Abstract
Oligodendrocyte precursor cells (OPCs) differentiate and mature into oligodendrocytes, which produce myelin in the central nervous system. Prior studies have shown that the mechanistic target of rapamycin (mTOR) is necessary for proper myelination of the mouse spinal cord and that bone morphogenetic protein (BMP) signaling inhibits oligodendrocyte differentiation, in part by promoting expression of inhibitor of DNA binding 2 (Id2). Here we provide evidence that mTOR functions specifically in the transition from early stage OPC to immature oligodendrocyte by downregulating BMP signaling during postnatal spinal cord development. When mTOR is deleted from the oligodendrocyte lineage, expression of the FK506 binding protein 1A (FKBP12), a suppressor of BMP receptor activity, is reduced, downstream Smad activity is increased and Id2 expression is elevated. Additionally, mTOR inhibition with rapamycin in differentiating OPCs alters the transcriptional complex present at the Id2 promoter. Deletion of mTOR in oligodendroglia in vivo resulted in fewer late stage OPCs and fewer newly formed oligodendrocytes in the spinal cord with no effect on OPC proliferation or cell cycle exit. Finally, we demonstrate that inhibiting BMP signaling rescues the rapamycin-induced deficit in myelin protein expression. We conclude that mTOR promotes early oligodendrocyte differentiation by suppressing BMP signaling in OPCs.
Keywords: BMP signaling, FKBP12, Id2, mTOR, OPC
1 |. INTRODUCTION
Myelin is an insulating sheath that wraps many axons of the nervous system to enable saltatory conduction of action potentials and provide trophic and metabolic support to neurons. Developmental myelination is a complex multistep process that begins when multipotent neural stem cells give rise to early stage oligodendrocyte precursor cells (OPCs), which in turn proliferate and migrate to populate the brain and spinal cord. These early stage OPCs form increasingly branched processes as they differentiate into late stage OPCs and then postmitotic immature oligodendrocytes. In response to both extrinsic cues and cell-intrinsic signaling, immature oligodendrocytes extend their processes to contact and ensheath axonal segments in concentric rings as the cells mature to form compact myelin.
Each step of developmental myelination is highly regulated through multiple signaling pathways to promote oligodendrocyte differentiation and myelination. Cellular functions are tightly controlled in order to express the necessary myelin genes at the appropriate time and to produce the cellular components essential to the lipid-rich myelin membrane. Many of the signaling pathways and molecules that regulate oligodendrocyte biology have been elucidated, but it is still unclear how these multiple levels of regulation coordinate to ensure that an OPC progresses through the stages of differentiation.
Of the known signaling pathways that regulate developmental myelination, some function to maintain OPCs in a proliferating precursor state, while others promote differentiation and maturation into oligodendrocytes. Bone morphogenetic protein (BMP) signaling modulates the activity of transcription factors functioning at myelin gene promoters and is a major negative regulator of oligodendrocyte differentiation (Cheng et al., 2007; Gomes, Mehler, & Kessler, 2003; Samanta & Kessler, 2004). BMP signaling through activation of Smad proteins increases expression of transcription factors such as the inhibitor of DNA binding (Id)2 and Id4, which in turn inhibit transcription of myelin genes (Chen, Zhang, Cai, & Yao, 2012; Marin-Husstege et al., 2006). Activated Smads form a heteromeric complex at the promoter regions of Id2 and Id4 along with other binding partners including Smad-interacting protein 1 (Sip1) and histone deacetylases (HDACs) (Emery & Lu, 2015; He et al., 2007). The presence or absence of components of this complex regulates expression of Id2 and Id4 (Weng et al., 2012). While this complex modulates the transcription machinery at the promoters, regulation of upstream components of BMP signaling in oligodendroglia is unclear, and how the BMP pathway is downregulated during OPC differentiation is not well understood.
Conversely, a major pathway that promotes oligodendrocyte differentiation and central nervous system (CNS) myelination is the PI3 kinase (PI3K)/Akt/mechanistic target of rapamycin (mTOR) pathway (recently reviewed: Figlia, Gerber, & Suter, 2018). Disruption of this pathway in mice compromises developmental myelination (Bercury et al., 2014; Lebrun-Julien et al., 2014; Wahl, McLane, Bercury, Macklin, & Wood, 2014). Specifically, these studies revealed that conditional deletion of mTOR in oligodendroglia results in a differentiation deficit and hypomyelination in the spinal cord. Interestingly, there is very little impact on developmental myelination in the brain, suggesting differential dependence on mTOR signaling. Our previous studies demonstrated that inhibiting mTOR during OPC differentiation in vitro induced mRNA expression of Id2 and Id4, suggesting that mTOR regulates BMP signaling either directly or indirectly (Tyler et al., 2009). Crosstalk between the PI3K/Akt/mTOR and BMP signaling pathways has been documented in other cell types including osteoblasts and hair follicle stem cells, further supporting a role for mTOR in inhibiting BMP signaling (Deng et al., 2015; Lee et al., 2010).
In this report, we present studies designed to test the hypothesis that mTOR promotes oligodendrocyte differentiation through suppression of BMP signaling. We demonstrate that mTOR is essential for early stage OPC differentiation in the mouse spinal cord and that oligodendrocyte-specific deletion of mTOR increases levels of Id2 in OPCs in vivo. We further provide evidence that the BMP pathway is upregulated in response to mTOR loss in vivo and to inhibition in primary rat OPCs in vitro, and we demonstrate that mTOR regulates the heteromeric Smad complex at the Id2 promoter. Finally, we show that blocking BMP activity concurrently with mTOR inhibition can rescue the myelin protein deficits caused by inhibition of mTOR, further demonstrating the critical relationship of mTOR and BMP signaling in regulating OPC differentiation.
2 |. MATERIALS AND METHODS
2.1 |. Experimental animals
All mouse protocols were conducted in accordance with the guidelines set forth by Rutgers University and the National Institutes of Health. Mice were housed in a barrier facility with a 12/12 light/dark cycle. Mice carrying a floxed-mTOR allele (Mtortm1.1Clyn) were obtained from Dr. Christopher Lynch (Pennsylvania State College of Medicine, Hershey, PA) (Carr, DiGiovanni, Lynch, & Shantz, 2012; C. H. Lang, Frost, Bronson, Lynch, & Vary, 2010; S. A. Lang, Hackl, et al., 2010). The floxed-mTOR mice were bred with a dual-reporter mouse line (B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J, The Jackson Laboratory). These mice contain the mT/mG reporter, which globally expresses tomato red, and, in cells with active Cre-recombinase, express GFP. CNP-Cre mice were obtained from K. Nave (Lappe-Siefke et al., 2003). All strains were on a C57/Bl/6 background. Mice homozygous for mTOR floxed- and mT/mG reporter alleles and heterozygous for CNP-Cre were used for breeding to generate Cre+ or Cre− littermates for experiments. The resultant mTORfl/fl mice expressing Cre (Cre+/−) were previously described (Wahl et al., 2014) and are hereafter referred to as mTOR cKO with mTORfl/fl/Cre−/− littermates used as controls. Both males and females were used in all analyses.
2.2 |. Flow cytometric analysis
Mice at postnatal day (PND) 10 were rapidly decapitated and spinal cords were dissected and dissociated into single cells using Neural Dissociation Kit (P) (Miltenyi). Live cells were identified using Molecular Probes Live/Dead Cell Stain Kit (Invitrogen). Cells were incubated with FcR blocking reagent and then stained with antibodies against oligodendroglial markers by incubating for 10 min at 4°C. Cells were then washed twice in PBS with 0.5% BSA and 2 mM EDTA and run on LSR II flow cytometer (BD Biosciences). For cell cycle analysis, cells were first stained with platelet-derived growth factor receptor (PDGFR)α antibody and then with Hoechst for 45 min at 37°C. Data were analyzed using FlowJo and ModFit LT software. The following antibodies were used: PDGFRα-BV421 rat anti-mouse antibody (1:100, BD Biosciences), NG2 (1:100, Millipore), O4-APC antibody (1:10, Miltenyi). Statistical analysis was performed using two-tailed unpaired t test in GraphPad Prism.
2.3 |. Phospho-histone H3 immunofluorescence
Mice at PND10 were rapidly decapitated, spinal cords were dissected and fixed overnight in 3% PFA. Tissue was then incubated in 30% sucrose for dehydration. Once dehydrated, spinal cords were frozen in Optimal cutting temperature compound on dry ice/ethanol and stored at −80°C. For immunofluorescence at least five sections of 20 μm, for each animal were stained for quantification. Slides were thawed for 10 min, washed in PBS, submerged in 10 mM sodium citrate (previously heated to 95°C) for 15 min for antigen retrieval and washed again in PBS. Sections were blocked in a solution containing 4% normal goat serum and 0.3% Triton X-100 in PBS for 1 hr. Primary antibody anti-phospho-histone H3 (1:300, Cell signaling, #3377) was diluted in block solution and incubated at 4°C overnight. After primary incubation, slides were washed in PBS 3 times for 5 min. Sections were then incubated in AlexaFluor secondary antibody goat anti-rabbit (1:200, Invitrogen) for 1 hr at room temperature and washed 3 times for 5 min in PBS. For nuclear staining, sections were incubated with DAPI for 3 min. Slides were washed again 3 times for 5 min in PBS and then coverslipped with Fluorogel (EMS).
2.4 |. Isolation of mouse OPCs
Mice at postnatal day (PND) 10 were rapidly decapitated and spinal cords were dissected and dissociated into single cells using Neural Dissociation Kit (P) (Miltenyi). We refer to this dissociation method as “crude glial preparation” because it preferentially yields oligodendroglia, with about 75% of the cells expressing markers of the oligodendrocyte lineage (Supporting Information Table S1). PDGFRα+ OPCs were isolated by magnetic-activated cell sorting using PDGFRα microbead kit (Miltenyi).
2.5 |. RNA isolation and reverse transcription
RNA was extracted from isolated PDGFRα+ cells using RNeasy Plus Mini Kit (Qiagen, Valencia, CA). For RNA isolation from spinal cord, tissues were homogenized immediately in 1 ml of Trizol reagent (Invitrogen). RNA was isolated according to the Trizol protocol provided by Invitrogen. Spinal cord RNA was further purified using chloroform (Sigma, St. Louis, MO) and precipitated with sodium acetate. RNA concentration was measured using a NanoDrop spectrophotometer (Thermo Scientific). A total of 1.5 μg of RNA was used to reverse transcribe cDNA using Superscript II (Invitrogen). Concentrations of cDNA were determined by spectrophotometry and working aliquots were stored at −20°C prior to RT-PCR.
2.6 |. Quantitative real-time PCR
One hundred nanograms of cDNA was used as a template in RT-PCR reactions containing 1X SYBR green detection master mix and 1X Amplification was normalized to expression levels of ß-actin (IDT) for each sample. RT-PCR was performed on the Applied Biosystems 7900B and (7500) (Carlsbad, CA) using the associated Sequence Detection System Version 2.2.2 and (2.0.1). The thermal reaction profiles were performed as previously described (Tyler et al., 2009).
BioRad plates and SYBR (#430001607) were used for these plates (BioRad, Hercules, CA).
QuantiTect Primers for quantitative real-time PCR for Cdk1 (QT00167734), E2f1 (QT01747977), cyclin B1 (QT01757007), Id2 (QT01038870), Id4 (QT00324352), Sox10 (QT01046451), Sox17 (QT00160720), Myrf (QT01061389), cyclin D1 (QT01548575), Enpp6 (QT00143493), Tcf4 (QT01658230), Sox5 (QT01758253), and Id1 (QT01743756) were purchased from Qiagen. Statistical analysis for each target was performed using two-tailed unpaired t test in GraphPad Prism.
2.7 |. TGFβ-BMP PCR array
PDGFRα+ cells isolated from spinal cords of control and mTOR cKO mice were obtained from three independent litters and combined. RNA was isolated using the RNeasy Plus Mini Kit (Qiagen). Genomic DNA elimination and cDNA synthesis were performed using the RT2 First Strand Kit (Qiagen). After the cDNA was synthesized an additional step was performed to preamplify the cDNA target templates using the RT2 PreAMP Pathway Primer Mix (Mouse TGFβ/BMP signaling path; Qiagen, #330241 PBM-035Z). The following steps for the real-time PCR reaction were performed using the reagents and protocols provided by Qiagen for the RT2 Profiler PCR Array Mouse TGFβ/BMP Signaling Pathway (#PAMM-035Z). After the PCR reaction, all samples passed the quality checks (PCR array reproducibility, RT efficiency and genomic DNA contamination) based on internal controls in the array. The CT values for the five housekeeping genes provided in the array were averaged and used for the ΔΔCT calculations. Three genes were discarded from the analysis due to CT values higher than 35.
2.8 |. In situ hybridization
Mice were intracardially perfused with 4% PFA in PBS, and spinal cords were dissected. The spinal cords were postfixed with 4% PFA overnight and cryoprotected with 30% sucrose-PBS buffer overnight and frozen. Mounted cryosections were prepared at 20 μm thickness with a cryostat. In situ hybridization was performed as described (Hashimoto et al., 2016) with slight modification. The following plasmid containing mouse cDNA was used to generate cRNA probes: Enpp6 (nucleotides 273–878 corresponding to NM_177304.4). Briefly, the sections were treated with proteinase K (2 μg/ml for 15 min at room temperature) and hybridized overnight at 63°C with DIG-labeled antisense riboprobes in a hybridization solution consisting of 50% formamide, 20 mM Tris-HCl (pH 7.5), 600 mM NaCl, 1 mM EDTA, 10% dextran sulfate, 200 μg/ml yeast tRNA, 1x Denhardt’s solution. After the sections were washed in buffers with decreasing stringency, they were incubated with an alkaline phosphatase-conjugated anti-DIG antibody (1:5000; Roche diagnostics). The color was developed in the presence of 4-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate (Roche diagnostics) in the dark at room temperature. Quantification of Enpp6+ cells was performed using Image J (U.S. National Institutes of Health, Bethesda, MD) on 63X control (n = 3) and mTOR cKO (n = 4) ventral white matter fields. Cells were counted on at least three sections per animal. Statistical analysis was performed using two-tailed unpaired t test in GraphPad Prism.
2.9 |. Rat OPC cultures
OPCs were purified from cortical mixed glial cultures by established methods (McCarthy & de Vellis, 1980; Tyler et al., 2009) and as for our prior studies. Briefly, brains were removed from postnatal day 0–2 Sprague Dawley rat pups and the cortices were dissected. Cortical pieces were enzymatically digested in 2.5% trypsin and Dnase I followed by mechanical dissociation. Cells were resuspended in MEM-C, which consisted of minimal essential media (MEM) supplemented with 10% FBS, L-glutamine, and 1% Pen-strep, and plated in T75 flasks. The resulting mixed glial cultures were maintained for 10 days. Purified OPC cultures were prepared by a differential shake. Purified OPCs were seeded onto poly-D-lysine coated T75 flasks at a density of 2 × 104 cells/cm2 in a chemically defined medium N2S. N2S consists of 66% N2B2 (DMEM/F12 supplemented with 0.66 mg/ml BSA, 10 ng/ml d-biotin, 5 μg/ml insulin, 20 nM progesterone, 100 μM putrescine, 5 ng/ml selenium, 50 μg/ml apo-transferrin, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.5% FBS) supplemented with 34% B104 conditioned media, 5 ng/ml FGF, and 0.5% FBS. Purified OPCs were amplified for 4–10 days in N2S, passaged once with papain, and plated for experiments. Cell culture media (MEM, DMEM/F12), FBS, trypsin, and insulin-selenium-transferrin (ITS) were purchased from GIBCO-BRL (Long Island, NY). Additional N2 supplements, triiodothyronine, and poly-d-lysine were purchased from Sigma. Recombinant human FGF-2 was purchased from R&D Systems (Minneapolis, MN).
Rat central glial (CG)-4 cells, an immortalized glial progenitor that gives rise to astrocytes and oligodendrocytes (Louis, Magal, Muir, Manthorpe, & Varon, 1992; Romanelli et al., 2007), were cultured and differentiated as for the primary rat OPCs and used for inhibitor experiments as indicated.
2.10 |. Differentiation paradigm and treatment with inhibitors
To initiate OPC differentiation for both primary rat OPCs and CG-4 cells, we followed an established mitogen withdrawal protocol (Tokumoto, Durand, & Raff, 1999; Tyler et al., 2009). Briefly, OPCs were seeded onto poly-D-lysine coated dishes, or coverslips for immunofluoresence assay, at a density of 2 × 104 cells/cm2 in N2S media and allowed to recover overnight. The following day, N2S media was replaced with mitogen free N2B2 media supplemented with 30 ng/ml triiodothyronine (T3). To inhibit mTOR, cells were treated with 15 nM rapamycin (Calbiochem, #553210) except for the noggin/rapamycin experiment where rapamycin was used at 10 nM to enhance primary cell viability with both inhibitors. Both doses of rapamycin are effective in inhibiting mTOR in differentiating oligodendrocytes based on our prior studies (Tyler et al., 2009; Tyler et al., 2011). To inhibit type 1 BMP receptors, cells were treated with 1 μM K02288 (Tocris, #4986). To inhibit BMP ligand activity, cells were treated with 500 ng/ml noggin (R&D Systems). Stock solutions of rapamycin and K02288 were prepared in ethanol; stock solution of noggin was prepared in PBS. Control cultures received vehicle alone. N2B2 + T3 differentiation media with or without inhibitors was replenished every 48 hr during the course of experiments.
2.11 |. Protein isolation from cultured or isolated cells and Western blot analysis
Following treatments or isolation, cells were washed twice with ice-cold PBS and total cell lysates were harvested in RIPA lysis buffer (Pierce) with 1/50 protease inhibitor (PI) cocktail (Sigma). The lysates were briefly sonicated and stored at −80°C prior to western analysis. The RC-DC protein assay (BioRad) was performed to determine protein concentration. Twenty microliters of total protein per sample was aliquoted, boiled for 5 min and separated by SDS-PAGE on Bis-Tris mini-gels (Invitrogen). Separated proteins were then transferred to nitrocellulose membranes and blocked in 5% milk/TBS-0.1% Tween for 1 hr at room temperature. Membranes were then incubated in the presence of primary antibodies diluted in 5% BSA/TBS-0.1% Tween overnight at 4°C. The following day, membranes were washed 3 times for 10 min with TBS-0.1% Tween and incubated for 1 hr at room temperature in 5% milk/TBS-0.1% Tween containing goat anti-rabbit or goat anti-mouse secondary antibodies at a dilution of 1:5000. The detection of HRP conjugated secondary antibodies was performed by enhanced chemiluminescence using the Ultra-LUM imaging device. Protein expression levels were quantified using Image J. The following antibodies were used: Id2 (Santa Cruz sc-489,1:1000), phospho-Smad (Cell Signaling 13820S, 1:1000), Smad (Santa Cruz sc-6,031, 1:1000), FKBP12 (Invitrogen PA1–026A, 1:500), MBP (Biolegend 808,402, 1:1000), MOG (Abcam ab32760, 1:1000). Statistical analysis was performed using GraphPad Prism. Unpaired two-tailed t test was performed when there were two groups. For more than two groups, one-way analysis of variance (ANOVA) was performed to test overall significance. Tukey’s post hoc test was used to determine significance of all pairwise comparisons.
2.12 |. MBP immunofluorescence
Cell cultures were fixed in 3% PFA for 15 min and permeabilized with 0.3% Triton X-100 for 15 min. Cells were blocked in 3% BSA and 3% goat serum in PBS for 1 hr and incubated overnight with primary antibody (MBP Abcam ab40390) diluted 1:500 in blocking buffer. Cells were washed and incubated in anti-rabbit secondary antibody 1:750 for 1 hr. Cells were incubated with DAPI 1:5000 for 5 min. Cells were washed and mounted using Prolong Gold mounting media. Images were acquired using an Olympus Provis AX70 microscope. Three coverslips per condition were imaged from each OPC isolation. Number of DAPI-positive nuclei and MBP-positive cells were counted, and one-way ANOVA was performed to determine significance.
2.13 |. Chromatin immunoprécipitation (ChIP) assay
Primary rat OPCs were plated in triplicate at 2.5 × 104 cells/cm2 in N2S media on poly-D-lysine coated 150 mm dishes. The following day, N2S was changed to differentiation media for control cells or differentiation media plus 15 nM rapamycin for treated cells. After 3 days cells were fixed in formaldehyde (1% final concentration) for 10 min followed by addition of glycine (125 mM final concentration) for 5 min at room temperature. Cells were harvested and washed in ice-cold PBS plus protease inhibitor (PI) and centrifuged. Pellet was resuspended in ChIP Lysis Buffer + PI and lysates from dishes with same treatment were combined. Lysates were sonicated (7 cycles of 10 min each) on ice and centrifuged to remove cellular debris. Supernatants were transferred to a new tube and a 50 μl aliquot (input) was taken and processed for DNA extraction to estimate chromatin concentration in the samples. Sonicated chromatin (25 μg DNA) was used for immunoprecipitation by incubation with 2 mg/ml of anti-Smad (Santa Cruz, SC-6031X), anti-HDAC1 (Santa Cruz, SC-7872X), anti-HDAC2 (Santa Cruz, SC-7899X), and anti-Sip1 (Santa Cruz, SC-48789). After incubation, beads were washed and eluted according to the protocol provided in the Millipore kit. All buffers and beads were obtained from Millipore, ChIP assay kit (# 17–371). Samples were incubated with NaCl at 65°C overnight, treated with RNase A at 37°C for 30 min and proteinase K at 45°C for 4 hr. DNA was purified by phenol:chloroform extraction. Glycogen (20 mg/ml), 3 M Na-Acetate and 1 ml cold 100% ethanol were added to the aqueous phase and incubated overnight at −20°C. Samples were centrifuged at 14,000 rpm for 15 min for DNA to precipitate, washed with cold 70% ethanol and resuspended in DNase-free water. PCR reaction was performed for ChIP analysis on Id2 promoter. The primers using in the reaction: Id2 forward, acagacccgcttggagttgc, Id2 reverse, gtcacgggcggaatggacac were previously published (Weng et al., 2012).
3 |. RESULTS
3.1 |. mTOR is critical for differentiation of early OPCs to late OPCs
Several studies have demonstrated that deletion of either mTOR or the mTORC1 component raptor from the oligodendrocyte lineage in mice results in a differentiation deficit and fewer mature oligodendrocytes in the spinal cord during developmental myelination (Bercury et al., 2014; Lebrun-Julien et al., 2014; Wahl et al., 2014). Here we investigated the role of mTOR specifically during early OPC differentiation in a mouse model with oligodendrocyte-specific knockdown of mTOR (mTOR cKO) in the CNS, using Cre-recombinase driven by the 2′,3′ cyclic nucleotide 3′ phosphohydrolase (CNP) promoter. We focused on the spinal cord for our studies since loss of mTOR has a greater impact on spinal cord myelination than on brain myelination, where very little impact was noted (Bercury et al., 2014; Wahl et al., 2014). To define the exact stage(s) when mTOR is necessary for oligodendrocyte lineage progression, we analyzed oligodendroglial populations from mTOR cKO and control (lacking Cre-recombinase) spinal cords by flow cytometry at postnatal day (PND)10. Early OPCs (PDGFRa+ and NG2+) were easily distinguished by flow cytometry from late OPCs/immature oligodendrocytes (O4+); there was little overlap between either progenitor population and O4+ cells (Figure 1a,b, Supporting Information Figure S1A,B). Many, but not all, OPCs co-express PDGFRα and NG2 at PND10. We observed that on average 71% of NG2+ OPCs also express PDGFRα, and on average 40% of PDGFRα+ cells co-express NG2 (Supporting Information Figure S1C). These findings are consistent with data suggesting that PDGFRα is expressed early in OPCs and is maintained in slightly more mature OPCs that express the proteoglycan NG2 (Li et al., 2017; Nishiyama, Boshans, Goncalves, Wegrzyn, & Patel, 2016).
FIGURE 1.
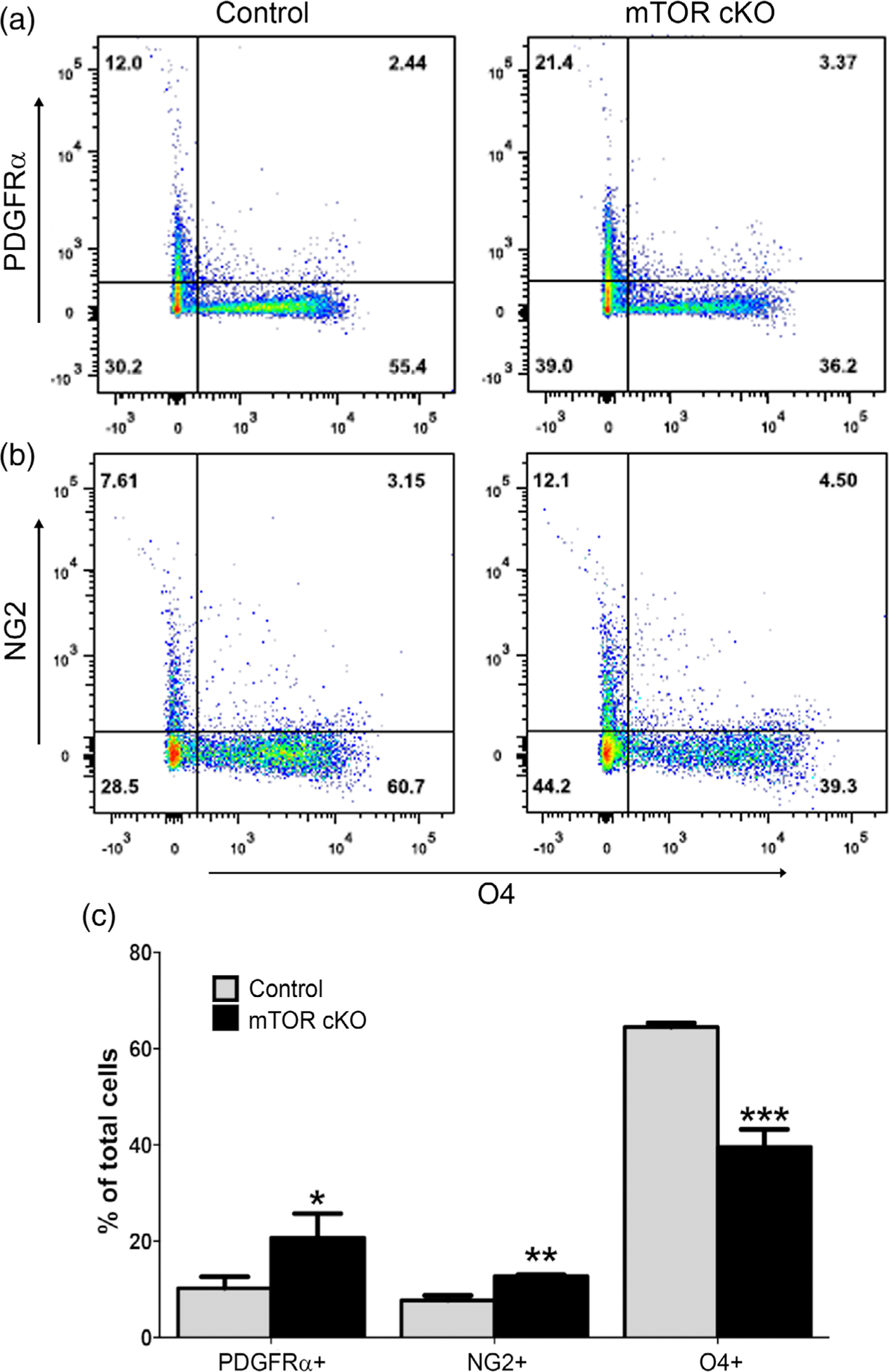
mTOR is critical for differentiation of early OPCs to late OPCs. Spinal cords from control and mTOR cKO mice at PND10 were dissociated and cell suspensions were analyzed by flow cytometry. PDGFRα and NG2 were used as markers for early OPCs and O4 as a marker for late OPCs and immature oligodendrocytes.
(a) Representative dot plots showing PDGFRα+ and O4+ populations in control and mTOR cKO.
(b) Representative dot plots showing NG2+ and O4+ populations in control and mTOR cKO. (c) Quantification of the percentage of PDGFRα+, NG2+ and O4+ cells in spinal cord of control and mTOR cKO animals. Values expressed as mean ± SEM in percentage of live cells. (n ≥ 5/genotype) *p < .05; **p < .01; ***p < .001
The early OPC populations (either PDGFRα+ or NG2+) were nearly twofold greater in mTOR cKO spinal cords when compared to control animals at PND10 (Figure 1c). In contrast, the O4+ population showed a clear reduction from an average of 64.5% in control to 39.4% in mTOR cKO spinal cords (Figure 1c). This is consistent with the decrease in CC1+ mature oligodendrocytes we observed by immunostaining at PND14 in our previous study (Wahl et al., 2014). Thus, mTOR is necessary for normal progression from an early OPC stage to a late OPC/immature oligodendrocyte in developing oligodendroglia.
3.2 |. mTOR deletion has no effect on cell proliferation or cell cycle exit
Since we observed an accumulation of the early OPC populations after mTOR deletion, we evaluated whether mTOR loss increased cell proliferation and/or prevented cell cycle exit, thereby inhibiting oligodendrocyte differentiation. We quantified the numbers of mitotically active cells by immunostaining for phospho-histone H3 (pH 3) at PND10 and saw no difference in the number of pH 3+ cells in spinal cord white matter of mTOR cKO compared to control animals (Figure 2a). Control and mTOR cKO spinal cords at PND10 were also dissociated and stained with anti-PDGFRα and Hoechst and then analyzed by flow cytometry. Loss of mTOR had no effect on the percentage of PDGFRα+ cells in G2-M, S or G0–G1 phases of the cell cycle as determined by DNA content (Figure 2b).
FIGURE 2.
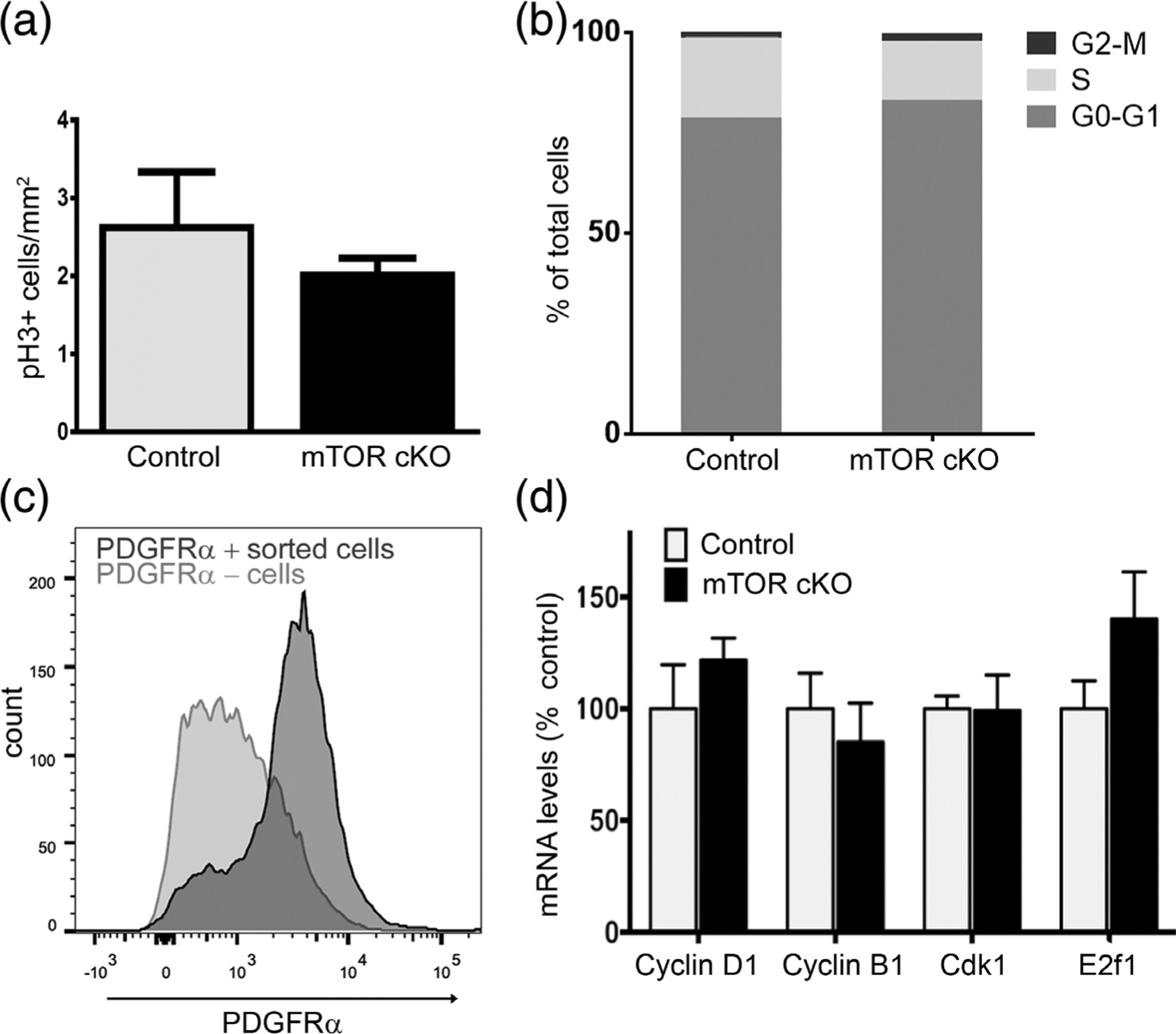
mTOR deletion does not alter cell proliferation or cell cycle exit. (a) Quantification of phospho-histone H3 (pH 3) immunofluorescence in spinal cord sections from control and mTOR cKO mice at PND10. Values expressed as mean ± SEM. (n = 3/genotype). (b) Cell cycle analysis of PDGFRα+ cells by flow cytometry. Spinal cords from control and mTOR cKO mice at PND10 were dissociated and stained with anti-PDGFRα and Hoescht. PDGFRα+ cell population was selected by gating, and cell cycle phase was determined based on the DNA content by Hoescht staining. (n = 3 controls, n = 5 mTOR cKO) (c) Representative graph showing sorting efficiency of PDGFRα+ cells. Dissociated cells from spinal cords at PND10 were incubated with PDGFRα antibody conjugated to magnetic microbeads and subjected to magnetic-activated cell sorting. Positive (dark gray) and negative (light gray) cell fractions were analyzed by flow cytometry to determine percentage of PDGFRα+ cells in each fraction. Eighty-three percent of the positive cell fraction is PDGFRα+; 37% of the negative fraction is PDGFRα+. (d) mRNA expression of genes involved in cell cycle regulation. Expression of cyclin D1, cyclin B1, Cdk1, E2f1 by quantitative real-time PCR in sorted PDGFRα+ cells from PND10 spinal cords. Data represent at least three independent experiments. In each experiment, cells were pooled from 3 to 4 mice/genotype. Values expressed as mean ± SEM
To analyze expression of genes involved in cell cycle regulation in the early OPC population, we isolated PDGFRα+ cells from control and mTOR cKO spinal cords at PND10 using magnetic-activated cell sorting. The OPC enrichment in the positive cell fraction was confirmed by flow cytometry (Figure 2c); on average 83% of cells in the positive fraction were PDGFRα+. We then extracted mRNA from sorted PDGFRα+ cells (Figure 2c) to determine expression of cell cycle genes by qPCR (Figure 2d). Loss of mTOR had no effect on cyclin D1, necessary for transition from G1 to S phase. Since our prior studies supported a function for mTOR activation in normal progression of OPCs through G2/M in vitro (Min, Singh, Fitzgerald-Bocarsly, & Wood, 2012), we further evaluated expression of cyclin B and Cdk1 in PDGFRα+ cells and saw no difference in the expression of either gene between the mTOR cKO and control PDGFRα+ cells. Consistent with these results, expression of E2f1, a transcription factor that is downregulated when cells exit cell cycle (Magri et al., 2014), was also unchanged in the OPCs by loss of mTOR. We conclude that the accumulation of early OPCs and decrease in late OPCs/immature oligodendrocytes with loss of mTOR is not due to increased OPC proliferation or to a deficit in cell cycle exit.
3.3 |. Loss of mTOR results in fewer newly formed oligodendrocytes in spinal cord white matter
To determine whether reduction of O4+ late OPCs/immature oligodendrocytes correlated with fewer newly formed oligodendrocytes, we analyzed expression of Enpp6 in control and mTOR cKO spinal cords. Enpp6 mRNA, recently identified as an early marker of differentiating oligodendrocytes in the CNS, is induced prior to the appearance of CC1 (Xiao et al., 2016). In situ hybridization for Enpp6 revealed fewer Enpp6+ cells in the ventral white matter of mTOR cKO compared to control spinal cords (Figure 3a–c). Enpp6 expression was also significantly reduced in sorted PDGFRα+ cells from mTOR cKO spinal cords (Figure 3d). These results support the conclusion that a differentiation deficit in mTOR cKO spinal cords leads to a reduction in the number of mature oligodendrocytes during development and, further, that mTOR is upstream of Enpp6 mRNA expression in OPCs.
FIGURE 3.
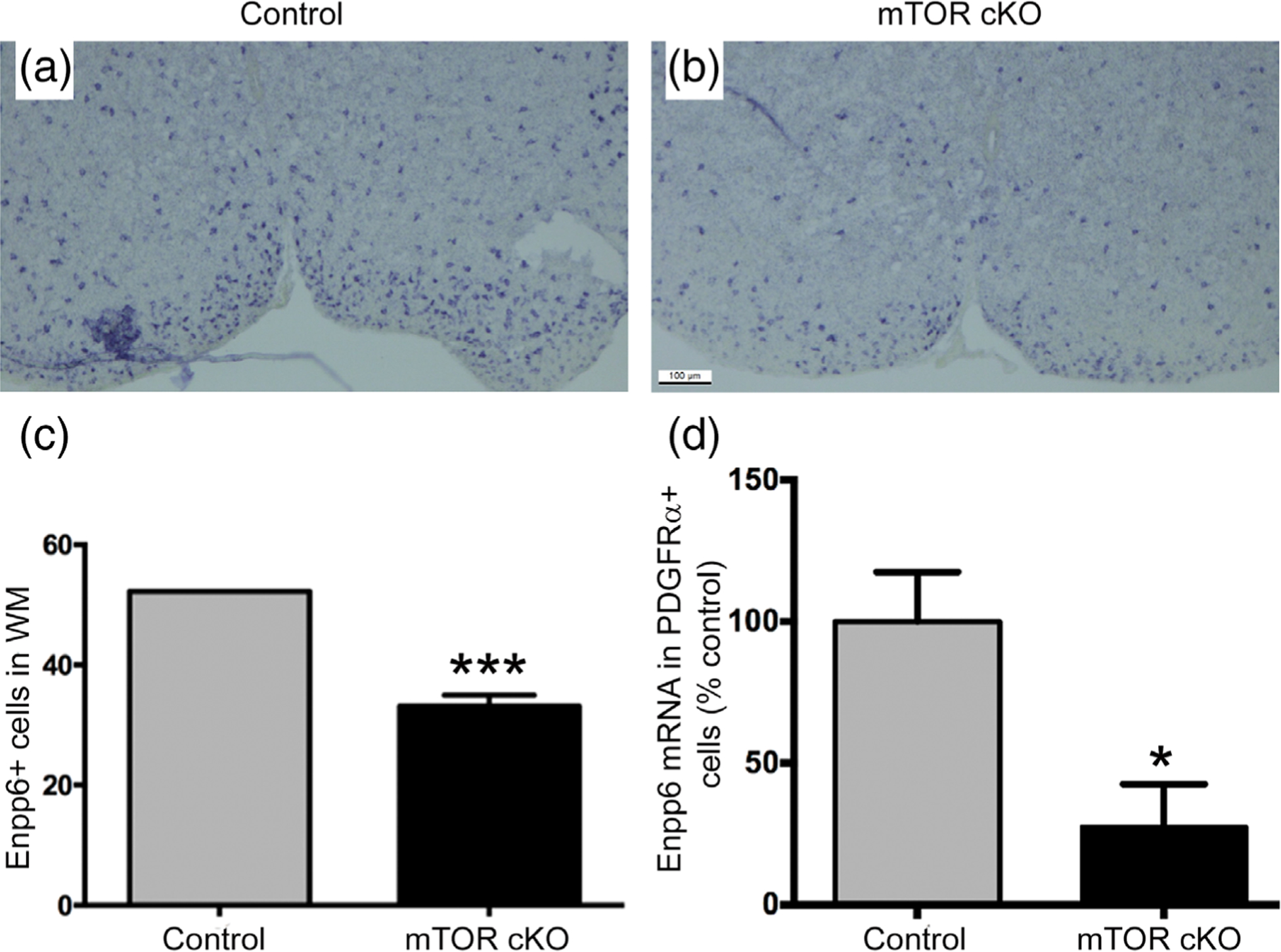
Loss of mTOR results in fewer newly formed oligodendrocytes in spinal cord. Enpp6 in situ hybridization in ventral spinal cord of (a) control and (b) mTOR cKO mice at PND7 (c) Quantification of Enpp6+ cells in ventral white matter fields for control (n = 3) and mTOR cKO (n = 4). (d) Enpp6 expression in sorted PDGFRα+ cells by quantitative real-time PCR at PND10 (n = 3). Values expressed as mean ± SEM. *p ≤ .05; ***p ≤ .001
3.4 |. Id2 expression is increased in the PDGFRα+ population in the mTOR cKO spinal cord
Early OPCs express negative transcriptional regulators of differentiation, which function to maintain the cell in its precursor state. As cells progress through differentiation, these negative regulators are suppressed and positive regulators of differentiation are activated and promote myelin gene expression (Emery & Lu, 2015; J. Liu & Casaccia, 2010). Several transcriptional repressors are known to prevent OPC maturation including Id2 and Id4; expression of both is upregulated by BMP signaling which inhibits oligodendrocyte differentiation (Cheng et al., 2007; Gomes et al., 2003; Samanta & Kessler, 2004). Overexpression of either Id2 or Id4 is sufficient to inhibit oligodendrocyte differentiation in vitro (Marin-Husstege et al., 2006; Samanta & Kessler, 2004; S. Wang, Sdrulla, Johnson, Yokota, & Barres, 2001). In mice, Id4 represses MBP expression through regulation at the promoter region, and Id4-null mice have premature oligodendrocyte differentiation (Marin-Husstege et al., 2006). Sox5 also inhibits OPC differentiation by promoting PDGFRα expression (Baroti et al., 2016). Positive regulators of oligodendrocyte maturation including Myrf and Sox10 induce myelin gene expression (Bujalka et al., 2013; Stolt et al., 2002), while the transcription factors Tcf4 and Sox17 have stage-specific functions and are also required for oligodendrocyte differentiation (Sohn et al., 2006; Zhao et al., 2016).
To determine if mTOR mediates oligodendrocyte differentiation through targeting either positive or negative regulators of differentiation, we initially analyzed mRNA expression of negative regulators Id4 and Id2, and positive regulators Myrf, Sox10, Sox17, and Tcf4 in whole spinal cord at PND7 by qPCR (Figure 4a). Myrf, Sox10, Sox17, Tcf4 and Id4 displayed similar expression levels in control and mTOR cKO spinal cords. In contrast, Id2 was significantly upregulated in mTOR cKO spinal cords. To determine whether the Id2 increase is specific to the early OPC population, we sorted PDGFRα+ and O4+ cells and analyzed RNA expression of transcription regulators in each population. We again observed a twofold increase in Id2 expression in the PDGFRα+ OPCs with mTOR deletion with no change in other factors analyzed (Figure 4b). In contrast, Id2 expression was unchanged in O4 + late OPCs/immature oligodendrocytes from mTOR cKO spinal cords (Figure 4c). We further confirmed upregulation of Id2 at the protein level in primary rat OPCs treated with rapamycin, an inhibitor of mTOR, during differentiation in vitro using Western blotting (Figure 4d). We verified these results in vivo by analyzing Id2 protein expression in cells acutely isolated from spinal cords of control or mTOR cKO animals at PND10. The dissociation method preferentially yields oligodendroglia, with about 75% of the cells expressing markers of the oligodendrocyte lineage (Supporting Information Table S1). The advantage of this crude glial cell preparation is that sufficient protein for analysis by western blotting can be obtained from individual animals, whereas the protein yield from isolated OPCs is low and would require pooling of multiple neonatal mice. Id2 protein expression is significantly increased in mTOR cKO spinal cords compared to control (Figure 4e,f). Taken together, these data suggest that mTOR regulates the transition from early to late OPCs in spinal cord by downregulating Id2 expression.
FIGURE 4.
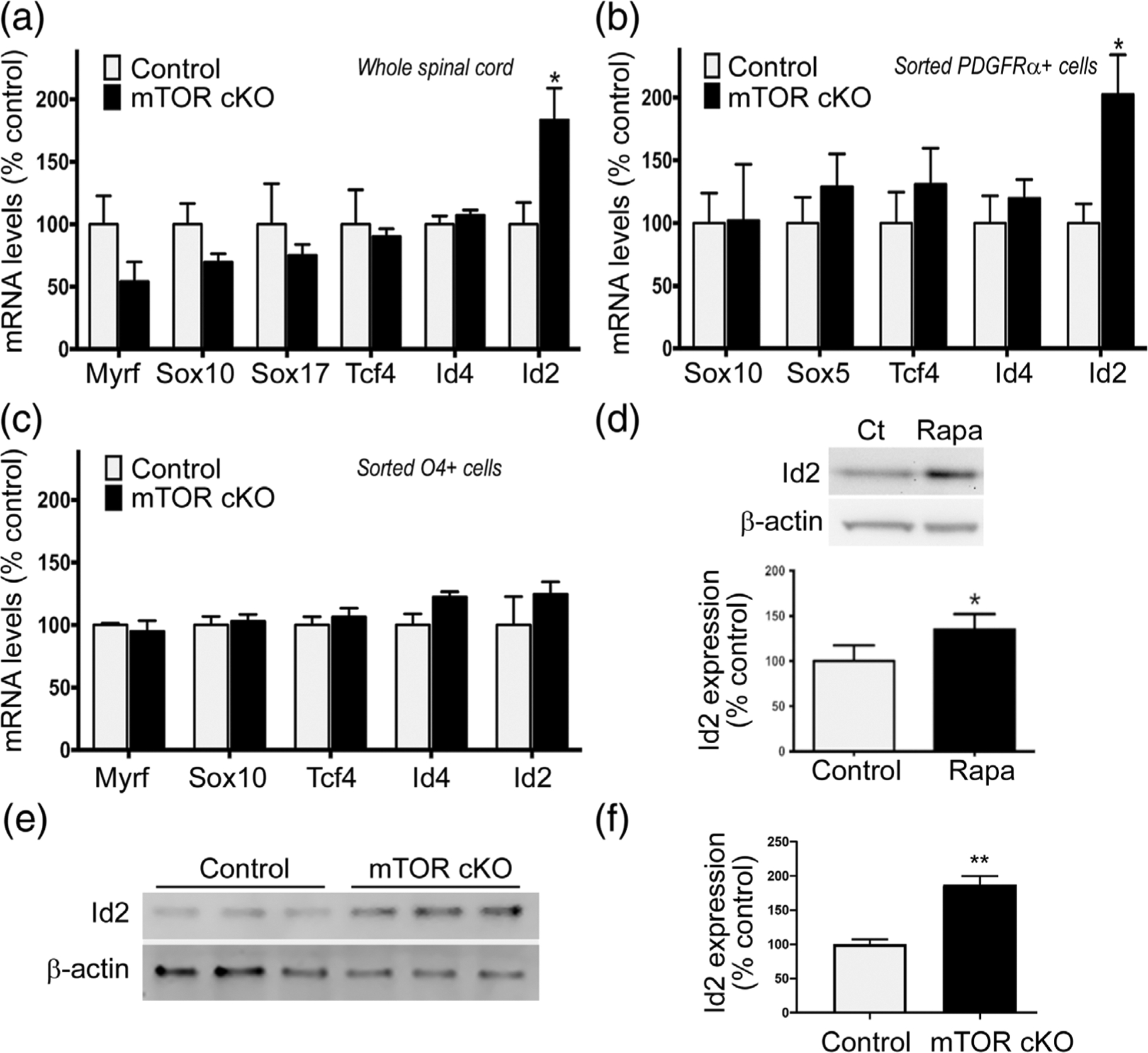
Loss of mTOR increases Id2 exclusively in the PDGFRα + population in the spinal cord. (a) Expression of Myrf, Sox10, Sox17, Tcf4, Id4, Id2 by quantitative real-time PCR in whole spinal cord at PND7 (n = 6 animals/genotype). (b) Expression of Sox10, Sox5, Tcf4, Id4, Id2 by quantitative real-time PCR in sorted PDGFRα+ cells from PND10 spinal cords. Data represent five independent experiments. In each experiment, cells were pooled from 3 to 4 mice/genotype. (c) Expression of Myrf, Sox10, Tcf4, Id4, Id2 by quantitative real-time PCR in sorted O4+ cells from PND10 spinal cords. Data represent three independent experiments. In each experiment, cells were pooled from 3 to 4 mice/genotype. (d) Representative Western blots and quantification of Id2 expression in primary rat OPCs after 24 hr of differentiation ±15 nM rapamycin (n = 5). Western blot analysis (e) and quantification (f) of Id2 expression in control and mTOR cKO spinal cords at PND10 (n = 3). Protein was extracted from dissociated whole spinal cords. All values expressed as mean ± SEM. *p < .05; **p < .01
3.5 |. mTOR deletion results in sustained BMP signaling in OPCs
Several studies have demonstrated that BMP signaling is activated in OPCs and is downregulated in differentiating oligodendrocytes; increased BMP signaling suppresses oligodendrocyte differentiation in vitro (Cheng et al., 2007; Gomes et al., 2003; Samanta & Kessler, 2004). Both in vitro and in vivo, increased levels of BMPs such as BMP2 and BMP4 cause neural progenitors to preferentially differentiate into astrocytes, resulting in decreased oligodendrogenesis (Gomes et al., 2003; Samanta & Kessler, 2004). BMPs activate BMP receptors, members of the TGF-β family of serine/threonine kinase receptors, which then phosphorylate receptor-activated Smad proteins (Smad1/5/8) and ultimately promote transcription of Id2, a known inhibitor of myelin gene expression (Chen et al., 2012; Nakahiro, Kurooka, Mori, Sano, & Yokota, 2010; S. Wang et al., 2001). Initial studies from our lab demonstrated that inhibiting mTOR with rapamycin during OPC differentiation in vitro resulted in upregulation of transcriptional targets of BMP signaling including Id2 (Tyler et al., 2009). Since we also observed increased Id2 expression in mTOR cKO spinal cord OPCs, we next assessed upstream components of the BMP pathway to determine whether BMP signaling is dysregulated in OPCs by the loss of mTOR.
To determine how loss of mTOR regulates BMP pathway components at the RNA level, we performed a TGFβ/BMP-focused RT2-PCR-Profiler PCR Array after isolating RNA from sorted PDGFRα + cells from control and mTOR cKO spinal cords. Complete results from all genes analyzed in the array are provided in Supporting Information Table S2. Gene expression analysis of the ligands, receptors and downstream targets of the BMP signaling pathway are shown in Figure 5a. Of these 15 genes, 4 genes were downregulated in PDGFRα+ cells with loss of mTOR and 11 are upregulated, including Id2. We selected three genes (in addition to Id2) that were upregulated in the mTOR cKO OPCs—BMP receptor type 1a (Bmpr-1a), BMP receptor type 1b (Bmpr-1b), and Id1—for validation by real-time PCR from four independent samples (Figure 5b). Id1 and Bmpr-1a expression levels were approximately 40% higher in mTOR cKO OPCs compared to control, whereas Bmpr-1b expression, although trending to an increase, was not significantly changed.
FIGURE 5.

BMP signaling is upregulated after mTOR loss. (a) Gene expression analysis in TGFβ-BMP signaling pathway using TGFβ-BMP RT2-Profiler PCR Array. mRNA was extracted from PDGFRα+ cells sorted from spinal cord of control and mTOR cKO mice at PND10. mRNA was combined from three independent sorting experiments. In each experiment, cells were pooled from 3 to 4 mice/genotype. (b) Expression of Id1, Bmpr-1a and Bmpr-1b by quantitative real-time PCR in sorted PDGFRα+ cells from PND10 spinal cords. Data represent four independent experiments. In each experiment, cells were pooled from 3 to 4 mice/genotype. Values expressed as mean ± SEM. (c) Representative western blots and (d) quantification of phosphorylated and total Smad1/5/8 in primary rat OPCs after 24 hr of differentiation ±15 nM rapamycin (n = 8). Values expressed as mean ± SEM. **p < .01; *p < .05
Activation of the serine/threonine kinase BMP receptors results in phosphorylation of the receptor Smad proteins, Smad1/5/8, that then translocate to the nucleus (Massague, Seoane, & Wotton, 2005). To measure Smad1/5/8 activation in OPCs with inactivation of mTOR, we treated primary rat OPCs with rapamycin during differentiation and measured levels of phospho/total-Smad1/5/8 by Western blot (Figure 5c,d). Pharmacological inhibition of mTOR for 24 hr resulted in increased Smad1/5/8 phosphorylation. The activation of Smad1/5/8 along with upregulation of Id2 in OPCs after mTOR inhibition support the hypothesis that mTOR promotes OPC differentiation during development by suppressing BMP signaling.
3.6 |. mTOR inhibition alters transcriptional machinery at Id2 promoter
The promoter region of Id2 contains BMP-responsive elements with Smad binding sites (Nakahiro et al., 2010). Activated Smad1/5/8, in a heteromeric complex, binds to the Id2 promoter and induces transcription. However, the presence of Smad-interacting protein-1 (Sip1) at the Id2 promoter acts as an inhibitor of transcription (Weng et al., 2012). The class I histone deacetylases HDAC1 and HDAC2 also suppress BMP-driven transcription of Id2 (Ye et al., 2009). Since the presence or absence of these specific components at the promoter region of Id2 can promote or inhibit transcription, we analyzed the transcriptional machinery at the Id2 promoter when mTOR is inhibited.
Primary rat OPCs were treated with rapamycin for 72 hr to inhibit mTOR during differentiation. Chromatin immunoprecipitation to analyze Id2 promoter binding to protein components of transcriptional machinery revealed no change in binding of Smad or HDAC1 to the Id2 promoter when mTOR was inhibited (Figure 6a,b). In contrast, the levels of both Sip1 and HDAC2 bound to the Id2 promoter decreased drastically in rapamycin-treated OPCs. The presence of Sip1 and HDAC2 normally inhibit transcription of Id2; thus, our data indicate that inhibition of mTOR alters the transcriptional machinery at the Id2 regulatory region, resulting in increased expression of Id2.
FIGURE 6.
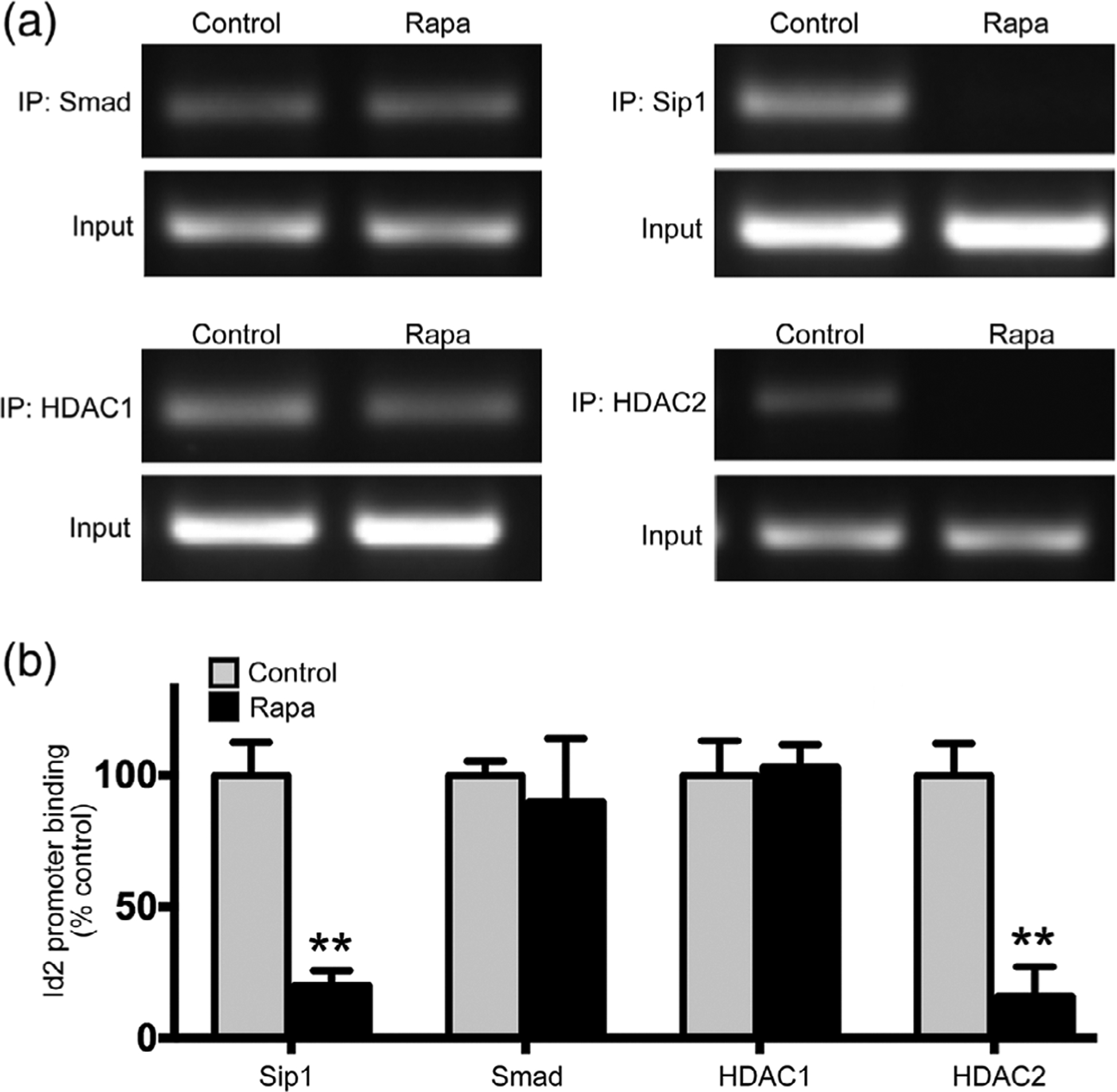
mTOR inhibition alters transcriptional machinery at Id2 promoter. ChIP assays performed in primary rat OPCs after 72 hr of differentiation ±15 nM rapamycin. (a) Representative PCR gels and (b) quantification of Id2 promoter bound to immunoprecipitated proteins (n = 3). Smad, Sip1, HDAC1, and HDAC2 were immunoprecipitated and Id2 promoter region was PCR amplified from bound chromatin. Input DNA was used as positive control. Values expressed as mean ± SEM. **p < .01
3.7 |. FKBP12 is reduced in mTOR cKO
Inhibition or deletion of mTOR results in OPCs with impaired differentiation, increased activation of Smad1/5/8, altered transcriptional machinery at the promoter of Id2, and increased expression of Id2, which in turn is a negative regulator of differentiation. We next asked whether mTOR regulates BMP signaling in part through modulating levels of an upstream component of BMP signaling, the FK506-binding protein 1A (FKBP12). FKPB12 is an immunophilin that can directly bind the GS domain of type I BMP receptors to suppress kinase activity (T. Wang et al., 1996). We analyzed FKBP12 protein expression in cells isolated from spinal cords of control or mTOR cKO animals at PND10 using the crude glial preparation described above. Expression of FKBP12 was reduced in cells from mTOR cKO spinal cords to 59% of control glial cells (Figure 7a,b). We conclude that mTOR signaling downregulates BMP receptor activity in part through promoting expression of FKBP12.
FIGURE 7.
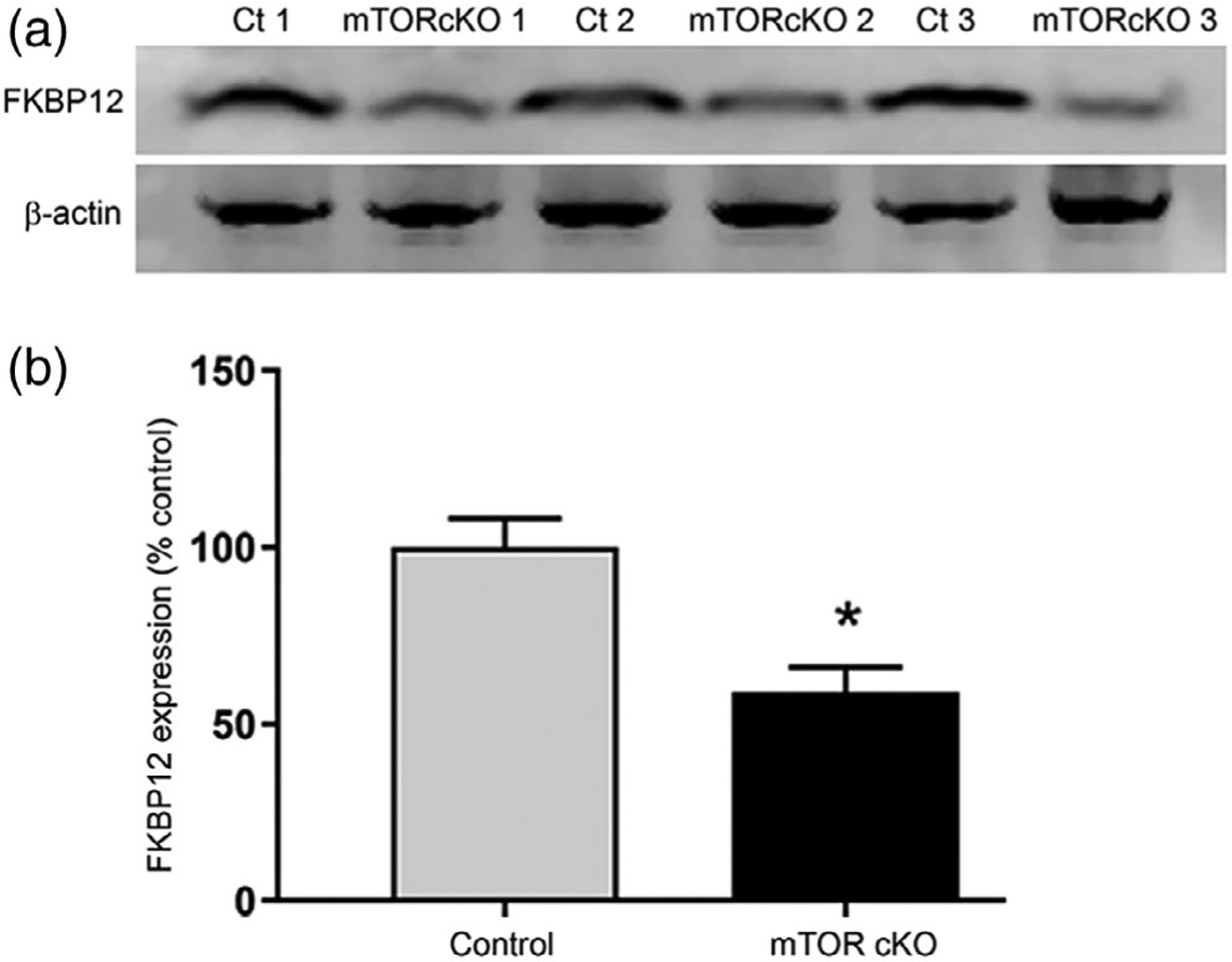
FKBP12 is reduced in mTOR cKO. Western blot analysis (a) and quantification (b) of FKBP12 expression in control and mTOR cKO spinal cords at PND10 (n = 3). Protein was extracted from dissociated whole spinal cords. Values expressed as mean ± SEM. *p < .05
3.8 |. Inhibition of BMP signaling rescues rapamycin-induced reduction in differentiation and myelin proteins
Based on the known actions of BMP signaling and Id2 in inhibiting OPC differentiation, we hypothesized that upregulation of this pathway might be sufficient to explain the block in differentiation with loss of mTOR signaling. To investigate whether downregulating BMP signaling is sufficient to rescue OPC differentiation under conditions where mTOR is inhibited, we blocked both signaling pathways concomitantly in OPCs in vitro, using noggin to antagonize BMP ligand activity and K02288 to inhibit BMP receptor activity. Treating primary rat OPCs in vitro with multiple pharmacological inhibitors negatively affected extended survival; therefore, for experiments involving K02288 we used CG-4 cells, a rat bipotential cell line with robust survival and differentiation potential. CG-4 cells were differentiated into oligodendroglia in the presence or absence of the mTOR inhibitor rapamycin and/or K02288, a pharmacological inhibitor of BMP type 1 receptor kinases that antagonizes downstream signaling. We then analyzed expression of myelin proteins in the cells after 4 days (Figure 8). As in prior studies, inhibiting mTOR with rapamycin almost completely prevented expression of both MBP and MOG, both of which are present in control cells by 4 days of differentiation. However, cotreatment with rapamycin and K02288 rescued expression of MBP and MOG to control levels.
FIGURE 8.
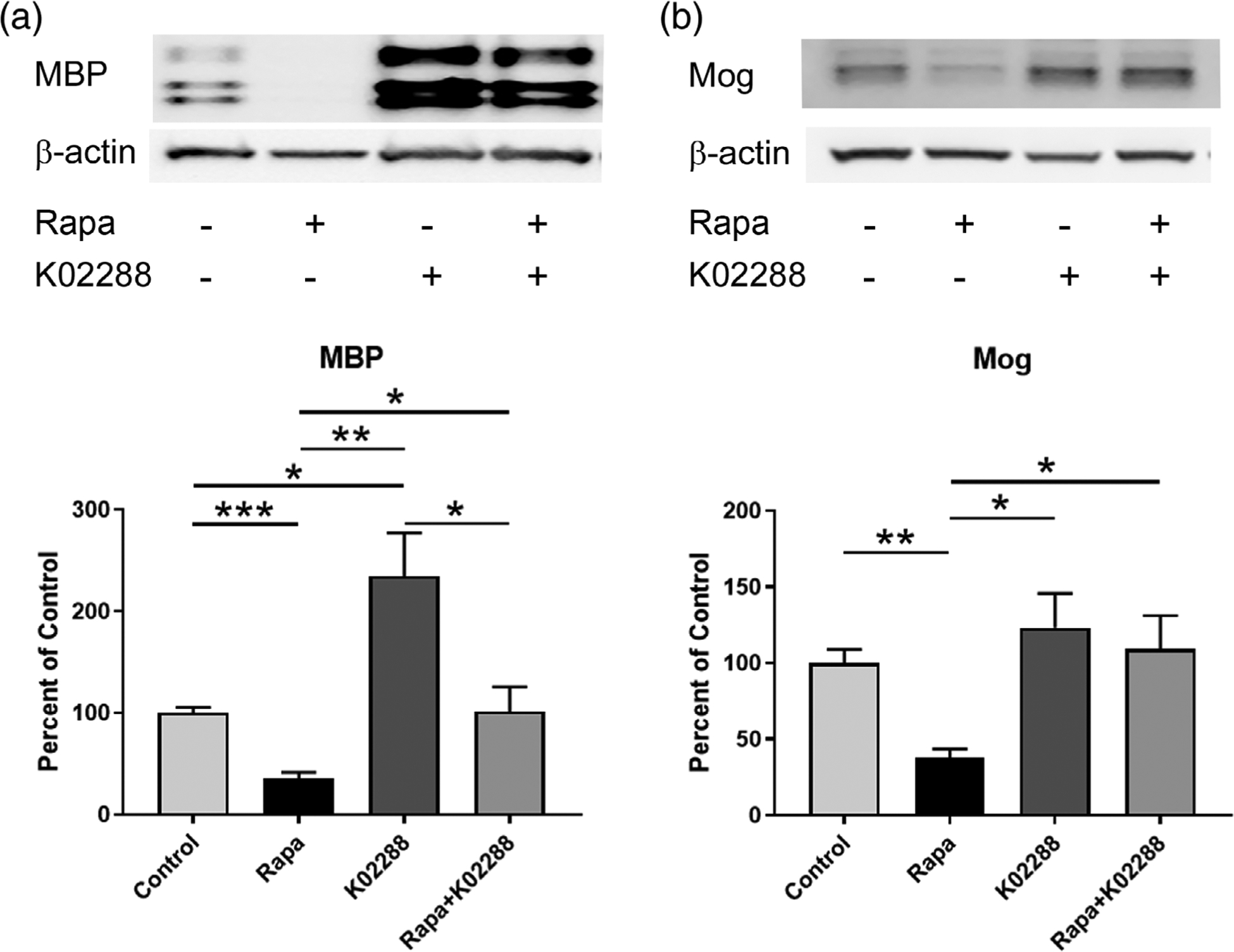
Inhibition of BMP receptor signaling rescues rapamycin-induced reduction in myelin proteins. CG-4 cells were differentiated for days ± rapamycin and/or BMP receptor inhibitor K02288. Following treatment protein was harvested and western blot performed to detect myelin proteins (a) MBP and (b) MOG. Representative western blot and quantification are shown (n = 4). Values expressed as mean ± SEM. *p < .05
To assess the effects of inhibiting mTOR and BMP ligands concurrently, we treated primary rat OPCs with rapamycin to inhibit mTOR in the presence or absence of noggin, a glycoprotein known to inhibit BMP ligand binding (Krause, Guzman, & Knaus, 2011; Zimmerman, De Jesus-Escobar, & Harland, 1996). Differentiating OPCs were treated for 3 days plus or minus inhibitors and then immunostained for MBP. At this time, 21% of control cells were MBP-positive, whereas only 11% of rapamycin-treated cells had progressed to an MBP-positive stage (Figure 9). Blocking BMP signaling with noggin rescued this differentiation deficit; cells treated with both rapamycin and noggin reached control levels of differentiation. These results suggest that decreasing BMP signaling in vitro, either at the receptor or ligand level, can rescue some of the differentiation deficits of mTOR inhibition.
FIGURE 9.
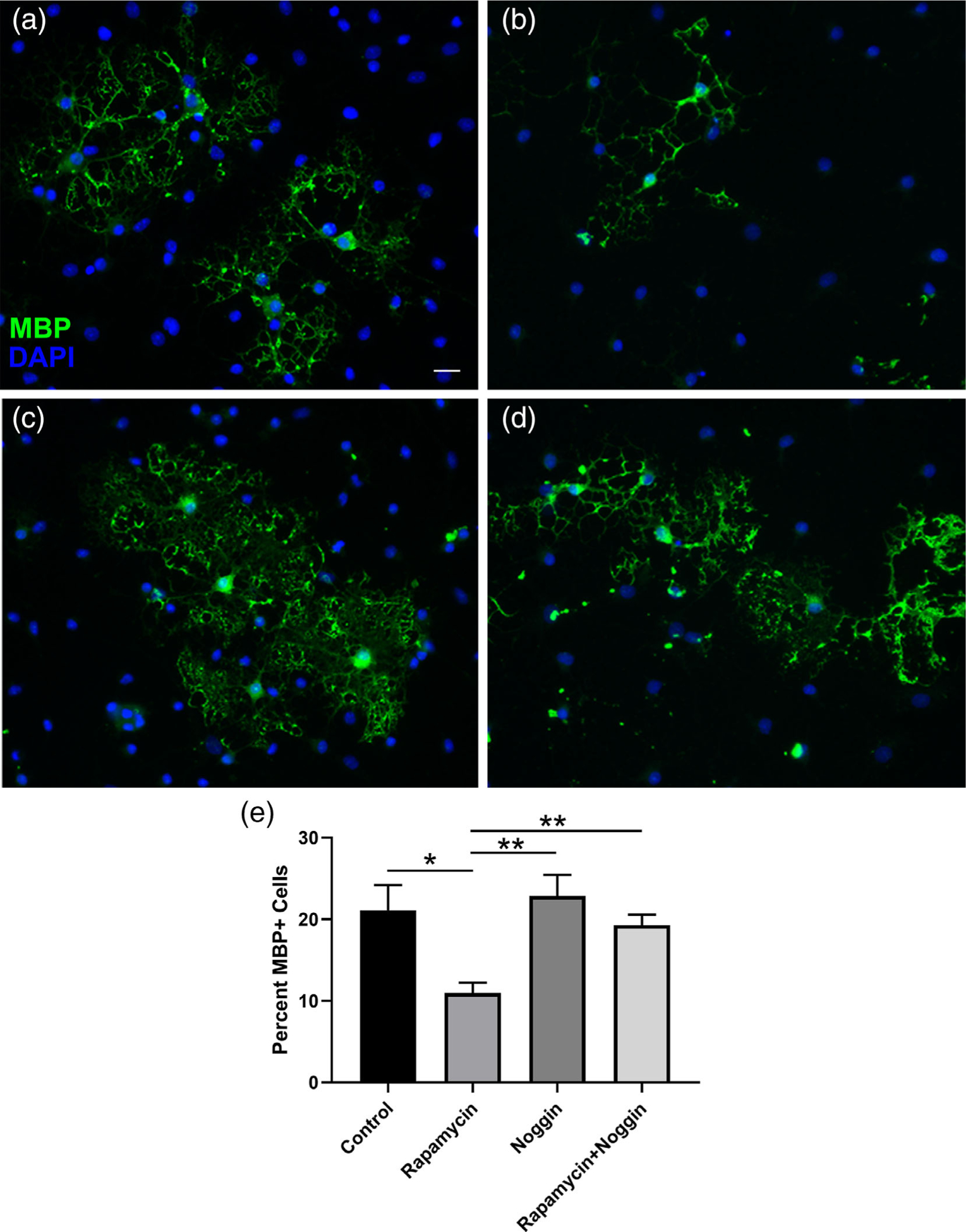
Inhibition of BMP ligands rescues rapamycin-induced reduction in MBP-positive oligodendroglia. Primary OPCs were differentiated for 3 days under (a) control conditions, or treated with (b) 10 nM rapamycin, (c) 500 ng/ml noggin, or (d) rapamycin and noggin. Cells were fixed and stained for MBP. Representative images and quantification (e) are shown (n = 6 over two independent rat litters for OPC prep). Scale bar 20 μm. Values expressed as mean ± SEM. *p < .05; **p < .01
Taken together, the data showing rescue of myelin protein expression by co-inhibiting BMP and mTOR signaling suggest that the differentiation deficit from inhibiting mTOR is predominantly due to sustained BMP signaling in early stage OPCs.
4 |. DISCUSSION
Myelin synthesis initially occurs during postnatal development in mammals and is necessary for normal nervous system function. In the mouse CNS, oligodendrocytes are produced through a highly regulated and sequential differentiation process during a peak of differentiation in the first 2 weeks of life. Multipotent neural progenitor cells differentiate to become committed early stage OPCs, which proliferate and migrate to populate the CNS. Bipolar early OPCs mature to become multipolar late stage OPCs and then immature oligodendrocytes with increasingly branched processes that extend to make contact with and myelinate axons. Multiple inhibitory and promoting cues must coordinate to determine whether an OPC will maintain its precursor state or develop into a postmitotic oligodendrocyte. We discuss here our findings that mTOR signaling, a positive regulator of OPC differentiation, promotes oligodendrogenesis by downregulating BMP signaling, a major negative regulator of OPC differentiation (Figure 10).
FIGURE 10.
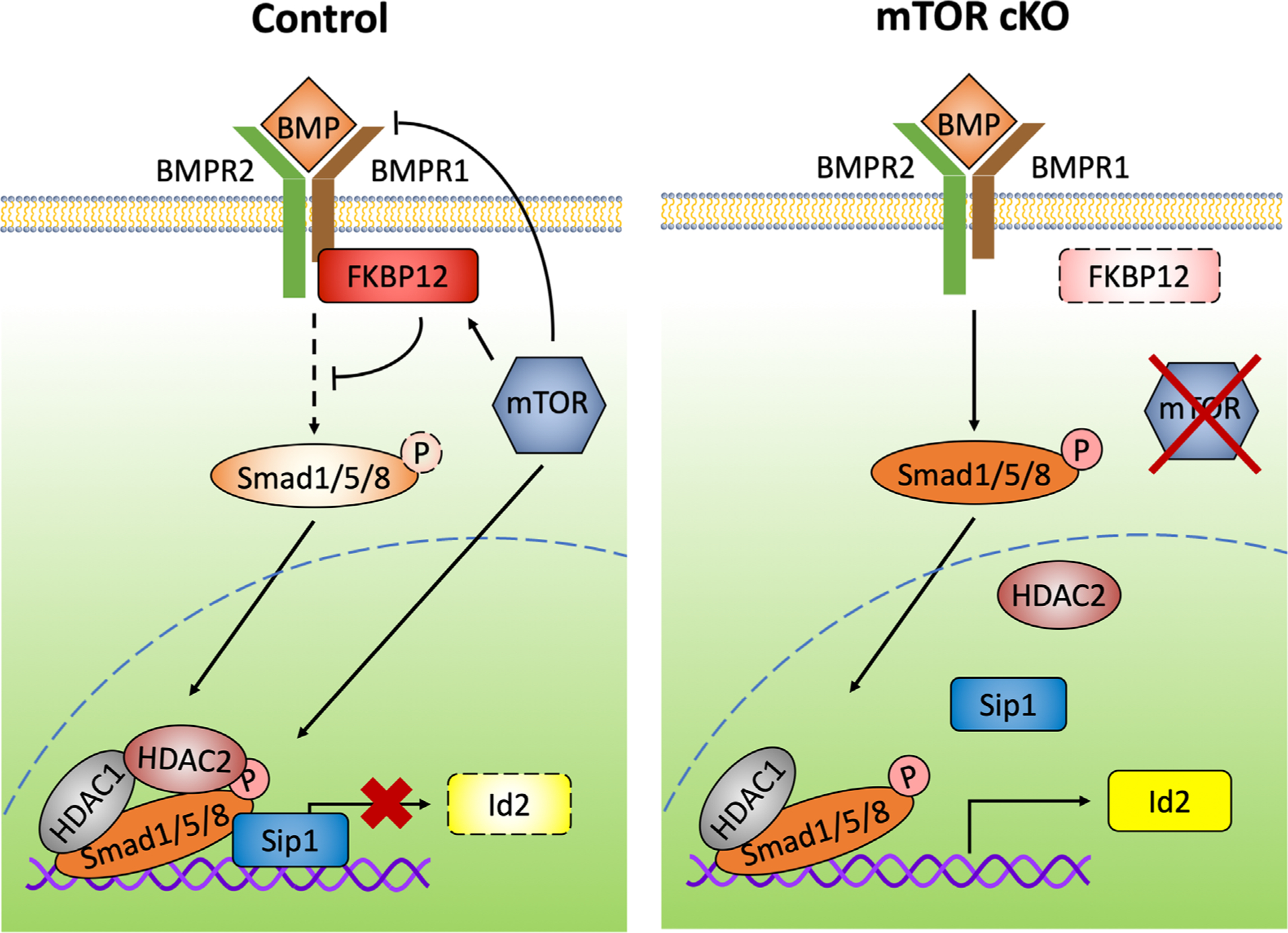
mTOR regulates BMP signaling in differentiation OPCs. Schematic illustrating mTOR regulation of BMP signaling in spinal cord OPCs. In control OPCs, mTOR either directly or indirectly inhibits BMPs and BMPR1. mTOR promotes FKBP12 expression and formation of the regulatory complex at the Id2 promoter. Compared to control cells, mTOR cKO OPCs have decreased expression of FKBP12, increased BMP signaling at the receptor, and increased downstream phosphorylation of Smad1/5/8. When mTOR is inhibited, the levels of Sip1 and HDAC2 bound to the Id2 promoter are reduced, resulting in increased expression of Id2, an inhibitor of oligodendrocyte differentiation
mTOR, as a component of the protein complexes mTORC1 and mTORC2, is a central regulator of cellular response to upstream pathways including growth factor receptor signaling and nutrient sensing. It is an ubiquitously expressed serine/threonine protein kinase and promotes myelination in the central and peripheral nervous systems (Figlia et al., 2018; Guardiola-Diaz, Ishii, & Bansal, 2012; Narayanan, Flores, Wang, & Macklin, 2009; Sherman et al., 2012; Tyler et al., 2009; Tyler et al., 2011; Wahl et al., 2014). mTOR hyperactivation in oligodendroglia, through either constitutive activation of Akt or deletion of PTEN, results in increased myelination (Flores et al., 2008; Goebbels et al., 2010; Harrington et al., 2010). Conversely, oligodendrocytes with an mTOR deficit have myelin defects in the spinal cord that include a delay in oligodendrocyte differentiation and initiation of myelination as well as sustained defects in myelin thickness (Wahl et al., 2014). These data suggest that mTOR functions early during OPC differentiation.
To define the function of mTOR in OPC differentiation, we analyzed differentiating OPCs in our previously reported mouse model carrying a deletion of mTOR in oligodendroglia driven by CNP-Cre (Wahl et al., 2014). In the CNS, the CNP promoter is specific to cells of the oligodendrocyte lineage and is first expressed early in differentiation (Vogel & Thompson, 1988; Yu, Collarini, Pringle, & Richardson, 1994). Our studies revealed that loss of mTOR results in a differentiation defect in the spinal cord, with many OPCs accumulating at an early PDGFRα+ stage, and a corresponding deficit in O4+ late OPCs and immature oligodendrocytes. We show by Enpp6 expression that these mTOR cKO mice also have fewer newly formed oligodendrocytes in the spinal cord during postnatal development.
As stated previously, BMP signaling is known to inhibit oligodendrogenesis. However, the effects of increased BMP signaling in committed OPCs in vivo, specifically during transition from early to late precursors, has not been well studied. We show that PDGFRα+ early OPCs from mTOR cKO mouse spinal cords have altered mRNA levels of BMPs, BMP receptors and upregulation of Id2 resulting in an accumulation of early OPCs and fewer late OPCs/immature oligodendrocytes. Our results support a necessity for BMP signaling to be suppressed for normal progression to O4+ oligodendroglia during development. Crosstalk between mTOR and BMP signaling has been demonstrated in other cell types; Lee et al. reported that rapamycin inhibition of mTOR upregulated Smad activity and expression of Id1–4 in human embryonic stem cells undergoing osteoblastic differentiation (Lee et al., 2010). Similar signaling regulation occurred in hair follicle stem cells during their transition from rest to regeneration; mTORC1 signaling was necessary to overcome suppressive signaling from the BMP pathway (Deng et al., 2015). It is apparent from our data presented here that mTOR suppression of BMP signaling is also an important mechanism in normal oligodendrocyte differentiation and development of myelin.
In addition to increased phosphorylation of Smad 1/5/8, our studies demonstrate that mTOR regulates the transcriptional machinery that binds to the promoter region of Id2 in OPCs. When mTOR is inhibited during OPC differentiation, there is less Sip1 and HDAC2 bound to the Id2 promoter. Sip1 is a Smad-interacting transcriptional repressor (Remacle et al., 1999; Verschueren et al., 1999) and promotes myelination in part by inhibiting BMP signaling through Smad1/5/8 (Weng et al., 2012). Sip1 forms a repressor complex with activated Smad1/5/8, Smad4, p300 and HDACs, translocates to the nucleus, and binds to a Sip1 consensus site in the promoter region of Id2, thereby repressing transcription (Miyazono, 2000; Miyazono, Maeda, & Imamura, 2005; Weng et al., 2012). Loss of Sip1 in the complex results in increased transcription of Id2.
Our observation that mTOR inhibition leads to loss of HDAC2, but not HDAC1, at the promoter of Id2 is interesting in the context of prior studies on these two family members in oligodendrocytes. Histone deacetylases remove histone acetyl groups leading to chromatin compaction and gene silencing, including genes which inhibit oligodendrocyte differentiation (Emery & Lu, 2015). While HDACs do not have DNA binding activity, they function by forming repressive complexes which bind chromatin. Id2 promoter activity is suppressed by overexpression of either HDAC1 or HDAC2 in vitro (Ye et al., 2009). HDAC inhibition has stage-specific effects on oligodendrocyte development, and in vivo studies have shown a functional redundancy between HDAC1 and HDAC2 since the presence of either alone is sufficient for normal development, but deletion of both results in a loss of oligodendrocyte formation (Ye et al., 2009). Differences between HDAC1 and 2 have been shown in Schwann cells where HDAC1 regulates β-catenin while HDAC2 activates SOX10 (Jacob, Lebrun-Julien, & Suter, 2011). Recent studies also point to some functional specificity in oligodendrocytes, since HDAC2, but not HDAC1, regulates expression of MCT1, a critical monocarboxylate transporter, during oligodendrocyte development (Lai et al., 2017). Our results demonstrate that increased Id2 transcription after mTOR deletion is specific to the early PDGFRα+ OPCs; once the oligodendroglia transition from early to late OPCs, Id2 is no longer upregulated in the mTOR cKO O4+ cells. These findings indicate that HDAC2 may be a specific regulator of Id2 during early stages of OPC differentiation and that its presence in the transcriptional complex at the Id2 promoter is downstream of mTOR signaling.
Our data showing rescue of myelin gene expression by co-inhibiting mTOR and BMP signaling support the hypothesis that suppression of BMP signaling during early OPC differentiation is regulated at least in part by mTOR. The specific mechanism(s) by which mTOR suppresses BMP signaling needs to be further investigated; however, the reduction of FKBP12 in mTOR cKO oligodendroglia suggests that mTOR directly modulates FKBP12 (either at the transcriptional or translational level) or acts further upstream. Our data also indicate that mTOR inhibits BMP signaling at the level of the receptors, since we see increased mRNA expression of Bmpr-1a in mTOR cKO spinal cord OPCs (Figure 5a,b, Supporting Information Table S2). Both type I and type II BMP receptors are expressed at very low levels throughout the oligodendrocyte lineage (Marques et al., 2016; Zhang et al., 2014), and increasing BMPR expression may contribute to altered downstream signaling. Treatment with Noggin, which inhibits several BMP ligands, rescues the rapamycin-induced differentiation deficit seen in primary OPCs, indicating that ligand activity is driving BMP signaling when mTOR is inhibited. While BMP2/4 are well described to inhibit oligodendrocyte differentiation, other BMP ligands may be involved as well. OPCs differentially express several Bmp ligands; the Barres and Castelo-Branco databases show that Bmp1, Bmp4 and Bmp7 are highly expressed in OPCs (Marques et al., 2016; Zhang et al., 2014). In contrast, Bmp2, Bmp3, Bmp5, and Bmp6 are expressed at low levels in OPCs so are less likely to be the ligands responsible for cell-autonomous regulation of differentiation. The BMP ligands expressed by OPCs that regulate early differentiation would be expected to decrease in expression when transitioning from OPC to newly formed oligodendrocyte, and we hypothesize these are likely regulated by mTOR signaling. Based on the RNA-Seq database (Zhang et al., 2014), Bmp1 and Bmp4, although highly expressed in OPCs, are increased further in newly differentiated oligodendrocytes (Supporting Information Figure S2B,C). The Castelo-Branco database, with its single-cell approach, further refines this to show that Bmp4 expression increases in committed oligodendrocyte precursors before declining in newly formed oligodendrocytes and myelin-forming oligodendrocytes (Marques et al., 2016). The reduction in Bmp4 only in more mature myelinating oligodendrocytes implies that downregulation of BMP4 is necessary at a later differentiation phase than the mTOR-regulated early transition studied in this manuscript. However, Bmp7 expression does decrease dramatically in newly differentiated oligodendrocytes compared to OPCs (Supporting Information Figure S2A; Zhang et al., 2014), during a transition stage that we find to be regulated by mTOR. Our data indicate that Bmp7 is increased in OPCs when mTOR is lost (Figure 5a). Moreover, BMP7 treatment inhibits myelin gene expression in primary rat Schwann cells (X. Liu et al., 2016). Therefore, BMP7 is a likely downstream mTOR target in the early differentiation phase of oligodendrogenesis. It will be interesting for future studies to determine whether mTOR has a single point of regulation or modulates multiple levels of the BMP signaling pathway.
Our animal model ablated mTOR in oligodendroglia and does not distinguish between specific functions regulated by mTORC1 or mTORC2. In early studies investigating the role of mTOR signaling, the loss of raptor, the subunit specific to mTORC1, had a significant impact on spinal cord myelination, similar to loss of mTOR (Bercury et al., 2014; Lebrun-Julien et al., 2014). mTORC1 function is necessary for normal oligodendrocyte development and myelin thickness in the spinal cord, and the loss of raptor essentially phenocopies mTOR loss (Bercury et al., 2014; Lebrun-Julien et al., 2014; Wahl et al., 2014). mTORC2 has been far less studied than mTORC1, but in studies directly comparing loss of mTORC1 vs. mTORC2 signaling using the Cnp promoter, the loss of rictor, the subunit specific to mTORC2, had little impact on spinal cord myelination (Bercury et al., 2014; Lebrun-Julien et al., 2014). More recent studies, however, in which rictor was deleted using the Olig2 promoter suggest that its loss at a potentially earlier stage in this lineage delays myelination in the corpus callosum (Grier et al., 2017). Additionally, recent studies in the Macklin laboratory using the Pdfra promoter identify a significant impact of rictor loss from OPCs, but with primary impact in corpus callosum and little impact in spinal cord (Macklin, unpublished). Thus, the role of mTORC2 in oligodendrocyte development is currently less clear than that of mTORC1, but there is no evidence that mTORC2 loss alters spinal cord myelination. Importantly, the current studies are consistent with those from the earlier studies (Bercury et al., 2014; Lebrun-Julien et al., 2014; Wahl et al., 2014), supporting the conclusion that mTOR function during transition from early to late OPC in spinal cord is primarily through mTORC1 signaling.
Our findings provide evidence that mTOR functions in early oligodendrocyte differentiation upstream of BMP signaling. Here we show that mTOR, through the inhibition of BMP signaling, may at least partially alleviate the BMP brake on differentiation, specifically during the transition from early OPC to immature oligodendrocyte.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institutes of Neurological Disorders and Stroke under award numbers (082203) to T.L.W. and W.B.M., and (076187) to S.E.W. I.M.O. was partially supported by CAPES Foundation, Ministry of Education of Brazil, Brasilia/DF 70040-020 Brazil (Proc. #BEX9283/13-7). The authors would like to thank Quan Shang for technical assistance.
Funding information
CAPES Foundation, Ministry of Education of Brazil, Grant/Award Number: #BEX9283/13-7; National Institutes of Health, Grant/Award Numbers: 076187, 082203
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supporting Information of this article.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- Baroti T, Zimmermann Y, Schillinger A, Liu L, Lommes P, Wegner M, & Stolt CC (2016). Transcription factors Sox5 and Sox6 exert direct and indirect influences on oligodendroglial migration in spinal cord and forebrain. Glia, 64(1), 122–138. 10.1002/glia.22919 [DOI] [PubMed] [Google Scholar]
- Bercury KK, Dai J, Sachs HH, Ahrendsen JT, Wood TL, & Macklin WB (2014). Conditional ablation of raptor or rictor has differential impact on oligodendrocyte differentiation and CNS myelination. The Journal of Neuroscience, 34(13), 4466–4480. 10.1523/JNEUROSCI.4314-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujalka H, Koenning M, Jackson S, Perreau VM, Pope B, Hay CM, … Emery B (2013). MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS Biology, 11(8), e1001625 10.1371/journal.pbio.1001625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr TD, DiGiovanni J, Lynch CJ, & Shantz LM (2012). Inhibition of mTOR suppresses UVB-induced keratinocyte proliferation and survival. Cancer Prevention Research (Philadelphia, PA), 5(12), 1394–1404. 10.1158/1940-6207.CAPR-12-0272-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XS, Zhang YH, Cai QY, & Yao ZX (2012). ID2: A negative transcription factor regulating oligodendroglia differentiation. Journal of Neuroscience Research, 90(5), 925–932. 10.1002/jnr.22826 [DOI] [PubMed] [Google Scholar]
- Cheng X, Wang Y, He Q, Qiu M, Whittemore SR, & Cao Q (2007). Bone morphogenetic protein signaling and olig1/2 interact to regulate the differentiation and maturation of adult oligodendrocyte precursor cells. Stem Cells, 25(12), 3204–3214. 10.1634/stemcells.2007-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Lei X, Zhang X, Zhang H, Liu S, Chen Q Duan E (2015). mTOR signaling promotes stem cell activation via counterbalancing BMP-mediated suppression during hair regeneration. Journal of Molecular Cell Biology, 7(1), 62–72. 10.1093/jmcb/mjv005 [DOI] [PubMed] [Google Scholar]
- Emery B, & Lu QR (2015). Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the central nervous system. Cold Spring Harbor Perspectives in Biology, 7(9), a020461 10.1101/cshperspect.a020461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlia G, Gerber D, & Suter U (2018). Myelination and mTOR. Glia, 66 (4), 693–707. 10.1002/glia.23273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G,… Macklin WB (2008). Constitutively active Akt induces enhanced myelination in the CNS. The Journal of Neuroscience, 28(28), 7174–7183. 10.1523/JNEUROSCI.0150-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, … Nave KA (2010). Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. The Journal of Neuroscience, 30(26), 8953–8964. 10.1523/JNEUROSCI.0219-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes WA, Mehler MF, & Kessler JA (2003). Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Developmental Biology, 255(1), 164–177 pii: S0012160602000374. [DOI] [PubMed] [Google Scholar]
- Grier MD, West KL, Kelm ND, Fu C, Does MD, Parker B, … Carson RP (2017). Loss of mTORC2 signaling in oligodendrocyte precursor cells delays myelination. PLoS One, 12(11), e0188417 10.1371/journal.pone.0188417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Ishii A, & Bansal R (2012). Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. Glia, 60(3), 476–486. 10.1002/glia.22281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EP, Zhao C, Fancy SP, Kaing S, Franklin RJ, & Rowitch DH (2010). Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Annals of Neurology, 68(5), 703–716. 10.1002/ana.22090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Ishino Y, Jiang W, Yoshimura T, Takeda-Uchimura Y, Uchimura K, … Ikenaka K (2016). Keratan sulfate regulates the switch from motor neuron to oligodendrocyte generation during development of the mouse spinal cord. Neurochemical Research, 41 (1–2), 450–462. 10.1007/s11064-016-1861-9 [DOI] [PubMed] [Google Scholar]
- He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, … Casaccia-Bonnefil P (2007). The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron, 55(2), 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C, Lebrun-Julien F, & Suter U (2011). How histone deacetylases control myelination. Molecular Neurobiology, 44(3), 303–312. 10.1007/s12035-011-8198-9 [DOI] [PubMed] [Google Scholar]
- Krause C, Guzman A, & Knaus P (2011). Noggin. The International Journal of Biochemistry & Cell Biology, 43(4), 478–481. 10.1016/j.biocel.2011.01.007 [DOI] [PubMed] [Google Scholar]
- Lai Q, Du W, Wu J, Wang X, Li X, Qu X, … Fan H (2017). H3K9ac and HDAC2 activity are involved in the expression of monocarboxylate transporter 1 in oligodendrocyte. Frontiers in Molecular Neuroscience, 10, 376 10.3389/fnmol.2017.00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Bronson SK, Lynch CJ, & Vary TC (2010). Skeletal muscle protein balance in mTOR heterozygous mice in response to inflammation and leucine. American Journal of Physiology. Endocrinology and Metabolism, 298(6), E1283–E1294. 10.1152/ajpendo.00676.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang SA, Hackl C, Moser C, Fichtner-Feigl S, Koehl GE, Schlitt HJ, … Stoeltzing O (2010). Implication of RICTOR in the mTOR inhibitor-mediated induction of insulin-like growth factor-I receptor (IGF-IR) and human epidermal growth factor receptor-2 (Her2) expression in gastrointestinal cancer cells. Biochimica et Biophysica Acta, 1803(4), 435–442. 10.1016/j.bbamcr.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, … Nave KA (2003). Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature Genetics, 33(3), 366–374. 10.1038/ng1095 [DOI] [PubMed] [Google Scholar]
- Lebrun-Julien F, Bachmann L, Norrmen C, Trotzmuller M, Kofeler H, Ruegg MA, … Suter U (2014). Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. The Journal of Neuroscience, 34(25), 8432–8448. 10.1523/JNEUROSCI.1105-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Yook JY, Son MY, Kim MJ, Koo DB, Han YM, & Cho YS (2010). Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway. Stem Cells and Development, 19 (4), 557–568. 10.1089/scd.2009.0147 [DOI] [PubMed] [Google Scholar]
- Li P, Li HX, Jiang HY, Zhu L, Wu HY, Li JT, & Lai JH (2017). Expression of NG2 and platelet-derived growth factor receptor alpha in the developing neonatal rat brain. Neural Regeneration Research, 12 (11), 1843–1852. 10.4103/1673-5374.219045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, & Casaccia P (2010). Epigenetic regulation of oligodendrocyte identity. Trends in Neurosciences, 33(4), 193–201. 10.1016/j.tins.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Peng S, Zhang S, Wang M, Chen Y, … Sun C (2016). BMP7 retards peripheral myelination by activating p38 MAPK in Schwann cells. Scientific Reports, 6, 31049 10.1038/srep31049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis JC, Magal E, Muir D, Manthorpe M, & Varon S (1992). CG-4, a new bipotential glial cell line from rat brain, is capable of differentiating in vitro into either mature oligodendrocytes or type-2 astrocytes. Journal of Neuroscience Research, 31(1), 193–204. 10.1002/jnr.490310125 [DOI] [PubMed] [Google Scholar]
- Magri L, Swiss VA, Jablonska B, Lei L, Pedre X, Walsh M, … Casaccia P (2014). E2F1 coregulates cell cycle genes and chromatin components during the transition of oligodendrocyte progenitors from proliferation to differentiation. The Journal of Neuroscience, 34(4), 1481–1493. 10.1523/JNEUROSCI.2840-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstege M, He Y, Li J, Kondo T, Sablitzky F, & Casaccia-Bonnefil P (2006). Multiple roles of Id4 in developmental myelination: Predicted outcomes and unexpected findings. Glia, 54(4), 285–296. [DOI] [PubMed] [Google Scholar]
- Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcao A, Xiao L, … Castelo-Branco G (2016). Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science, 352(6291), 1326–1329. 10.1126/science.aaf6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Seoane J, & Wotton D (2005). Smad transcription factors. Genes & Development, 19(23), 2783–2810. 10.1101/gad.1350705 [DOI] [PubMed] [Google Scholar]
- McCarthy KD, & de Vellis J (1980). Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. The Journal of Cell Biology, 85(3), 890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Singh S, Fitzgerald-Bocarsly P, & Wood TL (2012). Insulin-like growth factor I regulates G2/M progression through mammalian target of rapamycin signaling in oligodendrocyte progenitors. Glia, 60(11), 1684–1695. 10.1002/glia.22387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K (2000). TGF-beta signaling by Smad proteins. Cytokine & Growth Factor Reviews, 11(1–2), 15–22. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Maeda S, & Imamura T (2005). BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine & Growth Factor Reviews, 16(3), 251–263. 10.1016/j.cytogfr.2005.01.009 [DOI] [PubMed] [Google Scholar]
- Nakahiro T, Kurooka H, Mori K, Sano K, & Yokota Y (2010). Identification of BMP-responsive elements in the mouse Id2 gene. Biochemical and Biophysical Research Communications, 399(3), 416–421. 10.1016/j.bbrc.2010.07.090 [DOI] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, & Macklin WB (2009). Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. The Journal of Neuroscience, 29(21), 6860–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Boshans L, Goncalves CM, Wegrzyn J, & Patel KD (2016). Lineage, fate, and fate potential of NG2-glia. Brain Research, 1638(Pt. B), 116–128. 10.1016/j.brainres.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle JE, Kraft H, Lerchner W, Wuytens G, Collart C, Verschueren K, …. Huylebroeck D (1999). New mode of DNA binding of multi-zinc finger transcription factors: deltaEF1 family members bind with two hands to two target sites. The EMBO Journal, 18(18), 5073–5084. 10.1093/emboj/18.18.5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli RJ, LeBeau AP, Fulmer CG, Lazzarino DA, Hochberg A, & Wood TL (2007). Insulin-like growth factor type-I receptor internalization and recycling mediate the sustained phosphorylation of Akt. The Journal of Biological Chemistry, 282(31), 22513–22524. 10.1074/jbc.M704309200 [DOI] [PubMed] [Google Scholar]
- Samanta J, & Kessler JA (2004). Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development, 131(17), 4131–4142. [DOI] [PubMed] [Google Scholar]
- Sherman DL, Krols M, Wu LM, Grove M, Nave KA, Gangloff YG, & Brophy PJ (2012). Arrest of myelination and reduced axon growth when Schwann cells lack mTOR. The Journal of Neuroscience, 32(5), 1817–1825. 10.1523/JNEUROSCI.4814-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J, Natale J, Chew LJ, Belachew S, Cheng Y, Aguirre A, … Gallo V (2006). Identification of Sox17 as a transcription factor that regulates oligodendrocyte development. The Journal of Neuroscience, 26(38), 9722–9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, … Wegner M, (2002). Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes & Development, 16(2), 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumoto YM, Durand B, & Raff MC (1999). An analysis of the early events when oligodendrocyte precursor cells are triggered to differentiate by thyroid hormone, retinoic acid, or PDGF withdrawal. Developmental Biology, 213(2), 327–339. 10.1006/dbio.1999.9397 [DOI] [PubMed] [Google Scholar]
- Tyler WA, Gangoli N, Gokina P, Kim HA, Covey M, Levison SW, & Wood TL (2009). Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. The Journal of Neuroscience, 29(19), 6367–6378. 10.1523/JNEUROSCI.0234-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WA, Jain MR, Cifelli SE, Li Q, Ku L, Feng Y, … Wood TL (2011). Proteomic identification of novel targets regulated by the mammalian target of rapamycin pathway during oligodendrocyte differentiation. Glia, 59(11), 1754–1769. 10.1002/glia.21221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschueren K, Remacle JE, Collart C, Kraft H, Baker BS, Tylzanowski P, … Huylebroeck D (1999). SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5′-CACCT sequences in candidate target genes. The Journal of Biological Chemistry, 274(29), 20489–20498. [DOI] [PubMed] [Google Scholar]
- Vogel US, & Thompson RJ (1988). Molecular structure, localization, and possible functions of the myelin-associated enzyme 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Journal of Neurochemistry, 50(6), 1667–1677. [DOI] [PubMed] [Google Scholar]
- Wahl SE, McLane LE, Bercury KK, Macklin WB, & Wood TL (2014). Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. The Journal of Neuroscience, 34(13), 4453–4465. 10.1523/JNEUROSCI.4311-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Sdrulla A, Johnson JE, Yokota Y, & Barres BA (2001). A role for the helix-loop-helix protein Id2 in the control of oligodendrocyte development. Neuron, 29(3), 603–614. [DOI] [PubMed] [Google Scholar]
- Wang T, Li BY, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, … Donahoe PK (1996). The immunophilin FKBP12 functions as a common inhibitor of the TGF beta family type I receptors. Cell, 86(3), 435–444. [DOI] [PubMed] [Google Scholar]
- Weng Q, Chen Y, Wang H, Xu X, Yang B, He Q, … Lu QR (2012). Dual-mode modulation of Smad signaling by Smad-interacting protein Sip1 is required for myelination in the central nervous system. Neuron, 73(4), 713–728. 10.1016/j.neuron.2011.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, … Richardson WD (2016). Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nature Neuroscience, 19(9), 1210–1217. 10.1038/nn.4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, … Lu QR (2009). HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nature Neuroscience, 12(7), 829–838. 10.1038/nn.2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WP, Collarini EJ, Pringle NP, & Richardson WD (1994). Embryonic expression of myelin genes: Evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron, 12(6), 1353–1362. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, … Wu JQ (2014). An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. The Journal of Neuroscience, 34(36), 11929–11947. 10.1523/JNEURUSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng Y, Liu L, Yu K, Zhang L, Wang H, … Lu QR (2016). Dual regulatory switch through interactions of Tcf7l2/Tcf4 with stage-specific partners propels oligodendroglial maturation. Nature Communications, 7, 10883 10.1038/ncomms10883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, & Harland RM (1996). The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell, 86(4), 599–606. 10.1016/s0092-8674(00)80133-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


