Abstract
OBJECTIVE:
To study the molecular mechanism of warming and tonifying kidney-yang recipe (WTKYR) in the treatment of depression.
METHODS:
SD rats were divided into a control group, model group, WTKYR group, and fluoxetine group. Each group consisted of 21 rats. The chronic unpredictable mild stress model was used. Body weighing and SPT were performed regularly. After treatment, histopathology of the brain tissue was performed, and concentrations of 5-HT (5-hydroxytryptamine), NE (norepinephrine), and DA (dopamine) in the hippocampus were determined.
RESULTS:
The WTKYR group showed higher body weight and sucrose consumption than the control groups. Moreover, the concentrations of 5-HT, NE, and DA in the hippocampus were significantly different in the WTKYR group in comparison to those in the other groups. The hippocampus histomorphology of the WTKYR group exhibited less dematous pyramidal cells and mild inflammatory cell infiltration.
CONCLUSION:
The treatment effect of WTKYR in depression may be based on improvement in the content of 5-HT, NE, and DA in the hippocampus, extenuating edema of the cortical surface and pyramidal cells and decreasing the infiltration of inflammatory cells into hippocampus tissue.
Keywords: WTKYR, depression, kidney-yang, brain histopathology, monoamine neurotransmitters
1. Introduction
Depression is a type of mental disorder characterized by significant and persistent depression, decreased mobility, and sluggish thinking and cognition. As is reported by the World Health Organization, there are over 300 million persons around the world living with depression. Many studies have shown that some traditional Chinese medicine (TCM) remedies offer an antidepressant effect [1, 2, 3] and fewer side effects than Western antidepressants [4]. According to TCM theory, depression belongs to “Yu Zheng.” In addition to liver-qi stagnation, kidney-yang deficiency is an important pathogenesis of depression [5]. Kidney-yang can promote the operation of yang-qi, and kidney-yang deficiency is characterized by a low mood, lack of spirit, apathy, obtuse behavior, and decreased vitality. The warming and tonifying kidney-yang recipe (WTKYR) was developed to warm and tonify kidney-yang and was recorded in the “Golden Chamber.” Clinical applications have found that the abnormal emotional state of depression patients may be significantly improved by WTKYR. Previous animal experiments have also shown that WTKYR can diminish abnormal behavior in depression model rats and upregulate the AC – cAMP – PKA – CREB – BDNP signaling pathway [6].
Monoamine neurotransmitters such as 5-HT, NE, and DA transmit information between neurons or between nerves and effector cells, acting a major role in the regulation of emotion [7]. Dysfunction of the monoamine neurotransmitters’ metabolic system is the key to depression. Zhang et al. [8] found on autopsy that the abnormal neurotransmitter was mainly expressed as the decrease of 5-HT synthesis in the brain. Reduced NE concentration in the hypothalamus of depressed patients suggests that depression is associated with low central NE function [9]. DA dysfunction is also related to the pathophysiology of depression, [10] and Malhi and Berk [11] found that it is closely associated with psychomotor disorders. DA dysfunction can lead to damaged plasticity in hippocampus-frontal cortex synapses, resulting in cognitive dysfunction [12].
It is reported that depression is closely associated with changes in brain structure that regulate mood and cognitive function [13]. The hippocampus serves an important role in the brain by participating in the processes of memory and emotion, autonomic activity, and endocrine integration. In recent years, the hypothesis of adult hippocampal neurogenesis has dominated the study of depression [14, 15]. Numerous experiments have demonstrated that the hippocampus of depression patients is significantly reduced [16, 17].
2. Materials and methods
2.1. Experimental animals
Eighty-four male specific pathogen-free SD rats were bought from Chengdu Dashuo Experimental Animal Co., Ltd. in Chengdu (SCXK (Chuan) 2014-189). All the rats were 6–8 weeks old, weighed 200 20 g, and habituated for one week prior to any operation. The CUMS modeling procedure was used, and the protocol followed was similar to the protocol used in our previous study [10].
2.2. Open field test
An open field test [18] was conducted on the day after modeling, the fourteenth day of treatment, and on the day of sacrifice to judge whether or not the modeling was successful and to determine the therapeutic effect of the remedies. The experimental procedure was as follows: Rats were deposited in the center square of an open arena (0.8 m long by 0.8 m wide by 0.4 m deep) and were free to explore the field for three minutes. The ground of the open arena was divided into 25 pieces (0.16 m long by 0.16 m wide) with black lines. The number of squares spanned by the rat and its standing frequency were recorded to calculate the crossing score and rearing score. For each time that all the rat’s limbs crossed over a square or both forelimbs of the rat left the ground for more than 1 cm, one point was added to the score. Each rat was tested only once, between 7:30 am and 12:00 am in a quiet room.
2.3. Medicine and animal treatments
The WTKYR comprised of Rou Gui (Cinnamomum Cassia Presl) (1,000 g) and Ba Jitian (Morinda officinalis How) (3,000 g). The method of decoction was the same as in the previous study [6]. Before intragastric administration, fluoxetine hydrochloride was blended into conductivity water to a density of 0.000233 g/mL. The rats were arbitrarily distributed into four groups. Physiological saline at a dose of 2 mL/day were given to the control group ( 21) and the model group ( 21). The WTKYR group ( 21) was fed with a 2 mL/day dosage of the decoction, and the fluoxetine group ( 21) was given a 2 mL/day dose of fluoxetine hydrochloride. All the groups were treated for 21 days.
2.4. Sucrose preference test
The SPT is an important index for judging anhedonia. It was performed after modeling and after the weekly treatment. Rats were housed separately in cages and supplied a glass of pure water and a glass of 1% sucrose water. After 48 hours, the glasses were removed and the liquid consumption was recorded. To reduce the deviation from place preference, the locations of the two glasses were switched.
2.5. Sample preparation
Twenty-four rats (randomly taken from the four groups, 6 per group) were anesthetized and killed, and their blood was taken from the abdominal aortas. The hippocampus was collected to be dehydrated, trimmed, embedded, sectioned, stained with hematoxylin and eosin, mounted, and microscopically examined. All the pathological images were analyzed by Motic Images Advanced. Sixty rats (randomly chosen from the four groups, 15 per group) were killed after being anesthetized. The hippocampus was removed and added to a test tube containing iced normal saline, then homogenized using an electric homogenizer and centrifuged for 10 minutes (3,500 rpm). Supernatants were collected for detection of 5-HT, NE, and DA by ELISA.
2.6. ELISA assay
Rat 5-HT (cat. No. Rr1037XL), Rat DA (cat. No. Rr0425XL) and Rat NE (cat. No. Rr1371XL) Elisa kit were used to detect the concentrations of 5-HT, DA, and NE following the provided instructions.
2.7. Statistical analysis
Statistical Product and Service Solutions (SPSS) 20.0 software was performed to analyze the data. The data of body weight and SPT were analyzed by the repeated measures analysis of variance. The data of the open field test and the concentration of monoamine neurotransmitters were analyzed by single-factor analysis of variance. values were accepted as significant at 0.05.
3. Results
3.1. Rats’ body weight, SPT, and vitality of CUMS rats
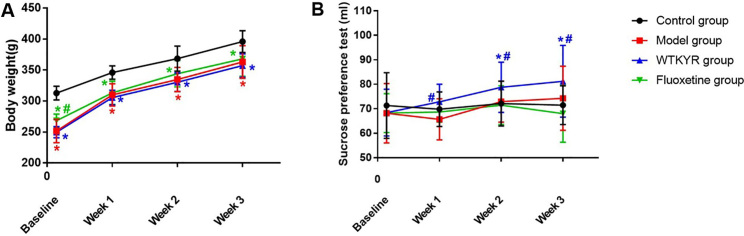
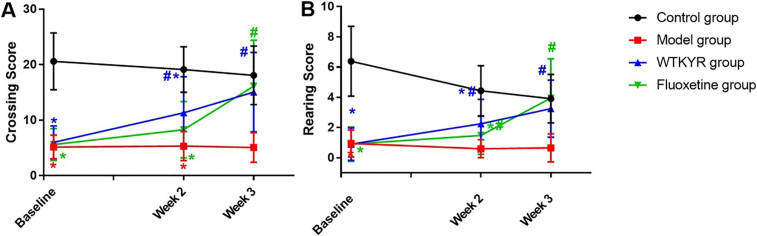
The rats’ weight (Fig. 1A) and SPT (Fig. 1B) were checked after modeling and weekly therapy. The results suggested that the body weight of the WTKYR group increased and was remarkably different than the control group ( 0.05). The SPT results displayed that the WTKYR group consumed more sucrose than the other groups at the last two weeks ( 0.05). When compared with the control group, the open field test result showed that the crossing scores (Fig. 2A) and the rearing scores (Fig. 2B) of the WTKYR, fluoxetine and model group had significantly decreased after modeling ( 0.05). After treatment, the fluoxetine and WTKYR groups displayed higher scores than the model group ( 0.05).
Figure 1.
Body weight (A) and SPT (B) results of all groups at baseline (the last day of modeling) and at the end of each treatment week. As shown in Fig. 1A, all groups that received CUMS modeling had lower body weights than the control group. The rats in the fluoxetine group and WTKYR group gradually gained weight during the treatment, but there remained an obvious discrepancy with the control group by the end of the therapy. The SPT data indicated no difference among the four groups at baseline. With the progress of treatment, rats in the WTKYR group consumed more sucrose than the model group. In the last two weeks of treatment, rats in the WTKYR group displayed remarkable higher sucrose consumption than the control group. The symbol ‘*’ indicated that -value is lower than 0.05 when compared with the control group, and the symbol ‘#’ expressed that -value is lower than 0.05 when compared with the model group.
Figure 2.
Crossing scores (A) and rearing scores (B) in open field tests of the four groups. The results displayed that the crossing scores of rats in the model, WTKYR, and fluoxetine groups were lower than the control group at baseline. A significant increase was displayed in the WTKYR group when compared with the model and fluoxetine groups at the second week of treatment. It was noted that both the WTKYR and fluoxetine group rats recorded higher crossing scores in the last week, and then no significant difference was observed among the WTKYR, fluoxetine group and the control group. Figure 2B displayed the rearing scores of the model, WTKYR, and fluoxetine groups decreased after modeling. After two weeks of treatment, the rearing scores of the fluoxetine group and WTKYR group were increased compared with the model group, but still lower than the control group. And no significant difference among the WTKYR, fluoxetine, and control groups were found until the last week of treatment.
3.2. The 5-HT, NE, and DA concentration of CUMS rats
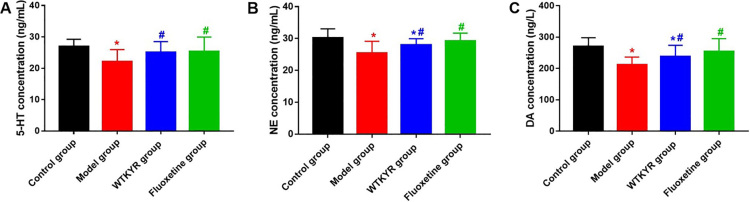
Compared to the model group, the concentration of hippocampus 5-HT (Fig. 3A) in the WTKYR group was notably higher ( 0.05), and no remarkable contrast was observed between the WTKYR and control groups. As shown in Fig. 3B and C, hippocampus NE and DA concentrations of the WTKYR group were found to be remarkably different from those of the control and model groups.
Figure 3.
Results of 5-HT (A), NE (B), and DA (C) of rats in all groups. As shown in Fig. 3A, the concentrations of hippocampus 5-HT in the fluoxetine group and the WTKYR group were remarkably higher compared with the model group, and there was no statistical difference when contrast the fluoxetine and WTKYR group with the control group. Hippocampus NE and DA content of the WTKYR group were higher than in the model group and lower than in the control group. The fluoxetine group showed a higher NE and DA concentration than the model group.
3.3. Hippocampus histomorphology of CUMS rats
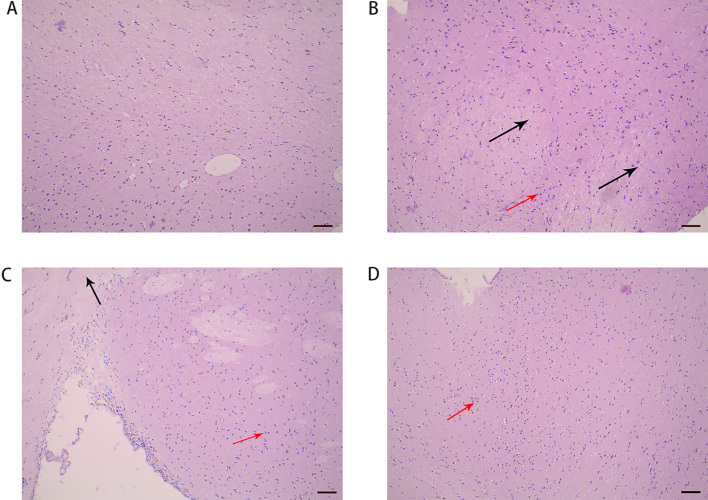
The hippocampus histomorphology of the control group (Fig. 4A) was normal, and no edema or inflammatory cell infiltration was observed. Rats of the model group (Fig. 4B) showed moderately edematous pyramidal cells, and the hippocampus histomorphology was basically normal. In the WTKYR group (Fig. 4C), slightly edematous pyramidal cells and mild inflammatory cell infiltration were found. The hippocampus histomorphology of the fluoxetine group (Fig. 4D) was structurally intact. Some rats in the group showed mild edema of pyramidal cells and mild inflammatory cell infiltration.
Figure 4.
Hippocampus histomorphology of the groups: control (A), model (B), WTKYR (C), and fluoxetine group (D). It was noted that hippocampus histomorphology of the control group was normal. Moderate pyramidal cell edema and infiltration of inflammatory cells were observed in the model group. There was lighter cell edema and inflammation in the rats’ hippocampus tissue of the fluoxetine and WTKYR groups. The black arrows in Fig. 4 point to the cell edema, and the red point to the inflammatory cell infiltration. Bar 100 m.
4. Discussion
In the study of the etiological factor of depression, the deficiency of monoamine neurotransmitters in the brain, for instance, NE, 5-HT, and DA, has been widely recognized [19]. According to the monoamine neurotransmitter theory, depression is caused by the insufficiency of the monoamine neurotransmitters NE and 5-HT in the brain. In 1965, Coppen [20] found that the lack of 5-HT in the central nervous system can cause depression, which was later confirmed by many scholars. Numerous studies have shown that DA may be involved in the pathogenesis of depression and that the therapeutic effect of drugs on depression may be achieved through enhancing the sensitivity of dopamine receptors [21]. During depression, the content and function of monoamine neurotransmitters may change. In the present study, hippocampus 5-HT, NE, and DA concentrations of the depression model rats were noticed to significantly decrease when compared with the control group, indicating that depression was related to the content of monoamine neurotransmitters. WKTYR may improve the content of 5-HT, NE, and DA of CUMS rats and ameliorate the depressive state of rats, suggesting that WKTYR may be effective in upregulating monoamine neurotransmitters. Anhedonia is one of the typical symptoms of depression, and it may be represented by a reduced preference for sucrose in SPT. In this study, increased consumption of sucrose was observed in the WTKYR group during the last two weeks, indicating that WTKYR may reverse the anhedonia of CUMS depression rats.
The cerebrum lesion is an important etiological factor of depression. Most depression patients have structural changes in the brain [22, 23] that might lead to depressive symptoms such as mental and emotional disorders [24]. As an important part of the brain that is closely related to memory and emotion, the hippocampus acts a considerable part in the pathogenesis of depression. The hippocampus of the depression model rats had changes in amino acid metabolism and impaired protein synthesis and degradation [25]. It has been reported that long-term stress can cause hippocampal neuronal degeneration and pyramidal cell edema in rats [26, 27]. This study found that the hippocampus was intact in the control group, with no pyramidal cell edema or inflammatory cell infiltration. Moderate pyramidal cell edema and infiltration of inflammatory cells were observed in the model group. Although pathological changes were observed in the WTKYR group, pyramidal cell edema and the infiltration of inflammatory cells were extenuated. This suggests that WKTYR may achieve therapeutic effects by mitigating pyramidal cell edema and infiltration of inflammatory cells into brain tissue.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (grant no. 81302900).
Conflict of interest
The authors have no conflict of interest to report.
Abbreviations
| SD | sprague dawley |
| PKA | protein kinase A |
| 5-HT | 5-hydroxytryptamine |
| NE | norepinephrine |
| cAMP | cyclic adenosine monophosphate |
| DA | dopamine |
| SPT | sucrose preference test |
| TCM | traditional Chinese medicine |
| AC | adenylyl cyclase |
| BDNF | brain derived neurotrophic factor |
| HE | hematoxylin and eosin |
| CREB | CAMP response element – binding protein |
| SPSS | statistical product and service solutions |
| CUMS | chronic unpredictable mild stress |
| WTKYR | warming and tonifying kidney-yang recipe |
References
- [1]. Wang Y, Fan R, Huang X. Meta-analysis of the clinical effectiveness of traditional Chinese medicine formula Chaihu-Shugan-San in depression. J Ethnopharmacol. 2012; 141(2): 571-7. doi: 10.1016/j.jep.2011.08.079. [DOI] [PubMed] [Google Scholar]
- [2]. Gao X, Sun P, Qiao M, Wei S, Xue L, Zhang H. ShuYu capsule, a Traditional Chinese Medicine formulation, attenuates premenstrual syndrome depression induced by chronic stress constraint. Mol Med Rep. 2014; 10(6): 2942-8. doi: 10.3892/mmr.2014.2599. [DOI] [PubMed] [Google Scholar]
- [3]. Zhang Y, Wang ZZ, Sun HM, Li P, Li YF, Chen NH. Systematic review of traditional chinese medicine for depression in Parkinson’s disease. Am J Chin Med. 2014; 42(5): 1035-51. doi: 10.1142/S0192415X14500657. [DOI] [PubMed] [Google Scholar]
- [4]. Yeung WF, Chung KF, Ng KY, Yu YM, Ziea ET, Ng BF. A systematic review on the efficacy, safety and types of Chinese herbal medicine for depression. J Psychiatr Res. 2014; 57: 165-75. doi: 10.1016/j.jpsychires.2014.05.016. [DOI] [PubMed] [Google Scholar]
- [5]. Chen ZH, Wang GH, Wang XP, Huo YX, Yang MH, Li L et al. Effect of Warm-Supplementing Kidney Yang (WSKY) added to risperidone on quality of life in patients with schizophrenia: A randomized controlled trial. Clin Rehabil. 2009; 23(11): 963-72. doi: 10.1177/0269215508101743. [DOI] [PubMed] [Google Scholar]
- [6]. Peng Y, Zhang C, Su Y, Wang Z, Jiang Y. Activation of the hippocampal AC-cAMP-PKA-CREB-BDNF signaling pathway using WTKYR in depression model rats. Electrophoresis. 2018. doi: 10.1002/elps.201800381. [DOI] [PubMed] [Google Scholar]
- [7]. Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998; 59(Suppl 14): 11-4. [PubMed] [Google Scholar]
- [8]. Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, et al. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005; 45(1): 11-6. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- [9]. Invernizzi RW, Garattini S. Role of presynaptic alpha2-adrenoceptors in antidepressant action: Recent findings from microdialysis studies. Prog Neuropsychopharmacol Biol Psychiatry. 2004; 28(5): 819-27. doi: 10.1016/j.pnpbp.2004.05.026. [DOI] [PubMed] [Google Scholar]
- [10]. Brody AL, Olmstead RE, Abrams AL, Costello MR, Khan A, Kozman D, et al. Effect of a history of major depressive disorder on smoking-induced dopamine release. Biol Psychiatry. 2009; 66(9): 898-901. doi: 10.1016/j.biopsych.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Malhi GS, Berk M. Does dopamine dysfunction drive depression? Acta Psychiatr Scand Suppl. 2007; (433): 116-24. doi: 10.1111/j.1600-0447.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- [12]. Jay TM, Rocher C, Hotte M, Naudon L, Gurden H, Spedding M. Plasticity at hippocampal to prefrontal cortex synapses is impaired by loss of dopamine and stress: Importance for psychiatric diseases. Neurotox Res. 2004; 6(3): 233-44. [DOI] [PubMed] [Google Scholar]
- [13]. Duman RS, Aghajanian GK. Synaptic dysfunction in depression: Potential therapeutic targets. Science. 2012; 338(6103): 68-72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000; 48(8): 732-9. [DOI] [PubMed] [Google Scholar]
- [15]. Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am J Psychiatry. 2004; 161(4): 598-607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- [16]. Vaidya VA, Fernandes K, Jha S. Regulation of adult hippocampal neurogenesis: Relevance to depression. Expert Rev Neurother. 2007; 7(7): 853-64. doi: 10.1586/14737175.7.7.853. [DOI] [PubMed] [Google Scholar]
- [17]. Thomas RM, Peterson DA. Even neural stem cells get the blues: Evidence for a molecular link between modulation of adult neurogenesis and depression. Gene Expr. 2008; 14(3): 183-93. [PMC free article] [PubMed] [Google Scholar]
- [18]. Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression. Neurosci Biobehav Rev. 1981; 5(2): 247-51. [DOI] [PubMed] [Google Scholar]
- [19]. Racagni G, Brunello N. Physiology to functionality: The brain and neurotransmitter activity. Int Clin Psychopharmacol. 1999; 14(Suppl 1): S3-7. [DOI] [PubMed] [Google Scholar]
- [20]. Coppen A, Turner P, Rowsell AR, Padgham C. 5-Hydroxytryptamine (5-HT) in the whole-blood of patients with depressive illness. Postgrad Med J. 1976; 52(605): 156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Healy E, McKeon P. Dopaminergic sensitivity and prediction of antidepressant response. J Psychopharmacol. 2000; 14(2): 152-6. doi: 10.1177/026988110001400204. [DOI] [PubMed] [Google Scholar]
- [22]. Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006; 26(1): 63-72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Tham MW, Woon PS, Sum MY, Lee TS, Sim K. White matter abnormalities in major depression: Evidence from post-mortem, neuroimaging and genetic studies. J Affect Disord. 2011; 132(1-2): 26-36. doi: 10.1016/j.jad.2010.09.013. [DOI] [PubMed] [Google Scholar]
- [24]. Dalby RB, Frandsen J, Chakravarty MM, Ahdidan J, Sorensen L, Rosenberg R, et al. Depression severity is correlated to the integrity of white matter fiber tracts in late-onset major depression. Psychiatry Res. 2010; 184(1): 38-48. doi: 10.1016/j.pscychresns.2010.06.008. [DOI] [PubMed] [Google Scholar]
- [25]. Zhang Y, Yuan S, Pu J, Yang L, Zhou X, Liu L, et al. Integrated metabolomics and proteomics analysis of hippocampus in a rat model of depression. Neuroscience. 2018; 371: 207-20. doi: 10.1016/j.neuroscience.2017.12.001. [DOI] [PubMed] [Google Scholar]
- [26]. Sakurai-Yamashita Y, Yamashita K, Niwa M, Taniyama K. Involvement of 5-hydroxytryptamine4 receptor in the exacerbation of neuronal loss by psychological stress in the hippocampus of SHRSP with a transient ischemia. Brain Res. 2003; 973(1): 92-8. [DOI] [PubMed] [Google Scholar]
- [27]. Wang MS, Zhang LN, Ji XY, Xu H, Liu H, Hou NG. Edema and neuronal apoptosis in the hippocampus and cortex of elderly rats following transient cerebral ischemia/reperfusion injury. Neural Regen Res. 2009; 4(12): 1013-8. [Google Scholar]