Abstract
Objective
To provide an update on key safety metrics after transfusion of convalescent plasma in hospitalized coronavirus 2019 (COVID-19) patients, having previously demonstrated safety in 5000 hospitalized patients.
Patients and Methods
From April 3 to June 2, 2020, the US Food and Drug Administration Expanded Access Program for COVID-19 convalescent plasma transfused a convenience sample of 20,000 hospitalized patients with COVID-19 convalescent plasma.
Results
The incidence of all serious adverse events was low; these included transfusion reactions (n=78; <1%), thromboembolic or thrombotic events (n=113; <1%), and cardiac events (n=677, ~3%). Notably, the vast majority of the thromboembolic or thrombotic events (n=75) and cardiac events (n=597) were judged to be unrelated to the plasma transfusion per se. The 7-day mortality rate was 13.0% (12.5%, 13.4%), and was higher among more critically ill patients relative to less ill counterparts, including patients admitted to the intensive care unit versus those not admitted (15.6 vs 9.3%), mechanically ventilated versus not ventilated (18.3% vs 9.9%), and with septic shock or multiple organ dysfunction/failure versus those without dysfunction/failure (21.7% vs 11.5%).
Conclusion
These updated data provide robust evidence that transfusion of convalescent plasma is safe in hospitalized patients with COVID-19, and support the notion that earlier administration of plasma within the clinical course of COVID-19 is more likely to reduce mortality.
Abbreviations and Acronyms: COVID-19, coronavirus disease 2019; EAP, Expanded Access Program; FDA, Food and Drug Administration; ICU, intensive care unit; IRB, Institutional Review Board; SAE, serious adverse event; TACO, transfusion-associated circulatory overload; TRALI, transfusion-related acute lung injury
Coronavirus disease 2019 (COVID-19) continues to be a worldwide pandemic, and the number of deaths attributed to COVID-19 in the United States at the time of this writing (~150,000) exceeds that of any other nation in the world.1 The overall case fatality rate for diagnosed COVID-19 ranges from approximately 4% to greater than 50%,2, 3, 4, 5, 6 with higher mortality rates observed in more critically ill patients. In response to the COVID-19 outbreak in the United States and reportedly high case-fatality rates, the US Food and Drug Administration (FDA) in collaboration with the Mayo Clinic and national blood banking community developed a national Expanded Access Program (EAP) to collect and distribute COVID-19 convalescent plasma. Historical precedent indicates that human convalescent plasma is a viable option for mitigation and treatment of COVID-19.7 , 8 The premise of human convalescent plasma therapy is that plasma of recently infected and currently recovered COVID-19 patients contains antiviral antibodies and other bioactive elements that can be used to treat patients with COVID-19. Convalescent plasma has a strong historical record of some efficacy during acute infectious pandemics.7 , 9 As recently summarized,7 convalescent plasma represents a promising treatment strategy with strong historical precedence, biological plausibility, and limited barriers for rapid development and deployment of this investigational therapy.
Recently, our investigation of key safety indicators in 5000 patients transfused with COVID-19 convalescent plasma showed an incidence of transfusion-related serious adverse events (SAEs) of less than 1% and a mortality rate of 14.9%.10 These early indicators suggest that transfusion of convalescent plasma is safe in hospitalized adults with COVID-19. Because an additional 15,000 hospitalized patients have been transfused with convalescent plasma under the purview of the EAP, a subsequent safety update is warranted. These new data may provide novel insights into the incidence of emerging adverse events associated with COVID-19, including thromboembolic11 , 12 and cardiac events.13 Further, these data may provide better understanding of the clinical features contributing to the 7-day mortality rate among transfused patients with COVID-19.
Thus, we analyzed key safety metrics following transfusion of convalescent plasma in 20,000 hospitalized adults with severe or life-threatening COVID-19. These data represent a deeper and larger analysis relative to our initial report of 5000 transfused patients under the EAP. We hypothesized that both the 7-day mortality rate and the number of SAEs related to the transfusion of convalescent plasma would continue to be low. Additionally, we hypothesized that higher mortality rates would be observed in more critically ill patients.
Patients and Methods
As described previously,10 the program is a FDA-initiated, national, multicenter, open-label EAP in hospitalized adults who had (or were judged to have high risk of progression to) severe or life-threatening COVID-19. The US COVID-19 Convalescent Plasma EAP was conducted as a pragmatic treatment study, empowering local acute care facilities to use the emerging best evidence for care while allowing for administration of convalescent plasma. It was conducted within a modified clinical trial framework: All participants were allowed access to convalescent plasma per the discretion of the treating physician/principal investigator given the nature of the pandemic and the lack of any effective therapies at the time of design. It was the intent a priori to create a control comparator group to determine potential efficacy using patients hospitalized with COVID-19 infections during the same period. This decision was made after collaboration with the US FDA. This report is only on safety of the convalescent plasma for the initial 20,000 subjects. A future publication will discuss potential efficacy.
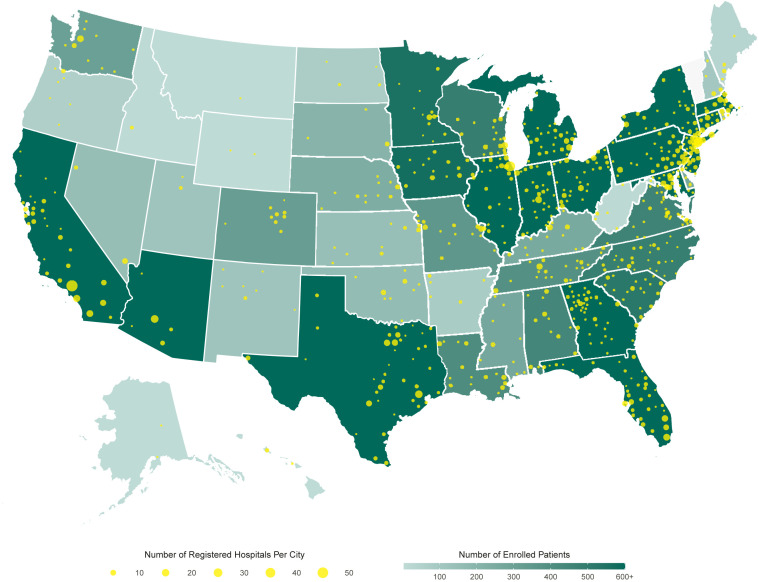
Between the date of initial Institutional Review Board (IRB) approval of the EAP (April 1, 2020) and June 11, 2020, more than 20,000 patients were transfused with COVID-19 convalescent plasma (Figure 1 ). The Mayo Clinic IRB served as the central IRB and empaneled an independent Data and Safety Monitoring Board to oversee the safety analyses. Written informed consent was obtained from the participant or a legally authorized representative before enrollment or with use of emergency consent procedures recommended by the US FDA and approved by the Mayo Clinic IRB. The protocol was modified to allow inclusion of incarcerated participants once it became clear that this group was especially vulnerable and at risk.
Figure 1.
Participation in the US COVID-19 Convalescent Plasma Expanded Access Program (EAP) including data extracted on June 11, 2020. Choropleth map displaying the number of cumulatively enrolled patients in the EAP within each state, with lower enrollment values displayed in a lighter hue of green and higher enrollment values displayed in a darker hue of green. Registered acute care facilities are represented as filled yellow circles, with larger circles indicating greater number of registered facilities within the metropolitan area of a city. The choropleth map does not display data from US territories, including registered facilities in Puerto Rico, Guam, and Northern Mariana Islands.
Eligible patients were aged 18 years or older, hospitalized with a laboratory-confirmed diagnosis of infection with severe acute respiratory syndrome coronavirus 2, and had (or were judged by a health care provider to be at high risk of progression to) severe or life-threatening COVID-19. The clinical symptoms defining severe or life-threatening COVID-19 are outlined in Table 1 .
Table 1.
Patient Characteristics Stratified by Month of COVID-19 Convalescent Plasma Transfusiona
| April | May | Total | |
|---|---|---|---|
| Characteristic | |||
| N | 6214 | 13,786 | 20,000 |
| Age, y | |||
| 18-39 | 449 (7.2) | 1083 (7.9) | 1,532 (7.7) |
| 40-59 | 2056 (33.1) | 4320 (31.3) | 6,376 (31.9) |
| 60-69 | 1798 (28.9) | 3611 (26.2) | 5,409 (27.0) |
| 70-79 | 1260 (20.3) | 2859 (20.7) | 4,119 (20.6) |
| ≥80 | 651 (10.5) | 1913 (13.9) | 2,564 (12.8) |
| Sex | |||
| Female | 2262 (36.4) | 5499 (39.9) | 7761 (38.8) |
| Male | 3924 (63.2) | 8241 (59.8) | 12,165 (60.8) |
| Intersex or transgender | 22 (0.4) | 35 (0.3) | 57 (0.3) |
| Undisclosed | 6 (0.1) | 11 (0.1) | 17 (0.1) |
| Weight statusb | |||
| Underweight | 61 (1.2) | 249 (1.8) | 310 (1.7) |
| Normal weight | 868 (17.3) | 2454 (18.0) | 3322 (17.8) |
| Overweight | 1502 (30.0) | 3802 (27.8) | 5304 (28.4) |
| Obese | 2587 (51.6) | 7166 (52.4) | 9753 (52.2) |
| Race | |||
| Asian | 408 (6.6) | 591 (4.3) | 999 (5.0) |
| Black | 1132 (18.2) | 2784 (20.2) | 3916 (19.6) |
| White | 2993 (48.2) | 6741 (48.9) | 9734 (48.7) |
| Other or unknown | 1681 (27.1) | 3670 (26.6) | 5351 (26.8) |
| Ethnicity | |||
| Hispanic or Latino | 2142 (34.5) | 4794 (34.8) | 6936 (34.7) |
| Not Hispanic or Latino | 4072 (65.5) | 8992 (65.2) | 13,064 (65.3) |
| Clinical status | |||
| Current severe or life-threatening COVID-19 | 4963 (79.9) | 9274 (67.3) | 14,237 (71.2) |
| High risk of severe or life-threatening COVID-19 | 1251 (20.1) | 4512 (32.7) | 5763 (28.8) |
| Intensive care unit admission | 4038 (65.0) | 7522 (55.0) | 11,560 (58.1) |
| Mechanical ventilationc | 2709 (48.5) | 4155 (30.4) | 6864 (35.6) |
| Clinical symptomsd | |||
| Respiratory failure | 3574 (72.0) | 6155 (66.4) | 9729 (68.3) |
| Dyspnea | 3152 (63.5) | 6561 (70.7) | 9713 (68.2) |
| Blood oxygen saturation ≤93% | 3092 (62.3) | 6663 (71.8) | 9755 (68.5) |
| Lung infiltrates >50% within 24 to 48 h | 2105 (42.4) | 4021 (43.4) | 6126 (43.0) |
| Respiratory frequency ≥30/min | 1937 (39.0) | 4014 (43.3) | 5951 (41.8) |
| PaO2:FiO2e <300 | 1642 (33.1) | 3014 (32.5) | 4656 (32.7) |
| Multiple organ dysfunction or failure | 936 (18.9) | 1212 (13.1) | 2148 (15.1) |
| Septic shock | 734 (14.8) | 987 (10.6) | 1721 (12.1) |
Values shown are n (%).
These data include a subset of the sample (n=18,689).
These data include a subset of the sample (n=19,256).
These data include a subset of the sample (n=14,237), only those patients that currently have severe or life-threatening COVID-19.
PaO2:FiO2 the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen ratio.
Procedures
As described previously,10 ABO-compatible COVID-19 convalescent plasma had no minimum neutralizing-antibody titer level and was donated by recently recovered COVID-19 survivors. Approximately 200 to 500 mL of convalescent plasma was administered intravenously according to institutional transfusion guidelines. Web-based, standardized data reporting surveys were completed to assess clinical status of patients at regular time intervals (4-hours and 7-days after convalescent plasma transfusion) using the Research Electronic Data Capture system (REDCap, v.9.1.15 Vanderbilt University, Nashville, TN).14 , 15
Serious Adverse Event Reporting
Separate REDCap data collection forms were used to report each SAE that occurred within 7 days following the convalescent plasma transfusion. Two primary data capture forms were used to report SAEs: a transfusion form and a SAE form. Serious adverse events which occurred during the time window beginning at the onset of the plasma transfusion and including the 4-hour period following the transfusion were reported on the transfusion form. By definition, the primary SAEs related to transfusion (including transfusion associated circulatory overload [TACO] and transfusion-related acute lung injury [TRALI]) occurred within 6 hours of the transfusion. All transfusion-related SAEs occurred within 4 hours of the transfusion and thus, were all reported on the transfusion form. All other SAEs were reported on the SAE form. The attribution scale used by treating physicians for evaluating SAE relatedness to convalescent plasma transfusion included unrelated, possibility related, probably related, or definitely related. All transfusion-related SAEs were independently adjudicated over the course of the study by the Investigational New Drug sponsor (M.J.J.) and trained designee (A.M.K.) using the National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol as a conceptual framework.16
Statistics
Data presented in this safety report may undergo additional data quality control measures as the study continues. The proportion of each of a series of SAEs was summarized using a point estimate and 95% score CI as outlined in Table 2 . The point estimates for mortality were estimated at day 7 based on the crude mortality and Wilson confidence interval calculation for binomial proportions. All analyses and graphics were produced with R version 3.6.2 (Vienna, Austria).
Table 2.
SAE Characteristics in Patients Transfused With COVID-19 Convalescent Plasma (N=20,000)a
| SAE: Transfusion reactions | Reported | Related | % Estimateb (95% CI) |
|---|---|---|---|
| Mortality within four hours of transfusion | 63 | 10 | 0.05 (0.03-0.09) |
| TACO | 36 | 36 | 0.18 (0.13-0.25) |
| TRALI | 21 | 21 | 0.10 (0.07-0.16) |
| Severe allergic transfusion reaction | 21 | 21 | 0.10 (0.07-0.16) |
| 7-day SAE reports | |||
| Thrombolic or thromboembolic complication | 113 | 38 | 0.19 (0.14-0.26) |
| Sustained hypotensionc | 457 | 54 | 0.27 (0.21-0.35) |
| Cardiac eventsd | 677 | 80 | 0.40 (0.32-0.50) |
| 7-day mortality | Reported | ||
| Crude Estimate | 2592 | 12.96 (12.50-13.44) | |
| Clinical status | |||
| No ICU admission (n=8323) | 772 | 9.28 (8.67-9.92) | |
| ICU admission (n=11,560) | 1806 | 15.62 (14.97-16.30) | |
| No mechanical ventilation (n=12,147) | 1220 | 9.85 (9.34-10.38) | |
| Mechanical ventilation (n=6864) | 1258 | 18.33 (17.43-19.26) | |
| Clinical symptoms | |||
| No MOF or septic shock (n=17,081) | 1952 | 11.45 (10.98-11.94) | |
| MOF or septic shock (n=2919) | 640 | 21.72 (20.27-23.24) | |
ICU = intensive care unit; MOF = multiple organ failure or dysfunction; SAE = severe adverse event; TACO = transfusion-associated circulatory overload; TRALI = transfusion-related acute lung injury.
Point estimate of related serious adverse event incidence relative to 20,000 transfusions.
Sustained hypotension included events requiring intravenous pressor support.
Cardiac events included ventricular or atrial fibrillation or arrhythmia requiring treatment, and cardiac arrest.
Results
From April 3 to June 11, 2020, a total of 30,117 patients were enrolled in the EAP and a total of 21,987 enrolled patients received a COVID-19 convalescent plasma transfusion (Figure 1). Data from the first 5000 transfused patients have been reported previously.10 This update reports data from 20,000 patients including the initial 5000 and subsequent 15,000 transfused patients. By June 2, 2020, a total of 20,000 patients had been transfused with COVID-19 convalescent plasma, thus, 7-day mortality data are presented for all 20,000 patients.
Patient Characteristics
Key patient characteristics at the time of enrollment are presented in Table 1. The patient enrollment indicates a wide age range of hospitalized patients with COVID-19, consistent with prior Centers for Disease Control and Prevention published data.17 The study population was diverse, with 20% of patients being African American, nearly 35% Hispanic, and 5% Asian. The recruitment of a diverse population has improved over the course of the study. Nearly 40% of our subjects were women. Most of the patients enrolled were overweight or obese, consistent with early reports of risk.18 Nearly all of the patients had severe or life-threatening COVID-19 by design of the investigational protocol. Nearly two-thirds had respiratory failure as well as dyspnea as a primary symptom. Most were hypoxic and nearly half had pulmonary infiltrates. At least one-third had severe respiratory compromise. Equal numbers appeared to have multiorgan failure or septic shock, but these were a small percentage of the total population.
Serious Adverse Events
Key SAEs related to the transfusion of convalescent plasma are reported in Table 2. Our report is not a comprehensive summary of all risks associated with hospitalization of COVID-19, but it did assume that convalescent plasma potentially could cause life-threatening cardiac events and thrombotic events, so these were collected with an underlying assumption of attribution. Within 4 hours of completion of the COVID-19 convalescent plasma transfusion, 141 SAEs classified as transfusion reactions were reported (<1% of all transfusions). Of these SAEs, there were 78 non-mortality events reported, with 36 reports of TACO, 21 reports of TRALI, and 21 reports of severe allergic transfusion reaction. Of the SAEs reported within 4 hours of plasma transfusion, there were 63 mortalities (0.3% of all transfusions) and 10 of these mortalities were judged as related (possibly, n=10; probably, n=0; definitely, n=0) to the transfusion of COVID-19 convalescent plasma.
Within 7 days of completion of the COVID-19 convalescent plasma transfusion, 1247 other SAEs were reported. Of these SAEs, 113 thromboembolic or thrombotic events were reported, 457 sustained hypotensive events requiring intravenous pressor support were reported, and 677 patients suffered a cardiac event. Notably, the vast majority of the thromboembolic or thrombotic complications (n=75) and cardiac events (n=597) were judged to be unrelated to the plasma transfusion.
Seven-Day Mortality
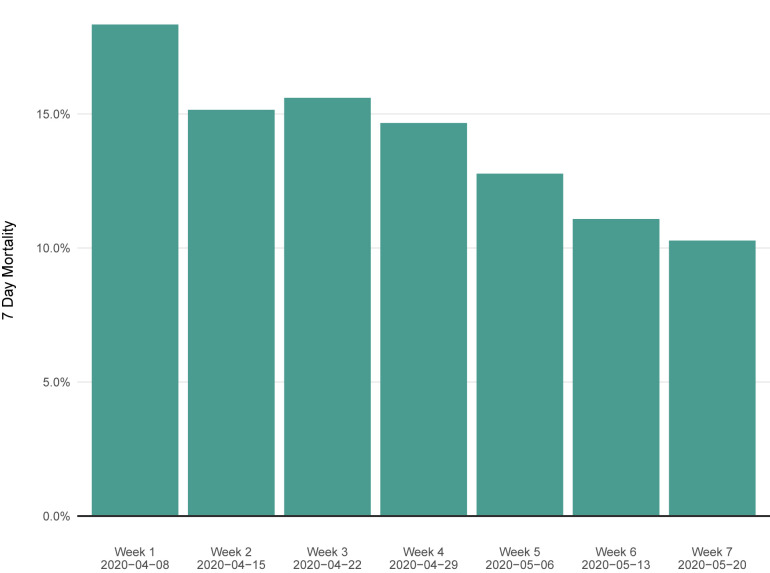
Seven-day mortality rates stratified by key clinical status indicators are presented in Table 2. Over the first 7 days after the COVID-19 convalescent plasma transfusion, a total of 2592 deaths were observed. The overall 7-day mortality rate was 12.96% (95% CI, 12.50% to 13.44%) (Figure 2 ). The 7-day mortality rate was higher among the sickest of our critically ill patients, including patients admitted to the intensive care unit (ICU) versus those not admitted to the ICU (15.6% vs 9.3%), mechanically ventilated versus not mechanically ventilated (18.3% vs 9.9%), and those with septic shock or multiple organ dysfunction/failure versus without septic shock or multiple organ dysfunction/failure (21.7% vs 11.5%).
Figure 2.
Seven-day mortality rate in patients transfused with COVID-19 convalescent plasma stratified by week since initiation of the US COVID-19 Convalescent Plasma Expanded Access Program (EAP). Each green bar indicates the 7-day mortality rate stratified by the patients enrolled during a given week since the initiation of the EAP.
Discussion
In this safety update of the US Convalescent Plasma Expanded Access Program of 20,000 hospitalized patients in the United States with severe or life-threatening COVID-19, the overall frequency of SAEs classified as attributable or likely secondary to convalescent plasma transfusion continued to be low (<1% of all transfusions) and the 7-day mortality rate in this extremely high-risk cohort was 13.0%. Despite the potential risks associated with plasma transfusion in critically ill patients,16 , 19 these data provide continued optimism for the safety of COVID-19 convalescent plasma.
Emerging COVID-19 Clinical Complications
Although thrombotic and thromboembolic events are emerging clinical complications of COVID-19,11 , 12 , 20 our data show a low rate (<1%) of these events within the first 7 days after COVID-19 convalescent plasma transfusion. Cardiac events represent another novel clinical concern of COVID-19,13 particularly in the context of open-label use of experimental treatments such as hydroxychloroquine.21 In aggregate, adverse cardiac events occurred in ~3% of patients transfused with COVID-19 convalescent plasma. The vast majority of adverse cardiac events of interest were deemed unrelated to the plasma transfusion (88%) by the treating physicians. Collectively, these data suggest that transfusion of COVID-19 convalescent plasma per se does not demonstrably increase the risk of adverse cardiac events. We note that the incidence of TRALI and TACO reported in this study of 0.10% and 0.10%, respectively, is significantly lower than those reported in previous studies, which ranged from 5% to 8% and 1% to 4%, respectively,22 despite the fact that many of the COVID-19 patients receiving plasma were critically ill and likely at risk for clinical conditions which mimic TACO.
Study Limitations
Although this study was not designed to evaluate efficacy of convalescent plasma, we note with optimism that after 20,000 transfused patients, the 7-day mortality rate (13.0%) is low (Table 2). Although the mortality rate has decreased (Figure 2), we note that the clinical characteristics of the transfused patients in the EAP have shifted toward less critically ill patients and lower proportions of apparent “rescue therapy.” No therapy has been introduced into clinical use during this period which reduces (to our knowledge) mortality in hospitalized patients with COVID-19. We postulate that several potential explanations may explain the observed decline in mortality. The ability and success of the US health care community at managing hospitalized COVID-19 patients is likely improving. Second, the blood banking communities have enhanced the availability of convalescent plasma such that more patients received plasma earlier in their hospital course. The more expeditious delivery of plasma to patients reflects improved logistics for the collection, deployment, and use of convalescent plasma, and a larger COVID-19 recovered population that may be potential plasma donors. In this regard it is remarkable that there was no system in place for convalescent plasma use in March 2020 and yet within months the nation is now able to meet most of the demand, despite complex logistics.23 Given the historical experience that antibody therapies are most effective when given earlier and that convalescent plasma has reduced mortality in prior epidemics,7 the lower mortality in more recently treated patients would be consistent with greater efficacy from earlier use. Finally, as recovered COVID-19 patients were more quickly recruited for convalescent plasma donation, the plasma may contain higher levels of neutralizing antibodies or other bioactive elements. However, additional data are required to evaluate these potential explanations for the observed trends.
Conclusion
Data from the first 20,000 patients transfused with COVID-19 convalescent plasma show that use of convalescent plasma is safe and carries no excess risk of complications. Indeed, convalescent plasma may be associated with improvement in survival; however, this report does not establish efficacy. Additionally, our data show that the US health care system is improving in its care for those hospitalized for COVID-19 including managing those critically ill patients with multiple comorbidities included in these analyses. Overall, the mortality observed has decreased with our observations and continued use of convalescent plasma. Given the accelerating deployment of this therapy, these emerging data provide early safety indicators of convalescent plasma for COVID-19 treatment and suggest research should shift focus from safety toward determining the efficacy of convalescent plasma.
Acknowledgments
The authors thank the dedicated members of the US Convalescent Plasma Expanded Access Program team — Machiko Anderson, Supriya Behl, Lori Bergstrom, Brian Butterfield, Grant Dubbels, Adam Eggert, Ree Erickson, Rebekah Frost, Daniel Gaz, Winston Guo, Starr Guzman, Karina Hex, Vidhu Joshi, Megan Knudson, Tessa Kroeninger, Frances Lynch, Tim Miksch, Lisa Muenkel, Ryan Oldenburg, Amy Olofson, Laura Pacheco-Spann, Dr Kelly Paulson, Dr Sumedha Penheiter, Melanie Peterson, Katrina Pierce, Nicloas Saikali, Jeffrey Schmoll, Pamela Skaran, Lindsay Stromback, Edward Swaray, Morgan Swope, Kristine Tree, Joe Wick, and Janelle Worthington; the members of the Mayo Clinic Institutional Review Board; the Mayo Clinic Office of Human Research Protection; the Mayo Clinic Office of Research Regulatory Support, and in particular Mark Wentworth. The authors thank the Executive Dean of Research at Mayo Clinic, Dr Gregory Gores, and the CEO of Mayo Clinic, Dr Gianrico Farrugia, for their support and assistance; the independent Data and Safety Monitoring Board for their work and oversight of the Expanded Access Program — Drs Allan S. Jaffe (chair), David O. Warner, William G. Morice II, Paula J. Santrach, Robert L. Frye, Lawrence J Appel, and Taimur Sher; the members of the National COVID-19 Convalescent Plasma Project (http://ccpp19.org) for their intellectual contributions and support; the participating medical centers and medical teams and blood centers for their rigorous efforts necessary to make this program possible; the donors for providing COVID-19 convalescent plasma; Drs Joyner, Bruno, Klassen, Kunze, Johnson, Lesser, Wiggins, Senefeld, and Klompas are co-first authors. Drs Paneth, Verdun, Marks, Casadevall, Fairweather, Carter, and Wright are co-senior authors.
Footnotes
For editorial comment, seepage 1813
Grant Support: This study was supported in part by a U.S. Department of Health and Human Services (HHS), Biomedical Advanced Research and Development Authority (BARDA) contract 75A50120C00096 (to M.J.J.), National Center for Advancing Translational Sciences (NCATS) grant UL1TR002377, National Heart, Lung, and Blood Institute (NHLBI) grant 5R35HL139854 (to MJJ), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 5T32DK07352 (to J.W.S. and C.C.W.), Natural Sciences and Engineering Research Council of Canada (NSERC) PDF-532926-2019 (to S.A.K.), National Institute of Allergy and Infectious Disease (NIAID) grants R21 AI145356 and R21 AI152318 (to D.F.), R01 AI152078 9 (to A.C.), National Heart, Lung, and Blood Institute RO1 HL059842 (to A.C.), National Institute on Aging (NIA) U54AG044170 (to S.E.B.), Schwab Charitable Fund (Eric E. Schmidt, Wendy Schmidt donors), United Health Group, National Basketball Association (NBA), Millennium Pharmaceuticals, Octapharma USA, Inc., and the Mayo Clinic.
Potential Competing Interests: The authors report no potential competing interests.
Supplemental Online Material
References
- 1.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed August 4, 2020.
- 2.Rajgor D.D., Lee M.H., Archuleta S., Bagdasarian N., Quek S.C. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–777. doi: 10.1016/S1473-3099(20)30244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang K., Zhang Z., Yu M., Tao Y., Xie M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. 2020. https://doi.org/10.1007/s00134-020-06047-w Intensive Care Med [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 4.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region — case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J.S., Chen J.T., Liu Y.X. A serological survey on neutralizing antibody titer of SARS convalescent sera. J Med Virol. 2005;77(2):147–150. doi: 10.1002/jmv.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 10.Joyner M.J. Early Safety Indicators of COVID-19 Convalescent Plasma in 5,000 Patients. 2020. https://doi.org/10.1172/JCI140200 J Clin Invest. [DOI] [PMC free article] [PubMed]
- 11.Wright F.L., Vogler T.O., Moore E.E. Fibrinolysis shutdown correlates to thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020 doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wichmann D., Sperhake J.P., Lutgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020 doi: 10.7326/M20-2003. [DOI] [PubMed] [Google Scholar]
- 13.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris P.A., Taylor R., Minor B.L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Centers for Disease Control and Prevention. The National Healthcare Safety Network (NHSN) Manual . Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases; Atlanta, GA: 2018. (Biovigilance Component v2.5). [Google Scholar]
- 17.Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzik S. COVID-19 Convalescent Plasma: Now Is the Time for Better Science. Transfus Med Rev. 2020 doi: 10.1016/j.tmrv.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klok F.A., Kruip M., van der Meer N.J.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clinic Proc. 2020;95(6):1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roubinian N. TACO and TRALI: biology, risk factors, and prevention strategies. Hematology Am Soc Hematol Educ Program. 2018;2018(1):585–594. doi: 10.1182/asheducation-2018.1.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloch E.M., Shoham S., Casadevall A. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.