Abstract
World is facing two major problems, day by day demand of energy and pollution on the planet increasing with the advancement of human activities. These are real problems not only for developing countries but also for developed civilization. Present energy sources are not enough to fulfill the demand of modern world these sources are limited and number of side effects from these. Other major problem pollution that is discussed in this article, very alarming number of population every year affected from pollution and death rate from pollution is very high. In this article, briefly review how photocatalytic technique help us to resolve these problem by environmental friendly, cost effective, less energy consumption and minimum side effect approach. This article cover the main concept about photo-catalysis technique and its related terms. The main feature of efficient photocatalytic activity is selection of photo-catalyst, briefly presentation for which types of nanomaterials are suitable for cost effective and efficient catalytic activity. An overview of application of photocatalytic activity for waste water splitting for H2 production, waste water treatment and air disinfection, which types of catalyst are for these application and briefly discussed factor affecting the catalytic activity.
Keywords: Energy, Environmental Issues, Photocatalysis, Pollution, Water splitting
Introduction
Pollution links to climate change and over population, and even COVID-19 are situations shared in both the developed and under-developed world. Second main problem now world faced that is energy crises, day by day with the increase of population demand of energy consumption increased but world have limited natural energy resources, According to World Energy Council in 2030 global energy demand compared to 2010 will increased 35% (pessimistic variant) or 45%–60% (optimistic variant). According to International Energy Agency (IEA) in 2000, 205 million barrel oil were consumed but in 2030 this will be increased as compared to 2010 1.5 time, in 2030 approximately 334 million barrel will be required (Tvaronavičienė et al., 2020). This is alarming situation for the world, we must focused on renewable, environmental friendly, long life energy resources. In-short world major problem pollution (water pollution, air pollution) and energy crises. In pollution there were in different form, such as water pollution, air pollution, land pollution, light pollution and noise pollution but in all of them water and air pollution cause more damage our environment, climate and all the living things, even death from these two in the world is very high. World focused on solution of these critical problem without solution survival of people on this planet very difficult, if world faced energy crises on future than very difficult to fulfill the demand of energy with present energy resources. On the other hand one of the biggest problem is pollution, from pollution more people died as compared to violence and war approximately 9 million people every year died from pollution according to WHO. Need of the world is fresh water and fresh air. What is the solution of all of these? Which method or experimental technique provided us green environmental friendly solution of all of these problem or not all the solution of these problem but at least some solution of these critical problem? “Photocatalytic technique” provided us some ground breaking results of above disused problem. We discussed, our need is environmental friendly, low cost, long life, renewable energy resource, pollution free, affordable solution. In energy sector photocatalytic technique provided us environmental friendly, long life hydrogen H2 production solution and in pollution this technique provided us environmental friendly and with no side effect solution of water treatment and air treatment.
Accelerated photo-reaction in the presence of a catalyst is called photo-catalysis. In the reaction, light is absorbed by the substrate. Reaction rate or activity of catalyst depends on the ease by which the catalyst is creating electron-hole pair. These electron-hole pairs react with the substrate to produce free radical. Secondary reactions takes place, these radicals reacts with reactant (water in our case) to produce different useful products. First Photo-catalysis was reported in back 1911, by a German chemist who used the technique to bleach dark blue pigment by using Zinc Oxide in the presence of sun light. His name was Dr. Alexander Eibnor. In 1913, two scientists, Kozak and Bruner wrote an article in which they discussed the breakdown of oxalic acid using a photo-catalyst i.e., uranyl salts. Landau described this process in his article. Then in 1921, photo-catalysis was used to formaldehyde using a photo-catalyst (ferric hydro-oxide and colloidal salts of uranium) (Hisatomi and Domen, 2019). In 1938, there was a major breakthrough in the photo-catalysis. TiO2 was discovered. It was highly stable metal oxide. It was used as a photo-catalyst for bleaching dyes. TiO2 is a non-toxic compound. Bleaching takes place under UV light in the presence of oxygen. Active Oxygen is produced by the catalyst in the presence of UV light, this active oxygen then react with the organic chemicals of diy to bleach it out. This was in fact the first time when we came to know that photocatalysis can be used as a water purification technique (Yatmaz et al., 2017). From 1938–1963, no work was done on this topic because there was a little interest in this field. But in 1964, photo oxidation of isopropanol was observed by TiO2 and ZnO. This was the work of V.N. Filimonov. In 1965, oxidation of organic solvents from Zinc Oxide was reported. Another major breakthrough took place in 1972, when Akira Fujishima and kenichi Honda reported the electrochemical photolysis of water using platinum and titanium di-oxide electrodes. UV light was absorbed during this process. End product was evolution of Hydrogen at cathode. It was the cleanest and cost effective method of producing hydrogen to meet our energy requirements (Wang et al., 2017, Hodges et al., 2018). Photo-catalysis is also very effective weapon against the water pollution. In photo-catalysis, a photo-catalyst decomposes the organic pollutants in the presence of light. In short photocatalytic reaction was take place in presence of light (Sunlight) and catalyst, produce free radical these radical react with pollutant molecule and decompose them. Photocatalytic reaction help us in energy sector and against pollution but here we need to increase the efficiency of photocatalytic reaction, instead of bulk material as a catalyst we can use nanotechnology to improve the efficiency of photocatalytic reaction.
Nanotechnology is new and emerging field of technology that deal at nanoscale less than 100 nm. From last century it becomes the field of research since the first lecture Richard Feynman in 1959 on “There’s Plenty at the Bottom” (Reiss and Hütten, 2005) Nanotechnology provided us ground-breaking results in every field of research. Basically, in nanotechnology we study materials at nanoscale 109, materials at bulk scale show different properties and at nanoscale different extraordinary properties, at nanoscale with size their surface to volume ratio increase. This surface to volume ratio property especially as a catalyst increase efficiency hundred percent as compared to bulk materials. In energy sector, for hydrogen production in water splitting process by using nanomaterials as a catalyst we can increase the efficiency of H2 production. In pollution, especially for water pollution nanotechnology for clean water is changing the business landscape in both the developed and the developing world. Nanomaterial have remarkable properties such as photo catalytic in nature, high surface area, highly reliable, high aspect ratio, electrostatic, compressible without change in surface area, tunable pore volume, magnetic, short intra-particle diffusion distance, hydrophobic and hydrophilic etc. High surface to volume ratio property of nanomaterials control the interaction with bacteria and pollutant. Nanotechnology help us to resolve the existing problem of conventional water purification method. Microbes in water are just thousand nanometer small while ordinary purifiers just lead with bacteria purifiers using nanotechnology are able to deal with virus without using any chemical, high temperature crushes and electricity. Photo-Impetuses and their studies on different fields is indicated in following studies (Shahzad et al., 2019, 2020; Sagir et al., 2014; Tahir et al., 2018a, Tahir et al., 2018b, Tahir et al., 2018c, Tahir et al., 2019a, Tahir et al., 2019b, Tahir et al., 2019c, Tahir et al., 2019d, Tahir et al., 2019e, Tahir et al., 2019f, Tahir and Sagir, 2019g, Tahir et al., 2019h, Tahir et al., 2020a, Tahir et al., 2020b, Tahir et al., 2020c, Tahir et al., 2020d).
General Mechanism for Photocatalytic Reaction
In photo-catalysis, a photo catalyst is used. Reaction rate mainly depends on the crystal structure of catalyst and the energy of incoming photons of visible or UV light. As a catalyst which materials are used, they acts as a sensitizer for the irradiation of light-stimulated redox processes depending on their electronic structure. Electronic structure is described by the filled valence and vacant conduction band.
If the band gap of the catalyst is equivalent or less than the energy of incident light, the electrons residing in valence band will absorb the photon and they will reach to the conduction band. Holes are left in valence band. They play the key role, Donor molecules are oxidized by these holes, also the hydroxyl (strong oxidizer) is produced when H2O react with these holes. Electron present in conduction band is absorbed by water to make superoxide ion, which is a reducing agent. So we can say that, this free electron is causing redox reactions to occur. These pairs of free electrons and holes can perform oxidation reduction reaction with any material which comes in contact with the catalyst and convert it into the desired products.
Oxidation Mechanism
When light is irradiated on the catalyst, electron hole pair is generated by the promotion of valance electron to the conduction band. The hole created on the catalyst captures water molecule to oxidize it into hydroxyl radical which has very strong oxidizing powers. If any organic pollutant is their react with these hydroxyls and then decomposes. If the whole process takes place in the presence of oxygen, than chain reactions takes place between organic molecule’s intermediate radicals and oxygen molecules. The end product is CO2 and H2O in case of organic pollutant.
Reduction Mechanism
Reduction of atmospheric oxygen occurs in the form of pairing reaction. Oxygen is easily reducible, so its reduction is an alternative to production of hydrogen. The electrons of conduction band reacts with oxygen to form superoxide ion. The anion can also attach themselves with the intermediate in oxidation reaction producing peroxides and then change into water. Reduction process can easily take place in Organic matter as compared to water. That’s why, high concentration of organic matter enhances the photocatalytic activity by increasing the probability of number of holes which eventually reduces the recombination rate of the carriers as shown in Fig. 1 .
Fig. 1.
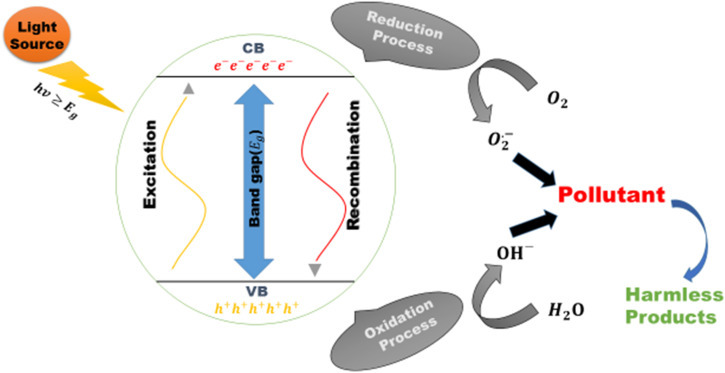
General reaction mechanism for photocatalytic process.
Material Selection for Nanomaterials as a Photo-Catalyst
In photocatalytic reaction, oxidation and reduction reaction occur simultaneously. We need such kind of materials or those catalyst for photocatalytic reaction which support both oxidation and reduction reaction. In general, on the base of electronic properties, materials are divided in three basic categories conductor, insulator and semiconductor.
In case of conductor, valence band and conduction band is overlap. For photocatalytic reaction necessary condition is oxidation and reduction simultaneously, but in conduction only free electron are available. Form conductor, we perform only oxidization reaction at a time not both reaction simultaneously. Best conductor are alkali, alkaline earth metals and transition metals. They have no suitable band gap or mostly they were overlap with each other in conduction and valence band. For catalytic activity they were not suitable for reaction.
In case of insulator, they have high band gap then high energy required to perform oxidation and reduction reaction. We cannot split water molecule by using insulator as a catalyst or high energy is required. We need, that type of catalyst which activates in visible region or in the ultraviolet region. Moreover, insulator is deficient of free electron so no oxidation take place that’s why insulator are not suitable for photolytic reaction. All gases in periodic like halogen and noble gases are insulator. Which were not suitable for the photocatalytic reaction.
In case of semiconductor, which have moderate band gap and they have capabilities of oxidation and reduction perform or support simultaneously. When light falls, free electron hole pairs is generated. Necessary condition for a semi-conductor to be a photo-catalyst, is the low recombination rate. Moreover, those semiconductor whose absorption wavelength (350–700 nm) in visible region or band gap in (1.5–3.5 eV) suitable for photocatalytic activity. Because they perform catalytic activity in the visible light. Generally semiconductors have a wide range of band gap but for a photo catalyst of UV visible region, we require only 1.5–3.5 eV bandgap semiconductor. Metal oxide generally falls in this category. Also they have some other properties which make them suitable as photo catalyst which are discussed below (Mano et al., 2015, Giannakis et al., 2017).
Metal oxides are very important for different electronic applications and in photo-catalysis. They full fill all of our requirements as a photo-catalyst. Metal oxides have auspicious light absorption, electronic structure, band gap and carrier transportation, which makes them suitable for this job. Most important and fundamental property a photo-catalyst should have, is its band gap. Band Gap should be in the range of UV–visible range for low cost. Other properties are stability of the structure. Morphology, reuse-ability, high surface area etc. Metal oxides for example oxides of chromium, zinc, vanadium, cerium and titanium have all these properties. So they are used as photo-catalysts. When photo-catalyst is exposed to the visible light, it absorbs the photon and electron in valance shell is excited the upper conduction band creating an electron and hole pair. This e−/h+ pair causes the redox reaction to occur on the surface of the metal oxide which decomposes the pollutants. That’s why metal oxide is used mostly as photo-catalyst (Giannakis et al., 2017).
On the bases of nanomaterials morphology, size, chemical and physical properties nanomaterials are classified in different categories such as carbon based, metal, ceramic, semiconductor and polymeric nanoparticles.
In carbon based nanoparticles divided into two main categories carbon-nanotubes and carbon-fullerenes. Carbon-nanotubes (CNTs) are basically graphene sheet that are rolled in the form of tubes. Graphene tubes are hundred time stronger than steel. Furthermore, CNTs are further divided in single wall carbon-nanotubes (SWCNTs) and multiwall carbon-nanotubes (MWCNTs), these tubes have specific property thermally-conductive along the length wise while the insulator across the tube. Second category of carbon based nanoparticles are carbon-fullerenes, these nanoparticles are basically allotropes of carbon, contain sixty or more than sixty carbon atoms and their structure like hollow football called Buckminsterfullerene (C-60). Due to their high electron affinity, strength, electrical-conductivity and structure have many commercial applications. Here, over focus is role of carbon based nanomaterials in photocatalytic application, they are used as a hydrogen production, treatment of organic and in-organic pollutant, environmental pollutant and photocatalytic disinfection. C60/g-C3N4.,TiO2@MWCNTs, C60/Bi2TiO4F2 reported against degradation of organic pollutant MB and RhB (Bai et al., 2014, Zhang et al., 2017, Li et al., 2013). Similarly C60-modified, MWCNT and CNF-TiNT used degradation of inorganic pollutant (Ju et al., 2017, Léonard et al., 2016, Kim et al., 2014).
Carbon based nanoparticles have application as a photo-catalyst but there is some limitation due to which these were not use commercially. These were very costly as a catalyst in photocatalytic reaction very difficult to used, we need at a time low cost and high efficient catalyst, different types of pollutant present in water or air such organic, inorganic, heavy metals etc., but these nanoparticles focused only single pollutant at a time or confined to aqueous pollutant (Dasgupta et al., 2017, Di et al., 2015, Han et al., 2014). Another limitation during the hydrogen production by using these photo-catalyst is H2 separation from O2, separation is achieved by using O2 trapping agent then cost of whole setup is increased (Li et al., 2017).
Ceramic nanoparticles are in-organic solid particles, they are made up of from oxide phosphate and carbonates. These types of nanoparticles have high chemical inertness and heat-resistance. These particles have applications in photocatalytic activity, photo-degradation of drug delivery, dyes and imaging. By controlling their shape, size. Surface to volume ratio and porosity make them good drug delivery agent (Thomas et al., 2015, Moreno-Vega et al., 2012) Some ceramic nanoparticle are semiconductor, in these semiconductor most of these are transition metal oxide which are suitable photo-catalyst for photocatalytic application. For example ceramic nanoparticles such as ZnO, TiO2 show lot application in photocatalytic process (Murugan et al., 2014, Chu et al., 2010, Hussain et al., 2010, Liu et al., 2011).
Metal nanoparticles include pure metal such as gold, platinum, silver, iron, zinc, cerium, thallium, nickel, cobalt etc., their compound contain hydroxide, oxide, chloride, phosphate, sulfides and fluoride of these metal. These nanoparticles are prepared from precursors of metals and synthesized by different methods such as photochemical, chemical and electrochemical etc., mostly metal nanoparticles band gap lies in infrared region and ultraviolet region not suitable as a photo-catalyst. Silver nanoparticles as a photo-catalyst show photocatalytic application band gap in visible region (Roy et al., 2015a, Roy et al., 2015b, Yang et al., 2013). Semiconductor nanoparticles found in group IV (Si, Ge), group VI (Se,Te) elements and group III–V, II–VI, I–VII, IV–VI, V–VI, II–V compound GaN, GaO, ZnS, CdSe, ZnO, TiO2, Mgo, AgO etc. Their properties like metal and non-metal, they have wide band gap by varying this show different properties. Semiconductor nanoparticles are best suitable photo-catalyst for photocatalytic application due to wide band and remarkable catalytic application, especially their band gap in visible region make them suitable for photocatalytic application (Liqiang et al., 2006, Marschall, 2014) Polymeric nanoparticles another category of nanoparticles, these are organic nanoparticles in nature. These nanoparticles structural shape like nano-spheres or nano-capsular, depend upon the synthesis techniques. Nano-capsular particles morphology like core shell, where nano-sphere have matrix like structure. These categories of nanoparticles have mostly applications in medical field in drug delivery and diagnoses (Elsabahy and Wooley, 2012, Guterres et al., 2007).
Application of Photo-Catalyst
Water Splitting
Photo catalysis can be applied for the production of hydrogen gas which is a potential energy source. Hence the energy extracted by this method is low cost and also it produces no harmful side products which could possibly pollute our environment. That’s many researchers are working on this method of obtaining clean energy. First time water splitting was discovered in 1972 by using TiO2 as a catalyst but that time gain less attention due to low efficiency, in 1973 oil crises and in 1979 energy crises in world cause lot of attention water splitting photocatalytic reaction (Boyjoo et al., 2017). When we discussed 21st century crises then energy is one of them, in present world we need such kind of energy resource which can be exchangeable with already available natural energy resources because number of problem with available energy sources most important in them is these resources are not long time available. Photocatalytic process is less energy consumption, long life and green process for hydrogen production. It work under the natural solar light and photo-catalyst.
Mechanism of photo-catalytic hydrogen production
Generating hydrogen from the process of photo-catalysis primarily depends on the photo-catalyst. Mainly, we use semiconductor metal oxides as photo-catalyst e.g., TiO2, ZnO, Ag2O etc. Reaction basically starts from a photon striking activating the catalyst. It will excite it and produce a free electron-hole pair which will initiate the reaction. Creation of electron-hole pair requires some specific properties of photo-catalyst. But the most important one is the Band Gap represented by Eg. In semiconductors, electrons and hole reside in valance band when exposed to photons having energy equal to or more than the band gap, excitation takes place promoting electron to the conduction band. The general reaction is
| (1) |
These electrons and holes generated by photon are most likely to recombine instantly releasing energy via heat or photon. Recombination takes place on surface of semi-conductor.
Electrons and holes that survives i.e., which did not recombined while reaching to the surface of catalyst reduces or oxidizes reactants. Electrons reduces the reactant and holes perform oxidation. Reduction reaction is the base of hydrogen evolution. Other key things includes the valence band and conduction band level. For efficient production of Hydrogen, conduction band of catalyst should be more negative as compared to production level of Hydrogen (). Similarly, valence band needs to be more positive than oxidation level of water () for evolution of oxygen.
Mechanism of hydrogen production by photo-catalysis is shown in Fig. 2 . Any semiconductor fulfilling the requirements mentioned above, can be used for hydrogen evolution by photo-catalysis. However, some semiconductors have a property of photo-corrosion which makes them imperfect for the photo-catalysis. Having high chemical stability, high catalytic activity and effective photo electron hole separation makes TiO2 an ideal photo-catalyst for evolution of Hydrogen. But still, the efficiency of converting solar energy to hydrogen by TiO2 is very low. Main factors for this low efficiency rate are.
-
•
Recombination rate: Electrons and holes generated by photons by activating catalyst have a huge tendency of recombining and releasing the excess energy in the form of photons and unproductive heat.
-
•
Reversible reaction: Splitting water into oxygen and hydrogen causes an increase in energy. Due to this increase of energy, the reverse reaction i.e., recombination easily proceeds. In the end, we get lower yield of our desired end product.
-
•
Visible light: TiO2 band gap lies in UV region and in sunlight, only 4% is UV whereas 50% of visible light stays untouched. This inability of utilizing visible light is a key factor in limiting the efficiency of the photo-catalytic process (Ni et al., 2007).
Fig. 2.

General mechanism for water splitting using TiO2 as a catalyst.
A lot of work has been done to resolve the problems mentioned above. Adding electron donors and carbonate salts as whole scavenger improves the efficiency. Several other techniques have been used to improve enhance the hydrogen production e.g., metal ion-implantation, anion doping, composite semiconductors etc.
Factors affecting photo-catalytic activity
Band gap energy
Energy difference of conduction and valence band of Photo-catalyst or semiconductor metal oxide is represented by Band Gap (Eg). To make hydrogen evolution feasible, thermodynamic potential of conduction band should be higher than potential level of acceptor. Band gap is responsible for charge separation to reduce recombination rate and activity under a specific wavelength. General intentions are to decrease the band gap so that photo-catalyst can be activated in visible region. This will enhance the efficiency of hydrogen production. It is reported that doping (metal or non-metal) reduces the band gap into the visible region (Bafaqeer et al., 2019, Tahir and Amin, 2013).
Surface area/structure
One method of lowering the band gap is by altering the surface texture of catalyst. Photo-catalytic activity is also affected by the different Crystal structures and surface area. Larger surface area contains higher number of active sites increasing the likelihood of reaction to take place. Moreover, for small size particle, it is easy for photo electron and hole to reach the surface by traveling shorter distance decreasing the recombination rate. This enhances the activity of a photo-catalyst (Wang and Gong, 2015, Zuo et al., 2014, Police et al., 2014).
Light intensity
Intensity of irradiated light is a key factor for the photo-catalytic efficiency since it has two regimes. Generally, activity is improved by increasing intensity of irradiated light having energy approximately equal or greater than band gap energy or threshold energy because the rate of forward reaction i.e., consumption of photo electron and hole to form hydrogen and oxygen, is higher than the backward reaction. This is the case of first regime. In the second regime, it is a half order reaction. Activity is decreased by increasing the intensity because the rate of backward reaction is greater than the forward reaction. We gain lesser hydrogen in the end (Puga, 2016, Al-Hamdi et al., 2017).
Temperature
Temperature enhances the photo-catalytic activity by facilitating the production of electron hole pair. At higher temperature, desorption of products from catalyst surface enhances the activity. Because it gives opportunity for more electron hole pairs to generate which are then utilized for oxidation and reduction reaction. At lower temperature, desorption rate of products decreases and it lowers the photo-catalytic activity (Ahmad et al., 2015, Dubey et al., 2014).
pH
Hydrogen production by water splitting depends on concentration of proton i.e., pH of solution, because the photo-generated electron reduces proton. Mostly in reported paper production rate of H2 is basic medium is large as compared to acidic mostly H2 in strong basic medium is large (pH>10) (Wu et al., 2009, Liu et al., 2018, Brahimi et al., 2007, Maeda, 2014). However, Nada et al. (2008) reported in their paper production of H2 in acidic medium is large as compared to basic medium, in case of strong acidic medium more H+ are absorbed on photo-catalyst then possibility of reduction of H+ to H2 by e- will be increased, they use TiO2/RuO2-MV2+ photo-catalyst in methanol water mixture. Overall at strong basic medium production of H2 are maximum (Fajrina and Tahir, 2019).
Waste Water Treatment
Our earth covered with water two-third of its surface almost it is 330 million cubic mile of water but we need fresh water. Only of water on our planet is fresh. Of that only surface water and rest of water is frozen or underground. One in six people on the planet do not have access to clean drinking water. World population is increase exponentially every year million people per year it is expected in 2020 world population is increased with 7.9 billion per year (Marcel, 2018). According to WHO report in 2019 cause of second most death is Diarrhea which were produce from water pollution, Cholera also water pollution diseases from this every year 95,000 death according to WHO (Salcedo, 2018). Moreover, still 2 billion people do not have simple sanitization-facilities, even one in fourth health-care facilities shortage water services. If this situation remain same then in 2025 half of the world population will be living in water stressed area (WHO, 2019). So according to these static, in current situation one of the biggest issue in our planet is water pollution, water purification and shortage of water. Especially in developing countries like Pakistan, India and Bangladesh where shortage of sources, population increase exponentially and problem like energy crises than water purification has great importance (Bhatti et al., 2017). In Pakistan 62% urban and 84% rural population have no proper water purification system. Hundred million diarrheal cases are registered in Pakistan hospital every year of death is due to the unviability of safe drinking water. It makes about 135,000 deaths per year (Shehzad et al., 2015). Pakistan is the third most effected country from pollution in the world after India and China which are at first and second spot respectively. Here terrorism is not a big issue water shortage and purification is main problem (Heinz et al., 2019). Different types of toxic containments are present in water which have adverse effect upon consumption and led to a waterborne diseases (Nyoni, 2019, Ataullahjan et al., 2019). That’s containments can be presents in the form of organic and inorganic chemicals or physical, biological, radiological or heavy metal substance. Industry waste is the major cause of water pollution because they discard about 20% of the annual dye consumption into the water bodies (Nairat et al., 2016, Dequigiovanni et al., 2018). We need a method to remove these organic pollutants form water. There are different water purification conventional methods are available like chemical transformation, distillation, biological treatments, reverse osmosis, coagulation and flocculation, microfiltration, ultraviolet treatment, ultrafiltration and many others (Ataullahjan et al., 2019, Smith and John, 2016). Each of these method have specific limitation present conventional method are not sufficient to remove the containments from water and to supply 100% drinking water. These were also expensive and energy required specially for developing countries not affordable. Here, new and developed water purification technology are tremendously important in present world crises situation, Nanomaterials as a photo-catalyst provided us ground breaking environmental friendly results.
Photocatalytic reaction is green technique for waste treatment, perform in the presence of light and suitable catalyst which degrade the pollutant in water. It is define the change the rate of chemical reaction that is initiate under the suitable ultra-violet, visible and infrared light energy depend upon the band of catalyst, this energy generate electron hole pair (e−h) then these generated (e−h) pair produce highly reactive reducing and/or oxidizing radical on conduction and valence band respectively these radicals in contaminated water react with organic or inorganic pollutant degrade them through some secondary reaction. For waste water treatment semiconductor nanoparticles are suitable catalyst due to wide band gap and in the visible region. Here, our focus is less energy consumption and low cost setup for this those whose band gap lies in visible region best for waste water treatment.
General mechanism
Photocatalytic reaction is surface phenomena, its general mechanism involve five basic steps, in first step on the surface of catalyst reactant diffusion, in second step reactant adsorption on surface of catalyst, in third step on surface of reaction basic take place, in fourth step production desorption and in final step once again product diffusion on surface of catalyst. General mechanism of degradation of organic pollutant in following 10 steps (Nalwa, 2003).
Electron hole pair generation
| (2) |
Aqueous dissociation of pollutant
| (3) |
Water splitting
| (4) |
Electrophilic dissolved O2 adsorption
| (5) |
Superoxide anion protonation
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
Recombination electron hole pair
| (11) |
Factors affecting photocatalytic water treatment
Different factor which play a vital role in photocatalytic activity against waste water treatment.
Electreon hole pair separation rate
One of the important factor that is electreon hole pair separation rate. Electron hole (e−h) pair in semiconductor catalyst have very small life. It is necessary for good catalyst before recombine utlize in secondary reaction before recombination. For example, in TiO2 hole is good oxidizing agent in valance band and electron is good reducing agent in valance band redox potential for hole and electron 1.0–3.5 V and 0.5 to −1.5 V respectively verses normal hydrogen electrode (Smith et al., 2007). For an ideal photocatalyst should have wide band gap and electron recombination as low as possible.
Structure of the catalyst
Photocatalytic activity directly depends on the structure of the catalyst. For example, titanium di-oxide (TiO2) is found the three different phases as rutile, brookite and anatase. In these three, the most effective one is anatase because it has the most stable structure, high adsorption power and its conduction band position. Morphology is an important factor in the degradation efficieny. It was reported earlier. For example, Zinc Oxide sample with spherical shape show higher efficiency than rod like structure because it has higher surface area. Photocatalyst is used in nano form instead of bulk form because we know that on nano scale, material has small size and higher surface area available for the reaction so the reaction rate increses or in other words we say that surface to volume ratio is increased which allows multiple reactants to react with the catalyst at a time e.g., Nano sized TiO2 is more effective than bulk TiO2 (Hao et al., 2013, Magdalane et al., 2018, Hafez, 2012).
pH
pH of the solution plays a vital role in the efficiency of a photocatalyst. pH affects the activity of photocatalyst by affecting its surface charges. It can be seen in term of electrostatic phenomenon between pollutant and charge particles. Result of observed photocatalytic activity for metal oxide semiconductor photocatalyst against dye, under different pH from litterature are discussed here. For pH less than 5 i.e., for acidic medium, lower efficiency of photocatalyst is observed from litterature due to the fact that concentration of proton is high in this pH so degradation of dye lags because less number of OH− are available. For pH range of 5–10, photoactivity of catalyst increases and for pH 10 (alkaline medium), efficiency id maximum because the OH− produced reacts with the organic pollutant and degrades it. For pH range 11–13, drop in the efficiency occurs here because hydroxyl ions concentration is very high and so they are scavenged. They don’t have the opportunity to react the dye. Hence the efficiency in this pH range is very low (Mohapatra et al., 2006, Suwarnkar et al., 2014, Turki et al., 2015).
Amount of catalyst
The relation of photocatalytic activity with the amount of catalyst is direct i.e., an increase in amount of catalyst will increase the degradation rate because with increase in amount of catalystm the number of radicals essential for the degradation also increases hence the reaction rate increases. But after the optimal limit is reached, the increase in catalyst amount does not favour the reaction because it is difficult for light to penetrate though so most of the catalyst surfaces remains in-active (Saikia et al., 2015).
Intensity of incident light
Photocatalytic degradation rate is proportional to the intensity of incident light. As the light intensity is increased, the quantum yield increses. Quantum yield is the ratio of reaction rate and absorption rate. Wavelength also effects the efficieny of the reaction. TiO2 has a bandgap of 3.2 eV. It absorbs UV light. For light intensity from 0 to 20 mW/cm2, degradation rate increases. This is half order reactions i.e., rate depends on square root of incident light. Above the intermediate intensity i.e., ~25 mW/cm2, reaction rate decreases because high intensity light promotes the recombination of electron-hole pairs (Seery et al., 2007, Prasad et al., 2009).
Temperature
Dependency of efficieny of photo-catalytic rate on temperature of reaction has been studied by many researchers. For TiO2, reaction rate is decreased at 80 ‘C because the recombination rate of electron and holes is increased and also the catalyst’s absorption rate is reduced at this temperature resulting decrease in the reaction rate. At the optimum temperature of 20–80′C, we obtain maximum reaction rate (Seery et al., 2007, Dong et al., 2011).
Role of photocatalytic process against different waste water pollutant
By using photocatalytic process lot of research work have been done against organic, inorganic, heavy metal and microbes present in a waste water. Organic pollutant present in waste water in the form of carboxylic acid, phenolic derivate, alcohol, chlorinated aromatic and in the form of pollutant dyes from leather and textile industry. Only Industry dyes have major role in waste water pollution because they discard about 20% of the annual dye consumption into the water bodies (Nairat et al., 2016, Dequigiovanni et al., 2018). Semiconductor metal oxide nanoparticles such as TiO2, ZnO, CuO etc, show great potential against these contaminated dyes (Danwittayakul et al., 2013, Baruah et al., 2010, Chan et al., 2011, Baruah et al., 2009). Photocatalytic treatment also show great potential against humic substance or natural organic matters. Humic substance have high molecular weight naturally occurring in yellow-brown, by using TiO2 80% humic acid reduction is reported (Liu et al., 2008), in another paper 65% reduction in humic acid reported (Valencia et al., 2018) In organic pollutant present in waste water in the form of nitrates, halides, ammonia, cyanide and thiocyanate these pollutant an effectively decompose by photocatalytic process. Different semiconductors nanoparticles such as TiO2 against AgNO3, TiO (López-Muñoz et al., 2016, Hong et al., 2009) ZnO against Cr(Vi) and potassium cyanides (Joshi and Shrivastava, 2011, Bagabas et al., 2013) and CdS/Titanate nanotubes reported against ammonia in water (Lee et al., 2002).
Another waste water pollutants are toxic heavy metals, their amount is vary depend upon the contaminated water. For maintaining the water quality and human health, it is necessary to remove these toxic metal from water but some of these metal are very costly, rare and precious then some time these heavy metals are preferred on contaminated water, so photocatalytic process help us to recover these metal from contaminated water, by suing photocatalytic process various metal recovered (Huang et al., 2014, Park et al., 2003, Slamet et al., 2010).
Microbes are another kind of pollutant that are present in waste water and directly effect on the human health, photocatalyst show anti-microbial effect and prevent the growth of microbes. During photocatalytic process highly reactive radicals are generated that help them in destruction of cell-wall of bacteria then which lead to destruction of complete bacteria/microbes. Harmful microbes that were present in waste water removed by heterogeneous photocatalyst such as S. mutans, S. natuss, S. cricetus, E. coli, S. cerevisisas, L. acidophilus, etc (Mahmood et al., 2012, Byrne et al., 2015). Other microbes Chlorella vulgaris inhibit using TiO2 photocatalyst (Shephard et al., 2002). Similarly photocatalyst ZnO used against E. coli and S. aureus (Baruah et al., 2012; Jaisai et al., 2012).
Air Treatment
Air quality in which we breathe has a vital impact on our health. Several international reports suggest that the air present inside our rooms is more polluted as compared to the air outside our houses. This is an alarming situation. Inhaling poor quality air can cause many respiratory diseases. So it is important to improve air quality effectively. According to WHO report in 2018 every year 7 million people were died, 4.2 million people from outdoor pollution, 3.8 million people from household pollution and 90% of world population live where air quality is not good, according to WHO report this is very alarming condition and serious issue for the world. Air pollution exist in two phase gases and solid phases. In gaseous organic and in-organic chemical-containments include carbon monoxide (CO), Volatile organic-compounds (VOCs), Nitrogen and Sulfur containing organic compounds (NOx), In pathogen include bacteria, fungi and viruses and in particles or solid phase include solid organic and inorganic pollutants and pathogens etc (Ren et al., 2017). These solid and gaseous phase pollutant in air treated by using prevention or removal approaches. At present most common removal of air pollution techniques includes such as gas-adsorption filtration, electrostatic-air purification, ventilation, air filtration (Zhang et al., 2011). These physical method have some side effects and costly, need continuous replacement of materials, disposal, physical removal, and disposal. However, other technique which are better than these which required no physical replacement such as UV-radiation and Ozone-disinfection but one of the major and most effecting disadvantage of these are harmful for the human health (Chen et al., 2010). Therefor, there is a serious need to use that technique for air disinfection which is environmental friendly, in-expensive, safe and less energy consumption helpful to decompose maximum number of air pollutant. There are several air filtration techniques but none of them is very much effective as each one has its own limitations. Recently, scientists have started working on purifying air using photo-catalysis. It is a green method of purifying air and it resembles with photosynthesis occurring in plants leaves. Photo-catalysis has an excellent disinfection properties. It is used for treating gaseous pollutants, photocatalytic process is environmental friendly, inexpensive and save process for air disinfection, nanomaterial as catalyst under the suitable wavelength of light in photocatalytic process produce reactive oxygen species which react with air pollutant and decomposed them. Mostly the air pollution is caused by chlorofluorocarbons, cigarette smoke, Nitrogen oxides etc. Photocatalytic process of TiO2 is used for purification of air as it can react with these pollutants and converts them into echo-friendly substances. So in polluted area, pollutants can be removed (Dong et al., 2011, Saif et al., 2014).
Disinfection
Disinfections means destroying micro-organisms by oxidation or by denaturing there DNA. UV light process works by targeting DNA of the micro-organisms, hydrogen peroxide and ozone oxidizes the target. Photo-catalysis also does oxidation of the viruses and bacteria and convert them into carbon dioxide and water. BY removing bacteria and viruses from our surroundings, we are much safer from many diseases.
Photon strikes the catalyst and produces electron hole pair, which react with water to produce free radicals which in the end gives us hydroxyls, strongest oxidizing agent known, to perform disinfection (Gamage and Zhang, 2010).
There are many factors which affect the efficiency of photo-catalyst and the efficiency or effectiveness of photo-catalyst is directly related to the disinfection rate. Some important factors are pH, catalyst loading, temperature, light intensity and wavelength. All these factors defines the practical and commercial use of this technology. For example take pH into consideration. It effects the efficiency of the reaction by changing charge carriers on the surface of photo-catalyst, altering the size of photo-catalyst and most importantly the position of valence and conduction band is displaced. pH also affects the isoelectric point of photo-catalyst TiO2. Isoelectric point is pH at which surface charge of catalyst becomes zero. Therefore, at iso-electric point, photo-catalyst will have no charge on its surface. This is also called point of zero charge (PZO). For TiO2, PZO lies between 4.5 and 7 pH (Chin et al., 2006, Chong et al., 2009, Toor et al., 2006).
PZO depends on which catalyst is used. At PZO, efficiency is minimal because there is a very little interaction between catalyst and contaminants due to unavailability if surface charge. When pH < PZO, catalyst surface becomes positively charged and starts developing an electrostatic force of attraction between anionic compounds. These interactions increases the absorption onto the activated catalyst. At pH > PZO, surface will become negatively charged and it will no longer be able to attract anionic compounds, it will repel them. Wavelength of irradiation light is related with the band gap of semi-conductor or photo-catalyst (Xu and Langford, 2000, Gogniat et al., 2006).
Photo-catalytic design for air purifier
Some factors should be taken under consideration for designing a photo-catalytic air purifier.
Proper or suitable band gap of photo-catalyst so that reactive species essential for the degradation of VOC can be made easily and in sufficient quantity. These essential reactive species are radicals (•OH, •O2 −, HO2•, etc.) which enhance oxidation. For a photo-catalyst, position of valence and conduction band is important for production of •OH and •O2 − radicals respectively. Degradation process of organic pollutants in water such as dyes resembles with the VOCs degradation via photo-catalysis (Zhao and Yang, 2003, Pepin et al., 2018).
Selectivity of photo-catalyst. For degradation of volatile organic compounds, end products are water and carbon dioxide. During the process, unwanted byproduct is coke which is deposited on the surface of photo-catalyst. If our target is removal of inorganic pollutants present in air, then we have to be selective about photo-catalyst. For example, in H2S removal or SOx removal, we want to oxidize sulfur into sulfur trioxide (SO3) or sulfate (SO4 2 –) because sulfur can block the photo-catalysts active sites. We need photo-catalyst with physiochemical properties to remove NOx. The end product is nitrate or nitrogen gas (Boyjoo et al., 2017, Sun et al., 2011).
For practical applications Stability of photo-catalyst is necessary for long term use. Otherwise, repeatedly changing the photo-catalyst could be very costly and irritating.
Visible light Response of photo-catalyst is also an important factor. We can efficiently use solar energy for purification purposes meaning that no electricity is required for the process. It will me a tremendous achievement if we can built such reactor which utilizes solar energy. It means that indoor air purification can be carried out by photo-catalyst under room light. No special light will be required (Boyjoo et al., 2014a, Boyjoo et al., 2014b).
Immobilization of Photo-catalyst is a key factor for making a photo-catalytic reactor for air purification. Unlink the photo-degradation of aqueous organic pollutants, continuous reactors are used for air purification. So it is necessary for photo-catalyst to be immobile. To achieve immobility, different approaches are used for example fabrication of thin photo-catalytic film, using porous material for supporting catalyst or advance material designing. All these factors are for immobilizing the photo-catalyst (Pham and Lee, 2015).
Conclusion
This article reported history of photocatalytic activity their importance in different application and role in current pollution and energy crises. Photocatalytic process initiate under the light and catalyst, nanomaterials are best suitable photo-catalyst for photocatalytic activity, in nanomaterials most efficient photo-catalyst are semiconductor due to their remarkable photocatalytic activity and wide band gap in visible region because our focus is cost effective and environmental friendly photo-catalyst. In energy sector, H2 production from photocatalytic technique becomes emerging and efficient technique in current situation. In pollution related problem, photocatalytic technique is cost effective, environmental friendly and most efficient approach against organic, inorganic, heavy metal and microbe’s pollutant. Different factors which affect the catalytic activity such electron hole pair separation and recombination rate, temperature, pH, catalytic structure and intensity of incident light. Overall, in the end we concluded photocatalytic technique in energy sector and in pollution related problem becomes emerging, efficient and green approach.
See also
Role of Photocatalysis in Green Energy Production Role of Photocatalysts in Air Purification Role of Photocatalysts in Organic Pollutants Degradation
References
- Ahmad H., et al. Hydrogen from photo-catalytic water splitting process: A review. Renewable and Sustainable Energy Reviews. 2015;43:599–610. [Google Scholar]
- Al-Hamdi A.M., Rinner U., Sillanpää M. Tin dioxide as a photocatalyst for water treatment: A review. Process Safety and Environmental Protection. 2017;107:190–205. [Google Scholar]
- Ataullahjan A., Mumtaz Z., Vallianatos H. Family planning in Pakistan: A site of resistance. Social Science & Medicine. 2019;230:158–165. doi: 10.1016/j.socscimed.2019.04.021. [DOI] [PubMed] [Google Scholar]
- Bafaqeer A., Tahir M., Amin N.A.S. Well-designed ZnV2O6/g-C3N4 2D/2D nanosheets heterojunction with faster charges separation via pCN as mediator towards enhanced photocatalytic reduction of CO2 to fuels. Applied Catalysis B: Environmental. 2019;242:312–326. [Google Scholar]
- Bagabas A., et al. Room-temperature synthesis of zinc oxide nanoparticles in different media and their application in cyanide photodegradation. Nanoscale Research Letters. 2013;8(1):516. doi: 10.1186/1556-276X-8-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X., et al. Enhanced oxidation ability of g-C3N4 photocatalyst via C60 modification. Applied Catalysis B: Environmental. 2014;152:262–270. [Google Scholar]
- Baruah S., et al. Photoreactivity of ZnO nanoparticles in visible light: Effect of surface states on electron transfer reaction. Journal of Applied Physics. 2009;105(7):074308. [Google Scholar]
- Baruah S., et al. Enhanced visible light photocatalysis through fast crystallization of zinc oxide nanorods. Beilstein Journal of Nanotechnology. 2010;1(1):14–20. doi: 10.3762/bjnano.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruah S., Jaisai M., Dutta J. Development of a visible light active photocatalytic portable water purification unit using ZnO nanorods. Catalysis Science & Technology. 2012;2(5):918–921. [Google Scholar]
- Bhatti S., et al. Mitochondrial DNA variation in the Sindh population of Pakistan. Australian Journal of Forensic Sciences. 2017;49(2):201–216. [Google Scholar]
- Boyjoo Y., et al. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chemical Engineering Journal. 2017;310:537–559. [Google Scholar]
- Boyjoo Y., Ang M., Pareek V. CFD simulation of a pilot scale slurry photocatalytic reactor and design of multiple-lamp reactors. Chemical Engineering Science. 2014;111:266–277. [Google Scholar]
- Boyjoo Y., Ang M., Pareek V. Lamp emission and quartz sleeve modelling in slurry photocatalytic reactors. Chemical Engineering Science. 2014;111:34–40. [Google Scholar]
- Brahimi R., et al. CuAlO2/TiO2 heterojunction applied to visible light H2 production. Journal of Photochemistry and Photobiology A: Chemistry. 2007;186(2–3):242–247. [Google Scholar]
- Byrne J.A., et al. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules. 2015;20(4):5574–5615. doi: 10.3390/molecules20045574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.H.S., et al. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste‐water. Journal of Chemical Technology & Biotechnology. 2011;86(9):1130–1158. [Google Scholar]
- Chen F., et al. Photocatalytic oxidation for antimicrobial control in built environment: A brief literature overview. Building and Environment. 2010;45(8):1747–1754. [Google Scholar]
- Chin S.S., Chiang K., Fane A.G. The stability of polymeric membranes in a TiO2 photocatalysis process. Journal of Membrane Science. 2006;275(1–2):202–211. [Google Scholar]
- Chong M.N., et al. Application of H-titanate nanofibers for degradation of Congo Red in an annular slurry photoreactor. Chemical Engineering Journal. 2009;150(1):49–54. [Google Scholar]
- Chu D., et al. Formation and photocatalytic application of ZnO nanotubes using aqueous solution. Langmuir. 2010;26(4):2811–2815. doi: 10.1021/la902866a. [DOI] [PubMed] [Google Scholar]
- Danwittayakul S., et al. Enhancement of photocatalytic degradation of methyl orange by supported zinc oxide nanorods/zinc stannate (ZnO/ZTO) on porous substrates. Industrial & Engineering Chemistry Research. 2013;52(38):13629–13636. [Google Scholar]
- Dasgupta A., et al. Covalent three-dimensional networks of graphene and carbon nanotubes: Synthesis and environmental applications. Nano Today. 2017;12:116–135. [Google Scholar]
- Dequigiovanni G., et al. New microsatellite loci for annatto (Bixa orellana), a source of natural dyes from Brazilian Amazonia. Crop Breeding and Applied Biotechnology. 2018;18(1):116–122. [Google Scholar]
- Di J., et al. Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight. Applied Catalysis B: Environmental. 2015;168:51–61. [Google Scholar]
- Dong F., et al. Enhancement of the visible light photocatalytic activity of C-doped TiO2 nanomaterials prepared by a green synthetic approach. The Journal of Physical Chemistry C. 2011;115(27):13285–13292. [Google Scholar]
- Dubey P.K., et al. Synthesis of reduced graphene oxide–TiO2 nanoparticle composite systems and its application in hydrogen production. International Journal of Hydrogen Energy. 2014;39(29):16282–16292. [Google Scholar]
- Elsabahy M., Wooley K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chemical Society Reviews. 2012;41(7):2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajrina N., Tahir M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. International Journal of Hydrogen Energy. 2019;44(2):540–577. [Google Scholar]
- Gamage J., Zhang Z. Applications of photocatalytic disinfection. International Journal of Photoenergy. 2010;2010 [Google Scholar]
- Giannakis S., et al. Iron oxide-mediated semiconductor photocatalysis vs. heterogeneous photo-Fenton treatment of viruses in wastewater. Impact of the oxide particle size. Journal of Hazardous Materials. 2017;339:223–231. doi: 10.1016/j.jhazmat.2017.06.037. [DOI] [PubMed] [Google Scholar]
- Gogniat G., et al. The bactericidal effect of TiO2 photocatalysis involves adsorption onto catalyst and the loss of membrane integrity. FEMS Microbiology Letters. 2006;258(1):18–24. doi: 10.1111/j.1574-6968.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- Guterres S.S., Alves M.P., Pohlmann A.R. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug target insights. 2007;2 (117739280700200002) [PMC free article] [PubMed] [Google Scholar]
- Hafez H.S. Highly active ZnO rod-like nanomaterials: Synthesis, characterization and photocatalytic activity for dye removal. Physica E: Low-Dimensional Systems and Nanostructures. 2012;44(7–8):1522–1527. [Google Scholar]
- Han Y., et al. Carbon quantum dots with photoenhanced hydrogen-bond catalytic activity in aldol condensations. ACS Catalysis. 2014;4(3):781–787. [Google Scholar]
- Hao J.-Y., et al. SiC nanomaterials with different morphologies for photocatalytic hydrogen production under visible light irradiation. Catalysis Today. 2013;212:220–224. [Google Scholar]
- Heinz E., et al. Resistance mechanisms and population structure of highly drug resistant Klebsiella in Pakistan during the introduction of the Carbapenemase NDM-1. Scientific Reports. 2019;9(1):2392. doi: 10.1038/s41598-019-38943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatomi T., Domen K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nature Catalysis. 2019;2(5):387–399. [Google Scholar]
- Hodges B.C., Cates E.L., Kim J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nature nanotechnology. 2018;13(8):642–650. doi: 10.1038/s41565-018-0216-x. [DOI] [PubMed] [Google Scholar]
- Hong Z.C., et al. Surface enhanced Raman scattering of nano diamond using visible‐light‐activated TiO2 as a catalyst to photo‐reduce nano‐structured silver from AgNO3 as SERS‐active substrate. Journal of Raman Spectroscopy: An International Journal for Original Work in all Aspects of Raman Spectroscopy, Including Higher Order Processes, and also Brillouin and Rayleigh Scattering. 2009;40(8):1016–1022. [Google Scholar]
- Huang S., et al. Heavy metal recovery from electroplating wastewater by synthesis of mixed-Fe3O4@SiO2/metal oxide magnetite photocatalysts. Green Chemistry. 2014;16(5):2696–2705. [Google Scholar]
- Hussain M., et al. Synthesis, characterization, and photocatalytic application of novel TiO2 nanoparticles. Chemical Engineering Journal. 2010;157(1):45–51. [Google Scholar]
- Jaisai M., Baruah S., Dutta J. Paper modified with ZnO nanorods–antimicrobial studies. Beilstein Journal of Nanotechnology. 2012;3(1):684–691. doi: 10.3762/bjnano.3.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi K., Shrivastava V. Photocatalytic degradation of Chromium (VI) from wastewater using nanomaterials like TiO2, ZnO, and CdS. Applied Nanoscience. 2011;1(3):147–155. [Google Scholar]
- Ju L., et al. Synthesis and characterization of Fullerene modified ZnAlTi-LDO in photo-degradation of Bisphenol A under simulated visible light irradiation. Environmental Pollution. 2017;228:234–244. doi: 10.1016/j.envpol.2017.05.038. [DOI] [PubMed] [Google Scholar]
- Kim S., et al. Core–shell-structured carbon nanofiber-titanate nanotubes with enhanced photocatalytic activity. Applied Catalysis B: Environmental. 2014;148:170–176. [Google Scholar]
- Lee J., Park H., Choi W. Selective photocatalytic oxidation of NH3 to N2 on platinized TiO2 in water. Environmental Science & Technology. 2002;36(24):5462–5468. doi: 10.1021/es025930s. [DOI] [PubMed] [Google Scholar]
- Léonard G.L.-M., Remy S., Heinrichs B. Doping TiO2 films with carbon nanotubes to simultaneously optimise antistatic, photocatalytic and superhydrophilic properties. Journal of Sol-Gel Science and Technology. 2016;79(3):413–425. [Google Scholar]
- Li G., et al. C60/Bi2TiO4F2 heterojunction photocatalysts with enhanced visible-light activity for environmental remediation. ACS Applied Materials & Interfaces. 2013;5(15):7190–7197. doi: 10.1021/am401525m. [DOI] [PubMed] [Google Scholar]
- Li Z., et al. Inhibition of hydrogen and oxygen recombination using oxygen transfer reagent hemin chloride in Pt/TiO2 dispersion for photocatalytic hydrogen generation. Applied Catalysis B: Environmental. 2017;203:408–415. [Google Scholar]
- Liqiang J., et al. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Solar Energy Materials and Solar Cells. 2006;90(12):1773–1787. [Google Scholar]
- Liu C., et al. Fabrication of graphene films on TiO2 nanotube arrays for photocatalytic application. Carbon. 2011;49(15):5312–5320. [Google Scholar]
- Liu S., et al. Removal of humic acid using TiO2 photocatalytic process–fractionation and molecular weight characterisation studies. Chemosphere. 2008;72(2):263–271. doi: 10.1016/j.chemosphere.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Liu S., et al. Multifunctional TiO2 overlayer for p-Si/n-CdS heterojunction photocathode with improved efficiency and stability. Nano Energy. 2018;53:125–129. [Google Scholar]
- López-Muñoz M.-J., et al. Adsorption of Hg (II) from aqueous solutions using TiO2 and titanate nanotube adsorbents. Applied Surface Science. 2016;367:91–100. [Google Scholar]
- Maeda K. Photocatalytic properties of rutile TiO2 powder for overall water splitting. Catalysis Science & Technology. 2014;4(7):1949–1953. [Google Scholar]
- Magdalane C.M., et al. Photocatalytic decomposition effect of erbium doped cerium oxide nanostructures driven by visible light irradiation: Investigation of cytotoxicity, antibacterial growth inhibition using catalyst. Journal of Photochemistry and Photobiology B: Biology. 2018;185:275–282. doi: 10.1016/j.jphotobiol.2018.06.011. [DOI] [PubMed] [Google Scholar]
- Mahmood M.A., et al. Heterogeneous photocatalysis for removal of microbes from water. Environmental Chemistry Letters. 2012;10(2):145–151. [Google Scholar]
- Mano T., et al. Water treatment efficacy of various metal oxide semiconductors for photocatalytic ozonation under UV and visible light irradiation. Chemical Engineering Journal. 2015;264:221–229. [Google Scholar]
- Marcel B. Criminal liability for water pollution. Criminology. 2018 Available at: http://www.cnaa.md/en/thesis/53933/. [Google Scholar]
- Marschall R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Advanced Functional Materials. 2014;24(17):2421–2440. [Google Scholar]
- Mohapatra P., Mishra T., Parida K. Effect of microemulsion composition on textural and photocatalytic activity of titania nanomaterial. Applied Catalysis A: General. 2006;310:183–189. [Google Scholar]
- Moreno-Vega A.-I., et al. Polymeric and ceramic nanoparticles in biomedical applications. Journal of Nanotechnology. 2012;2012 [Google Scholar]
- Murugan R., et al. Synthesis and photocatalytic application of ZnO nanoarrows. Materials Letters. 2014;128:404–407. [Google Scholar]
- Nada A., et al. Enhancement of photocatalytic hydrogen production rate using photosensitized TiO2/RuO2-MV2+ International Journal of Hydrogen Energy. 2008;33(13):3264–3269. [Google Scholar]
- Nairat M., et al. Controlling S2 population in cyanine dyes using shaped femtosecond pulses. The Journal of Physical Chemistry A. 2016;120(11):1876–1885. doi: 10.1021/acs.jpca.6b01835. [DOI] [PubMed] [Google Scholar]
- Nalwa H.S. Vol. 1. American Scientific Publishers; 2003. Handbook of Photochemistry and Photobiology: Inorganic Photochemistry. [Google Scholar]
- Ni M., et al. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renewable and Sustainable Energy Reviews. 2007;11(3):401–425. [Google Scholar]
- Nyoni, T., 2019. A Box-Jenkins ARIMA approach to the population question in Pakistan: A reliable prognosis.
- Park S., et al. Photocatalytic ZnO nanopowders prepared by solution combustion method for noble metal recovery. Journal of Materials Science. 2003;38(22):4493–4497. [Google Scholar]
- Pepin P.A., et al. Thermal and photocatalytic reactions of methanol and acetaldehyde on Pt-modified brookite TiO2 nanorods. ACS Catalysis. 2018;8(12):11834–11846. [Google Scholar]
- Pham T.-D., Lee B.-K. Novel adsorption and photocatalytic oxidation for removal of gaseous toluene by V-doped TiO2/PU under visible light. Journal of Hazardous Materials. 2015;300:493–503. doi: 10.1016/j.jhazmat.2015.07.048. [DOI] [PubMed] [Google Scholar]
- Police A.K.R., et al. CaFe2O4 sensitized hierarchical TiO2 photo composite for hydrogen production under solar light irradiation. Chemical Engineering Journal. 2014;247:152–160. [Google Scholar]
- Prasad G., et al. Photocatalytic inactivation of Bacillus anthracis by titania nanomaterials. Journal of Hazardous Materials. 2009;165(1–3):506–510. doi: 10.1016/j.jhazmat.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Puga A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coordination Chemistry Reviews. 2016;315:1–66. [Google Scholar]
- Reiss G., Hütten A. Magnetic nanoparticles: Applications beyond data storage. Nature Materials. 2005;4(10):725. doi: 10.1038/nmat1494. [DOI] [PubMed] [Google Scholar]
- Ren H., et al. Photocatalytic materials and technologies for air purification. Journal of Hazardous Materials. 2017;325:340–366. doi: 10.1016/j.jhazmat.2016.08.072. [DOI] [PubMed] [Google Scholar]
- Roy K., Sarkar C., Ghosh C. Photocatalytic activity of biogenic silver nanoparticles synthesized using yeast (Saccharomyces cerevisiae) extract. Applied Nanoscience. 2015;5(8):953–959. [Google Scholar]
- Roy K., Sarkar C., Ghosh C. Photocatalytic activity of biogenic silver nanoparticles synthesized using potato (Solanum Tuberosum) infusion. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2015;146:286–291. doi: 10.1016/j.saa.2015.02.058. [DOI] [PubMed] [Google Scholar]
- Sagir M., Naz M.Y., Sulaiman S.A., Shukrullah S. Investigation of air quality and suspended particulate matter inside and outside of university research laboratories. Trends in Applied Sciences Research. 2014;9:43–53. [Google Scholar]
- Saif M., et al. Evaluation of the photocatalytic activity of Ln3+–TiO2 nanomaterial using fluorescence technique for real wastewater treatment. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2014;128:153–162. doi: 10.1016/j.saa.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Saikia L., et al. Photocatalytic performance of ZnO nanomaterials for self sensitized degradation of malachite green dye under solar light. Applied Catalysis A: General. 2015;490:42–49. [Google Scholar]
- Salcedo, C.M., 2018. Modification of the treatment protocol as a strategy in the control of the cholera epidemic in Haiti 2016–2017.
- Seery M.K., et al. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. Journal of Photochemistry and Photobiology A: Chemistry. 2007;189(2–3):258–263. [Google Scholar]
- Shahzad K., Tahir M.B., Sagir M., Kabli M.R. Role of CuCo2S4 in Z-scheme MoSe2/BiVO4 composite for efficient photocatalytic reduction of heavy metals. Ceramics International. 2019;45(17):23225–23232. [Google Scholar]
- Shahzad K., Tahir M.B., Sagir M. Utilization of Bi2WO6-encapsulated polyaniline-based redox reactions for the efficient detoxification of organic pollutants. Applied Nanoscience. 2020 doi: 10.1007/s13204-020-01265-6. [DOI] [Google Scholar]
- Shehzad W., et al. Forest without prey: Livestock sustain a leopard Panthera pardus population in Pakistan. Oryx. 2015;49(2):248–253. [Google Scholar]
- Shephard G., Stockenstro M.S., De Villiers D., Engelbrecht W.J., Wessels G.F.S. Degradation of microcystin toxins in a falling film photocatalytic reactor with immobilized titanium dioxide catalyst. Water Res. 2002;36:140–146. doi: 10.1016/s0043-1354(01)00213-5. [DOI] [PubMed] [Google Scholar]
- Slamet S., Arbianti R., Daryanto D. Simultaneous treatment of organic (Phenol) and heavy metal (Cr6+ or Pt4+) wastes over TiO2, ZnO-TiO2 and CdS-TiO2 photocatalysts. Makara Journal of Technology. 2010;9(2):66–71. [Google Scholar]
- Smith R., John G. Azo dye toxicity: A measure of toxic effect metabolized azo dyes have on the body. Research Reports from Life Science Freshmen Research Scholars. 2016;2:1. [Google Scholar]
- Smith T., Stevenson K., Zoski C. Elsevier; Amsterdam: 2007. Handbook of Electrochemistry; pp. 73–110. [Google Scholar]
- Sun H., et al. Room-light-induced indoor air purification using an efficient Pt/N-TiO2 photocatalyst. Applied Catalysis B: Environmental. 2011;108:127–133. [Google Scholar]
- Suwarnkar M., et al. Enhanced photocatalytic activity of Ag doped TiO2 nanoparticles synthesized by a microwave assisted method. Ceramics International. 2014;40(4):5489–5496. [Google Scholar]
- Tahir M., Amin N.S. Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Conversion and Management. 2013;76:194–214. [Google Scholar]
- Tahir M.B., Sagir M., Zubair M., et al. WO3 nanostructures-based photocatalyst approach towards degradation of RhB dye. Journal of Inorganic and Organometallic Polymers. 2018;28:1107–1113. [Google Scholar]
- Tahir M.B., Nabi G., Iqbal T., Sagir M., Rafique M. Role of MoSe2 on nanostructures WO3-CNT performance for photocatalytic hydrogen evolution. Ceramics International. 2018;44(6):6686–6690. [Google Scholar]
- Tahir M.B., Rafique M., Khan M.I., et al. Enhanced photocatalytic hydrogen energy production of g-C3N4-WO3 composites under visible light irradiation. International Journal of Energy Research. 2018;42(15):4667–4673. [Google Scholar]
- Tahir M.B., Sagir M., Shahzad K. Removal of acetylsalicylate and methyl-theobromine from aqueous environment using nano-photocatalyst WO3-TiO2 @g-C3N4 composite. Journal of Hazardous Materials. 2019;363:205–213. doi: 10.1016/j.jhazmat.2018.09.055. [DOI] [PubMed] [Google Scholar]
- Tahir M.B., Aasir A.M., Sagir M., et al. In: Encyclopedia of Renewable and Sustainable Materials. Hashmi S., Choudhury I.A., editors. Elsevier; 2019. Hydrogen evolution using advanced technologies based on photocatalysis and plasma; pp. 126–131. [Google Scholar]
- Tahir M.B., Sagir M., Abbas N. Enhanced photocatalytic performance of CdO-WO3 composite for hydrogen production. International Journal of Hydrogen Energy. 2019;44(45):24690–24697. [Google Scholar]
- Tahir M.B., Zahra T., Iqbal T., et al. In: Encyclopedia of Renewable and Sustainable Materials. Hashmi S., Choudhury I.A., editors. Elsevier; 2019. Hydrogen production through water splitting using nanomaterials under solar energy; pp. 132–135. [Google Scholar]
- Tahir M.B., Rafique M., Sagir M., et al. Reference Module in Materials Science and Materials Engineering. 2019. Nanomaterials for photocatalytic applications. Elsevier. [Google Scholar]
- Tahir M.B., Khan M.I., Pervaiz M., et al. In: Encyclopedia of Renewable and Sustainable Materials. Hashmi S., Choudhury I.A., editors. Elsevier; 2019. Green energy fuel from biomass and sea water; pp. 114–119. [Google Scholar]
- Tahir M.B., Sagir M. Carbon nanodots and rare metals (RM?=?La, Gd, Er) doped tungsten oxide nanostructures for photocatalytic dyes degradation and hydrogen production. Separation and Purification Technology. 2019;209:94–102. [Google Scholar]
- Tahir M.B., Asiri A.M., Nabi G., Rafique M., Sagir M. Fabrication of heterogeneous photocatalysts for insight role of carbon nanofibre in hierarchical WO3/MoSe2 composite for enhanced photocatalytic hydrogen generation. Ceramics International. 2019;45(5):5547–5552. [Google Scholar]
- Tahir M.B., Nawaz T., Nabi G., et al. Role of nanophotocatalysts for the treatment of hazardous organic and inorganic pollutants in wastewater. International Journal of Environmental Analytical Chemistry. 2020 doi: 10.1080/03067319.2020.1723570. [DOI] [Google Scholar]
- Tahir M.B., Akhtar J., Sagir M., Bamufleh H.S. Improved photocatalytic performance of Gd and Nd co-doped ZnO nanorods for the degradation of methylene blue. Ceramics International. 2020;46(8):11955–11961. [Google Scholar]
- Tahir M.B., Nawaz T., Nabi G., et al. Recent advances on photocatalytic nanomaterials for hydrogen energy evolution in sustainable environment. International Journal of Environmental Analytical Chemistry. 2020 doi: 10.1080/03067319.2019.1691188. [DOI] [Google Scholar]
- Tahir M.B., Sagir M., Muhammad S., et al. Hierarchical WO3@ BiVO4 nanostructures for improved green energy production. Applied Nanoscience. 2020;10:1183–1190. doi: 10.1007/s13204-019-01180-5. [DOI] [Google Scholar]
- Thomas C., Kumar Mishra P., Sharma H., Talegaonkar S. Ceramic nanoparticles: Fabrication methods and applications in drug delivery. Current Pharmaceutical Design. 2015;21(42):6165–6188. doi: 10.2174/1381612821666151027153246. [DOI] [PubMed] [Google Scholar]
- Toor A.P., et al. Photocatalytic degradation of Direct Yellow 12 dye using UV/TiO2 in a shallow pond slurry reactor. Dyes and Pigments. 2006;68(1):53–60. [Google Scholar]
- Turki A., et al. Phenol photocatalytic degradation over anisotropic TiO2 nanomaterials: Kinetic study, adsorption isotherms and formal mechanisms. Applied Catalysis B: Environmental. 2015;163:404–414. [Google Scholar]
- Tvaronavičienė M., et al. Energy Transformation Towards Sustainability. Elsevier; 2020. Global energy consumption peculiarities and energy sources: Role of renewables; pp. 1–49. [Google Scholar]
- Valencia S., Marín J., Restrepo G. Photocatalytic degradation of humic acids with titanium dioxide embedded into polyethylene pellets to enhance the postrecovery of catalyst. Environmental Engineering Science. 2018;35(3):185–193. [Google Scholar]
- Wang T., Gong J. Single‐crystal semiconductors with narrow band gaps for solar water splitting. Angewandte Chemie International Edition. 2015;54(37):10718–10732. doi: 10.1002/anie.201503346. [DOI] [PubMed] [Google Scholar]
- Wang W., et al. Single-step one-pot synthesis of graphene foam/TiO2 nanosheet hybrids for effective water treatment. Scientific Reports. 2017;7:43755. doi: 10.1038/srep43755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; 2019. Water, Sanitation, Hygiene and Health: A Primer for Health Professionals. [Google Scholar]
- Wu Y., Lu G., Li S. The role of Cu (I) species for photocatalytic hydrogen generation over CuOx/TiO2. Catalysis Letters. 2009;133(1–2):97. [Google Scholar]
- Xu Y., Langford C.H. Variation of Langmuir adsorption constant determined for TiO2-photocatalyzed degradation of acetophenone under different light intensity. Journal of Photochemistry and Photobiology A: Chemistry. 2000;133(1–2):67–71. [Google Scholar]
- Yang Y., et al. Preparation and enhanced visible-light photocatalytic activity of silver deposited graphitic carbon nitride plasmonic photocatalyst. Applied Catalysis B: Environmental. 2013;142:828–837. [Google Scholar]
- Yatmaz H.C., Dizge N., Kurt M.S. Combination of photocatalytic and membrane distillation hybrid processes for reactive dyes treatment. Environmental Technology. 2017;38(21):2743–2751. doi: 10.1080/09593330.2016.1276222. [DOI] [PubMed] [Google Scholar]
- Zhang X., et al. Enhanced photocatalytic activity towards degradation and H2 evolution over one dimensional TiO2@ MWCNTs heterojunction. Applied Surface Science. 2017;402:360–368. [Google Scholar]
- Zhang Y., et al. Can commonly-used fan-driven air cleaning technologies improve indoor air quality? A literature review. Atmospheric Environment. 2011;45(26):4329–4343. doi: 10.1016/j.atmosenv.2011.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Yang X. Photocatalytic oxidation for indoor air purification: A literature review. Building and Environment. 2003;38(5):645–654. [Google Scholar]
- Zuo F., Wang L., Feng P. Self-doped Ti3+@TiO2 visible light photocatalyst: Influence of synthetic parameters on the H2 production activity. International Journal of Hydrogen Energy. 2014;39(2):711–717. [Google Scholar]


