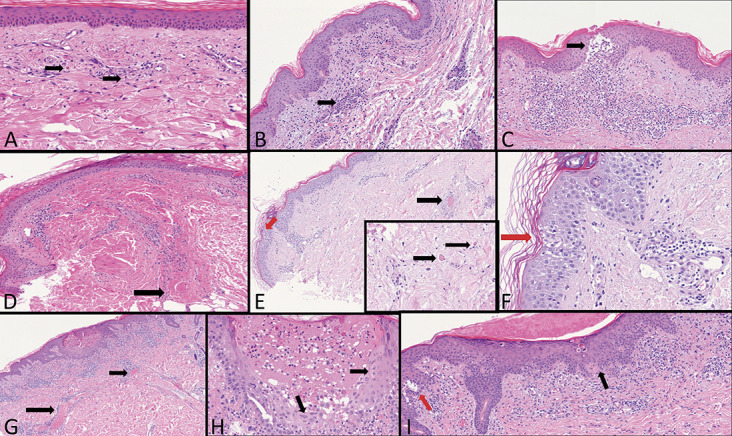
First group: COVID-19 active infection with PCR positive test: A, Highly dilated capillaries, initial blood extravasations, and some rare eosinophils (arrows). B, Dense perivascular lymphocytes cuffs around small capillary vessels (arrow). C, A large nest of Langerhans cells (arrow) in the superficial dermis dense perivascular lymphoid infiltration with extravasated red blood cells. D, A large thrombus in the deep vascular plexus (arrow). E, A large thrombus in the mid dermis (black arrow). Intraepidermal Langerhans cell nest (red arrow). Inset: Small thrombi in the deep dermis (arrows). F, Nest of Langerhans cells (red arrow). G, Extremely dilated vessels in the mid dermis in a Grover-like dermatosis (arrows). H, A vesicle-pustule surrounded by keratinocytes with cytopathic changes similar to herpetic infections (arrows). I, Sovra-epidermal clefts (red arrow), dyskeratotic keratinocytes, and patchy band-like infiltration with exocytosis (black arrow).
In the papular phase, there was slight exocytosis with minimal vacuolar changes near the dermal–epidermal junction. The papillary dermis was frankly edematous with abnormally swollen and dilated capillaries. Eosinophils were increased, blood extravasation was more prominent, and there were also collections of perivascular lymphocytes (Fig. 4B). In one of these patients (Fig. 1C), there were nests of intraepidermal Langerhans cells associated with signs of vasculitis and extravasation of red blood cells (Fig. 4C).
Two of the patients were hospitalized in the intensive care unit with severe systemic and pulmonary symptoms and had a livedoid exanthematous eruption (Fig. 1D). Serial sections (data not previously published) showed the nest of Langerhans cells in the epidermis. In the deep dermis and occasionally in the superficial dermis, there were microthrombi admixed with nuclear and eosinophilic debris (Figs. 4D–F). Diffuse livedoid exanthema seems to be related to a multiorgan involvement.
Finally, 2 patients, one of whom was under home quarantine, had been clinically diagnosed with chickenpox eruption (Fig. 1B). Curiously, he presented with an acantholytic dermatitis resembling Grover disease. The biopsy showed the characteristic clefts in the lower epidermis, as well as dyskeratotic keratinocytes in the granular layer and also near the basement membrane. Some of the keratinocytes were multinucleated and had nuclear inclusions. The second patient displayed more prominent histological changes. In the superficial and mid dermis, the capillary vessels were abnormally dilated and engulfed with erythrocytes. A band-like, patchy perivascular dermatitis, marked exocytosis, necrotic keratinocytes, combined to junctional lymphocytic satellitosis were present. There was also a vesicle-pustule surrounded by keratinocytes with cytopathic changes simulating a herpetic infections (Figs. 4G–I).
Second Group: Most Probably COVID-19 Infection—Negative PCR Test
Histopathological and immunohistochemical findings of this group have now completed. The findings of 4 of these patients have not been previously reported. Histologically, the lesions clinically diagnosed as erythema multiforme showed an urticarial pattern with full-thickness diffuse edema of the dermis, dilated vessels, and interstitial lymphocytic infiltration with numerous eosinophils. An interface dermatitis was absent, and necrotic keratinocytes were not observed (Figs. 5A, B). Cases diagnosed clinically with pernio features were characterized by a dense coat-sleeve–like perivascular lymphoid infiltrate with a prevalence of cytotoxic CD8+ lymphocytes (Figs. 5D, G). There were also inflammatory infiltrates with many eosinophils involving the secretory portion of the sweat glands (Fig. 5I). In some cases, there were microthrombi in the small dermal vessels associated with secondary epithelial damage (Figs. 5E, F, H). In one case, scattered necrotic keratinocytes were observed but no evidence of epithelial damage by cytotoxic lymphocytes. Epidermal cells underwent necrosis following severe destruction of the papillary plexus and prominent blood extravasation (Fig. 5E). Finally, acute edema and severe vascular damage led in one case to a large bullous junctional detachment, with lymphocytes and red blood cells floating inside the blisters (Fig. 5C).
FIGURE 5.
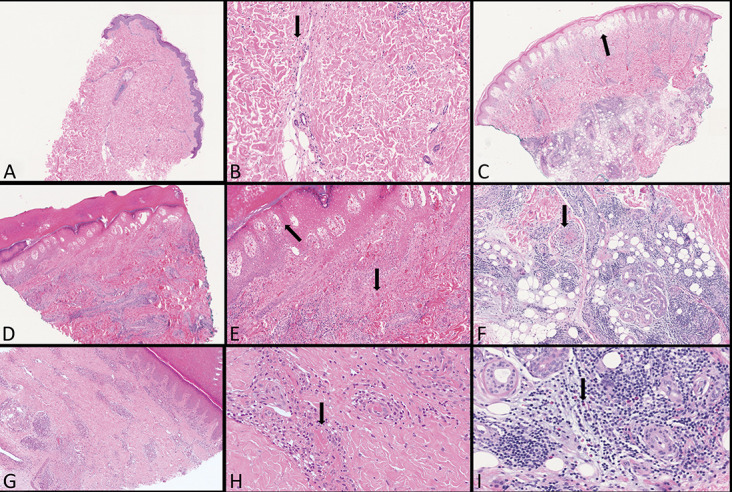
Second group: most probably COVID-19 infection with a negative PCR test. A and B, Urticarial pomphoid stage with swollen vessels and few eosinophils (arrow). C, Acute edema and severe vascular damage led to a large bullous junctional detachment (arrow). D and E, Diffuse dense CD8+ lymphocytes around the vascular plexus. Epithelial necrosis following severe destruction of the papillary plexus with diffuse blood extravasations (arrows). F, A thrombus in a deep dermis vessel (arrow). G and H, Diffuse perivascular cuffs in a chilblains with a small thrombus (arrow). I, Clusters of eosinophils around deep glomerular glands (arrow).
Third Group: Highly Suspicious for COVID-19 Active Infection—PCR Test Not Performed
Two immunodeficient patients with previous gastric and pulmonary carcinoma showed a diffuse papular macular rash on the trunk and upper limbs (Fig. 3A). The histopathological examination revealed a superficial perivascular spongiotic dermatitis with minimal lymphocytic exocytosis and intraepidermal nests of Langerhans cells. The dermis was edematous with dilated capillaries surrounded by lymphocytes and scattered interstitial eosinophils (Fig. 6A). In cases with more conspicuous papular lesions, there was a massive perivascular infiltrate with numerous extravasated red blood cells and large Langerhans nests. An HIV-positive patient (Fig. 3B) presented with vesicular lesions resembling erythema multiforme and pernio-like lesions. Histologically, there was a patchy band-like, superficial and deep perivascular infiltrates, widespread spongiosis with vesicle formations, epidermotropic CD8+ lymphocytes, focal necrotic keratinocytes, and lymphocytic microsatellitosis (Figs. 6F, G). In the dermis, the vessels were dilated and surrounded by prominent lymphocytic infiltrates (Fig. 6H). There were numerous eosinophils and extravasated red blood cells. Deeply, the eccrine glomeruli were infiltrated by lymphocytes (Fig. 6I).
FIGURE 6.
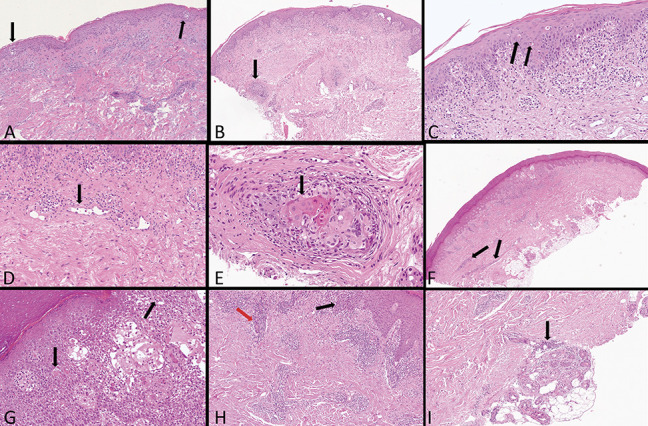
Third group: highly suspicious for COVID-19 infection—PCR test not performed. A, Nests of Langerhans cells (arrows). B, Band-like infiltration diffusely epidermo and folliculotropic (arrow). C, Diffuse necrotic intraepidermal keratinocytes with satellitosis (double arrows). D, Abnormally swollen vessels in the superficial dermis (arrow). E, Large ballooning keratinocytes with nuclear features resembling a cytopathic viral infection in a hair follicle (arrow). F, Superficial and deep perivascular vesiculospongiotic dermatitis with cuff of lymphocytes around deep vessels (arrows). G, Necrotic keratinocytes with lymphocytic satellitosis (arrows). H, Marked exocytosis (black arrow) and periductal lymphocytosis (red arrow). I, Deep glomerular glands surrounded by lymphocytes (arrow).
The most intriguing case concerned a 51-year-old woman with polycystic kidney who had clinically polymorphic dermatitis associated with cough, asthenia, and ageusia. The dermatological picture was complex. On the trunk, we could see a reticulated pigmented dermatitis reminiscent of prurigo pigmentosa by Nagashima4 (Fig. 3F). On the elbows, the buttocks (Fig. 3C), and capillitium, psoriasiform lesions were noticed. On the arms, there were papular confluent lesions in plaques (Fig. 3D), whereas in the lower limbs, erythematous macular lesions similar to vasculitis were visible (Fig. 3E). Histologically, a lichenoid dermatitis with marked epidermotropism, numerous necrotic keratinocytes, and conspicuous signs of lymphocytic satellitosis were present (Figs. 6B, C). The superficial dermis was edematous combined with dilated capillaries, surrounded by lymphocytes and eosinophils throughout the dermis (Fig. 6D). Surprisingly, large ballooning keratinocytes with nuclear features resembling a cytopathic viral infection were evident in a hair follicle (Fig. 6E). Ten days after the biopsy, the lesions regressed. Finally, a 78-year-old woman who had suffered from guttate psoriasis for many years abruptly presented with erythrodermic psoriasis associated with fever and cough. Psoriasis was superimposed on a maculopurpuric rash (Fig. 3H, I, J). Histopathology showed classical epidermal features of psoriasis. In the superficial dermis we observed edema, swollen and dilated vessels surrounded by lymphocytes and eosinophils.
DISCUSSION
The pathogenetic mechanisms of the systemic damage induced by the COVID-19 virus represent an enigma and challenge for researchers. Dermatologists and dermatopathologists have an advantage, however, in that the skin never lies. Diseases of the skin cannot be hidden from the dermatologist. The cutaneous pathology always manifests true to the character and provides the clues needed to solve the diagnostic puzzle. Clinicians and dermatopathologists can collect these clues and put them together like pieces of a puzzle to find the pathogenic action of the virus.
What can we learn from the skin of patients with COVID-19 to understand how the virus affects other organs? From autopsy and skin biopsies performed to date, we are sure that SARS-CoV-2 travels quickly through the vascular system.5,6 During this journey, it may leave a devastating trail or conversely pass through causing little damage even while alerting the cutaneous immune system. A consistent finding in all skin biopsies is the extreme vasodilation with swollen endothelial cells and vessels engulfed with red blood cells. Perivascular cuffing is also found primarily caused by cytotoxic CD8+ lymphocytes and eosinophils.1,2
There have been reports of a possible association of the COVID-19 infection with Kawasaki syndrome, a systemic vasculitis of unknown etiology that characteristically presents coat-sleeve–like perivascular infiltrate composed mainly of cytotoxic CD8+ lymphocytes.7
Hypereosinophilia appears to be a characteristic feature of Kawasaki syndrome,8,9 and many authors point out the eosinophils as one of the activators of the blood coagulation cascade. A prothrombotic state has recently been demonstrated in Churg–Strauss syndrome, suggesting that the eosinophil-derived tissue factor activates the coagulation cascade and may play a role in the mechanism leading to thrombosis in patients with Churg–Strauss syndrome, in patients with hypereosinophilic syndromes,10,11 as well as in patients with bullous pemphigoid.12 Furthermore, the histopathological examination of scabies lesions has shown the presence of thrombosis in the small vessels associated with dense eosinophilic infiltrates.13 The exhaustive work of Magro et al6 differs from our findings. These authors do not believe that eosinophils produce the activation of the coagulation cascade; however, in one of the pictures (N5) depicted in their research article, it is possible to appreciate a thrombosed cutaneous vessel surrounded by nuclear debris and occasional eosinophils. The article suggests that the activation of the mannose-binding lectin pathway could be engaged by the COVID-19 spike glycoprotein throughout a direct interaction on the endothelium, which contains the viral spike protein in endocytosed microparticles. This interaction seems to activate the coagulation cascade. Second, the authors emphasize the effects of increased angiotensin II on the complement system due to ACE2 deactivation.6 At the moment, the mechanism that the virus uses to trigger the complement cascade by inducing diffuse multiorgan thrombosis has not been clarified.
Our heuristic theory imagines a rapid viral passage in the cutaneous vascular system, which stimulates the immune system activating the Langerhans cells from the skin to the lymph node. The presence of possible circulating immune complexes created by the virus can induce brief urticarial reactions or urticarial vasculitis, as seen in our histological cases. If the event stops at this stage, the clinical manifestation is of urticaria, just lasting 1 week. If the pathological process continues along its natural course, the next step would be the creation of immunocomplexes leading CD4+ T helper lymphocytes to produce cytokines, such as IL-1, IFN-γ, and TNF-α, and recruiting eosinophils, CD8+ cytotoxic T cells, B cells, and natural killer cells, leading to lymphocytic thrombophilic arteritis, as already demonstrated.14 At this point, the clinical picture is of a livedo vasculopathy with a variable, but recurrent vascular damage and formation of microthrombi in patients with severe systemic signs. Fortunately, in young patients, the event seems to be limited only to the cutaneous vessels with the formation of pernio erythema–like lesions.3 Our analytical discussion, however, lacks the clinical and histological features not related to vascular damage and instead directed to keratinocytes, such as cases that showed Grover disease or erythema multiforme–like lesions. The question we must ask ourselves is the following: “Is ‘SARS-CoV-2’ a virus with cytopathic capability?”
In our previous articles,1,2 we highlighted that in the dermatological literature, there are inflammatory pathologies strongly suspected of having a viral etiology, but unfortunately not yet demonstrated. In lichen striatus, a viral alteration of clones of keratinocytes along the Blaschko's lines has been hypothesized.15–17 In fact, in its classic form, histologically, it can be observed band-like dermatitis with lymphocyte satellitosis destroying the keratinocytes and Langerhans cells. Our 2 cases of COVID+ patients who had typical Grover disease alterations should not be considered incidental. One case, in particular, showed a vesicle-pustule surrounded by keratinocytes with nuclear alterations similar to those found in herpetic vesicles. In fact, overlapping features mimicking a viral infection and Grover disease have already been described in patients with simultaneous Grover and Kaposi varicelliform eruption.18 What we can assert with certainty is that 2 of our patients in the “third highly suspicious group” showed an invisible dermatitis. In particular, in one of the cases, there were diffused necrotic keratinocytes with large ballooned and multinucleated cells in the epithelium of a hair follicle. We can also not forget that some viral infections, especially herpes simplex, are suspected of provoking stimulation of immunopathological mechanisms in erythema multiforme. The herpes simplex virus could play a role in autoimmune cross-reactivity,19 triggering the keratinocyte that activates IL-1, IFN-γ, and TNF-α and recruiting cytotoxic and natural killer cells that target the keratinocytes itself. Histopathological examination of lung biopsies of COVID + pneumonia cases indicates severe damage of the alveolar epithelial cell floating in the alveolar space, just like in bullous severe erythema multiforme in which ballooning keratinocytes detach from the spinous layer.5 These data, on the other hand, would be in contrast with what was found in the autopsy cases of Magro et al6 in which direct damage to the alveolar epithelial cells was not seen. Finally, young age, swab negativity, and the absence of other symptoms appear to be common features of the “second most probably COVID-19 infection group.” The swab negativity could be explained with the disappearance of detectable viral presence after a brief asymptomatic course: according to this hypothesis, the observed skin lesions would represent late manifestations of the COVID-19 infection in young healthy subjects.3,20
Currently, we can assert that SARS-CoV-2 can provoke a systemic coagulopathy with a yet unclear pathogenetic mechanism. As for the cytopathic capacity of the virus on epithelial cells, we have only clues warning us. Our case history should induce clinical dermatologists to suspect, in this tragic period, the possibility of COVID-19 infection if they encounter patients with fever and cough who show urticarial skin lesions, chilblains, targetoid lesions (erythema multiforme–like lesions), exanthema, maculohemorrhagic rash, or chickenpox-like lesions.21
ACKNOWLEDGMENTS
This article is dedicated to his father Ferdinando who described for the first time, as the solo author, the papular acrodermatitis of childhood in 1955. Warm thanks to his first histopathology teacher Prof. Elvio Alessi and later Bernie Ackerman. Finally, he would like to mention Heinz Kutzner who in 2000 believed in him and joined the historic enterprise of creating a computer expert system in the diagnosis of dermatitis, Lampyris 101.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;23;100:adv00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gianotti R, Zerbi P. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. 2020;30:S0923-1811(20)30143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020. doi: 10.1111/jdv.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagashima M. Prurigo pigmentosa—clinical observations of our 14 cases. J Dermatol. 1978;5:61–67. [DOI] [PubMed] [Google Scholar]
- 5.Yao XH, Li TY, He ZC, et al. A pathological report of three COVID-19 cases by minimally invasive autopsies [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. [DOI] [PubMed] [Google Scholar]
- 6.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;15:S1931-5244(20)30070-0. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi M, Matsumoto Y, Ohya M, et al. Histologic and immunohistochemical evaluation of infiltrating inflammatory cells in Kawasaki disease arteritis lesions. Appl Immunohistochem Mol Morphol. 2020. doi: 10.1097/PAI.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 8.Terai M, Yasukawa K, Honda T, et al. Peripheral blood eosinophilia and eosinophil accumulation in coronary microvessels in acute Kawasaki disease. Pediatr Infect Dis J. 2002;21:777–781. [DOI] [PubMed] [Google Scholar]
- 9.Kuo HC, Wang CL, Liang CD, et al. Association of lower eosinophil-related T helper 2 (Th2) cytokines with coronary artery lesions in Kawasaki disease. Pediatr Allergy Immunol. 2009;20:266–272. [DOI] [PubMed] [Google Scholar]
- 10.Ames PR, Margaglione M, Mackie S, et al. Eosinophilia and thrombophilia in churg strauss syndrome: a clinical and pathogenetic overview. Clin Appl Thromb Hemost. 2010;16:628–636. [DOI] [PubMed] [Google Scholar]
- 11.Maino A, Rossio R, Cugno M, et al. Hypereosinophilic syndrome, Churg-Strauss syndrome and parasitic diseases: possible links between eosinophilia and thrombosis. Curr Vasc Pharmacol. 2012;10:670–675. [DOI] [PubMed] [Google Scholar]
- 12.Marzano AV, Tedeschi A, Fanoni F, et al. Activation of blood coagulation in bullous pemphigoid: role of eosinophils, and local and systemic implications. Br J Dermatol. 2009;160:266–272. [DOI] [PubMed] [Google Scholar]
- 13.Elwood H, Berry RS, Gardner JM. Superficial fibrin thrombi … and other findings: a review of the histopathology of human scabietic infections. J Cutan Pathol. 2015;42:346–352. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Kossard S, McGrath MA. Lymphocytic thrombophilic arteritis: a newly described medium-sized vessel arteritis of the skin. Arch Dermatol. 2008;144:1175–1182. [DOI] [PubMed] [Google Scholar]
- 15.Gianotti R, Restano L, Grimalt R, et al. Lichen striatus—a chameleon: an histopathological and immunohistological study of forty-one cases. J Cutan Pathol. 1995;22:18–22. [DOI] [PubMed] [Google Scholar]
- 16.Hafner C, Landthaler M, Vogt T. Lichen striatus (blaschkitis) following varicella infection. J Eur Acad Dermatol Venereol. 2006;20:1345–1347. [DOI] [PubMed] [Google Scholar]
- 17.Jones J, Marquart JD, Logemann NF, et al. Lichen striatus-like eruption in an adult following hepatitis B vaccination: a case report and review of the literature. Dermatol Online J. 2018;24. [PubMed] [Google Scholar]
- 18.Kosann MK, Fogelman JP, Stern RL. Kaposi's varicelliform eruption in a patient with Grover's disease. J Am Acad Dermatol. 2003;49:914–915. [DOI] [PubMed] [Google Scholar]
- 19.Lucchese A. From HSV infection to erythema multiforme through autoimmune crossreactivity. Autoimmun Rev. 2018;17:576–581. [DOI] [PubMed] [Google Scholar]
- 20.Colonna C, Monzani NA, Rocchi A, et al. Chilblains-like lesions in children following suspected Covid-19 infection. Pediatric Dermatol. doi: 10.1111/pde.14210. [DOI] [PMC free article] [PubMed]
- 21.Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


