Abstract
Objective
To describe the epidemiology of herpes simplex virus type 1 (HSV-1) in Europe.
Methods
We systematically reviewed HSV-1 related publications, conducted various meta-analyses and meta-regressions, assessed pooled mean seroprevalence, and estimated pooled mean proportions of HSV-1 viral detection in clinically diagnosed genital ulcer disease (GUD) and in genital herpes.
Results
We extracted, from 142 relevant records, 179 overall (622 stratified) seroprevalence measures, 4 overall proportions of HSV-1 in GUD and 64 overall (162 stratified) proportions of HSV-1 in genital herpes. Pooled mean seroprevalence was 67.4% (95% CI 65.5% to 69.3%) with 32.5% (95% CI 29.4% to 35.7%) of children and 74.4% (95% CI 72.8% to 76.0%) of adults infected. Pooled seroprevalence increased steadily with age, being lowest in those aged <20 years (39.3%, 95% CI 35.9% to 42.7%) and highest in those aged >50 years (82.9%, 95% CI 78.8% to 86.6%). Pooled seroprevalence decreased yearly by 0.99-fold (95% CI 0.99 to 1.00). Pooled mean proportion of HSV-1 detection was 13.6% (95% CI 4.1% to 27.1%) in GUD, 34.1% (95% CI 31.7% to 36.5%) in genital herpes and 49.3% (95% CI 42.2% to 56.4%) in first episode genital herpes. Pooled proportion of HSV-1 detection in genital herpes increased yearly by 1.01-fold (95% CI 1.00 to 1.02), with higher detection in women (42.0%, 95% CI 37.4% to 46.7%) than men (24.1%, 95% CI 19.8% to 28.6%).
Conclusions
HSV-1 epidemiology is transitioning away from its historical pattern of oral acquisition in childhood. Every year, seroprevalence is declining by 1% and the proportion of HSV-1 in genital herpes is increasing by 1%. As many as two-thirds of children are reaching sexual debut unexposed, and at risk of HSV-1 genital acquisition in adulthood.
Keywords: herpes, genital ulcer disease, seroprevalence, prevalence, meta-analysis, meta-regression, europe, region
Key questions.
What is already known?
Herpes simplex virus type 1 (HSV-1) infection is typically acquired through oral transmission during childhood.
Recent data from North America and Europe suggest a decrease in acquisition of HSV-1 in childhood, a decline in seroprevalence in youth and an increase in genital herpes cases that are caused by HSV-1.
What are the new findings?
Only two-thirds of the population in Europe are HSV-1 seropositive, far lower than the historical level of universal infection in childhood.
Two-thirds of European children are reaching sexual debut unexposed to this infection, and at risk of genital acquisition in adulthood.
Half of first episode genital herpes cases in Europe are already due to HSV-1, as opposed to HSV-2 infection.
Seroprevalence in Europe is declining by 1% per year, and the contribution of HSV-1 to genital herpes is increasing, also by 1% per year.
What do the new findings imply?
HSV-1 epidemiology in Europe is in transition and shifting away from its historical pattern of oral acquisition in childhood.
HSV-1 transition in Europe is leading to more heterogeneous and variable transmission by age and geography, and an increasing role for HSV-1 in genital herpes and as a sexually transmitted disease.
The findings highlight the importance of disease surveillance and monitoring of HSV-1 seroprevalence and genital herpes aetiology, and strengthen the case for an HSV-1 vaccine to limit transmission.
Introduction
Herpes simplex virus type 1 (HSV-1) causes a latent and mostly asymptomatic infection, and is typically acquired orally during childhood.1 2 Infection is lifelong, with most viral shedding occurring through subclinical short-duration reactivations on the oral mucosa.3 When symptomatic, HSV-1 can result in a number of adverse outcomes and sequelae such as mucocutaneous conditions and central nervous system complications.1 2 The historical pattern of HSV-1 epidemiology appears to be changing, at least in a few regions.4–12 Studies show a decrease in early acquisition of HSV-1, a decline in seroprevalence among youths and an increase in genital herpes cases caused by HSV-1.4 5 11 13–17 The disease burden of this infection, alongside its evolving epidemiology, has drawn the attention of the World Health Organization (WHO) and global partners, who are leading an international multisectorial effort focused on understanding the epidemiology of the virus and developing a HSV vaccine.18–20
Under this guise, we conducted a comprehensive systematic review to characterise HSV-1 epidemiology in Europe. We also used meta-analytical methods to provide robust estimates for HSV-1 seroprevalence across different populations, as well as proportions of HSV-1 detection in genital ulcer disease (GUD) and in genital herpes. We further assessed associations and temporal trends for these outcome measures.
Methods
The methodology of this study was adapted from a previously conducted systematic review investigating the epidemiology of HSV-1 in Asia.9 Details of the methodology are described in table 1.
Table 1.
Detailed methodology for this study
| Methodology | Detailed description |
| Data source and search strategy |
|
| Study selection and inclusion and exclusion criteria |
|
| Data extraction and data synthesis |
|
| Quality assessment | The Cochrane’s approach for risk of bias assessment included:
|
| Meta-analyses |
|
| Meta-regressions |
|
ELISA, enzyme linked immunosorbent type specific assay; GUD, genital ulcer disease; HSV-1, herpes simplex virus type 1; HSV-2, herpes simplex virus type 2.
Patient and public involvement
Patients were not involved in this study.
Data sources and search strategy
This systematic review followed the Cochrane Collaboration Handbook21 and reported its findings in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (see online supplementary table s1).22 We conducted a systematic literature search to identify HSV-1 related publications in Europe. Europe’s definition and subregional classification were informed by the WHO and the United Nations Geoscheme, respectively23 24 (see details in table 1). The search strategies used can be found in online supplementary box s1.
bmjgh-2020-002388supp001.pdf (2.3MB, pdf)
Study selection and inclusion and exclusion criteria
WY and HI conducted the initial screening, and MH conducted the double screening, as detailed in table 1. In this review, the term 'publication' refers to a document reporting one or several overall outcome measures in one or several (different) populations. 'Study' or 'measure' refers to a specific outcome measure and its details.
Data extraction and data synthesis
Extraction and double extraction of relevant data were conducted. Extracted variables are listed in table 1. Overall outcome measures and their strata were extracted based on a preset hierarchy, provided the sample size in each stratum was ≥10 (table 1). Strata of outcome measures were extracted for more statistical power in assessing predictors of heterogeneity in effect size.
Quality assessment
An initial quality assessment of relevant publications was conducted to assess the validity of the diagnostic assay in each study, given documented limitations.25 26 Professor Rhoda Ashley-Morrow, an expert advisor from the University of Washington, evaluated the validity, sensitivity, and specificity of the assays. Only studies with valid and reliable assays were included in the systematic review. The precision and quality of each study were subsequently evaluated using the Cochrane approach for risk of bias (ROB) assessment21 (table 1).
Meta-analyses
We conducted meta-analyses in R V.3.4.127 using the DerSimonian–Laird random effects models and the Freeman–Tukey double arcsine transformation,28 29 as listed in the meta package,30 whenever ≥3 measures were available. This methodology was selected as it accounts for sampling variation and heterogeneity in effect size28 (see table 1).
A sensitivity analysis was conducted using logit transformation instead of the Freeman–Tukey double arcsine transformation, in view of the recently identified issue for the latter transformation.31 A second sensitivity analysis was conducted using a multilevel meta-analytic model32 to account for potential dependence in measures extracted from the same study.
Meta-regressions
We regressed log transformed seroprevalence and log transformed GUD/genital herpes proportions in Stata/SE V.13,33 using the metareg package.34 We used log transformation because we needed to estimate the risk ratios and not the odds ratios—HSV-1 seroprevalence is very high and thus the odds ratios may not be as meaningful. Univariable and multivariable random effects meta-regression analyses were conducted to identify sources of between study heterogeneity and predictors of outcomes (table 1). A sensitivity analysis was conducted using a multilevel meta-analytic model32 to account for potential dependence in measures extracted from the same study.
Results
Search results and scope of evidence
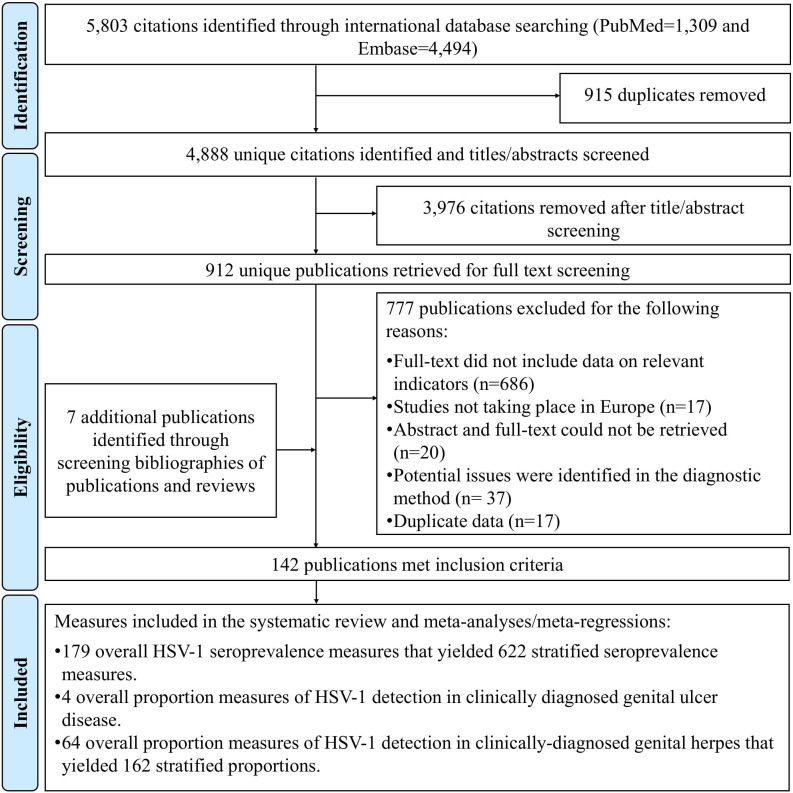
Figure 1 describes the study selection process based on PRISMA guidelines.22 The search identified 5803 citations (PubMed=1309 and Embase=4494). After performing the first two stages of the screening process, 912 citations were relevant or potentially relevant. The full text screening of these citations identified 135 relevant publications, and a further 7 relevant publications were identified through bibliography screening, including country level reports and articles in non-indexed journal.35–41
Figure 1.
Flowchart of article selection for the systematic review of herpes simplex virus type 1 (HSV-1) infection in Europe, according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.22
In total, 142 publications met the inclusion criteria. The extracted outcome measures were: 179 overall and 622 stratified HSV-1 seroprevalence measures; 4 overall proportions of HSV-1 detection in GUD, and 62 overall and 161 stratified proportions of HSV-1 detection in genital herpes.
HSV-1 seroprevalence overview
Online supplementary tables S2, S3 and S4 list the overall seroprevalence measures (number of measures (n)=179). The earliest publication was published in 1972. Studies were mainly cross sectional (n=117; 65.4%) and based on convenience sampling (n=133; 74.3%).
Stratified seroprevalence measures across all studies (n=622) ranged between 0.0% and 100% with a median of 70.0% (table 2). In healthy general populations, HSV-1 seroprevalence ranged between 0.0% and 82.0% with a median of 31.0% among children (n=101), and between 20.0% and 100% with a median of 73.6% among adults (n=402). In clinical populations, HSV-1 seroprevalence ranged between 31.0% and 52.0% with a median of 36.0% among children (n=3), and between 0.0% and 100% with a median of 73.3% among adults (n=59). A summary of HSV-1 seroprevalence measures across various populations and subpopulations is shown in table 2.
Table 2.
Pooled mean estimates for herpes simplex virus type 1 seroprevalence in Europe
| Population | Outcome measure | Sample | HSV-1 seroprevalence | Pooled mean HSV-1 seroprevalence | Heterogeneity measures | |||
| Total N | Total N |
Range | Median | Mean (95% CI) | Q* (p value) |
I²† (%) (95% CI) |
Prediction interval‡ (%) | |
| Healthy general populations | ||||||||
| Children | 101 | 23 948 | 0.0–82.0 | 31.0 | 32.4 (29.2 to 35.6) | 2689.7 (p<0.001) | 96.3 (95.9 to 96.7) | 7.0–65.3 |
| Adults | 402 | 105 523 | 20.0–100 | 73.6 | 73.7 (71.9 to 75.4) | 13 302.3 (p<0.001) | 97.5 (97.4 to 97.7) | 36.0–98.1 |
| Age mixed | 13 | 5985 | 30.4–68.6 | 53.7 | 54.3 (47.4 to 61.0) | 301.1 (p<0.001) | 96.0 (94.5 to 97.1) | 27.4–79.9 |
| All healthy general populations | 516 | 135 456 | 0.0–100 | 68.0 | 65.5 (63.3 to 67.6) | 35 415.7 (p<0.001) | 98.5 (98.5 to 98.6) | 17.8–98.9 |
| Clinical populations | ||||||||
| Clinical children | 3 | 149 | 31.0–52.0 | 36.0 | 37.8 (28.3 to 47.8) | 2.5 (p=0.281) | 21.3 (0.0 to 91.8) | 0.0–100 |
| Clinical adults | 59 | 11 071 | 0.0–100 | 73.3 | 73.8 (68.9 to 78.5) | 1770.0 (p<0.001) | 96.7 (96.2 to 97.1) | 33.3–99.0 |
| All clinical populations | 62 | 11 071 | 0.0–100 | 72.5 | 72.4 (67.4 to 77.2) | 1847.3 (p<0.001) | 96.7 (96.2 to 97.1) | 31.3–98.8 |
| Other populations | ||||||||
| HIV positive patients | 19 | 2493 | 76.0–97.0 | 90.1 | 89.0 (86.3 to 91.5) | 65.2 (p<0.001) | 72.4 (56.4 to 82.6) | 77.2–97.1 |
| Female sex workers | 6 | 1062 | 60.9–99.0 | 78.3 | 83.2 (66.9 to 94.8) | 171.7 (p<0.001) | 97.1 (95.4 to 98.1) | 18.5–100 |
| Men who have sex with men | 10 | 6074 | 52.1–91.0 | 68.0 | 67.0 (59.9 to 73.8) | 286.9 (p<0.001) | 96.9 (95.6 to 97.8) | 39.3–89.5 |
| Prisoners | 9 | 357 | 67.0–86.4 | 77.0 | 80.4 (75.8 to 84.6) | 5.7 (p=0.680) | 0.0 (0.0 to 50.6) | 74.9–84.4 |
| European subregion/country | ||||||||
| Northern Europe | 161 | 47 202 | 13.0–100 | 67.2 | 57.7 (54.4 to 60.9) | 7721.3 (p<0.001) | 97.9 (97.8 to 98.1) | 19.3–91.4 |
| Eastern Europe | 64 | 12 260 | 0.0–100 | 85.5 | 78.7 (74.1 to 83.0) | 2153.3 (p<0.001) | 97.1 (96.7 to 97.4) | 37.8–100 |
| Southern Europe | 77 | 16 063 | 3.6–100 | 81.4 | 77.2 (71.7 to 82.3) | 4677.4 (p<0.001) | 98.4 (98.2 to 98.5) | 24.8–100 |
| Western Europe | 264 | 68 556 | 0.0–95.7 | 67.9 | 66.1 (63.1 to 69.0) | 17 258.0 (p<0.001) | 98.5 (98.4 to 98.5) | 18.8–98.9 |
| Israel | 35 | 7060 | 22.2–94.9 | 67.9 | 64.8 (58.8 to 70.7) | 910.9 (p<0.001) | 96.3 (95.5 to 96.9) | 28.3–93.6 |
| Turkey | 17 | 3076 | 30.4–99.0 | 92.3 | 87.9 (79.8 to 94.2) | 557.2 (p<0.001) | 97.1 (96.3 to 97.8) | 43.4–100 |
| Mixed subregions | 4 | 2295 | 55.3–76.7 | 71.7 | 68.9 (56.9 to 79.8) | 101.7 (p<0.001) | 97.0 (94.8 to 98.3) | 13.5–100 |
| Sex | ||||||||
| Women | 258 | 62 162 | 2.0–100 | 72.1 | 69.5 (66.5 to 72.5) | 16 531.4 (p<0.001) | 98.4 (98.4 to 98.5) | 20.8–99.8 |
| Men | 194 | 49 887 | 7.5–100 | 65.5 | 63.3 (59.7 to 66.7) | 12 372.9 (p<0.001) | 98.4 (98.3 to 98.5) | 16.8–97.9 |
| Mixed sexes | 170 | 44 463 | 0.0–100 | 70.0 | 68.8 (65.3 to 72.2) | 9860.3 (p<0.001) | 98.3 (98.2 to 98.4) | 23.0–99.1 |
| Age group (years) | ||||||||
| <20 | 147 | 32 492 | 0.0–100 | 36.4 | 39.3 (35.9 to 42.7) | 5530.3 (p<0.001) | 97.4 (97.1 to 97.6) | 6.8–78.5 |
| 20–30 | 73 | 13 156 | 32.0–100 | 66.7 | 66.7 (62.0 to 71.1) | 2204.1 (p<0.001) | 96.7 (96.3 to 97.1) | 26.8–96.1 |
| 30–40 | 60 | 9594 | 40.0–96.9 | 73.0 | 72.9 (69.3 to 76.3) | 854.6 (p<0.001) | 93.1 (91.8 to 94.2) | 44.1–94.0 |
| 40–50 | 25 | 5188 | 49.0–96.6 | 76.0 | 74.5 (68.5 to 80.0) | 487.7 (p<0.001) | 95.1 (93.7 to 96.1) | 42.3–96.4 |
| >50 | 47 | 16 363 | 57.1–100 | 84.0 | 82.9 (78.8 to 86.6) | 1546.3 (p<0.001) | 97.0 (96.5 to 97.4) | 52.2–99.6 |
| Mixed | 270 | 79 719 | 0.0–100 | 78.2 | 77.1 (75.1 to 79.1) | 11 355.6 (p<0.001) | 97.6 (97.5 to 97.8) | 41.1–98.9 |
| Year or publication range | ||||||||
| <2000 | 127 | 23 076 | 0.0–100 | 71.6 | 70.0 (66.0 to 73.9) | 4826.5 (p<0.001) | 97.4 (97.2 to 97.6) | 25.2–99.2 |
| 2000–2010 | 361 | 86 175 | 6.0–100 | 72.0 | 69.3 (67.0 to 71.5) | 1891.5 (p<0.001) | 97.1 (98.0 to 98.2) | 24.7–98.8 |
| >2010 | 134 | 47 261 | 0.0–100 | 62.7 | 59.7 (55.1 to 64.3) | 13 668.2 (p<0.001) | 99.0 (99.0 to 99.1) | 11.0–98.2 |
| Age bracket | ||||||||
| All children | 104 | 24 097 | 0.0–82.0 | 31.5 | 32.5 (29.4 to 35.7) | 2698.1 (p<0.001) | 96.2 (95.7 to 96.6) | 7.1–65.4 |
| All adults | 505 | 126 430 | 0.0–100 | 76.0 | 74.4 (72.8 to 76.0) | 19 321.3 (p<0.001) | 97.4 (97.3 to 97.5) | 37.1–98.3 |
| All age mixed | 13 | 5985 | 30.4–68.6 | 53.7 | 54.3 (47.4 to 61.0) | 301.1 (p<0.001) | 96.0 (94.5 to 97.1) | 27.4–79.9 |
| All studies | 622 | 156 512 | 0.0–100 | 70.0 | 67.4 (65.5 to 69.3) | 39 384.9 (p<0.001) | 98.4 (98.4 to 98.5) | 20.2–99.1 |
*The Cochran’s Q statistic is a measure assessing the existence of heterogeneity in pooled outcome measures (here, HSV-1 seroprevalence).
†I2 is a measure assessing the magnitude of between study variation that is due to true differences in HSV-1 seroprevalence across studies rather than sampling variation.
‡Prediction interval is a measure quantifying the 95% interval of the distribution of true HSV-1 seroprevalence around the estimated pooled mean.
HSV-1, herpes simplex virus type 1.
Pooled mean estimates for HSV-1 seroprevalence
Table 2 shows the seroprevalence meta-analyses. Overall pooled mean seroprevalence (across all measures, n=622) was 67.4% (95% CI 65.5% to 69.3%). The pooled mean seroprevalences for healthy (n=101) and for clinical (n=3) children were 32.4% (95% CI 29.2% to 35.6%) and 37.8% (95% CI 28.3% to 47.8%), respectively. The pooled mean for healthy (n=402) and for clinical (n=59) adults was 73.7% (95% CI 71.9% to 75.4%) and 73.8% (95% CI 68.9% to 78.5%), respectively.
Across age groups, pooled mean seroprevalence increased gradually from 39.3% (n=147, 95% CI 35.9% to 42.7%) in those aged <20 years, followed by 66.7% (n=73, 95% CI 62.0% to 71.1%) in 20–30 year olds, 72.9% (n=60, 95% CI 69.3% to 76.3%) in 30–40 year olds, 74.5% (n=25, 95% CI 68.5% to 80.0%) in 40–50 year olds, to 82.9% (n=47, 95% CI 78.8% to 86.6%) in those aged >50 years.
Across European subregions/countries, the pooled mean seroprevalence was lowest at 57.7% (n=161, 95% CI 54.4% to 60.9%) in Northern Europe, followed by 64.8% (n=35, 95% CI 58.8% to 70.7%) in Israel, 66.1% (n=264, 95% CI 63.1% to 69.0%) in Western Europe, 77.2% (n=77, 95% CI 71.7% to 82.3%) in Southern Europe, 78.7% (n=64, 95% CI 74.1%–83.0%) in Eastern Europe and 87.9% (n=17, 95% CI 79.6% to 94.2%) in Turkey.
Heterogeneity was evident in the majority of meta-analyses (p value <0.001; table 2), and affirmed by wide prediction intervals. Variation in seroprevalence was due to true variation in seroprevalence as opposed to sampling variation (I2 >50%). Forest plots for meta-analyses across age groups can be found in online supplementary figure s1. Sensitivity analyses using the logit transformation and the multilevel meta-analytic model generated overall similar results (online supplementary table s5).
Predictors of HSV-1 seroprevalence
Table 3 and online supplementary table s6 show seroprevalence meta-regression analyses. Four multivariable models were conducted due to collinearity between age bracket and age group, as well as between year of publication as a categorical variable and year of publication as a continuous linear term. Each multivariable model included nine eligible variables (yielding a p value <0.1 in the univariable analysis).
Table 3.
Univariable and multivariable meta-regression models for herpes simplex virus type 1 seroprevalence in Europe, with time trend assessed categorically by decade
| Outcome measure | Sample | Univariable analysis | Multivariable analysis* | |||||||
| Model 1* | Model 2† | |||||||||
| Total n | Total N | RR (95% CI) | P value | LR test p value | Adjusted R2 (%) | ARR (95% CI) | P value | ARR (95% CI) | P value | |
| Population characteristics | ||||||||||
| Age bracket | ||||||||||
| Children | 104 | 24 097 | 1.00 | – | <0.001 | 50.16 | 1.00 | – | – | – |
| Adults | 505 | 126 430 | 2.23 (2.07 to 2.4) | <0.001 | 2.11 (1.98 to 2.26) | <0.001 | – | – | ||
| Age mixed | 13 | 5985 | 1.67 (1.38 to 2.02) | <0.001 | 1.43 (1.21 to 1.69) | <0.001 | – | – | ||
| Age group (years) | ||||||||||
| <20 | 147 | 32 492 | 1.00 | – | <0.001 | 48.69 | – | – | 1.00 | – |
| 20–30 | 73 | 13 156 | 1.70 (1.54 to 1.87) | <0.001 | – | – | 1.62 (1.49 to 1.76) | <0.001 | ||
| 30–40 | 60 | 9594 | 1.91 (1.73 to 2.11) | <0.001 | – | – | 1.82 (1.67 to 1.99) | <0.001 | ||
| 40–50 | 25 | 5188 | 1.95 (1.70 to 2.24) | <0.001 | – | – | 1.93 (1.70 to 2.18) | <0.001 | ||
| >50 | 47 | 16 363 | 2.17 (1.94 to 2.42) | <0.001 | – | – | 2.29 (2.06 to 2.53) | <0.001 | ||
| Mixed | 270 | 79 719 | 1.99 (1.86 to 2.13) | <0.001 | – | – | 1.91 (1.79 to 2.04) | <0.001 | ||
| Sex | ||||||||||
| Women | 258 | 62 162 | 1.00 | – | 0.027 | 1.27 | 1.00 | – | 1.00 | – |
| Men | 194 | 49 887 | 0.91 (0.83 to 0.98) | 0.020 | 0.93 (0.87 to 0.98) | 0.006 | 0.91 (0.86 to 0.96) | 0.001 | ||
| Mixed | 170 | 44 463 | 1.01 (0.93 to 1.10) | 0.773 | 0.98 (0.92 to 1.05) | 0.633 | 0.93 (0.87 to 1.00) | 0.039 | ||
| Population type | ||||||||||
| Healthy | 516 | 135 456 | 1.00 | – | <0.001 | 3.71 | 1.00 | – | 1.00 | – |
| Clinical | 62 | 11 071 | 1.16 (1.03 to 1.30) | 0.014 | 1.05 (0.96 to 1.15) | 0.256 | 1.06 (0.97 to 1.16) | 0.179 | ||
| Other | 44 | 9985 | 1.33 (1.16 to 1.52) | <0.001 | 1.24 (1.13 to 1.36) | <0.001 | 1.22 (1.11 to 1.34) | <0.001 | ||
| European subregion/country | ||||||||||
| Northern Europe | 161 | 47 202 | 1.00 | – | <0.001 | 8.78 | 1.00 | – | 1.00 | – |
| Eastern Europe | 64 | 12 260 | 1.42 (1.26 to 1.61) | <0.001 | 1.54 (1.39 to 1.72) | <0.001 | 1.54 (1.39 to 1.72) | <0.001 | ||
| Southern Europe | 77 | 16 063 | 1.34 (1.19 to 1.51) | <0.001 | 1.37 (1.25 to 1.49) | <0.001 | 1.36 (1.25 to 1.49) | <0.001 | ||
| Western Europe | 264 | 68 556 | 1.16 (1.06 to 1.26) | 0.001 | 1.24 (1.16 to 1.32) | <0.001 | 1.20 (1.12 to 1.28) | <0.001 | ||
| Israel | 35 | 7060 | 1.17 (0.99 to 1.37) | 0.053 | 1.29 (1.16 to 1.44) | <0.001 | 1.22 (1.09 to 1.37) | 0.001 | ||
| Turkey | 17 | 3076 | 1.56 (1.26 to 1.93) | <0.001 | 1.37 (1.12 to 1.67) | 0.002 | 1.38 (1.15 to 1.72) | 0.001 | ||
| Mixed subregions | 4 | 2295 | 1.28 (0.84 to 1.95) | 0.239 | 1.14 (0.86 to 1.51) | 0.339 | 1.13 (0.85 to 1.50) | 0.386 | ||
| Country’s income | ||||||||||
| UMIC | 46 | 8893 | 1.00 | – | <0.001 | 3.68 | 1.00 | – | 1.00 | – |
| HIC‡ | 576 | 147 619 | 0.74 (0.65 to 0.84) | <0.001 | 0.92 (0.80 to 1.05) | 0.245 | 0.92 (0.81 to 1.06) | 0.274 | ||
| Study methodology characteristics | ||||||||||
| Assay type | ||||||||||
| Western blot | 52 | 6551 | 1.00 | – | 0.021 | 0.92 | 1.00 | – | 1.00 | – |
| ELISA | 518 | 137 318 | 0.84 (0.74 to 0.96) | 0.011 | 0.94 (0.86 to 1.03) | 0.258 | 1.00 (0.91 to 1.10) | 0.977 | ||
| Others | 52 | 12 643 | 0.80 (0.67 to 0.95) | 0.013 | 0.85 (0.73 to 0.98) | 0.031 | 0.89 (0.77 to 1.03) | 0.131 | ||
| Sample size§ | ||||||||||
| <100 | 46 | 1795 | 1.00 | – | 0.016 | 1.19 | 1.00 | – | 1.00 | – |
| ≥100 | 576 | 154 717 | 0.84 (0.73 to 0.96) | 0.016 | 0.91 (0.82 to 1.01) | 0.090 | 0.94 (0.84 to 1.04) | 0.240 | ||
| Sampling method | ||||||||||
| Probability based | 267 | 67 601 | 1.00 | – | 0.395 | 0.00 | – | – | – | – |
| Non- probability based | 355 | 88 911 | 1.03 (0.96 to 1.11) | 0.395 | – | – | – | – | ||
| Response rate | ||||||||||
| ≥80 | 37 | 11 985 | 1.00 | – | 0.083 | 0.42 | 1.00 | – | 1.00 | – |
| <80 | 94 | 29 203 | 0.84 (0.71 to 0.99) | 0.049 | 0.96 (0.84 to 1.10) | 0.606 | 0.90 (0.79 to 1.02) | 0.123 | ||
| Unclear | 491 | 115 324 | 0.93 (0.80 to 1.08) | 0.326 | 0.98 (0.89 to 1.09) | 0.768 | 0.96 (0.86 to 1.06) | 0.387 | ||
| Year of publication range | ||||||||||
| <2000 | 127 | 23 076 | 1.00 | – | 0.001 | 2.22 | 1.00 | – | 1.00 | – |
| 2000–2010 | 361 | 86 175 | 0.97 (0.88 to 1.06) | 0.482 | 0.89 (0.83 to 0.96) | 0.003 | 0.91 (0.84 to 0.98) | 0.010 | ||
| >2010 | 134 | 47 261 | 0.83 (0.74 to 0.93) | 0.001 | 0.85 (0.78 to 0.93) | <0.001 | 0.87 (0.80 to 0.95) | 0.002 | ||
*Variance explained by the final multivariable model 1 (adjusted R2)=63.80%.
†Variance explained by the final multivariable model 2 (adjusted R2)=63.69%.
‡High income country category includes one measure representing 12 European countries.
§Sample size denotes the sample size of the study population found in the original publication.
ARR, adjusted risk ratio; ELISA, enzyme linked immunosorbent type specific assay; HIC, high income country; HSV-1, herpes simplex virus type 1; RR, risk ratio; UMIC, upper middle income country.
The first model in table 3 included age bracket, sex, population type, European subregion/country, country’s income, assay type, sample size, response rate and year of publication range. This model explained 63.80% of the seroprevalence variation. HSV-1 seroprevalence was 2.11-fold (95% CI 1.98 to 2.26) higher in adults compared with children, and 0.93-fold (95% CI 0.87 to 0.98) lower in men compared with women. Compared with Northern Europe, HSV-1 seroprevalence was 1.24-fold (95% CI 1.16 to 1.32) higher in Western Europe, 1.29-fold (95% CI 1.16 to 1.44) higher in Israel, 1.36-fold (95% CI 1.25 to 1.49) higher in Southern Europe, 1.37-fold (95% CI 1.12 to 1.67) higher in Turkey and 1.54-fold (95% CI 1.39 to 1.72) higher in Eastern Europe. Evidence of a decline in seroprevalence over time was significant. Compared with the years before 2000, HSV-1 seroprevalence was 0.89-fold (95% CI 0.83 to 0.96) and 0.85-fold (95% CI 0.78 to 0.93) lower between the years 2000–2010 and the years after 2010, respectively.
The second model incorporated age group in proxy of age bracket, explained 63.69% of the seroprevalence variation and yielded similar results (table 3). Compared with those aged <20 years, HSV-1 seroprevalence was 1.62-fold (95% CI 1.49 to 1.76) higher in 20–30 year olds, 1.82-fold (95% CI 1.67 to 1.99) higher in 30–40 year olds, 1.93-fold (95% CI 1.70 to 2.18) higher in 40–50 year olds and 2.29-fold (95% CI 2.06 to 2.53) higher in those aged >50 years.
Online supplementary table s6 includes the univariable and multivariable analyses using year of publication as a continuous variable. The results were similar to those observed in the two models listed above. The models showed evidence of a decline in HSV-1 seroprevalence of 0.99-fold (95% CI 0.99 to 1.00) per year.
These analyses were conducted using year of publication instead of year of data collection for completeness—13% of studies had no specified year of data collection. With imputation using year of publication adjusted for median difference with year of data collection, the declining trend did not reach statistical significance. Sensitivity analyses using the multilevel meta-analytic model supported the findings of the baseline analysis results, but some of the effects had wider CI (online supplementary table s7).
HSV-1 isolation in genital ulcer disease and in genital herpes: overview and meta-analyses
Online supplementary table s8 and table 4 summarise the extracted proportion measures of HSV-1 detection in GUD and in genital herpes; table 4 also includes the results of the meta-analyses.
Table 4.
Pooled proportions of herpes simplex virus type 1 virus isolation in clinically diagnosed genital ulcer disease and in clinically diagnosed genital herpes in Europe
| Population | Outcome measure | Sample | Proportion of HSV-1 detection | Pooled proportion of HSV-1 detection | Heterogeneity measures | |||
| Total N | Total N |
Range | Median | Mean (95% CI) |
Q* (p-value) |
I²† (%) (95% CI) |
Prediction interval‡ (%) | |
| Patients with clinically diagnosed GUD | ||||||||
| All patients with GUD | 4 | 800 | 4.7–39.8 | 8.5 | 13.6 (4.1 to 27.1) | 58.1 (p<0.001) | 94.8 (89.0 to 97.4) | 0.0–85.5 |
| Patients with clinically diagnosed genital herpes | ||||||||
| Sex | ||||||||
| Women | 62 | 4933 | 6.0–89.6 | 43.5 | 42.0 (37.4 to 46.7) | 615.3 (p<0.001) | 90.2 (88.0 to 91.8) | 11.6–76.0 |
| Men | 56 | 3578 | 0.0–75.0 | 26.6 | 24.1 (19.8 to 28.6) | 461.1 (p<0.001) | 88.1 (85.3 to 90.3) | 1.8–58.4 |
| Mixed | 44 | 32 570 | 4.6–81.8 | 45.0 | 35.8 (32.1 to 39.6) | 1778.5 (p<0.001) | 97.6 (97.2 to 97.9) | 14.7–60.3 |
| Age group (years) | ||||||||
| <20 | 3 | 157 | 45.4–66.6 | 53.3 | 52.1 (39.1 to 64.9) | 3.2 (p=0.197) | 38.5 (0.0 to 80.8) | 0.0–100 |
| 20–30 | 6 | 643 | 27.3–54.9 | 38.5 | 39.9 (32.2 to 47.8) | 18.3 (p=0.003) | 72.7 (37.1 to 88.1) | 17.1–65.2 |
| 30–40 | 2§ | 102 | 34.0–51.8 | 43.3 | 43.5 (27.4 to 60.3)§ | – | – | – |
| Mixed | 151 | 40 179 | 0.0–89.6 | 33.3 | 33.4 (30.9 to 39.6) | 3497.8 (p<0.001) | 95.7 (95.3 to 96.1) | 9.5–62.8 |
| Genital herpes episode status | ||||||||
| First episode genital herpes | 13 | 1366 | 31.0–75.0 | 49.0 | 49.3 (42.2 to 56.4) | 66.1 (p<0.001) | 81.8 (70.1 to 89.0) | 24.5–74.2 |
| Recurrent genital herpes | 11 | 893 | 1.0–77.3 | 10.0 | 13.7 (5.8 to 24.1) | 152.7 (p<0.001) | 92.5 (90.2 to 95.6) | 0.060.5 |
| Unspecified status | 138 | 38 822 | 0.0–89.6 | 34.7 | 34.7 (32.3 to 37.1) | 2730.0 (p<0.001) | 95.0 (94.4 to 95.5) | 12.3–61.2 |
| European subregion/country | ||||||||
| Northern Europe | 131 | 38 843 | 1.0–89.6 | 34.8 | 35.0 (32.5 to 37.6) | 2854.7 (p<0.001) | 95.4 (95.0 to 95.9) | 12.1–62.2 |
| Eastern Europe | 2§ | 100 | 34.9–63.1 | 49.0 | 49.3 (22.7 to 76.0)§ | – | – | – |
| Southern Europe | 8 | 286 | 0.0–50.0 | 15.4 | 18.2 (7.3 to 23.1) | 39.1 (p<0.001) | 82.1 (66.0 to 90.6) | 0.0–68.9 |
| Western Europe | 5 | 462 | 7.5–37.1 | 25.0 | 21.8 (11.7 to 33.8) | 33.2 (p<0.001) | 88.0 (74.4 to 94.3) | 0.0–70.2 |
| Israel | 4 | 605 | 21.0–72.7 | 54.0 | 50.3 (20.8 to 79.7) | 142.4 (p<0.001) | 97.9 (96.5 to 98.7) | 0.0–100 |
| Mixed subregions | 12 | 785 | 6.0–57.5 | 37.9 | 30.8 (19.5 to 43.4) | 140.4 (p<0.001) | 92.2 (88.2 to 94.8) | 0.0–79.5 |
| Year or publication range | ||||||||
| <2000 | 74 | 5072 | 0.0–81.8 | 34.8 | 33.4 (28.3 to 38.8) | 1099.7 (p<0.001) | 93.4 (92.3 to 94.3) | 1.7–78.1 |
| 2000–2010 | 53 | 8727 | 4.0–77.3 | 25.0 | 29.4 (24.6 to 34.5) | 1196.4 (p<0.001) | 95.7 (94.9 to 96.3) | 3.0–67.1 |
| >2010 | 35 | 27 282 | 18.7–89.6 | 42.8 | 46.0 (43.6 to 48.3) | 280.0 (p<0.001) | 87.9 (84.1 to 90.7) | 35.6–56.5 |
| All patients with genital herpes | 162 | 41 081 | 0.0–89.7 | 34.7 | 34.1 (31.7 to 36.5) | 3529.8 (p<0.001) | 95.4 (95.0 to 95.8) | 10.1–63.2 |
*The Cochran’s Q statistic is a measure assessing the existence of heterogeneity in pooled outcome measures (here, proportions of HSV-1 virus isolation).
†I2 is a measure assessing the magnitude of between study variation that is due to true differences in proportions of HSV-1 virus isolation across studies rather than sampling variation.
‡Prediction interval is a measure quantifying the 95% interval of the distribution of true proportions of HSV-1 virus isolation around the estimated pooled mean.
§No meta-analysis was done as number of studies was <3. The two study samples were merged to yield one sample size, for which the 95% CI was calculated.
GUD, genital ulcer disease; HSV-1, herpes simplex virus type 1.
In GUD cases (n=4), proportion measures ranged between 4.7% and 39.8% with a median of 8.5% and a pooled proportion of 13.6% (95% CI 4.1% to 27.1%). In genital herpes cases (n=162), proportion measures ranged between 0.0% and 89.7% with a median of 34.7% and a pooled proportion of 34.1% (95% CI 31.7% to 36.5%) (table 4).
Among women, proportions of HSV-1 detection in genital herpes ranged between 6.0% and 89.6% with a median of 43.5% and a pooled mean of 42.0% (n=62, 95% CI 37.4% to 46.7%), and among men, between 0.0% and 75.0% with a median of 26.6% and a pooled mean of 24.1% (n=56, 95% CI 19.8% to 28.6%).
In first episode genital herpes cases, proportions of HSV-1 detection ranged between 31.0% and 75.0% with a median of 49.0% and a pooled mean of 49.3% (n=13, 95% CI 42.2% to 56.4%). In recurrent genital herpes cases, proportions ranged between 1.0% and 77.3% with a median of 10.0% and a pooled mean of 13.7% (n=11, 95% CI 5.8% to 24.1%)
Table 4 lists summaries for other population classifications. The majority of meta-analyses showed evidence of heterogeneity with I2 >50%, and large prediction intervals. The two key forest plots (all GUD and all genital herpes) are available in online supplementary figure s2. Sensitivity analyses using the logit transformation and the multilevel meta-analytic model generated overall similar results (online supplementary table s9).
Predictors of HSV-1 detection in genital herpes
Table 5 shows meta-regression analyses for proportion measures of HSV-1 virus isolation in genital herpes. In the univariable analyses, sex, genital herpes episode status, year of publication and year of publication range had a p value<0.1 and thus were included in the multivariable analyses. Two multivariable models were constructed due to collinearity between year of publication range (categorical variable) and year of publication (continuous variable).
Table 5.
Univariable and multivariable meta-regression models for proportion measures of herpes simplex virus type 1 virus isolation in clinically diagnosed genital herpes in Europe
| Outcome measure | Sample | Univariable analysis | Multivariable analysis | |||||||
| Model 1* | Model 2† | |||||||||
| Total n | Total N | RR (95% CI) | P value | LR test p value | Adjusted R2 (%) | ARR (95% CI) | P value | ARR (95% CI) | P value | |
| Age group (years) | ||||||||||
| <20 | 3 | 157 | 1.00 | – | 0.394 | 0.00 | – | – | – | – |
| 20–30 | 6 | 643 | 0.71 (0.29 to 1.71) | 0.447 | – | – | – | – | ||
| 30–40 | 2 | 102 | 0.78 (0.25 to 2.44) | 0.675 | – | – | – | – | ||
| Mixed | 151 | 40 179 | 0.58 (0.28 to 1.21) | 0.147 | – | – | – | – | ||
| Sex | ||||||||||
| Women | 62 | 4933 | 1.00 | – | 0.001 | 11.27 | 1.00 | – | 1.00 | – |
| Men | 56 | 3578 | 0.63 (0.50 to 0.80) | <0.001 | 0.62 (0.50 to 0.76) | <0.001 | 0.61 (0.49 to 0.75) | <0.001 | ||
| Mixed | 44 | 32 570 | 0.63 (0.65 to 1.02) | 0.074 | 0.81 (0.65 to 1.00) | 0.061 | 0.81 (0.66 to 1.01) | 0.067 | ||
| Genital herpes episode status | ||||||||||
| First episode genital herpes | 13 | 1366 | 1.00 | – | <0.001 | 12.19 | 1.00 | – | 1.00 | – |
| Recurrent genital herpes | 11 | 893 | 0.27 (0.17 to 0.47) | <0.001 | 0.29 (0.49 to 0.93) | 0.016 | 0.27 (0.17 to 0.44) | <0.001 | ||
| Unspecified status | 138 | 38 822 | 0.68 (0.49 to 0.95) | 0.026 | 0.67 (0.49 to 0.92) | 0.016 | 0.66 (0.48 to 0.89) | 0.008 | ||
| European subregion/country | ||||||||||
| Northern Europe | 131 | 38 843 | 1.00 | – | 0.397 | 0.61 | – | – | – | – |
| Eastern Europe | 2 | 100 | 1.44 (0.59 to 3.46) | 0.417 | – | – | – | – | ||
| Southern Europe | 8 | 286 | 0.79 (0.44 to 1.40) | 0.424 | – | – | – | – | ||
| Western Europe | 5 | 462 | 0.63 (0.34 to 1.13) | 0.119 | – | – | – | – | ||
| Israel | 4 | 605 | 1.38 (0.74 to 2.60) | 0.304 | – | – | – | – | ||
| Mixed subregions | 12 | 785 | 0.89 (0.60 to 1.34) | 0.600 | – | – | – | – | ||
| Sample sizec‡ | ||||||||||
| <100 | 56 | 3905 | 1.00 | – | 0.591 | 0.00 | – | – | – | – |
| ≥100 | 106 | 37 176 | 1.06 (0.85 to 1.32) | 0.591 | – | – | – | – | ||
| Year of publication range | ||||||||||
| <2000 | 74 | 5072 | 1.00 | – | 0.021 | 3.95 | 1.00 | – | – | – |
| 2000–2010 | 53 | 8727 | 0.86 (0.68 to 1.08) | 0.193 | 0.94 (0.76 to 1.16) | 0.555 | – | – | ||
| >2010 | 35 | 27 282 | 1.26 (0.98 to 1.63) | 0.070 | 1.26 (1.00 to 1.58) | 0.049 | – | – | ||
| Year of publication | 162 | 41 081 | 1.01 (1.00 to 1.02) | 0.043 | 0.043 | 2.57 | – | – | 1.01 (1.00 to 1.02) | 0.014 |
*Variance explained by the final multivariable model 1 (adjusted R2)=26.10%.
†Variance explained by the final multivariable model 2 (adjusted R2)=28.38%.
‡Sample size denotes the sample size of the study population found in the original publication.
ARR, adjusted risk ratio; HSV-1, herpes simplex virus type 1; RR, risk ratio.
The first model, including year of publication range, explained 26.10% of the proportion variation. Compared with women, the proportion of HSV-1 virus isolation in genital herpes was 0.62-fold (95% CI 0.50 to 0.79) lower in men. Genital herpes episode status showed a 0.29-fold (95% CI 0.49 to 0.93) lower proportion of HSV-1 detection in recurrent genital herpes compared with first episode genital herpes. Compared with the years before 2000, evidence of a 1.28-fold (95% CI 1.02 to 1.66) increase in HSV-1 detection in genital herpes was observed in the years after 2010. Similar results were observed when using imputed year of data collection as a categorical term in the model.
The second model, including year of publication as a linear term, had similar results and explained 14.99% of the proportion variation. Evidence of a 1.01-fold (95% CI 1.00 to 1.02) yearly increase in the proportion of HSV-1 detection in genital herpes was observed. Similar results were observed when using imputed year of data collection as a linear term in the model. Sensitivity analyses using the multilevel meta-analytic model supported the findings of the baseline analysis results, but some of the effects had wider CI (online supplementary table s10).
Quality assessment
Online supplementary table s11 summarises the quality assessment of seroprevalence studies (n=179). A total of 149 studies (83.2%) had high precision, 45 studies (25.1%) had low ROB in the sampling methodology domain and 12 studies (6.7%) had low ROB in the response rate domain. Only five studies (2.8%) had low ROB in both quality domains. Despite this, the meta-regression analyses (table 3) did not demonstrate an association between these study quality variables and HSV-1 seroprevalence, suggesting a minimal impact on the findings of this study.
Discussion
This comprehensive systematic review presented a detailed assessment of the epidemiology of HSV-1 in Europe. The results demonstrated that the epidemiology is in transition, with seroprevalence declining by 1% per year (online supplementary table s6), and HSV-1 detection in genital herpes increasing concurrently by 1% per year (table 5). With only two-thirds of the population being seropositive (table 2), HSV-1 seroprevalence in Europe is lower than that in most other regions,6–10 12 and far lower than its historical level of nearly universal childhood infection still seen in other parts of the world,6 9 12 notably Africa.7
The epidemiology of HSV-1 in Europe appears to be traversing a path seen already in North America,17 42 with the declining seroprevalence possibly attributable to the general decrease in both family size and school crowding, as well as improved hygiene.43 44 Although a seroprevalence decline is a positive development, there is cause for concern as a larger proportion of youth (as much as two-thirds) are reaching sexual debut uninfected (table 2), and are thus at risk of acquiring the infection genitally, through (mostly) oral–genital sex or genital–genital sex (genital herpes),4 with a range of psychosexual adverse outcomes, such as effects on sexual relations and quality of life, depression, anxiety and shame.45–48 This outcome is affirmed by the concurrency of seroprevalence decline with HSV-1 genital herpes increase (table 3 and online supplementary table s6 vs table 5).
HSV-1 seroprevalence increased with age, reflecting cumulative exposure, with age alone explaining about 50% of the seroprevalence variation (table 3). This confirms the general global age pattern seen in other regions,6 7 9 12 42 where age typically explained half of the seroprevalence variation,6 9 12 with the notable exception of Africa where age explained 80% of the variation.7 There was also substantial difference in seroprevalence among those aged <20 years and >20 years (Tables 2 and 3), suggesting that older cohorts had higher exposure in their youth, compared with the current young cohort, and affirming the decreasing trend in seroprevalence in recent decades.
Seroprevalence also varied by geographical location, with Eastern and Southern Europe as well as Turkey exhibiting higher seroprevalence rates compared with Northern and Western Europe (tables 2 and 3), possibly mirroring the individual level association between HSV-1 infection and lower socioeconomic status.43 49 This within region variation has also been observed in other regions,6 9 12 17 42 49 with the exception of Africa7 where the infection is homogenous and universal. Seroprevalence in Europe was also lower in men than women, in contrast with all other regions,6 9 12 a finding that remains to be explained. Remarkably, clinical condition and country’s income (after adjustment for European subregion) as well as study characteristics, did not appear to affect seroprevalence (table 3), probably highlighting that HSV-1 is a truly general population infection.
HSV-1 (vs HSV-2) detection in genital herpes was high at 34% and increasing (table 4), similar to that observed in North America,5 11 50 but substantially higher than that observed in other regions.7 9 12 This affirms that Europe has progressed in its epidemiological transition towards less oral acquisition and more genital acquisition, and that HSV-1 infection plays an increasing role as a sexually transmitted disease. Our results also demonstrated that women are more affected by HSV-1 genital herpes than men, possibly reflecting the age gap in sexual mixing, with younger women partnering with older men, or possibly reflecting biological susceptibility differences by sex.51 52 Our results also demonstrated a much higher detection of HSV-1 in first episode genital herpes (at 49%) than in recurrent herpes (at only 10%) (tables 4 and 5), supporting the fact that HSV-2 reactivates (in the genital tract) for a longer duration than HSV-1.47
This systematic review had limitations, primarily the unavailability of data for 25 of 53 European countries, and comparatively less data for GUD and genital herpes than for seroprevalence. For example, we expected to observe variation in HSV-1 detection in genital herpes by age or subregion, but no significant effect was found (table 5), possibly because of an insufficient number of studies. Included studies exhibited heterogeneity (table 2), but most seroprevalence variation (64%) was subsequently explained through meta-regressions (table 3 and online supplementary table s6). Studies differed by assay type, sample size, sampling method and response rate, but none of these study characteristics appeared to affect seroprevalence (table 3 and online supplementary table s6). Thus while these remain theoretical limitations, they do not appear to pose a barrier to the interpretation of the results of the study. It is also important to note that even though this study identified an increasing role for HSV-1 in genital herpes, this possibly could have been caused by a decreasing seroprevalence of the other competing cause, that is HSV-2 infection.
Conclusions
The epidemiology of HSV-1 in Europe is in transition and shifting away from its historical pattern of oral acquisition in childhood. As many as two-thirds of children are reaching sexual debut unexposed to this infection, and at risk of genital acquisition in adulthood. The transition is leading to more heterogeneous and variable transmission by age and geography, and an increasing role for HSV-1 in genital herpes. Seroprevalence is declining by 1% per year, and the contribution of HSV-1 to genital herpes is increasing, also by 1% per year. At present, half of first episode genital herpes is due to HSV-1 as opposed to HSV-2 infection. These findings highlight the importance of disease surveillance and monitoring of HSV-1 seroprevalence and genital herpes aetiology, and strengthen the case for a HSV-1 vaccine to limit transmission.
Acknowledgments
The authors gratefully acknowledge Professor Emeritus Rhoda Ashley Morrow, University of Washington, for her support in assessing the quality of the study diagnostic methods. The authors are also grateful for Ms Adona Canlas for administrative support. This publication was made possible by NPRP grant No 9-040-3-008 from the Qatar National Research Fund (a member of Qatar Foundation). The findings achieved herein are solely the responsibility of the authors. The authors are also grateful for pilot funding by the Biomedical Research Program and infrastructure support provided by the Biostatistics, Epidemiology and Biomathematics Research Core, both at Weill Cornell Medicine, Qatar.
Footnotes
Handling editor: Alberto L Garcia-Basteiro
WY, HI and MH contributed equally.
Contributors: WY, HI, and MH conducted the systematic search, data extraction, data analysis and wrote the first draft of the paper. FAH conducted the double extraction. LA-R conceived the study and led the data analysis and interpretation of the results. All authors contributed to drafting and revision of the article, and read and approved the final manuscript.
Funding: This work was supported by the Qatar National Research Fund (NPRP 9-040-3-008), and through pilot funding by the Biomedical Research Program at Weill Cornell Medicine, Qatar.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. All data are fully available without restriction.
References
- 1. Brady RC, Bernstein DI. Treatment of herpes simplex virus infections. Antiviral Res 2004;61:73–81. 10.1016/j.antiviral.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 2. Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol 2007;57:737–63. 10.1016/j.jaad.2007.06.027 [DOI] [PubMed] [Google Scholar]
- 3. Ramchandani M, Kong M, Tronstein E, et al. Herpes simplex virus type 1 shedding in tears and nasal and oral mucosa of healthy adults. Sex Transm Dis 2016;43:756–60. 10.1097/OLQ.0000000000000522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ayoub HH, Chemaitelly H, Abu-Raddad LJ. Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: model-based predictions. BMC Med 2019;17:57. 10.1186/s12916-019-1285-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis 2013;56:344–51. 10.1093/cid/cis891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaabane S, Harfouche M, Chemaitelly H, et al. Herpes simplex virus type 1 epidemiology in the middle East and North Africa: systematic review, meta-analyses, and meta-regressions. Sci Rep 2019;9:1136 10.1038/s41598-018-37833-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harfouche M, Chemaitelly H, Abu-Raddad LJ. Herpes simplex virus type 1 epidemiology in Africa: systematic review, meta-analyses, and meta-regressions. J Infect 2019;79:289–99. 10.1016/j.jinf.2019.07.012 [DOI] [PubMed] [Google Scholar]
- 8. James C, Harfouche M, Welton NJ, et al. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ 2020;98:315–29. 10.2471/BLT.19.237149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khadr L, Harfouche M, Omori R, et al. The epidemiology of herpes simplex virus type 1 in Asia: systematic review, meta-analyses, and meta-regressions. Clin Infect Dis 2019;68:757–72. 10.1093/cid/ciy562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Looker KJ, Magaret AS, May MT, et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One 2015;10:e0140765 10.1371/journal.pone.0140765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis 2003;30:797–800. 10.1097/01.OLQ.0000092387.58746.C7 [DOI] [PubMed] [Google Scholar]
- 12. Sukik L, Alyafei M, Harfouche M, et al. Herpes simplex virus type 1 epidemiology in Latin America and the Caribbean: systematic review and meta-analytics. PLoS One 2019;14:e0215487 10.1371/journal.pone.0215487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lowhagen G-B, et al. First episodes of genital herpes in a Swedish STD population: a study of epidemiology and transmission by the use of herpes simplex virus (HSV) typing and specific serology. Sex Transm Infect 2000;76:179–82. 10.1136/sti.76.3.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nilsen A, Myrmel H. Changing trends in genital herpes simplex virus infection in Bergen, Norway. Acta Obstet Gynecol Scand 2000;79:693–6. [PubMed] [Google Scholar]
- 15. Samra Z, Scherf E, Dan M. Herpes simplex virus type 1 is the prevailing cause of genital herpes in the TEL Aviv area, Israel. Sex Transm Dis 2003;30:794–6. 10.1097/01.OLQ.0000079517.04451.79 [DOI] [PubMed] [Google Scholar]
- 16. Gilbert M, Li X, Petric M, et al. Using centralized laboratory data to monitor trends in herpes simplex virus type 1 and 2 infection in British Columbia and the changing etiology of genital herpes. Can J Public Health 2011;102:225–9. 10.1007/BF03404902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chemaitelly H, Nagelkerke N, Omori R, et al. Characterizing herpes simplex virus type 1 and type 2 seroprevalence declines and epidemiological association in the United States. PLoS One 2019;14:e0214151. 10.1371/journal.pone.0214151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Broutet N, Fruth U, Deal C, et al. Vaccines against sexually transmitted infections: the way forward. Vaccine 2014;32:1630–7. 10.1016/j.vaccine.2014.01.053 [DOI] [PubMed] [Google Scholar]
- 19. Gottlieb SL, Deal CD, Giersing B, et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: update and next steps. Vaccine 2016;34:2939–47. 10.1016/j.vaccine.2016.03.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gottlieb SL, Giersing B, Boily M-C, et al. Modelling efforts needed to advance herpes simplex virus (HSV) vaccine development: key findings from the World Health Organization consultation on HSV vaccine impact modelling. Vaccine 2019;37:7336-7345. 10.1016/j.vaccine.2017.03.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins J, Green S. Cochrane Handbook for systematic reviews of interventions. John Wiley & Sons, 2011. [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization WHO regional offices, 2017. Available: http://www.who.int/about/regions/en/ [Accessed on September, 2019].
- 24. World Atlas "Europe Countries and Regions.". Available: www.worldatlas.com/articles/the-four-european-regions-as-defined-by-the-united-nations-geoscheme-for-europe.html [Accessed on September, 2019].
- 25. Ashley-Morrow R, Nollkamper J, Robinson NJ, et al. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect 2004;10:530–6. 10.1111/j.1469-0691.2004.00836.x [DOI] [PubMed] [Google Scholar]
- 26. Ashley RL. Performance and use of HSV type-specific serology test kits. Herpes 2002;9:38–45. [PubMed] [Google Scholar]
- 27. RStudio Team RStudio: Integrated Development for R : RStudio, Inc. Boston, MA, 2015. http://www.rstudio.com/ [Google Scholar]
- 28. Borenstein M H L, Higgins JPT, Rothstein HR, et al. Front matter, in introduction to meta-analysis. Chichester, UK: John Wiley & Sons, Ltd, 2009. [Google Scholar]
- 29. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist 1950;21:607–11. 10.1214/aoms/1177729756 [DOI] [Google Scholar]
- 30. Schwarzer G. Meta: an R package for meta-analysis. R news 2007;7:40–5. [Google Scholar]
- 31. Schwarzer G, Chemaitelly H, Abu‐Raddad LJ, et al. Seriously misleading results using inverse of Freeman‐Tukey double arcsine transformation in meta‐analysis of single proportions. Research Synthesis Methods 2019;10:476–83. 10.1002/jrsm.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Assink M, Wibbelink CJM. Fitting three-level meta-analytic models in R: a step-by-step tutorial. Quant Method Psychol 2016;12:154–74. 10.20982/tqmp.12.3.p154 [DOI] [Google Scholar]
- 33. StataCorp Stata statistical software: release 14. College Station, TX: StataCorp LP, 2015. [Google Scholar]
- 34. Harbord RM, Higgins JPT. Meta-regression in Stata. Stata J 2008;8:493–519. 10.1177/1536867X0800800403 [DOI] [Google Scholar]
- 35. Arvaja M, Lehtinen M, Koskela P, et al. Serological evaluation of herpes simplex virus type 1 and type 2 infections in pregnancy. Sex Transm Infect 1999;75:168–71. 10.1136/sti.75.3.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barton IG, Kinghorn GR, Walker MJ, et al. Association of HSV-1 with cervical infection. Lancet 1981;318:1108 10.1016/S0140-6736(81)91304-0 [DOI] [PubMed] [Google Scholar]
- 37. Potts A, Wallace L, Shaw Primrose L, et al. Genital herpes simplex, genital Chlamydia and gonorrhoea infection in Scotland: laboratory diagnoses 2004-2013 : Genital herpes simplex, genital Chlamydia and gonorrhoea infection in Scotland. Glasgow (Scotland): Health Protection Scotland, 2014. [Google Scholar]
- 38. Potts A, Wallace L, Shaw Primrose L, et al. Genital herpes simplex, genital Chlamydia and gonorrhoea infection in Scotland: laboratory diagnoses 2005–2014. Genital herpes simplex, genital chlamydia and gonorrhoea infection in Scotland Glasgow (Scotland): Health Protection Scotland, 2015. [Google Scholar]
- 39. Rodgers CA, O'Mahony C. High prevalence of herpes simplex virus type 1 in female anogenital herpes simplex. Int J STD AIDS 1995;6:144 10.1177/095646249500600218 [DOI] [PubMed] [Google Scholar]
- 40. Van Doornum GJ, Slomka MJ, Buimer M, et al. Comparison of a monoclonal antibody-blocking enzyme-linked immunoassay and a strip immunoblot assay for identifying type-specific herpes simplex virus type 2 serological responses. Clin Diagn Lab Immunol 2000;7:641–4. 10.1128/CDLI.7.4.641-644.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilson P, Cropper L, Sharp I, et al. Apparent increase in the prevalence of herpes simplex virus type 1 genital infections among women. Sex Transm Infect 1994;70:228–28. 10.1136/sti.70.3.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McQuillan G, Kruszon-Moran D, Flagg EW. Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14–49: United States, 2015–2016 : NCHS data brief, no 304. Hyattsville, MD: National Center for Health Statistics, 2018. [PubMed] [Google Scholar]
- 43. Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2--United States, 1999-2010. J Infect Dis 2014;209:325–33. 10.1093/infdis/jit458 [DOI] [PubMed] [Google Scholar]
- 44. Korr G, Thamm M, Czogiel I, et al. Decreasing seroprevalence of herpes simplex virus type 1 and type 2 in Germany leaves many people susceptible to genital infection: time to raise awareness and enhance control. BMC Infect Dis 2017;17:471. 10.1186/s12879-017-2527-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mindel A, Marks C. Psychological symptoms associated with genital herpes virus infections: epidemiology and approaches to management. CNS Drugs 2005;19:303–12. [DOI] [PubMed] [Google Scholar]
- 46. Mark H, Gilbert L, Nanda J. Psychosocial well-being and quality of life among women newly diagnosed with genital herpes. J Obstet Gynecol Neonatal Nurs 2009;38:320–6. 10.1111/j.1552-6909.2009.01026.x [DOI] [PubMed] [Google Scholar]
- 47. Gupta R, Warren T, Wald A. Genital herpes. Lancet 2007;370:2127–37. 10.1016/S0140-6736(07)61908-4 [DOI] [PubMed] [Google Scholar]
- 48. Fisman DN. Health related quality of life in genital herpes: a pilot comparison of measures. Sex Transm Infect 2005;81:267–70. 10.1136/sti.2004.011619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis 2002;186 Suppl 1:S3–28. 10.1086/343739 [DOI] [PubMed] [Google Scholar]
- 50. Dabestani N, Katz DA, Dombrowski J, et al. Time trends in first-episode genital herpes simplex virus infections in an urban sexually transmitted disease clinic. Sex Transm Dis 2019;46:795–800. 10.1097/OLQ.0000000000001076 [DOI] [PubMed] [Google Scholar]
- 51. Fleming DT, McQuillan GM, Johnson RE, et al. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med 1997;337:1105–11. 10.1056/NEJM199710163371601 [DOI] [PubMed] [Google Scholar]
- 52. Garland SM, Steben M. Genital herpes. Best Pract Res Clin Obstet Gynaecol 2014;28:1098–110. 10.1016/j.bpobgyn.2014.07.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2020-002388supp001.pdf (2.3MB, pdf)