Background
Diaphragm dysfunction could be induced by sepsis with subsequent ventilatory pump failure that is associated with local infiltration of inflammatory factors in the diaphragm. It has been shown that the administration of anticonvulsant agent, magnesium sulfate (MgSO4) could decrease systematic inflammatory response. We recently reported that MgSO4 could inhibit macrophages high mobility group box 1 (HMGB1) secretion that confirms its anti-inflammatory properties. Toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB) signal pathway appears to be involved in the pathology of septic experimental animal’s inflammatory response and involve in the pathogenic mechanisms of sepsis-induced diaphragm dysfunction. Thus, in this study, we are aiming to explore whether MgSO4 could ameliorate sepsis-induced diaphragm dysfunction via TLR4/NF-κB pathway in a rodent model with controlled mechanical ventilation (CMV) and subsequent septic challenge.
Methods
Rats were randomly assigned into (1) control group: having an identical laparotomy but without ligation or puncture in the cecum; (2) CLP group: cecal ligation and puncture (CLP) with continuous saline infusion; (3) CLP + MgSO4 group: CLP with continuous MgSO4 administration; and (4) MgSO4 group: a sham surgery with MgSO4 administration. After surgery, all rats were submitted to CMV for 18 h. After completion of the study protocol, blood inflammatory cytokine/chemokine was detected by ELISA, as well as diaphragm contractility, TLR4, NF-κB (p65), phospho-NF-κB (p65) and HMGB1 protein expression.
Results
The level of inflammatory cytokine/chemokine includes interleukin-6, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2) and HMGB1 in blood were significantly increased at 18-h post-CLP compared with the control group. We found that rats in the CLP group had substantial diaphragm dysfunction with a distinct downshift of the force–frequency curve. Furthermore, expression of HMGB1, TLR4, NF-κB (p65) and phospho-NF-κB (p65) in diaphragm were significantly increased in the CLP group. In contrast, MgSO4 attenuated the septic inflammation reaction in diaphragm and serum and preserved diaphragm function.
Conclusion
MgSO4 protects against sepsis-induced diaphragm dysfunction. This may be associated with its anti-inflammatory effect on HMGB1/TLR4/NF-κB signal pathway
Keywords: diaphragm dysfunction, high mobility group box 1, magnesium sulfate, sepsis
Introduction
Severe diaphragm dysfunction could be caused by sepsis [1–5] to delay mechanical ventilation weaning of those critically ill patients [4,6,7], resulting in higher mortality rate and longer ICU stay [4,8]. Moreover, sepsis-induced diaphragm dysfunction can increase muscle exposure to those proinflammatory cytokines [9,10], whilst weakening the inflammation process can ameliorate sepsis-induced diaphragm dysfunction [11].
Magnesium sulfate (MgSO4), widely administrated in the treatment of placenta previa or eclampsia [12], encompasses prominent anti-inflammatory properties [13–17]. More recently, MgSO4 has been found in an effect of inhibiting inflammatory molecules, for example, inflammatory cytokine/chemokine, prostaglandin E2 and nitric oxide [14,18]. Furthermore, MgSO4 has also been found to mitigate sepsis-related acute lung injury, as well as the protective effect on ischemia-reperfusion injury [19,20]. Meanwhile, we also found that MgSO4 could inhibit high mobility group box 1 (HMGB1) secretion from lipopolysaccharide-activated RAW264.7, proving its anti-inflammatory property [21].
HMGB1, a nonhistone chromosomal protein responsive to damage or stress, acts as gatekeeper within the immune system for cell survival/death [22]. In the circumstance of severe sepsis, cytokine/chemokine released by inflammatory cells could be stimulated by HMGB1 through binding with transmembrane receptors, for example, toll-like receptors (TLRS) and receptor for advanced glycation end-products [23]. HMGB1 can trigger downstream signals by activating TLR4 through MyD88 or non-MyD88 dependent signaling, and induce inflammatory cytokine secretion through nuclear factor-kappa B (NF-κB), MAPKs or PI3K pathway [24–26]. In-vivo murine study also found that TLR4/NF-κB signal was involved in the course of endotoxin and mechanical ventilation-induced diaphragm injury [27].
However, the anti-inflammatory effect of MgSO4 against sepsis-induced diaphragm dysfunction is far from fully understood. The aim of this study was to investigate the protective function of MgSO4 on diaphragm dysfunction in septic rat model, and the role of HMGB1/TLR4/NF-κB signaling in the mechanism of the protective effects.
Materials and methods
Animals and groups
In accordance with the National Institutes of Health guidelines, animal experiments were implemented with the approval from Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine. Sprague–Dawley rats aged 9–10 weeks were randomized into four groups with 10 in each: (1) control group: having an identical laparotomy whilst without ligation or puncture in cecum; (2) CLP group: cecal ligation and puncture (CLP) with continuous saline infusion; (3) CLP + MgSO4 group: CLP with continuous MgSO4 administration; and (4) MgSO4 group: a sham surgery with MgSO4 administration. After surgery, all rats were adopted to controlled mechanical ventilation (CMV) for 18 h.
Experimental protocol
Sepsis would be generated by CLP technique as described earlier [28]. In brief, rats were anesthetized by pentobarbital through midline abdominal incision. After that, cecum had been ligated with 5-0 silk thread and punctured twice with a 21-gauge needle, following with preparation of a small amount fecal material to induce polymicrobial peritonitis. Then, the cecum was replaced, whilst the abdominal wall was closed in two layers. Rodents were then resuscitated with pre-warmed Ringer’s lactate subcutaneously (5 mL/100 g body weight) with or without loading dose of MgSO4 (270 mg/kg, Sigma-Aldrich, St. Louis, Missouri, USA).
The approach of experimental controlled mechanical ventilation has been described before [29]. Briefly, tracheotomy was performed either after CLP or sham operation. The carotid artery and left lateral tail vein were cannulated for the administration of pentobarbital sodium (10 mg/kg/h), MgSO4 (27 mg/kg/h) or NaCl 0.9%, in parallel with maintaining fluid homeostasis and temperature. Rats were ventilated with a volume-driven small-animal ventilator (V8S, Alcott Biotech, Shanghai, China) for 18 h. Hourly continuous care in terms of bladder expression, removal of airway mucus and eye lubrication were maintained throughout the whole experimental period.
With 18-h CLP and mechanical ventilation, rats were euthanatized through overdose sodium pentobarbital intraperitoneal injection. Blood samples (0.5 mL) were obtained from abdominal aorta to explore inflammatory cytokine/chemokine. Left costal diaphragm segment was excised to investigate contractile properties in vitro; right costal diaphragm was frozen at −80°C for further analysis.
Measurement of in vitro diaphragmatic contractile properties
The diaphragm muscles were taken out rapidly and contractile properties were determined [28]. Briefly, with 15 min equilibration in pre-warmed Krebs solution, diaphragm muscle strips were stimulated by a 250-ms stimulus train at different frequencies (10, 20, 40, 80 and 120 Hz), in parallel with the contractile forces recorded by a data collection system (MPA 2000; Alcott Biotech, Shanghai, China). The value of muscle forces was normalized by cross-sectional areas. After the completion of all measurements, muscle strips were weighed to obtain ‘wet’ muscle mass with completion of all measurements. Subsequently, strips were placed in a desiccator for 48 h to obtain ‘dry weight’. The wet-to-dry ratio of each strip was calculated by dividing the wet and the dry weight.
Cytokine/chemokine level measurements
Several inflammatory cytokine/chemokines consisting of interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2) and HMGB1 in serum are explored by ELISA kits (Uscn Life Science, Wuhan, China). All assays have been performed according to the manufacturer’s guidelines and instructions.
Western blot analysis
Right costal diaphragm tissue samples were homogenized in lysis buffer containing protease inhibitors and liaised on ice for 30 min. The homogenate was centrifuged at 12 000 rpm for 5 min at 4°C and supernatant was removed as the total protein. Diaphragm protein samples (30 μg each lane) were subjected to SDS-PAGE using a 12% SDS-PAGE gel and then transblotted onto polyvinylidene fluoride membranes. The protein levels of TLR4 (Toll-like receptor 4), NF-κB (p65), Phospho-NF-κB (p65) and HMGB1 were determined using specific antibodies (1:1000; Cell Signaling, Danvers, Massachusetts, USA). Beta-actin was used as loading control, and the amount of protein amount in the blots was quantified using a densitometer and Image Lab 6.0 software (Bio-Rad Laboratories, Hercules, California, USA).
Statistical analysis
Data are presented as mean ± SE and tested for normality and equality of variance by using SPSS version 17.0. Statistical differences were analyzed by one-way analysis of variance with a post hoc Newman–Keuls multiple comparison test. P < 0.05 is considered as statistical significance.
Results
Animal characteristics
At 18-h post-CLP, animals’ weight was unchanged compared with baseline, whilst a comparative weigh within four groups was also recorded at study end. As shown in Fig. 1, all experimental rats show similar diaphragm mass and diaphragm wet-to-dry weight ratios, which act as premises supporting the argument that diaphragm weight not being changed by 18-h post-CLP with or without MgSO4 administration.
Fig. 1.
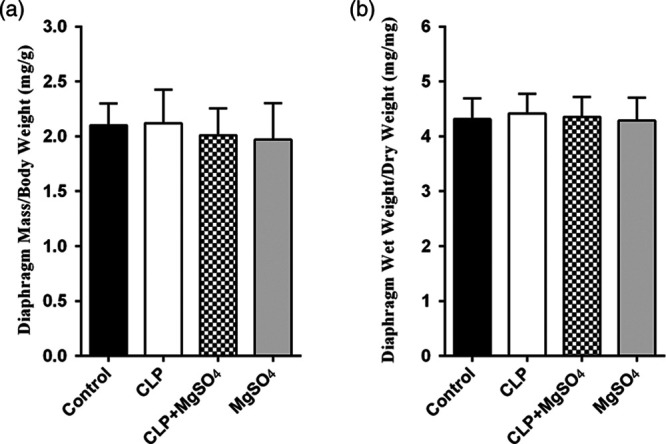
Diaphragm weight/animal weight ratios on the left and wet diaphragm weight/dry diaphragm weight ratios on the right for control, CLP, CLP + MgSO4 and MgSO4 groups. Values for both parameters were similar for all groups. CLP, cecal ligation and puncture; MgSO4, magnesium sulfate.
The change of diaphragm contractile properties
In vitro, as shown in Fig. 2, CLP could sharply reduce diaphragm force-generating capacity, whilst the entire force-frequency curve of diaphragm was downshifted 18-h post-CLP. Compared with the control group, those could sharply reduce in specific force at all stimulation frequencies tested (i.e. 10–120 Hz). Moreover, MgSO4 could prevent sepsis-induced deterioration in diaphragm muscle force generation whilst MgSO4 + CLP group was significantly improved with force at all stimulation frequencies when compared with CLP alone group.
Fig. 2.
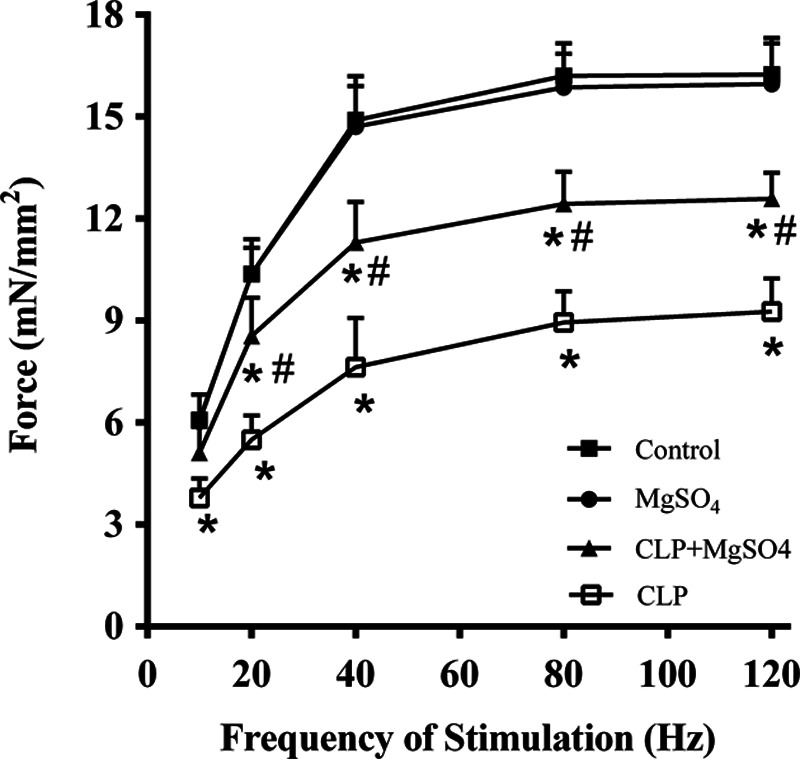
Force-frequency curves from the diaphragm strips. Force-frequency curves demonstrate that CLP reduces the diaphragm-specific force production at different stimulation frequencies compared to a sham operation. The loss of force generation induced by CLP was partially attenuated by the administration of MgSO4. *P < 0.05 compared to the control group; #P < 0.05 compared to the CLP group. CLP, cecal ligation and puncture; MgSO4, magnesium sulfate.
Serum inflammatory cytokine/chemokine expression levels
Serum IL-6, MCP-1, MIP-2 and HMGB1 were tested at 18 h after the introduction of sepsis. As shown in Fig. 3, serum inflammatory cytokine/chemokine in terms of IL-6 (Fig. 3a), MCP-1 (Fig. 3b), MIP-2 (Fig. 3c) and HMGB1 (Fig. 3d) were higher at 18-h post-CLP compared with the control group (P < 0.05). However, CLP associated with high inflammatory cytokine/chemokine could be significantly hampered by MgSO4 (P < 0.05).
Fig. 3.
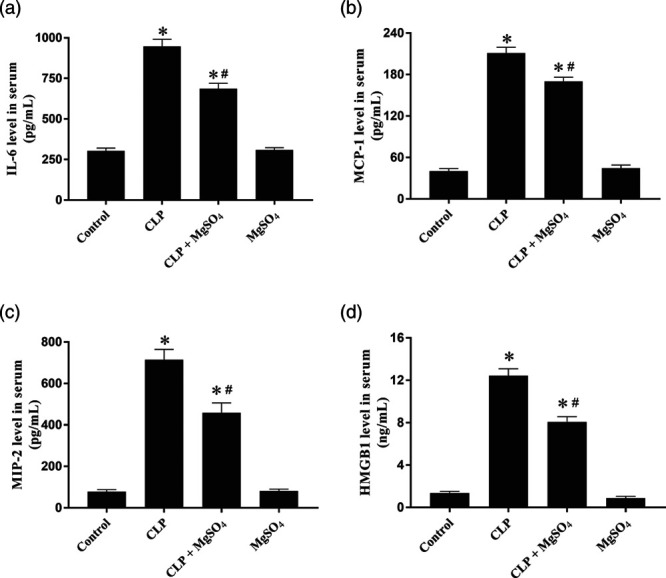
Quantification of inflammatory cytokine/chemokine levels in serum after CLP in vivo. Serum levels of (a) IL-6, (b) MCP-1, (c) MIP-2 and (d) HMGB1 were determined in the control, CLP, CLP + MgSO4 and MgSO4 groups. Data are presented as the means ± SE. *P < 0.05 compared to the control group; #P < 0.05 compared to the CLP group. CLP, cecal ligation and puncture; HMGB1, high-mobility group box 1; IL-6, interleukin-6; MgSO4, magnesium sulfate; MCP-1, monocyte chemoattractant protein-1; MIP-2, macrophage inflammatory protein-2.
Magnesium sulfate treatment reduces sepsis-exacerbated HMGB1 expression via inhibiting HMGB1/TLR4/NF-κB pathway in the diaphragm
Diaphragm HMGB1 was significantly higher in CLP group compared with the control group (Fig. 4), in contrast to the down-regulated HMGB1 protein level captured in CLP + MgSO4 group when compared with CLP group (P < 0.05). In order to justify the correlation between CLP responsive systemic inflammation and NF-κB activity, we explored NF-κB expression in diaphragm muscle of septic rats. As a result, an upregulated NF-κB phosphorylation could be seen in rats with sepsis. Administration of MgSO4 could substantially reduce the sepsis-induced NF-κB (Fig. 5a). In addition, western blot analyses were performed to identify the effects of MgSO4 on sepsis-induced TLR4 upregulation. As shown in (Fig. 5b), TLR4 in the CLP + MgSO4 group were lower than CLP group, but higher than the control group and MgSO4 group.
Fig. 4.
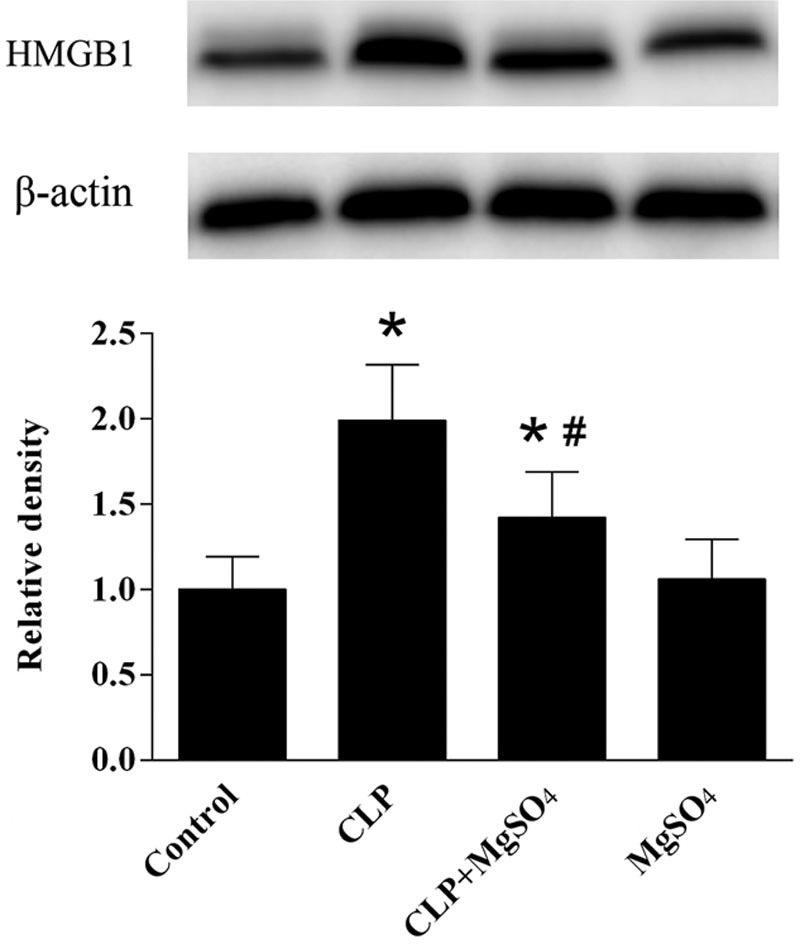
Changes in the expression of HMGB1 in rat diaphragm. A comparison of the relative densities of HMGB1 and β-actin are shown for the control, CLP, CLP + MgSO4 and MgSO4 groups. Data are shown as the means ± SE (n = 10). *P < 0.05 compared to the control group; #P < 0.05 compared to the CLP group. CLP, cecal ligation and puncture; HMGB1, high-mobility group box 1; MgSO4, magnesium sulfate.
Fig. 5.
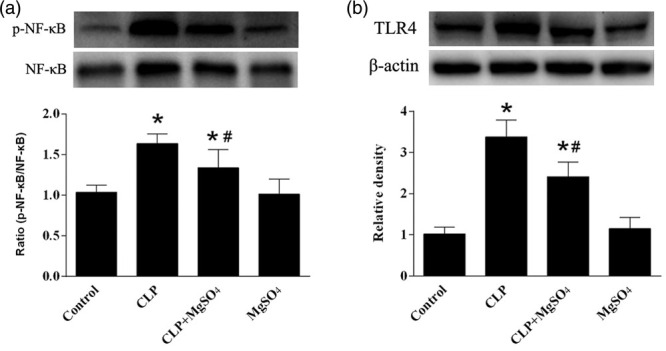
Effect of MgSO4 on the TLR4/NF-κB signaling pathway in diaphragmatic muscle tissues. (a and b) Western blots were conducted using antibodies that recognize the expression levels of phosphorylated NF-κB, total NF-κB, TLR4 and β-actin from diaphragm tissue in the control, CLP, CLP + MgSO4 and MgSO4 groups, expressed as fold change of control group. Data are presented as the means ± SE (n = 10). *P < 0.05 compared to the control group; #P < 0.05 compared to the CLP group. CLP, cecal ligation and puncture; MgSO4, magnesium sulfate.
Discussion
Consistent with existing knowledge [1,2,30–32], our study found that sepsis with following CMV could make significant diaphragm injury in rat model. Moreover, since diaphragm contractile properties improvement with MgSO4 administration after CLP was recorded in our study, we, therefore, assume the MgSO4 therapeutic potential for sepsis-induced diaphragmatic dysfunction. On the other hand, the protective effect of MgSO4 might be inclusive of alleviation of systematic and diaphragm inflammation that could be justified by significant decreased serum inflammatory cytokine/chemokine and diaphragmatic expression of HMGB1 through inhibiting the TLR4/NF-κB signal pathway.
Rodent model of septic challenges with following controlled mechanical ventilation can approximate the corresponding aspects in critically injured patients. Our preceding study had shown that early administration of cisatracurium, one of the most widely adopted nondepolarizing neuromuscular blocking drugs, can attenuate sepsis-induced diaphragm dysfunction [28]. In clinical practice, there is an increasing trend of adopting magnesium for the treatment of acute myocardial infarction, arrhythmia, asthma, pheochromocytoma, and postoperative analgesia [33,34]. Moreover, it was reported that high magnesium therapy provides protective effect on septic rats [35], whilst hypomagnesemia was contrastingly correlated with poor clinical outcomes [36]. The major finding in our study is that MgSO4 (18-h period post-CLP) administration could ameliorate sepsis-induced diaphragmatic injury, which was evident in terms of increased diaphragm contractility when compared to CLP rats.
HMGB1 protein, a nonhistone chromosomal protein responsive to damage or stress, acts as sentinel in the immune system playing critical role in cell survival and death pathway [22]. HMGB1 is a potent pro-inflammatory cytokine which act as a ‘late’ mediator in the course of systemic inflammatory response [37]. Inhibiting HMGB1 can dramatically reduce not only endotoxin-induced lethal endotoxemia but concurrent acute tissue damage [38–41]. Thus, HMGB1 could presumably be the treatment target, inflammatory-related biomarker and bioindicator [22]. On that note, combined with our previous findings [28], the diaphragm and systemic HMGB1 level was associated with deteriorated sepsis-induced diaphragmatic dysfunction.
The diaphragm function was affected by sepsis-induced over-activation on multiple pro-inflammatory pathways, here TLR4/NF-κB signal pathway might act as a crucial interpretation because of its role as a central coordinator responsive to inflammatory response in kinds organs and diverse cells. TLR4, a key factor in inflammatory response [42], can activate NF-κB signal pathway [43] where the latter modulates inflammatory response of diaphragm muscle fibers [44–46]. Phosphorylated NF-κB coordinates the induction of several genes encoding corresponding proinflammatory cytokines. Upregulated TLR4 has been reported to modulate skeletal muscle atrophy in terms of increased cytokines in the diaphragm [47]. Moreover, another study also reported the emergence of endotoxin-augmented mechanical ventilation-induced diaphragmatic injury via activated TLR4/NF-κB signal pathway [27]. Enhancement of NF-κB activity and TLR4 expression has also been found in our study. Therefore, we speculate that MgSO4 could inhibit the activation of TLR4/NF-κB signal pathway induced by CLP and may, in turn, inhibit inflammatory mediators’ expression and improve diaphragm contractile properties.
Additional mechanisms regarding protective effect of MgSO4 against sepsis lie in the functions of immunomodulatory, anti-aggregatory and free oxygen radical cleanse from MgSO4 [48]. However, the underlying mechanism of MgSO4 as inflammatory modulator is unclear despite relentless efforts in past decades.
One of our study limitations is the lack of MgSO4 gradient doses in the murine model, so the dose-dependent potential effects of MgSO4 are still unknown. In this study, we observed a protective effect of MgSO4 used a loading dose (270 mg/kg) then continuous infusion at the rate of 27 mg/kg/h for 18 h refer to our preexperiment and other’s article [16,49]. Further studies are needed to evaluate the dosage dependent-context effect of MgSO4 on respiratory muscle and involved potential anti-inflammatory signal pathway.
Conclusion
Our current findings indicate that MgSO4 has beneficial effects on ameliorating sepsis-induced diaphragm dysfunction, identifying HMGB1/TLR4/NF-κB as one of the possible signal pathways. Further studies should include clinical effective intervention doses of MgSO4 and ways to protect diaphragm function in sepsis.
Acknowledgements
This work was supported by Shanghai Songjiang District Programs for Science and Technology Development (18sjkjgg57 to Jihong Jiang), Xinchen Foster Fund for Anesthesiologists in Shanghai to Jihong Jiang, Basic science and advanced technology foundation of Chongqing Science and Technology Commission (cstc2016jcyjA0158 to Bin Yang), Performance incentive and guidance project of scientific research institutes of Chongqing Science and Technology Commission (cstc2018jxjl130028 to Bin Yang), High-level Medical Reserved Personnel Training Project of Chongqing (2019GDRC017 to Bin Yang) and Foundation of Chongqing Health and Family Planning Commission(ZY201702036 to Bin Yang)
In accordance with the National Institutes of Health guidelines, animal experiments were implemented with the approval from Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine
Y.B.: guarantor of integrity of the entire study. Y.B. and L.S.T.: study concepts. Y.B. and J.J.H.: study design. J.J.H. and Y.B.: definition of intellectual content. C.Q. and C.X.: literature research. J.J.H. and L.S.T.: experimental studies. C.Q. and J.J.H.: data acquisition. C.X. and L.J.Q.: data analysis. C.Q. and Y.B.: statistical analysis. J.J.H. and L.J.B.: manuscript preparation. C.Q. and J.J.H.: manuscript editing. L.S.T. and Y.B.: manuscript review.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Shitong Li and Bin Yang contributed equally to the writing of this article.
References
- 1.Supinski GS, Wang W, Callahan LA. Caspase and calpain activation both contribute to sepsis-induced diaphragmatic weakness. J Appl Physiol (1985). 2009; 107:1389–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Supinski GS, Callahan LA. Calpain activation contributes to endotoxin-induced diaphragmatic dysfunction. Am J Respir Cell Mol Biol. 2010; 42:80–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung B, Moury PH, Mahul M, de Jong A, Galia F, Prades A, et al. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016; 42:853–861 [DOI] [PubMed] [Google Scholar]
- 4.Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, et al. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact – a prospective study. Am J Respir Crit Care Med. 2013; 188:213–219 [DOI] [PubMed] [Google Scholar]
- 5.Celik Kavak E, Gulcu Bulmus F, Bulmus O, Kavak SB, Kocaman N. Magnesium: does it reduce ischemia/reperfusion injury in an adnexal torsion rat model? Drug Des Devel Ther. 2018; 12:409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Z, Xu Q, Yuan Y, Zhang G, Guo F, Ge H. Diaphragmatic dysfunction is characterized by increased duration of mechanical ventilation in subjects with prolonged weaning. Respir Care. 2016; 61:1316–1322 [DOI] [PubMed] [Google Scholar]
- 7.Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care. 2010; 14:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care. 2013; 17:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shindoh C, Hida W, Ohkawara Y, Yamauchi K, Ohno I, Takishima T, Shirato K. TNF-alpha mRNA expression in diaphragm muscle after endotoxin administration. Am J Respir Crit Care Med. 1995; 152:1690–1696 [DOI] [PubMed] [Google Scholar]
- 10.van Hees HW, Schellekens WJ, Linkels M, Leenders F, Zoll J, Donders R, et al. Plasma from septic shock patients induces loss of muscle protein. Crit Care. 2011; 15:R233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Supinski GS, Callahan LA. β-hydroxy-β-methylbutyrate (HMB) prevents sepsis-induced diaphragm dysfunction in mice. Respir Physiol Neurobiol. 2014; 196:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar J, Ben-Haroush A, Feldberg D, Hod M. The pharmacologic approach to the prevention of preeclampsia: from antiplatelet, antithrombosis and antioxidant therapy to anticonvulsants. Curr Med Chem Cardiovasc Hematol Agents. 2005; 3:181–185 [DOI] [PubMed] [Google Scholar]
- 13.Rochelson B, Dowling O, Schwartz N, Metz CN. Magnesium sulfate suppresses inflammatory responses by human umbilical vein endothelial cells (HuVECs) through the NFkappaB pathway. J Reprod Immunol. 2007; 73:101–107 [DOI] [PubMed] [Google Scholar]
- 14.Lin CY, Tsai PS, Hung YC, Huang CJ. L-type calcium channels are involved in mediating the anti-inflammatory effects of magnesium sulphate. Br J Anaesth. 2010; 104:44–51 [DOI] [PubMed] [Google Scholar]
- 15.Esen F, Erdem T, Aktan D, Orhan M, Kaya M, Eraksoy H, et al. Effect of magnesium sulfate administration on blood-brain barrier in a rat model of intraperitoneal sepsis: a randomized controlled experimental study. Crit Care. 2005; 9:R18–R23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CY, Jan WC, Tsai PS, Huang CJ. Magnesium sulfate mitigates acute lung injury in endotoxemia rats. J Trauma. 2011; 70:1177–85; discussion 1185 [DOI] [PubMed] [Google Scholar]
- 17.El-Tanbouly DM, Abdelsalam RM, Attia AS, Abdel-Aziz MT. Pretreatment with magnesium ameliorates lipopolysaccharide-induced liver injury in mice. Pharmacol Rep. 2015; 67:914–920 [DOI] [PubMed] [Google Scholar]
- 18.Aryana P, Rajaei S, Bagheri A, Karimi F, Dabbagh A. Acute effect of intravenous administration of magnesium sulfate on serum levels of interleukin-6 and tumor necrosis factor-α in patients undergoing elective coronary bypass graft with cardiopulmonary bypass. Anesth Pain Med. 2014; 4:e16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen SD, Chen YB, Peng Y, Xu J, Chen SS, Zhang JL, et al. Role of PI3K/Akt signaling in the protective effect of magnesium sulfate against ischemia-perfusion injury of small intestine in rats. Chin Med J (Engl). 2010; 123:1447–1452 [PubMed] [Google Scholar]
- 20.Gormus ZI, Ergene N, Toy H, Baltaci AK, Mogulkoc R. Preventive role of magnesium on skeletal muscle ischemia-reperfusion injury-an experimental study. Biol Trace Elem Res. 2009; 127:183–189 [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Zhang J, Huang X, Huang L, Li S, Wang Z. Magnesium sulfate inhibits the secretion of high mobility group box 1 from lipopolysaccharide-activated RAW264.7 macrophages in vitro. J Surg Res. 2013; 179:e189–e195 [DOI] [PubMed] [Google Scholar]
- 22.VanPatten S, Al-Abed Y. High Mobility Group Box-1 (HMGb1): current wisdom and advancement as a potential drug target. J Med Chem. 2018; 61:5093–5107 [DOI] [PubMed] [Google Scholar]
- 23.Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014; 507:109–113 [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y, Wang D, Wang B, Li H, Xiong J, Xu S, et al. HMGB1 translocation and release mediate cigarette smoke-induced pulmonary inflammation in mice through a TLR4/MyD88-dependent signaling pathway. Mol Biol Cell. 2017; 28:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006; 26:174–179 [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Wu S, Chen C, Xie B, Fang Z, Hu W, et al. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation. 2017; 14:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li LF, Liu YY, Chen NH, Chen YH, Huang CC, Kao KC, et al. Attenuation of ventilation-induced diaphragm dysfunction through toll-like receptor 4 and nuclear factor-kappaB in a murine endotoxemia model. Lab Invest. 2018; 98:1170–1183 [DOI] [PubMed] [Google Scholar]
- 28.Jiang J, Yang B, Han G, Yang M, Li S. Early administration of cisatracurium attenuates sepsis-induced diaphragm dysfunction in rats. Inflammation. 2015; 38:305–311 [DOI] [PubMed] [Google Scholar]
- 29.Yang M, Wang H, Han G, Chen L, Huang L, Jiang J, Li S. Phrenic nerve stimulation protects against mechanical ventilation-induced diaphragm dysfunction in rats. Muscle Nerve. 2013; 48:958–962 [DOI] [PubMed] [Google Scholar]
- 30.Supinski GS, Alimov AP, Wang L, Song XH, Callahan LA. Calcium-dependent phospholipase A2 modulates infection-induced diaphragm dysfunction. Am J Physiol Lung Cell Mol Physiol. 2016; 310:L975–L984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao G, Hao L, Wang M, Zhong B, Yu M, Zhao S, et al. Upregulation of endoplasmic reticulum stress is associated with diaphragm contractile dysfunction in a rat model of sepsis. Mol Med Rep. 2017; 15:366–374 [DOI] [PubMed] [Google Scholar]
- 32.Supinski GS, Callahan LA. Double-stranded RNA-dependent protein kinase activation modulates endotoxin-induced diaphragm weakness. J Appl Physiol (1985). 2011; 110:199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James MF. Magnesium: an emerging drug in anaesthesia. Br J Anaesth. 2009; 103:465–467 [DOI] [PubMed] [Google Scholar]
- 34.Khalilzadeh M, Abdollahi A, Abdolahi F, Abdolghaffari AH, Dehpour AR, Jazaeri F. Protective effects of magnesium sulfate against doxorubicin induced cardiotoxicity in rats. Life Sci. 2018; 207:436–441 [DOI] [PubMed] [Google Scholar]
- 35.Salem M, Kasinski N, Munoz R, Chernow B. Progressive magnesium deficiency increases mortality from endotoxin challenge: protective effects of acute magnesium replacement therapy. Crit Care Med. 1995; 23:108–118 [DOI] [PubMed] [Google Scholar]
- 36.Velissaris D, Karamouzos V, Pierrakos C, Aretha D, Karanikolas M. Hypomagnesemia in critically ill sepsis patients. J Clin Med Res. 2015; 7:911–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu XM, Yao YM, Liang HP, Xu CT, Dong N, Yu Y, Sheng ZY. High mobility group box-1 protein regulate immunosuppression of regulatory T cells through toll-like receptor 4. Cytokine. 2011; 54:296–304 [DOI] [PubMed] [Google Scholar]
- 38.Bangert A, Andrassy M, Müller AM, Bockstahler M, Fischer A, Volz CH, et al. Critical role of RAGE and HMGB1 in inflammatory heart disease. Proc Natl Acad Sci U S A. 2016; 113:E155–E164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang W, Zhao F, Fang Y, Li X, Shen L, Cao T, Zhu H. Glycyrrhizin protects against porcine endotoxemia through modulation of systemic inflammatory response. Crit Care. 2013; 17:R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Li J, Sama AE, Wang H. Carbenoxolone blocks endotoxin-induced protein kinase R (PKR) activation and high mobility group box 1 (HMGB1) release. Mol Med. 2013; 19:203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hidaka S, Iwasaka H, Hagiwara S, Noguchi T. Gabexate mesilate inhibits the expression of HMGB1 in lipopolysaccharide-induced acute lung injury. J Surg Res. 2011; 165:142–150 [DOI] [PubMed] [Google Scholar]
- 42.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001; 2:675–680 [DOI] [PubMed] [Google Scholar]
- 43.Binker-Cosen MJ, Richards D, Oliver B, Gaisano HY, Binker MG, Cosen-Binker LI. Palmitic acid increases invasiveness of pancreatic cancer cells AsPC-1 through TLR4/ROS/NF-κB/MMP-9 signaling pathway. Biochem Biophys Res Commun. 2017; 484:152–158 [DOI] [PubMed] [Google Scholar]
- 44.Smuder AJ, Hudson MB, Nelson WB, Kavazis AN, Powers SK. Nuclear factor-κB signaling contributes to mechanical ventilation-induced diaphragm weakness*. Crit Care Med. 2012; 40:927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okazaki T, Liang F, Li T, Lemaire C, Danialou G, Shoelson SE, Petrof BJ. Muscle-specific inhibition of the classical nuclear factor-κB pathway is protective against diaphragmatic weakness in murine endotoxemia. Crit Care Med. 2014; 42:e501–e509 [DOI] [PubMed] [Google Scholar]
- 46.Friedrichsen M, Ribel-Madsen R, Wojtaszewski J, Grunnet L, Richter EA, Billestrup N, et al. Dissociation between skeletal muscle inhibitor-kappaB kinase/nuclear factor-kappaB pathway activity and insulin sensitivity in nondiabetic twins. J Clin Endocrinol Metab. 2010; 95:414–421 [DOI] [PubMed] [Google Scholar]
- 47.Schellekens WJ, van Hees HW, Vaneker M, Linkels M, Dekhuijzen PN, Scheffer GJ, et al. Toll-like receptor 4 signaling in ventilator-induced diaphragm atrophy. Anesthesiology. 2012; 117:329–338 [DOI] [PubMed] [Google Scholar]
- 48.Bora S, Erdoğan MA, Yiğittürk G, Erbaş O, Parlak İ. The effects of lipid emulsion, magnesium sulphate and metoprolol in amitriptyline-induced cardiovascular toxicity in rats. Cardiovasc Toxicol. 2018; 18:547–556 [DOI] [PubMed] [Google Scholar]
- 49.Hallak M, Hotra JW, Kupsky WJ. Magnesium sulfate protection of fetal rat brain from severe maternal hypoxia. Obstet Gynecol. 2000; 96:124–128 [DOI] [PubMed] [Google Scholar]


