Supplemental Digital Content is available in the text.
Keywords: amniotic fluid embolism, extracorporeal life support, extracorporeal membrane oxygenation, hemorrhagic shock
Objectives:
Amniotic fluid embolism is a rare obstetric emergency that can be accompanied by profound hypoxemia, coagulopathy, hemorrhage, and cardiogenic shock. Extracorporeal membrane oxygenation may provide a rescue strategy in amniotic fluid embolism with cardiopulmonary collapse. Approaches to anticoagulation must be balanced against the risk of hemorrhage with concomitant coagulopathy. Although extracorporeal membrane oxygenation has been described for cardiopulmonary collapse in the setting of amniotic fluid embolism, its initiation as a bridge to hemostasis and cardiopulmonary recovery in amniotic fluid embolism–induced hemorrhagic and cardiogenic shock remains a novel resuscitation strategy.
Design, Subject, and Intervention:
We present a case detailing the initiation of extracorporeal life support with veno-arterio-venous extracorporeal membrane oxygenation in a patient with hemorrhagic shock and cardiopulmonary failure due to amniotic fluid embolism. The patient was ultimately discharged home 19 days after presentation free from neurologic or other significant disability.
Main Results and Conclusion:
Through this case, we describe a tailored approach to extracorporeal life support initiation and advanced extracorporeal membrane oxygenation management as a bridge to recovery in patients with mixed shock. Additionally, we discuss how the culmination of prehospital, outpatient and inpatient provider teamwork, easily portable extracorporeal membrane oxygenation equipment, and multispecialty collaboration can afford promising therapeutic options for patients who were previously deemed ineligible for extracorporeal life support.
BACKGROUND
Amniotic fluid embolism (AFE) is a rare but potentially life-threatening obstetric emergency with a reported incidence of 5.5 per 100,000 parturients in the United States and a mortality as high 60% in some studies (1, 2). The clinical manifestations are characterized by the sudden onset of respiratory distress, cardiovascular collapse, and disseminated intravascular coagulation. The hypothesized pathophysiology of AFE involves unregulated activation of proinflammatory and vasoactive mediators leading to a consumptive coagulopathy, severe pulmonary hypertension, and ultimately right ventricular (RV) failure (3).
As a result of these profound systemic changes, the optimal management of AFE can be markedly challenging. Guidelines recommend high-quality advanced cardiac life support (ACLS), although this may prove inadequate in cases of refractory circulatory collapse (4, 5). Veno-venous, veno-arterial ECMO, and other forms of extracorporeal life support (ECLS) have been described as rescue modalities for obstetrical complications—including AFE (5–15). The initiation of ECLS in the context of profound coagulopathy and hemorrhagic shock requiring activation of a massive transfusion protocol (MTP) have been described in the setting of trauma (9–12). ECLS has also been safely used in the setting of postpartum bleeding (11, 16). However, ECLS in the setting of profound disseminated intravascular coagulation (DIC) with hemorrhagic shock owing to AFE has yet to be reported. Traditionally, the anticoagulation required to maintain ECMO circuit patency and oxygenator function limited its applicability in patients with profound coagulopathy. As the associated technology has evolved, the conduct of “heparin-free” ECMO has become more commonplace, and the potential role of extracorporeal support even in the setting of coagulopathy appears promising but has yet to be fully explored (17).
In this case report, we describe the utilization of ECLS in a patient with mixed cardiogenic and hemorrhagic shock secondary to AFE. We additionally discuss the potential advantages of advanced ECMO configurations in this context and address anticoagulation strategies in the ECMO patient with coagulopathy. This patient’s course is detailed from clinical presentation through discharge from the ICU.
This article adheres to the Consensus-based Clinical Case Reporting Guidelines (18). Written informed consent was obtained from the patient for publication of this case report.
CASE PRESENTATION
A 25-year-old multiparous woman at 18 weeks gestation presented to the emergency department (ED) from an outpatient surgery center in respiratory distress after a dilatation and evacuation. According to the report from the referring surgery center, she became tachypneic and unresponsive when transferring to a recovery room stretcher at the end of the procedure. She subsequently developed profuse vaginal bleeding and was transported by ambulance to the ED. On arrival, she was minimally responsive with cool extremities. Vitals signs included a heart rate of 158 beats/min, respiratory rate 32 breaths per min, blood pressure 110/60 mm Hg, and oxygen saturation of 99% on a nonrebreather face mask. She was subsequently intubated for airway protection and worsening hypoxic respiratory failure.
Following intubation, she became hypotensive. Physical examination revealed significant vaginal bleeding without evidence of retained products of conception or uterine atony. The estimated blood loss was 2 L within the first hour of presentation, and she required ongoing protocolized massive transfusion for hemorrhagic shock. Despite initial resuscitation efforts, she remained hypoxemic and hemodynamically unstable with increasing doses of vasopressors, inotropes, and massive transfusion. In order to better characterize her shock state, a bedside transthoracic echocardiogram (TTE) was performed, which demonstrated severe biventricular hypokinesis.
Given evidence of medically refractory cardiogenic shock, preparations were undertaken to emergently initiate veno-arterial ECMO. While preparing, the patient sustained cardiac arrest with pulseless electrical activity. ACLS was promptly initiated with high-quality cardiopulmonary resuscitation continuing until peripheral cannulation for veno-arterial ECMO (via the right femoral vein and right common femoral artery) was completed. Cannulation and initiation of veno-arterial ECMO support were completed within 30 minutes of cardiac arrest. A right superficial femoral artery distal reperfusion cannula was additionally placed according to institutional protocol.
The patient was transferred to the operating room (OR) for an anticipated hysterectomy. Prior to transport to the OR, the patient had received the following: 58 units of packed RBCs, 32 units of fresh frozen plasma (FFP), 10 units of pooled platelets, 30 units of cryoprecipitate, and 3 L of crystalloid. Laboratory studies revealed a pH of 6.98, base deficit of –20.9, lactate of 12 mmol/L, international normalized ratio (INR) 3.22, d-dimer 60,000 ng/mL, fibrinogen 61 mg/dL, and platelet count of could use 51,000 alternatively for reader ease/comprehension. She was transported to the OR with ongoing veno-arterial ECMO, MTP, and high-dose vasopressor (norepinephrine, epinephrine, and vasopressin) support.
Once in the OR, the patient received an additional 2 units of packed RBCs, 10 units of cryoprecipitate, and 8 units of FFP. Upon reevaluation, the patient’s vaginal bleeding had slowed considerably with marked improvement in her coagulopathy. Persistent hypoxemia as measured by a right radial arterial catheter raised concerns for differential hypoxia and prompted conversion to veno-arterial-venous ECMO. A right internal jugular venous outflow (i.e., return) cannula was placed to ensure adequate upper body and cerebral oxygenation. Serial reevaluations of coagulopathy and vaginal bleeding continued to demonstrate interval improvement, INR downtrended to 1.0 from 3.0, and therefore hysterectomy was deferred. Inhaled epoprostenol was initiated after perioperative transesophageal echocardiography demonstrated significant right-ventricular dysfunction with an associated left-ventricular ejection fraction (LVEF) of 25%. She was transferred to the ICU for further management.
Within 24 hours of admission to ICU, a repeat TTE demonstrated decreased left-ventricular function with an LVEF of 20% and moderately dilated RV with severely reduced systolic function. Chest x-ray at that time showed diffuse bilateral pulmonary edema despite appropriate urine output and creatinine of 0.89; therefore, the decision was made to initiate continuous renal replacement therapy for aggressive fluid removal (Fig. 1). Anticoagulation was withheld due to high risk of hemorrhage, and ECMO flow rates were maintained at approximately 5 L/min. Flows for venous return, arterial, and distal perfusion cannulas were 4.5, 0.5, and 0.2 L/min, respectively.
Figure 1.
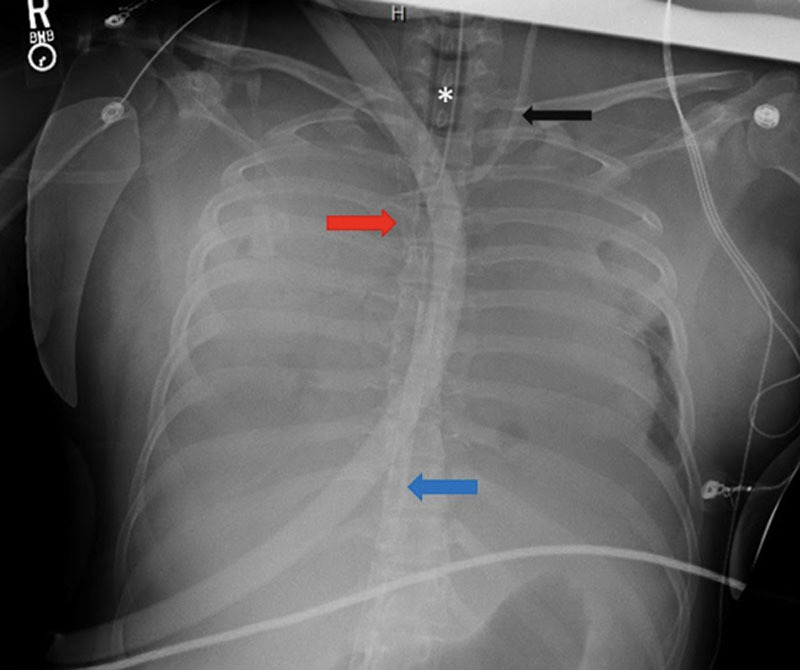
Portable chest radiograph from hospital day 1. This radiograph demonstrates diffuse bilateral coalescent opacities with complete and near complete opacification of right and left lungs respectively. Inflow (blue arrow) and outflow cannula (red arrow) from initial VA ECMO configuration can be additionally visualized. Endotracheal tube (*); Percutaneous sheath introducer (black arrow).
Over the next 48 hours, her lactic acidosis resolved, and her vasopressor requirements were minimal. On day 3 of admission, repeat TTE showed marked improvement in cardiac contractility with normalization of RV systolic function and a LVEF of 45–50%, prompting femoral artery decannulation. Due to persistent hypoxemia, she was transitioned to veno-venous ECMO (Fig. 2). Six days after admission, the patient was decannulated, and veno-venous ECMO support discontinued. She was weaned off mechanical ventilation 10 days after her admission and transferred to the surgical floor after a 14-day ICU stay.
Figure 2.
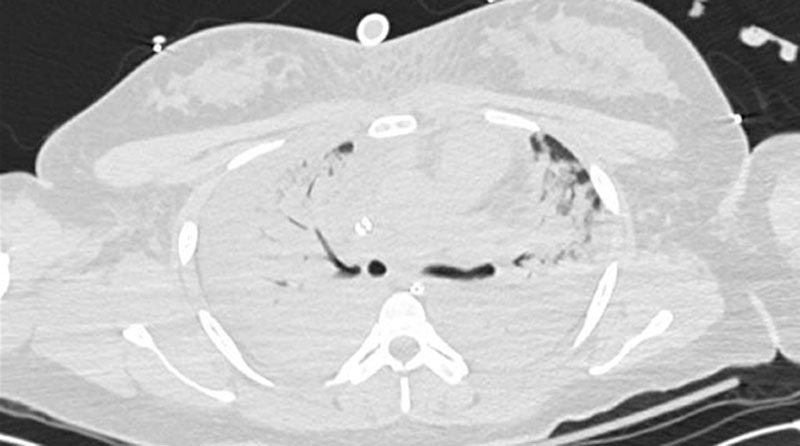
Selected images from computed tomographic imaging of the chest during hospital day 2. Computed tomographic imaging demonstrated diffuse, near complete, consolidative opacities in the bilateral lungs fields.
Prior to discharge from the hospital, TTE demonstrated improvement in LVEF to 55%, normal diastolic function, and normal RV systolic function (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A232). She was discharged home on day 19, neurologically intact, with recovered renal function, and without a supplemental oxygen requirement.
DISCUSSION
The case exemplifies not only the diagnostic and pathophysiologic challenges in managing a patient with an AFE but also the advanced resources required for the successful resuscitation and longitudinal management of such a patient. In this case with combined shock with associated coagulopathy, we were able to use veno-arterial-venous ECMO to promote both cardiopulmonary and eventual functional recovery following timely diagnosis and intervention. In addition, we were able to demonstrate that advanced ECMO configurations can still be used to support cardiopulmonary function and perfusion while attempts are made at obtaining hemostasis and correcting coagulopathy.
Early diagnostic recognition of AFE and associated hallmark cardiopulmonary manifestations (i.e. RV failure from increased pulmonary vascular resistance and associated impairment in LV filling and cardiac output) was crucial to mobilization of additional care teams. Expansion to veno-arterial-venous ECMO allowed for RV offloading in the setting of severe dysfunction while also supporting systemic oxygenation and perfusion. The resultant decrease in myocardial oxygen demand and improved delivery allowed time for cardiopulmonary recovery.
The ability of our team to implement ECLS while managing this patient’s severe hemorrhage, coagulopathy, cardiovascular collapse, and respiratory failure was the result of the rapid mobilization of a multidisciplinary and multidepartmental team. Our ECMO consultants—consisting of a critical care attending and fellow—were notified promptly after the realization that initial prehospital and ED resuscitation efforts were becoming futile. Our ECMO procedural team—including representatives from critical care medicine, cardiothoracic surgery and perfusion medicine—responded quickly to subsequent activation, and the decision to cannulate was reached based on discussions between the ECMO team, obstetricians, and emergency medicine providers. The successful response would not have been possible without physicians accustomed to cannulating in periprocedural locations, perfusionists, portable ECMO cannulation equipment, a MTP, and on-call OR capabilities.
Veno-arterial ECMO initially accomplished two primary objectives: offloading the RV through diversion of systemic venous return and augmentation of systemic perfusion. Additional improvements in cardiac function can be seen through correction of acid/base and gas exchange abnormalities. As biventricular function improved, differential hypoxemia (i.e. Harlequin or north-south) syndrome was observed. Improving cardiac function with simultaneous respiratory failure shifts the transition point between deoxygenated blood from the patient’s native cardiac output, which is why arterial blood sampling (and/or pulse oximetry) from the right upper extremity is typically undertaken. When encountered, one potential response is conversion to veno-arterial-venous ECMO, wherein an additional postoxygenator outflow limb and venous cannula are used to deliver oxygenated blood to the RV to partially supplant pulmonary function. In this instance, the patient required relatively little arterial outflow early in her ECMO course after correction of factors that contributed to her initial cardiogenic shock, which highlights the need to continuously reassess the interactions between native cardiac function, native pulmonary function, and ECLS modalities. After recovery of biventricular function, we converted solely to veno-venous ECMO to promote ongoing respiratory support.
The risk of thromboembolic events (e.g., circuit thrombosis, oxygenator failure, and native vasculature thromboembolism) during ECMO must be weighed against the risk of major bleeding during systemic anticoagulation. Advancements in oxygenator design alongside heparin-bonded and other coated circuits have allowed for the safe conduct of ECMO without systemic anticoagulation. Risks of circuit failure can be further mitigated by maintaining high circuit flows (thereby avoiding stasis), simplifying circuit configurations (e.g., excluding sampling ports), and monitoring the flow of bifurcated circuit limbs. Moreover, the need for systemic anticoagulation may be further diminished in the setting of superimposed coagulopathy. Recent cohorts demonstrate a lack of increase in thromboembolism rates and overall decreased bleeding-related complications in patients who are not anticoagulated while on ECMO (19). This strategy was initially described in the trauma literature (17, 20, 21). During the time that this patient was on veno-arterial-venous ECMO, anticoagulation was initially deferred in light of her hemorrhagic shock and DIC. Her relatively rapid recovery allowed us to decannulate her quickly and defer anticoagulation completely. She demonstrated no thrombotic complication on routine post decannulation venous duplex ultrasound.
A high index of suspicion by outpatient and prehospital providers, first responders, and inpatient providers from various disciplines alike is crucial in timely diagnosis of AFE. Awareness of advanced management options (e.g., veno-arterial-venous ECMO) when AFE is associated with persistent hemorrhagic shock, profound hypoxemia, and cardiopulmonary arrest can promote rapid mobilization of resources (22–25). As additionally highlighted in this case, a multidepartmental and multidisciplinary team is needed to initiate ECLS and longitudinally manage ECLS, but through timely recognition and group efforts, the potential for significant reduction in AFE-associated morbidity and mortality may be possible.
CONCLUSIONS
As demonstrated, morbidity-free survival can be achieved through timely utilization of veno-arterial-venous ECMO and without systemic anticoagulation in a patient suffering from combined cardiopulmonary failure and hemorrhagic shock. There continues to be a need for additional exploration of advanced mechanical support options in young, comorbid free patients suffering from hemorrhagic shock and cardiopulmonary failure, such as ours. Application of a successful extracorporeal treatment strategy is contingent on prompt resource mobilization and care coordination between practiced, interdisciplinary healthcare professionals.
Supplementary Material
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Clark SL, Hankins GDV, Dudley DA, et al. Amniotic fluid embolism: Analysis of the national registry. Am J Obstet Gynecol. 1995; 1724 Pt 11158–1167 [DOI] [PubMed] [Google Scholar]
- 2.Knight M, Berg C, Brocklehurst P, et al. Amniotic fluid embolism incidence, risk factors and outcomes: A review and recommendations. BMC Pregnancy Childbirth. 2012; 12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014; 123:337–348 [DOI] [PubMed] [Google Scholar]
- 4.Pacheco LD, Saade G, Hankins GDV; Society for Maternal-Fetal Medicine (SMFM) Amniotic fluid embolism: Diagnosis and management. Am J Obstet Gynecol. 2016; 215:B16–B24 [DOI] [PubMed] [Google Scholar]
- 5.Ho C-H, Chen K-B, Liu S-K, et al. Early application of extracorporeal membrane oxygenation in a patient with amniotic fluid embolism. Acta Anaesthesiol Taiwan. 2009; 47:99–102 [DOI] [PubMed] [Google Scholar]
- 6.Seong GM, Kim SW, Kang HS, et al. Successful extracorporeal cardiopulmonary resuscitation in a postpartum patient with amniotic fluid embolism. J Thorac Dis. 2018; 10:E189–E193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh Y-Y, Chang C-C, Li P-C, et al. Successful application of extracorporeal membrane oxygenation and intra-aortic balloon counterpulsation as lifesaving therapy for a patient with amniotic fluid embolism. Am J Obstet Gynecol. 2000; 183:496–497 [DOI] [PubMed] [Google Scholar]
- 8.Tincrès F, Conil JM, Crognier L, et al. Veno-arterial extracorporeal membrane oxygenation in a case of amniotic fluid embolism with coexisting hemorrhagic shock: Lessons learned. Int J Obstet Anesth. 2018; 33:99–100 [DOI] [PubMed] [Google Scholar]
- 9.Shen H-P, Chang W-C, Yeh L-S, Ho M. Amniotic fluid embolism treated with emergency extracorporeal membrane oxygenation: A case report. J Reprod Med. 2009; 54:706–708 [PubMed] [Google Scholar]
- 10.Firstenberg MS, Abel E, Blais D, et al. Temporary extracorporeal circulatory support and pulmonary embolectomy for catastrophic amniotic fluid embolism. Heart Surg Forum. 2011; 14:E157–E159 [DOI] [PubMed] [Google Scholar]
- 11.Viau-Lapointe J, Filewod N. Extracorporeal therapies for amniotic fluid embolism. Obstet Gynecol. 2019; 134:989–994 [DOI] [PubMed] [Google Scholar]
- 12.Depondt C, Arnaudovski D, Voulgaropoulos A, et al. Venoarterial extracorporeal membrane oxygenation as supportive therapy after cardiac arrest after amniotic fluid embolism: a case report. A A Pract. 2019; 13:74–77 [DOI] [PubMed] [Google Scholar]
- 13.Skolnik S, Ioscovich A, Eidelman LA, et al. Anesthetic management of amniotic fluid embolism -- a multi-center, retrospective, cohort study. J Matern Fetal Neonatal Med. 2019; 32:1262–1266 [DOI] [PubMed] [Google Scholar]
- 14.Gitman R, Bachar B, Mendenhall B. Amniotic fluid embolism treated with veno-arterial extracorporeal membrane oxygenation. Case Rep Crit Care. 2019; 2019:4589636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wise EM, Harika R, Zahir F. Successful recovery after amniotic fluid embolism in a patient undergoing vacuum-assisted vaginal delivery. J Clin Anesth. 2016; 34:557–561 [DOI] [PubMed] [Google Scholar]
- 16.Huang K-Y, Li Y-P, Lin S-Y, et al. Extracorporeal membrane oxygenation application in post-partum hemorrhage patients: Is post-partum hemorrhage contraindicated? J Obstet Gynaecol Res. 2017; 43:1649–1654 [DOI] [PubMed] [Google Scholar]
- 17.Bonacchi M, Spina R, Torracchi L, Harmelin G, Sani G, Peris A. Extracorporeal life support in patients with severe trauma: An advanced treatment strategy for refractory clinical settings. J Thorac Cardiovasc Surg. 2013; 145:1617–1626 [DOI] [PubMed] [Google Scholar]
- 18.Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: Consensus-based clinical case reporting guideline development. Glob Adv Health Med. 2013; 2:38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood KL, Ayers B, Gosev I, et al. Venoarterial-extracorporeal membrane oxygenation without routine systemic anticoagulation decreases adverse events. Ann Thorac Surg. 2020; 10951458–1466 [DOI] [PubMed] [Google Scholar]
- 20.Torre VD, Della Torre V, Robba C, et al. Extra corporeal membrane oxygenation in the critical trauma patient. Curr Opin Anaesthesiol. 2019; 322234–241 [DOI] [PubMed] [Google Scholar]
- 21.Wu MY, Chou PL, Wu TI, et al. Predictors of hospital mortality in adult trauma patients receiving extracorporeal membrane oxygenation for advanced life support: A retrospective cohort study. Scand J Trauma Resusc Emerg Med. 2018; 26:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma NS, Wille KM, Bellot SC, et al. Modern use of extracorporeal life support in pregnancy and postpartum. ASAIO J. 2015; 611110–114 [DOI] [PubMed] [Google Scholar]
- 23.McDonald C, Laurie J, Janssens S, et al. Successful provision of inter-hospital extracorporeal cardiopulmonary resuscitation for acute post-partum pulmonary embolism. Int J Obstet Anesth. 2017; 30:65–68 [DOI] [PubMed] [Google Scholar]
- 24.Moore SA, Dietl CA, Coleman DM. Extracorporeal life support during pregnancy. J Thorac Cardiovasc Surg. 2016; 151:1154–1160 [DOI] [PubMed] [Google Scholar]
- 25.Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020; 222148–52 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


