Abstract
Objective
To investigate chest computed tomography (CT) findings associated with severe COVID-19 pneumonia in the early recovery period.
Methods
We retrospectively analyzed the cases of patients diagnosed with severe COVID-19 pneumonia at a single center between January 12, 2020, and March 16, 2020. The twelve ICU patients studied had been diagnosed SARS-CoV-2 (COVID-19) nucleic acid positive. Patient clinical symptoms were relieved or disappeared, and basic clinical information and laboratory test results were collected. The study focused on the most recent CT imaging characteristics.
Results
The average age of the 12 patients was 58.8 ± 16.2 years. The most prevalent symptoms were fever (100%), dyspnea (100%), and cough (83.3%). All patients experienced acute respiratory distress syndrome (ARDS), of which 9 were moderate to severe. Six patients used noninvasive ventilators, and 4 patients used mechanical ventilation. One patient was treated with extracorporeal membrane oxygenation (ECMO). The lymphocyte count decreased to 0.67 ± 0.3 (× 10 9/L). The average day from illness onset to the last follow-up CT was 56.1 ± 7.7 d. The CT results showed a decrease in ground glass opacities (GGO), whereas fibrosis gradually increased. The common CT features included GGO (10/12, 83.3%), subpleural line (10/12, 83.3%), fibrous stripes (12/12, 100%), and traction bronchiectasis (10/12, 83.3%). Eight patients (66.7%) showed predominant reticulation and interlobular thickening. Four patients (33.3%) showed predominant GGO. Lung segments involved were 174/216 (80.6%).
Conclusions
Fibrous stripes and GGO are common CT signs in critically ill patients with COVID-19 pneumonia in the early recovery period. Signs of pulmonary fibrosis in survivors should be carefully monitored.
Keywords: COVID-19, Pneumonia: Critical care, Spiral CT scan, Pulmonary fibrosis
Highlights
-
•
Treatment of critically ill patients with COVID-19 pneumonia is challenging.
-
•
Fibrous stripes are common CT signs in critically ill patients with COVID-19 pneumonia in the early recovery period.
-
•
More focus should be placed on the signs of pulmonary fibrosis in survivors.
1. Introduction
Since mid-December 2019, some hospitals in Wuhan, Hubei Province, China, have identified many patients with an unknown pneumonia, now known as a new type of acute respiratory infection caused by a coronavirus infection [1,2]. On February 12, 2020, the International Virus Classification Commission officially classified the new coronavirus as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. Subsequently, the World Health Organization (WHO) named the disease Corona Virus Disease 2019 (COVID-19) [4]. Since then, COVID-19 has attracted attention worldwide [5,6].
Computed tomography (CT) plays an important role in the diagnosis of respiratory diseases [7]. Previous radiological studies have reported that a typical CT feature of COVID-19 pneumonia is bilateral ground-glass opacification (GGO), which can be combined with peripheral and posterior lung distributions [[7], [8], [9], [10]]. Similar to other coronavirus pneumonia, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), COVID-19 can cause acute respiratory distress syndrome (ARDS) [1,11]. In a retrospective study of critically ill patients, the authors reported that 67% of patients had ARDS, and 61.5% of patients died within 28 days [12]. The treatment of critically ill patients remains challenging, and the long-term quality of life of these patients has attracted increased attention. Pulmonary fibrosis was reported in 62% of survivors of coronavirus-SARS pneumonia during follow-up [13]. This study explores the CT findings of lungs in critically ill survivors during the early recovery period, focuses on patient lung imaging, and provides more information for diagnosis and treatment.
2. Methods
2.1. Patients and data sources
The study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and complies with the 1964 Helsinki Statement and subsequent amendments or similar ethical standards. We retrospectively analyzed cases of critically ill patients diagnosed with COVID-19 pneumonia at a single center (Tongji Hospital) between January 12, 2020, and March 16, 2020. The patients in this unit were those who had been diagnosed SARS-CoV-2 nucleic acid positive and transferred from other hospitals or our general ward to the ICU. The study recorded patients who had successfully been rescued to the general ward from this ward. Severe cases were defined as: 1. Respiratory distress, respiratory rates ≥30 breaths/min; 2. SpO2 ≤ 93% at a rest state; 3. PaO2/FIO2 ratio ≤ 300; 4. Patients with >50% lesions progression within 24 to 48 h in pulmonary imaging. Critically ill cases were defined as: 1. Respiratory failure and mechanical ventilation required; 2. Shock occurs; 3. Complications with other organ failures that require monitoring and treatment in ICU [14]. If accompanied by ARDS disease, ARDS classification will be based on reported criteria [15].
We collected CT imaging data, clinical characteristics (age, gender, and chronic medical histories: hypertension, diabetes, COPD, immunosuppression, heart disease, and asthma), symptoms from onset to hospital admission (fever, cough, fatigue, expectoration, chest pain, myalgia, dyspnea, abdominal pain and diarrhea, pharyngeal discomfort, and headache), vital signs at ICU admission (heart rate, respiratory rate, and blood pressure), the time before ICU transfer, ARDS levels, the presence and type of shock (septic shock, cardiogenic shock, and hypovolemic shock), type of respiratory support, length of ICU stay and ADL score leaving ICU. Moreover, laboratory tests were conducted at admission and included leukocyte, lymphocyte, and neutrophil counts and measurements of C-reactive protein (CRP), D-dimer, lactate dehydrogenase (LDH), procalcitonin, and erythrocyte sedimentation rate (ESR).
2.2. Chest CT protocols
Chest CT images were obtained on one of three CT systems (LightSpeed Plus-GE, Aquilion ONE, and UCT 780) with patients in the supine position. The scanning parameters were as follows: tube voltage = 100–120 kV, automatic tube current modulation (30–70 mAs), pitch = 0.99–1.22 mm, matrix = 512 × 512, slice thickness = 10 mm, and field of view =350 mm × 350 mm. All images were reconstructed with a slice thickness of 1.0–1.25 mm with the same increment. According to the recommendations of the National Health and Health Commission, patients also underwent follow-up CT scans before transferring out of the ICU.
2.3. Chest CT evaluation
We focused on the most recent CT imaging of each patient and compared it with the previous film. Three radiologists (X.D, J.Z, and G.H.L with 11, 10 and 6 years of experience in interpreting chest CT imaging, respectively) described the main CT features, i.e., ground-glass opacity, subpleural line, interlobular septal thickening, fibrous stripes, small nodule, traction bronchiectasis, lymph node enlargement, pericardial effusion, pleural effusion and lesion distribution (central/peripheral/both central and peripheral). To quantify the extent of lesions, lung segments were assigned based on all abnormal areas. The CT scans were assessed for the presence and distribution of parenchyma abnormalities. Locations of lung involvement were reported as one or more of 18 segments in the lungs. The following rules were used to assess the involvement of the lung segment: if more than half, then record the segment (1 point). If the part involved does not exceed half, record 0.5 points. The final score was determined by consensus among the three radiologists. Finally, for each CT scan, the radiologist was required to determine the main types of CT image: 1. GGO predominant, or 2. Reticulation predominant.
2.4. Statistical analysis
Statistical analysis and calculations were performed using SPSS version 18.0 (Chicago SPSS, Inc.). Continuous variables were expressed as the means ± standard deviation, and categorical variables were recorded as frequencies and percentages.
3. Results
3.1. Characteristics of the patients
Data were collected for 12 patients who were transferred to the general ward after ICU treatment. The average age of the 9 male and 3 female patients was 58.8 ± 16.2 years (range, 26–81 years old). The length of time before transfer to the ICU ward was 15.9 ± 6.8 d (Including general ward treatment time). Hypertension (58.0%) and diabetes (33.3%) were the most common concomitant diseases. The most prevalent presenting symptoms were fever (100%), cough (83.3%), and dyspnea (100%). Less common symptoms were diarrhea, expectoration, and myalgia. The average heart rate was 102.1 ± 12.6 bpm, and the average respiratory rate was 34.2 ± 6.1 bpm. All patients experienced ARDS, of which 9 were moderate to severe. All patients underwent oxygen therapy, of which 6 patients used noninvasive ventilators and 4 patients were treated with mechanical ventilation. One patient was treated with extracorporeal membrane oxygenation (ECMO), whereas two patient experienced septic shocks. All patients were alive with high ADL scores (97.1 ± 4.0) and transferred to the general ward with intermittent oxygen therapy. The length of stay in the ICU ward was 21.8 ± 8.6 d (Table 1 ). Regarding laboratory data, the average leukocyte count was 11.1 ± 8.2 (× 109/L), the average neutrophil count was 10.2 ± 8.1 (× 10 9/L), and the average lymphocyte count was 0.67 ± 0.3 (× 109/L). The average C-reactive protein, ESR, D-dimer and LDH levels increased to 90.7 ± 74.5 (mg/L), 51.0 ± 29.5 (mm/H), 9.5 ± 8.9 (mg/L), and 566.3 ± 325.2 (U/L) respectively. The average procalcitonin level was 0.49 ± 0.56 (ng/mL) (Table 2 ).
Table 1.
Characteristics of the 12 critical patients with COVID-19.
| Characteristics | Patients (N = 12) |
|---|---|
| Age, years | 58.8 ± 16.2 |
| Male | 9 (75.0%) |
| Time before transfer ICU, day | 15.9 ± 6.8 |
| Comorbidity | |
| Hypertension | 7 (58.0%) |
| Diabetes | 4 (33.3%) |
| COPD | 0 (0%) |
| Immunosuppression | 0 (0%) |
| Heart disease | 1 (8.3%) |
| Asthma | 0 (0%) |
| ARDS level | |
| Mild | 3 (25.0%) |
| Moderate | 5 (41.7%) |
| Severe | 4 (33.3%) |
| Shock | 2 (16.7%) |
| Respiratory support | |
| High flow oxygen therapy | 12 (100%) |
| Non-invasive ventilator | 6 (50.0%) |
| Ventilator | 4 (33.3%) |
| ECMO | 1 (8.3%) |
| Length of stay ICU, day | 21.8 ± 8.6 |
| ADL scores | 97.1 ± 4.0 |
Data are n (%), n/N (%), Mean ± SD, where N is the total number of patients with available data.
ECMO: Extracorporeal membrane oxygenation.
Table 2.
Laboratory findings of the 12 critical patients with COVID-19.
| Laboratory findings | Patients (n = 12) |
|---|---|
| White blood cell count, × 10 9 /L | 11.1 ± 8.2 |
| >10 | 5 (41.7%) |
| 4–10 | 7 (58.3%) |
| ≤4 | 0 (0%) |
| Neutrophil count, × 10 9 /L | 10.2 ± 8.1 |
| >6.3 | 7 (58.3%) |
| 1.8–6.3 | 5 (41.7%) |
| ≤1.8 | 0 (0%) |
| Lymphocyte count, × 10 9 /L | 0.67 ± 0.3 |
| >3.2 | 0 (0%) |
| 1.1–3.2 | 1 (8.1%) |
| ≤1.1 | 11 (91.9%) |
| C-reactive protein, mg/L | 90.7 ± 74.5 |
| >10 | 10 (83.3%) |
| ≤10 | 2 (16.7%) |
| ESR, mm/H | 51.0 ± 29.5 |
| >15 | 10 (83.3%) |
| ≤15 | 2 (16.7%) |
| D-dimer, mg/L | 9.5 ± 8.9 |
| >0.5 | 12 (100%) |
| ≤0.5 | 0 (0%) |
| LDH, U/L | 566.3 ± 325.2 |
| >225 | 12 (100%) |
| ≤225 | 0(0%) |
| Procalcitonin, ng/mL | 0.49 ± 0.56 |
| >0.5 | 3 (25.0%) |
| ≤0.5 | 9 (75.0%) |
Data are n (%), n/N (%), mean ± SD, where N is the total number of patients with available data.
3.2. CT findings in the early recovery stage
The average time from illness onset to the most recent CT scan was 56.1 ± 7.7 days (Table 3 ). The CT images showed that most patients showed decreased GGO and gradually increased fibrosis (Fig. 1 ). Common chest CT features in the early recovery stage included GGO (10/12, 83.3%), subpleural line (10/12, 83.3%), interlobular septal thickening (12/12, 100%), and fibrous stripes (12/12, 100%). The GGO showed peripheral distribution in 3 cases (25%) and both central and peripheral distribution in 7 cases (58.3%). The fibrous stripes showed peripheral distribution in 3 cases (25.0%) and both central and peripheral distribution in 9 cases (75.0%). Three (25.0%) patients had consolidation, 10 (83.3%) patients had traction bronchiectasis, 2 (16.7%) patients had pleural effusion, 7 (58.3%) patients had lymph node enlargement, and one patient had a small fibrous nodule. No patients had pericardial effusion. As the predominant CT feature, eight patients (66.7%) showed reticulation predominant and interlobular thickening (Fig. 2 a1–a2), and 4 (33.3%) showed GGO predominant (Fig. 2 b1–b2). Lung segments involved were 174/216 (80.6%). We also found that some lung fibrotic lesions were absorbed slowly when comparing an older CT examination on the 49th day with that on the 59th day, which indicated low alleviation in lung fibrosis (Fig. 3 ).
Table 3.
Characteristics of the most recent CT in early recovery of the 12 patients with COVID-19.
| CT features | Patients (n = 12) |
|---|---|
| Days from illness onset to follow-up CT(d) | 56.1 ± 7.7 |
| GGO | 10 (83.3%) |
| Central | 0 (0%) |
| Peripheral | 3 (25.0%) |
| Both central and peripheral | 7 (58.3%) |
| Subpleural line | 10 (83.3%) |
| Interlobular septal thickening | 12 (100%) |
| Fibrous stripes | 12 (100%) |
| Central | 0 (0%) |
| Peripheral | 3 (25.0%) |
| Both central and peripheral | 9 (75.0%) |
| Consolidation | 3 (25.0%) |
| Traction brochiectasis | 10 (83.3%) |
| Small nodule | 1 (8.3%) |
| Lymph node enlargement | 7 (58.3%) |
| Pericardial effusion | 0 (0%) |
| Pleural effusion | 2 (16.7%) |
| Main CT manifestation | |
| GGO predominance | 4 (33.3%) |
| Reticulation predominance | 8 (66.7%) |
| Lung Segments involved | 174/216(80.6%) |
Data are n (%), n/N (%), mean ± SD, where N is the total number of patients with available data.
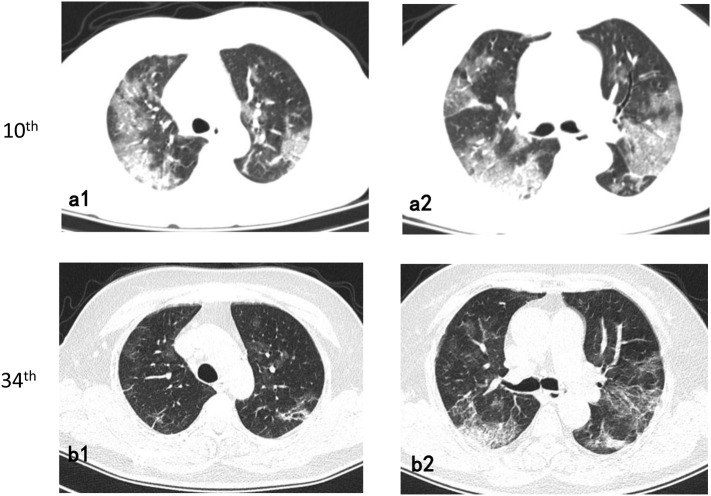
Fig. 1.
72-year-old/Female.
Follow-up axial chest CT images (34th day from onset) (b1-b2) showed a decrease in the extent of ground-glass opacities than the 10th day CT images and an increase in fibrotic lesions (a1-a2).
Fig. 2.
26-year-old/Male.
The main performance of chest CT images (61st day from onset) was reticulation predominant and interlobular thickening (a1–a3).
60-year-old/Male.
The main performance of chest CT images (41st day from onset) was GGO (b1–b3).
Fig. 3.
72-year-old/Female.
Follow-up axial chest CT images (59th day from onset) (b1-b2) showed slight decrease in the extent of ground-glass opacities than the 49th day CT images, with negligible changes in fibrotic lesions (a1–a2).
4. Discussion
In the study, most patients with COVID-19 pneumonia were diagnosed with moderate to severe ARDS. The main clinical manifestations were moderate fever, cough, and dyspnea. Many laboratory tests were abnormal, especially a significantly reduced lymphocyte count, indicating severe disease [16]. The disease was and remains difficult to treat. Oxygen supportive therapy was often used, and some patients were treated with ventilation. One patient was treated with ECMO in the ICU, which indicated that the disease was extremely critical when considered severe. The treatment was very challenging, and surviving patients suffered from many psychological and physiological injuries. Therefore, it is necessary to improve the quality of life for survivors.
This study directed more focus on pulmonary imaging after clinical symptoms alleviated. Earlier reports indicated that lung abnormalities on chest CT showed the greatest severity approximately 10 days after the initial onset of symptoms and only began to reduce after fourteen days when entering an absorption phase [17]. The 12 patients were able to achieve normal living ability, as indicated by CT examinations nearly two months after onset. However, the lesions in lung CT images were still apparent. CT findings in the early recovery stage showed reduced GGO and reduced consolidation, but pulmonary fibrosis significantly increased with multiple lung lobes involved, even diffuse changes. Pulmonary fibrosis was manifested as fibrous shadows (such as fibrous stripes, subpleural line, and traction bronchiectasis) and a developed network advantage. As reported, pulmonary fibrosis may develop early in patients with SARS who had been discharged after treatment [18]. In patients recovered from SARS, follow-up lung CT scans showed persistent lung fibroses, suggesting the possibility of long-term fibrosis [13]. We also observed pulmonary fibrosis absorption appeared to be slow during recovery (Fig. 3). Whether these lesions would completely disappear requires further observation. Similarly, whether lung fibrosis will affect lung function requires further research.
5. Limitations
There are shortcomings in this study. Because critically ill patients have a low survival rate and the number of patients was limited, it was difficult to arrive at any definitive conclusion on the effects of pulmonary fibrosis and pulmonary function in the disease. Second, the observation time of the patients was limited; the patients require a longer follow-up period.
6. Conclusion
Pulmonary fibrous stripes and GGO are common CT signs in critically ill patients with COVID-19 pneumonia in the early recovery period. We need to pay more attention to the signs of pulmonary fibrosis in survivors.
Funding
None.
Author contributions
Study concept and design (Y·F, S.S·Y), analysis and interpretation of the data (X.D, J.Z, G.H.L), drafting of the manuscript (Y·F, S.S.Y), and critical revision of the manuscript for important intellectual content (S.S.Y).
Credit author statement
Pulmonary fibrosis in critically ill patients recovered from COVID-19 pneumonia: Preliminary Experience.
Yu Fang: Study concept and design, Drafting of the manuscript.
Jun Zhou, Xun ding, Gonghao Ling: Analysis and interpretation of the data.
Shanshan Yu: Study concept and design, critical revision of the manuscript for important intellectual content.
Each of the coauthors has seen and agrees with each of the changes made to this manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.V. Coronaviridae Study Group of the International Committee on Taxonomy of The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang S., Shi Z., Shu Y., Song J., Gao G.F., Tan W. A distinct name is needed for the new coronavirus. Lancet. 2020;395(10228):949. doi: 10.1016/S0140-6736(20)30419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahase E. China coronavirus: WHO declares international emergency as death toll exceeds 200. BMJ. 2020 doi: 10.1136/bmj.m408. 368:m408. [DOI] [PubMed] [Google Scholar]
- 6.Chavez S., Long B., Koyfman A., Liang S.Y. Coronavirus disease (COVID-19): a primer for emergency physicians. Am J Emerg Med. 2020;S0735-6757(20):30178–30179. doi: 10.1016/j.ajem.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Y., Guan H., Zhou S., Wang Y., Li Q., Zhu T. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): A study of 63 patients in Wuhan, China. Eur Radiol. 2020;30(6):3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan Y., Guan H. Imaging changes in patients with 2019-nCov. Eur Radiol. 2020;30(7):3612–3613. doi: 10.1007/s00330-020-06713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song F., Shi N., Shan F., Zhang Z., Shen J., Lu H. Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2019;2020(295):210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT Imaging Features of 2019 Novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11:836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan K.S., Zheng J.P., Mok Y.W., Li Y.M., Liu Y.N., Chu C.M. SARS: prognosis, outcome and sequelae. Respirology. 2003;8(Suppl):S36–S40. doi: 10.1046/j.1440-1843.2003.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. [Google Scholar]
- 15.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 16.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;12:ciaa248. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan F., Ye T., Sun P., Gui S., Liang B., Li L. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020:200370. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonio G.E., Wong K.T., Hui D.S., Wu A., Lee N., Yuen E.H. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. 2003;228:810–815. doi: 10.1148/radiol.2283030726. [DOI] [PubMed] [Google Scholar]