Structured Abstract
Purpose
Despite data demonstrating safety of omitting axillary surgery in older women with early stage breast cancer, the incidence of axillary surgery remains high. We hypothesized that the prevalence of nodal positivity would decrease with advancing age.
Patients and Methods
The National Cancer Database was used to construct a cohort of adult women with early stage, clinically node negative, estrogen receptor positive (ER+), HER2 negative breast cancer treated between 2013 and 2015. Multivariable logistic regression was used to assess the relationship between age and nodal positivity, stratified by axillary surgery category. Modified Poisson regression was used to estimate the proportion of women receiving adjuvant therapy according to age and nodal status.
Results
Incidence of axillary surgery among women over 70 (n=51,917) remained high nationwide (86%). There was a significant decrease in nodal positivity with advancing age in women with early stage, ER+, clinically node negative breast cancer, from the youngest cohort up to patients aged 70–89, independent of histologic subtype (ductal versus lobular), race, comorbidities and socioeconomic factors. Overall, <10% of women over age 70 who underwent surgery were node positive, regardless of axillary surgery type, and almost 95% of node positive patients over age 70 were pathological stage N1mi or N1.
Conclusion
Axillary surgery may be safely omitted in many older women with ER+ clinically node negative early stage breast cancer. Nodal positivity declines with advancing age, suggestive of varied biology in older versus younger patients.
Keywords: Hormone receptor positive breast cancer, early breast cancer, sentinel lymph node biopsy, geriatric oncology
Precis for use in Table of Contents
Nodal positivity declines with advancing age in early stage estrogen receptor positive breast cancer. This suggests varied biology in older patients, making traditional nodal staging less important in an era of genomic predictors of tumor behavior.
Introduction
In the past decade, considerable efforts have been made to limit overtreatment of women with breast cancer, especially older women. For example, the Society of Surgical Oncology’s Choosing Wisely Campaign, introduced in 2016, discourages routine use of sentinel lymph node biopsy in clinically node negative women over 70 years of age with estrogen receptor positive (ER+) early stage breast cancer1. This recommendation is based upon data from randomized trials and a retrospective study demonstrating equivalent overall survival when axillary surgery is omitted in select women over 70 2–4, even in the absence of adjuvant post-operative radiation.5,6
Despite excellent data and guidelines supporting omission of axillary surgery in women over 70 with breast cancer, its use remains high nationwide. In a study using 2004–2013 National Cancer Database (NCDB) and Surveillance, Epidemiology, and End Results Program (SEER) data, 80.2% and 79.8% of women over 70 with clinical stage T1-T3 ER+ breast cancer underwent axillary surgery, and incidence of axillary surgery increased overy time. The incidence of axillary surgery was 76.4% in a similar study utilizing NCDB data during a similar time period 7,8. In single institution studies of similar populations at academic medical centers, axillary surgery incidence was over 90%9,10.
Slow de-escalation of axillary surgery for older women with ER+ breast cancer is multifactorial. We sought to provide context for the multidisciplinary teams treating older women with breast cancer to improve de-escalation of axillary surgery in this group. We hypothesized that in women with early stage (T1), clinically node negative (N0) ER+ Her-2 non-over-expressing breast cancer, the prevalence of nodal positivity would decrease with advancing age.
Methods
Data source
The National Cancer Database (NCDB) breast-cancer site-specific 2015 Participant User File (PUF) was used to construct a cohort of women with early stage, estrogen receptor positive (ER+) disease. The NCDB is jointly implemented by the Commission on Cancer of the American College of Surgeons and the American Cancer Society and is the largest hospital-based cancer registry program in the United States. It captures more than 70% of all annual incident cancers in the United States.
Study population
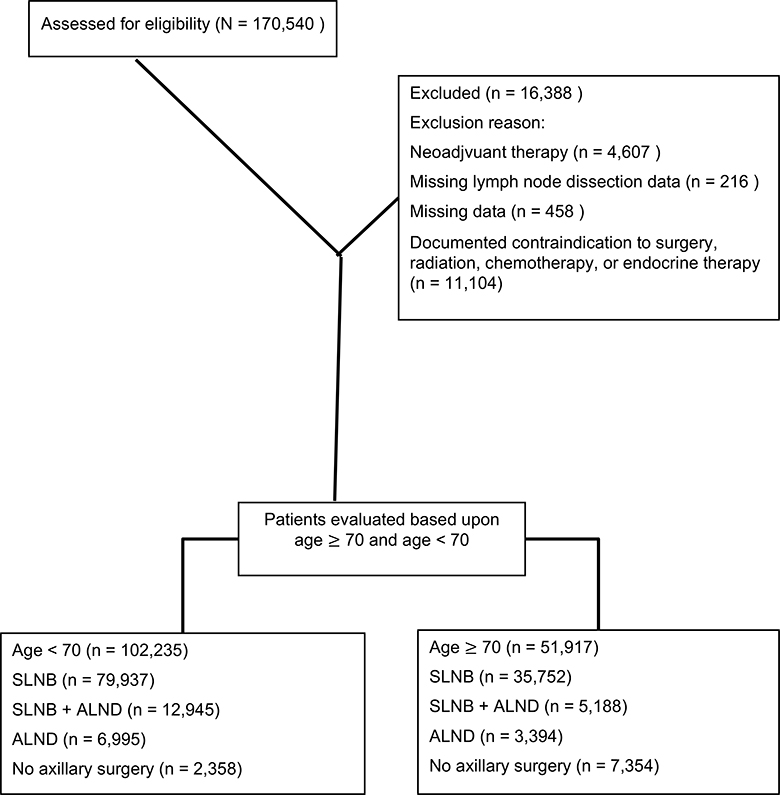
The study population consisted of women ≥18 years of age with a diagnosis of early stage (adjusted AJCC 6th edition Clinical T1, Clinical N0 or NX, and Clinical M0), ER+, HER2 negative invasive breast cancer (ductal, lobular, tubular, mucinous, intraductal papillary adenocarcinoma with invasion, cribiform) diagnosed between January 1, 2012 and December 31, 2015 treated by lumpectomy. Initially, 170,540 patients were identified. Women who had radiation or systemic therapy prior to surgery were excluded (n=4,607) as well as those with missing data regarding the scope of axillary surgery (n=219). Finally, women who had missing data (n=458) or a documented contraindication for surgery, radiation, chemotherapy, or endocrine therapy (n=11,104) were excluded, resulting in a final cohort of 154,152 women. (Figure 1). The study period began after the NCDB revised coding directives on the scope of regional lymph node surgery (2012) to accurately differentiate between patients undergoing SLNB and ALND.
Figure 1:
CONSORT Diagram
Statistical analysis
The research team made an a priori decision to consider decade of age as the representation of the main exposure, with the 50–59 age group serving as the reference category. Patients were categorized as having axillary lymph node dissection (ALND), sentinel lymph node biopsy (SLNB), or no axillary surgery. Patients who underwent both SLNB and ALND were grouped with ALND patients. The primary outcome measure, prevalence of lymph node positivity, was assessed as a dichotomous outcome (≥1 positive lymph node vs. 0 positive lymph nodes). Secondary outcome measures included being offered adjuvant systemic therapy or radiation therapy.
Crude and multivariable logistic regression models were used to assess the association between age, histology, and lymph node positivity, stratified by axillary surgery category. The multivariable model adjusted for age, histology, year of diagnosis, race, Hispanic ethnicity, Charlson Comorbidity Index (CCI), insurance type, residential urbanicity, median residential income, and residential education level. Residential median income and education level were captured through the 2012 American Community Survey and reported by NCDB based on patient ZIP code at time of diagnosis. Adjusted poisson regression was used to estimate the proportions of women receiving adjuvant therapy—including chemotherapy, radiation, and endocrine therapy—by nodal status and age group. This approach is recommended for outcomes with a high occurrence because linear-risk models often fail to converge in such a setting; where both linear-risk and poisson models fail to converge the convential logistic regression is appropriate 11. Interaction terms between nodal positivity and age were included in the model to assess whether the association between age and adjuvant therapy (offered - regardless of receipt, or received) was modified by nodal status. A p-value of <0.05 was considered statistically significant. All analyses were performed in SAS 9.4 (SAS Institute, Cary, NC).
Results
Demographics
There were 102,235 (66%) women <70 years old and 51,917 (34%) ≥70 years old included in the final cohort. Notably, older patients were more likely to have a CCI score ≥1 (22% vs. 15%), and have Medicare or Medicaid as their primary insurance compared to younger women (89% vs. 33%, Table 1). Other socioeconomic variables were relatively equal between groups. Older women were also more likely to have lobular or special histologic subtype cancers (18% vs. 14%) and were slightly less likely to have poorly differentiated tumors (9% vs. 11%).
TABLE 1.
Patient demographic and clinical characteristics, by age group
| <70 years old 102,235 (66%) |
≥70 years old 51,917 (34%) |
|
|---|---|---|
| Race, n (%) | ||
| White | 88,402 (87) | 47,096 (91) |
| Black | 82,96 (8) | 3,122 (6) |
| Asian | 3,475 (3) | 995 (2) |
| Other race | 1,207 (1) | 378 (1) |
| Missing | 855 | 326 |
| Hispanic, n (%) | 4,792 (5) | 1,443 (3) |
| Charlson score, n (%) | ||
| 0 | 87,297 (85) | 40,614 (78) |
| 1 | 12,444 (12) | 8,991 (17) |
| 2 | 2,026 (2) | 1,809 (4) |
| 3+ | 468 (1) | 503 (1) |
| Primary insurance type, n (%) | ||
| Uninsured | 1,487 (2) | 152 (<1) |
| Private insurance/managed care | 66,823 (66) | 5,435 (11) |
| Medicare/Medicaid | 32,905 (33) | 45,852 (89) |
| Missing | 1,020 | 478 |
| Residential type, n (%) | ||
| Metro | 86,293 (87) | 43,624 (86) |
| Urban | 11,807 (12) | 6,154 (12) |
| Rural | 1,396 (1) | 742 (1) |
| Missing | 2,739 | 1,397 |
| Median residential incomea, n (%) | ||
| <$38,000 | 12,275 (12) | 6,387 (12) |
| $38,000 – $47,999 | 19,997 (20) | 11,011 (21) |
| $48,000 – $62,999 | 27,377 (27) | 14,595 (28) |
| ≥$63,000 | 42,430 (42) | 19,825 (38) |
| Missing | 156 | 99 |
| Residential educational levelb, n (%) | ||
| ≥21% | 12,019 (12) | 5,615 (11) |
| 13% – 20.9% | 22,443 (22) | 11,580 (22) |
| 7% – 12.9% | 34,626 (34) | 18,384 (36) |
| <7% | 33,019 (32) | 16,265 (31) |
| Missing | 128 | 73 |
| Histologic type, n (%) | ||
| Ductal | 88,137 (86) | 42,484 (82) |
| Lobular | 9,914 (10) | 6,301 (12) |
| Special histologic subtypes | 4,184 (4) | 3,132 (6) |
| Tumor Grade, n (%) | ||
| Well differentiated; differentiated, NOS | 38,334 (38) | 20,035 (39) |
| Moderately differentiated; moderately well differentiated; intermediate differentiation | 46,725 (46) | 24,671 (48) |
| Poorly differentiated | 11,657 (11) | 4,490 (9) |
| Undifferentiated; anaplastic | 49 (<1) | 27 (<1) |
| Cell type not determined | 5,470 (5) | 2,694 (5) |
Median residential household income of each patient’s ZIP code was estimated using the 2012 American Community Survey
Proportion of adults in patient’s ZIP code who did not complete high school, measured in the 2012 American Community Survey
Axillary Surgery Incidence
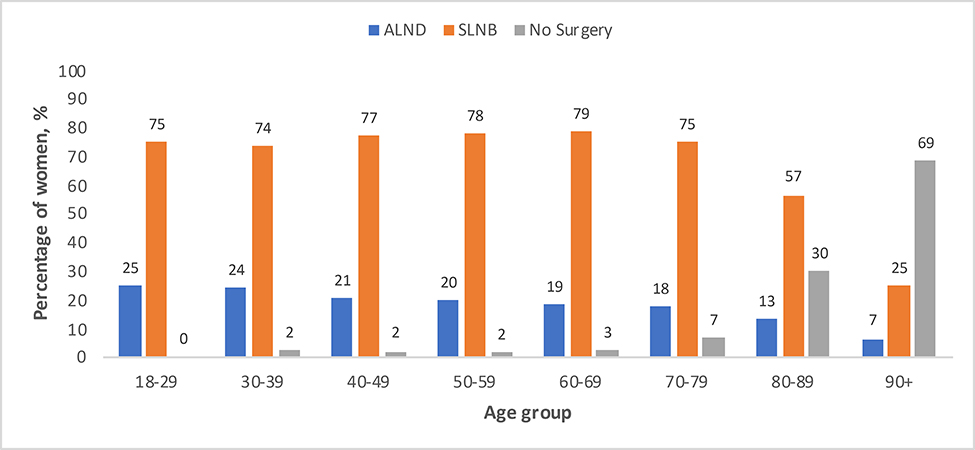
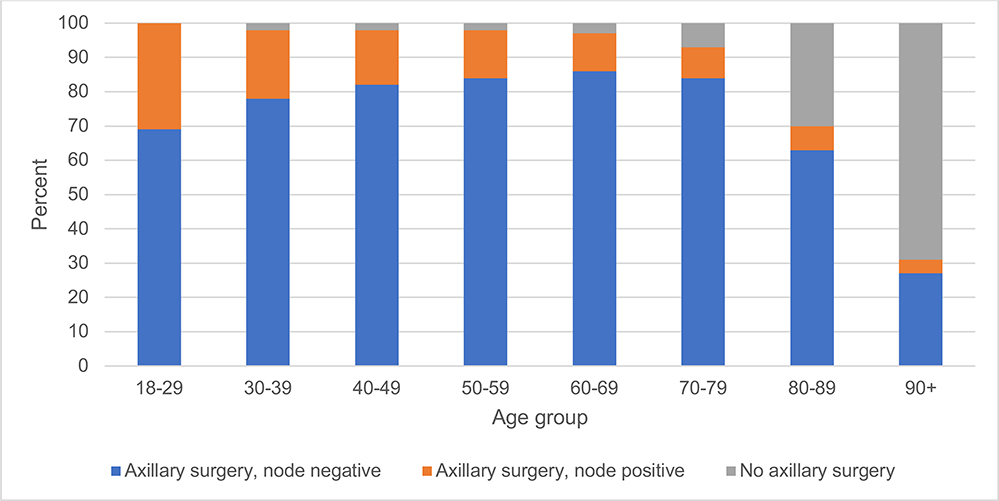
Overall, 115,918 (75%) of patients underwent SLNB alone, 10,389 (7%) underwent ALND alone, 18,133 (12%) underwent SLNB and ALND, and 9,712 (6%) of patients did not undergo either surgery. Almost all (98%) women aged 18 to 69 years old underwent axillary surgery (either SLNB and/or ALND), (Figure 2). Women 70–79 years old were slightly less likely (93% incidence), and women 80–89 and ≥90 years old were substantially less likely to undergo axillary surgery (70% and 32% respectively). In patients ≥70 years old, 35,981 (69%) underwent SLNB alone, 3,394 (7%) underwent ALND alone, 5,188 (10%) underwent both SLNB and ALND, and 7354 (14%) of patients did not undergo either surgery.
Figure 2:
Percentage of adult women (y-axis) with clinical T1N0, ER+, HER2- breast cancer receiving axillary surgery, stratified by age (x-axis).
Prevalence of Nodal Positivity by Age
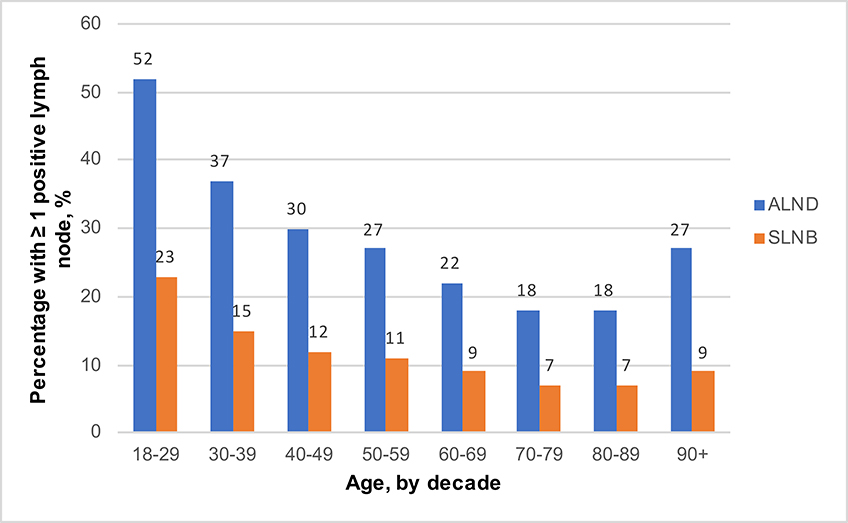
In women who underwent axillary surgery, the prevalence of nodal positivity decreased with increasing age among women 18–89 years old (Figure 3). After adjustment, patients aged 60–69 (ALND: OR 0.76, 95% CI 0.70, 0.82; SLNB: OR 0.77, 95% CI 0.73, 0.82), 70–79 (ALND: OR 0.59, 95% CI 0.54, 0.65; SLNB: OR 0.65, 95% CI 0.59, 0.69), and 80–89 years old (ALND: OR 0.64, 95% CI 0.55, 0.74; SLNB: OR 0.66, 95% CI 0.60, 0.74) were significantly less likely to have at least one positive lymph node compared to women 50–59 years old who underwent axillary surgery. (Table 2, Figure 4). Patients aged 40–49 (ALND: OR 1.19, 95% CI 1.08, 1.31; SLNB: OR 1.13, 95% CI 1.05, 1.21), 30–39 (ALND: OR 1.54, 95% CI 1.22, 1.96; SLNB: OR 1.51, 95% CI 1.27, 1.79), and 18–29 (ALND: OR 2.17, 95% CI 0.97, 4.87; SLNB: OR 2.21, 95% CI 1.27, 3.86) were more likely to have at least one positive lymph node compared to women 50–59 years old who underwent axillary surgery.
Figure 3:
Nodal positivity (any axillary surgery), stratified by age in women with clinical T1N0, ER+, HER2- breast cancer
TABLE 2.
Association between age and histologic type on having ≥1 positive lymph node, stratified by axillary surgery category
| Age group, years | Positive LN N (%) | Total N | OR (95% CI)a | p-value |
|---|---|---|---|---|
| ALND | ||||
| 18–29 | 14 (52) | 27 | 2.17 (0.97, 4.87) | 0.06 |
| 30–39 | 131 (37) | 353 | 1.54 (1.22, 1.96) | 0.0003 |
| 40–49 | 937 (30) | 3,095 | 1.19 (1.08, 1.31) | 0.0005 |
| 50–59 | 1,847 (27) | 6,857 | REF | -- |
| 60–69 | 2,078 (22) | 9,528 | 0.76 (0.70, 0.82) | <0.0001 |
| 70–79 | 1,174 (18) | 6,682 | 0.59 (0.54, 0.65) | <0.0001 |
| 80–89 | 324 (18) | 1,780 | 0.64 (0.55, 0.74) | <0.0001 |
| ≥90 | 18 (27) | 67 | 1.18 (0.66, 2.10) | 0.57 |
| SLNB | ||||
| 18–29 | 19 (23) | 81 | 2.21 (1.27, 3.86) | 0.005 |
| 30–39 | 163 (15) | 1,079 | 1.51 (1.27, 1.79) | <0.0001 |
| 40–49 | 1,409 (12) | 11,594 | 1.13 (1.05, 1.21) | 0.0008 |
| 50–59 | 2,931 (11) | 26,691 | REF | -- |
| 60–69 | 3,518 (9) | 40,121 | 0.77 (0.73, 0.82) | <0.0001 |
| 70–79 | 2,046 (7) | 28,014 | 0.64 (0.59, 0.69) | <0.0001 |
| 80–89 | 557 (7) | 7,499 | 0.66 (0.60, 0.74) | <0.0001 |
| ≥90 | 23 (9) | 249 | 0.75 (0.47, 1.19) | 0.22 |
| Any Axillary Surgery | ||||
| 18–29 | 33 (31) | 108 | 2.26 (1.45, 3.53) | 0.0003 |
| 30–39 | 294 (21) | 1432 | 1.57 (1.37, 1.80) | <0.0001 |
| 40–49 | 2346 (16) | 14689 | 1.15 (1.09, 1.22) | <0.0001 |
| 50–59 | 4778 (14) | 33548 | REF | -- |
| 60–69 | 5596 (11) | 49649 | 0.76 (0.73, 0.80) | <0.0001 |
| 70–79 | 3220 (9) | 34696 | 0.62 (0.58, 0.66) | <0.0001 |
| 80–89 | 881 (9) | 9279 | 0.64 (0.59, 0.70) | <0.0001 |
| ≥90 | 41 (13) | 316 | 0.87 (0.61, 1.23) | 0.4138 |
Abbreviations: ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; LN, lymph node, OR, odds ratio; CI, confidence interval; REF, reference
Adjusted age, histology, year of diagnosis, race/ethnicity, Charlson score, insurance type, residential urbanicity, median residential income, and residential education level
Figure 4:
Prevalence of nodal positivity (≥1 positive lymph node) (y-axis) among adult women diagnosed with clinical T1N0 ER+ HER2- breast cancer, stratified by age (x-axis) and axillary surgery type.
Of the patients who underwent any axillary surgery (SLNB or ALND), >90% of patients over ≥70 years old were pathologically node negative (Table 2, Figure 3). Among patients ≥70 that had at least 1 positive node, 93.5% had a pathologic nodal status of N1mi or N1. (Supplemental Table 1). As expected, increasing primary tumor size measured by T1 substage (T1mic, T1a, T1b, T1c) and poor differentiation were both associated with nodal positivity (both chi-square tests p<0.001, Supplemental Table 2).
Notably, in women aged 70–79, 80–89 and 90+ who underwent ALND as their primary axillary staging surgery (<7% of all older women), 68%, 85% and 73% were pathologically node negative respectively. Additionally, among the small percentage of women in these age deciles who underwent SLNB followed by ALND (11%, 8%, and 3% by age decile), 80%, 80% and 87% were pathologically node negative. (Supplemental Table 3)
Nodal Positivity and Recommendation for Adjuvant Therapy
After adjustment, among node positive patients, women aged 60–69 (RD −0.09, 95% CI −0.11, −0.07), 70–79 (RD −0.26, 95% CI −0.29, −0.24), and 80–89 years old (RD −0.41, 95% CI −0.44, −0.37) were significantly less likely to be recommended adjuvant chemotherapy, compared to women 50–59 years old. In contrast, women 40–49 (RD 0.05, 95% CI 0.03, 0.08), 30–39 (RD 0.14, 95% CI 0.10, 0.19), and 18–29 (RD 0.19, 95% CI 0.07, 0.31) were more likely to be recommended adjuvant chemotherapy (Table 3). Additionally, adjuvant radiation recommendation was less likely for node positive women ≥60 years old, although the difference was smaller than that observed for chemotherapy (Table 3). Among node positive women, there was no difference in recommendation for adjuvant endocrine therapy across age. Among women 70–79 and 80–89 years old, those with nodal positivity were significantly more likely to be recommended to undergo adjuvant radiation, compared to node negative women in the same age group (RD 0.15, 95% CI 0.11, 0.18, and RD 0.26, 95% CI 0.19, 0.32, respectively).
TABLE 3.
Association between age and receipt of adjuvant therapy amongst women with clinical T1N0, ER+, HER2− breast cancer, stratified by nodal status.
| NODE POSITIVE | ||||||||
| Age group | Chemotherapy N (%) | RD (95% CI)a | Radiation N (%) | RD (95% CI)a | Hormonal Therapy N (%) | RD (95% CI)a | Any Adjuvant N (%) | RD (95% CI)a |
| 18–29 | † | 0.19 (0.07, 0.31) | † | −0.05 (−0.17, 0.06) | † | 0.00 (−0.07, 0.07) | † | 0.01 (0.00, 0.01) |
| 30–39 | 252 (86) | 0.14 (0.10, 0.19) | 274 (93) | −0.01 (−0.04, 0.02) | 278 (95) | −0.02 (−0.05, 0.00) | † | 0.00 (−0.02, 0.01) |
| 40–49 | 1,769 (75) | 0.05 (0.03, 0.08) | 2197 (94) | −0.01 (−0.02, 0.00) | 2,282 (97) | 0.00 (0.00, 0.01) | 2,331 (99) | 0.00 (0.00, 0.00) |
| 50–59 | 3,358 (70) | REF | 4519 (95) | REF | 4,627 (97) | REF | 4,750 (99) | REF |
| 60–69 | 3,357 (60) | −0.09 (−0.11, −0.07) | 5238 (94) | −0.01 (−0.02, 0.00) | 5,422 (97) | 0.00 (−0.01, 0.01) | 5,553 (99) | 0.00 (−0.01, 0.00) |
| 70–79 | 1,359 (42) | −0.26 (−0.29, −0.24) | 2845 (88) | −0.06 (−0.07, −0.04) | 3,108 (97) | 0.00 (−0.01, 0.01) | 3,188 (99) | 0.00 (−0.01, 0.00) |
| 80–89 | 238 (27) | −0.41 (−0.44, −0.37) | 683 (78) | −0.16 (−0.19, −0.13) | 834 (95) | −0.02 (−0.04, 0.00) | 862 (98) | −0.02 (−0.03, −0.01) |
| ≥90 | † | −0.52 (−0.64, −0.39) | 28 (68) | −0.29 (−0.44, −0.13) | † | 0.01 (−0.04, 0.06) | † | 0.01 (0.00, 0.01) |
| NODE NEGATIVE | ||||||||
| Age group | Chemotherapy N (%) | RD (95% CI)a | Radiation N (%) | RD (95% CI)a | Hormonal Therapy N (%) | RD (95% CI)a | Any Adjuvant N (%) | RD (95% CI)a |
| 18–29 | 41 (55) | 0.27 (0.16, 0.39) | † | −0.03 (−0.10, 0.03) | † | 0.01 (−0.04, 0.05) | † | 0.00 (−0.03, 0.02) |
| 30–39 | 565 (50) | 0.22 (0.19, 0.25) | 1,052 (92) | −0.03 (−0.04, −0.01) | 1,068 (94) | −0.01 (−0.03, −0.00) | 1,121 (99) | 0.00 (−0.01, 0.00) |
| 40–49 | 3,799 (31) | 0.04 (0.03, 0.05) | 11,730 (95) | 0.00 (−0.01, 0.00) | 11,851 (96) | 0.01 (0.00, 0.01) | 12,218 (99) | 0.00 (0.00, 0.00) |
| 50–59 | 7,668 (27) | REF | 27,400 (95) | REF | 27,480 (96) | REF | 28,483 (99) | REF |
| 60–69 | 9,656 (22) | −0.04 (−0.05, −0.04) | 41,129 (93) | −0.01 (−0.01, −0.01) | 41,873 (95) | 0.00 (−0.01, 0.00) | 43,526 (99) | 0.00 (0.00, 0.00) |
| 70–79 | 5,402 (17) | −0.08 (−0.09, −0.07) | 23,280 (74) | −0.20 (−0.21, −0.19) | 29,159 (93) | −0.03 (−0.03, −0.02) | 30,499 (97) | −0.02 (−0.023, −0.02) |
| 80–89 | 1,208 (12) | −0.11 (−0.12, −0.09) | 4,388 (52) | −0.42 (−0.43, −0.41) | 7,337 (87) | −0.08 (−0.09, −0.07) | 7,812 (93) | −0.06 (−0.07, −0.05) |
| ≥90 | 47 (17) | −0.07 (−0.12, −0.02) | 61 (22) | −0.72 (−0.77, −0.67) | 207 (75) | −0.19 (−0.25, −0.14) | 230 (84) | −0.15 (−0.20, −0.11) |
Adjusted histology, year of diagnosis, race/ethnicity, Charlson Comorbidity Index, insurance type, residential urbanicity, median residential income, and residential education level
Cell value suppressed for patient number
When analyzed by results of axillary surgery (node positive, node negative, or no axillary surgery), the association of axillary surgery outcome and recommendation for adjuvant therapy was stronger for those under 70 than those over 70 (Supplemental Table 4). Women over 70 with at least one positive node were 21% (RD 0.21, 95% CI 0.2, 0.23) more likely to be recommended adjuvant chemotherapy than those with negative nodes. In contrast, women <70 with at least one positive node were 42% (RD 0.42, 95% CI 0.41, 0.42) more likely to be recommended to undergo adjuvant chemotherapy than those with negative nodes. In both age groups, those who did not have any axillary surgery were about as likely as those who had negative nodes to be recommended to undergo adjuvant chemotherapy. In the over 70 group, a positive axillary node was associated with a 17% (RD 0.17, 95% CI 0.16, 0.18) increase in adjuvant radiation recommendation compared to those with negative nodes; however, a positive axillary node had no effect on the rate of adjuvant radiation recommendation in those under 70, a group in which radiation recommendation was high (94%) regardless of nodal positivity. In patients in whom axillary surgery was omitted, recommendation for adjuvant radiation was significantly less likely than those with a negative node (40% decrease in those over 70 and 22% decrease in those under 70).
Discussion
The results of our study demonstrate a significant decrease in the prevelance of of nodal positivity with age in women with T1, ER+, clinically node negative breast cancer who underwent axillary surgery, from the youngest cohort up to patients aged 70–89, independent of histologic subtype (ductal versus lobular), race, comorbidities and socioeconomic factors. Overall, <10% of older women were node positive, regardless of axillary surgery type, 95% of node positive patients were pathological stage N1mi or N1.
Despite the well-published safety of omission of axillary surgery in this patient population (with and without adjuvant radiation) 2–6 and retrospective studies confirming no impact on both regional control and overall survival 12,13 axillary surgery remains a routine part of the surgical care of older breast cancer patients. Similar to previous reports, we found a high incidence of surgical evaluation of the axilla in older women with early stage, ER+ breast cancer, despite restricting our study period to after CALGB 9343 study was published (though notably Choosing Wisely Guidelines were pubished after our study period). 6 Previous studies of NCDB and SEER have demonstrated axillary surgery use of over 76% and 80 % respectively in the decade preceding our study period 7,8, further suggesting that the change in recommendations has had minimal impact on axillary surgery use.
The decline in nodal positivity seen with age in this large, diverse cohort of patients suggests the possibility of varying biology by age, despite similarities in currently measurable predictors of disease behavior such as grade or size. Single-institution studies have had mixed results, showing decreased nodal positivity in older patients (women >66 years old)14 as well as a decreasing incidences of nodal metastases across age in women ≤70 years old, but increasing among women >70 15. Our data demonstrates a steady decline in nodal positivity prevalence with increasing age in early stage, ER+ breast cancer, among those who underwent axillary surgery. In addition to understanding cancer behavior in terms of the traditional anatomic based staging system, we must incorporate new knowledge about tumor biology in our treatment decisions. While we anxiously await the results of RxPONDER to guide adjuvant therapy decisions in one to three node-positive, ER+ patients, we do know that substantial biologic heterogeneity exists among patients, regardless of nodal status, as was illustrated by Bello et al.16 In this 2018 study, the Oncotype Dx recurrence score distribution was similar among node negative, N1mi and node positive patients, supporting the concept that anatomic status is not the sole predictor or tumor behavior. Until the results of RxPONDER are reported, we do have limited data to suggest that Oncotye Dx can be used to predict both the benefit of chemotherapy and breast cancer specific mortality in node positive patients 17,18 and retrospective data confirms that there is little to no chemotherapy benefit (versus endocrine therapy) in patients with 1–3 positive nodes and luminal A cancers19. While axillary staging is certainly not an integral part of local control in this patient population, it is also no longer the key prognostic indicator it has been historically. The use of more biologically driven determinants of prognosis may soon prove to more relevant in a population of ER+ patients.
The majority of elderly patients in our study who underwent axillary staging were node negative (90%) and among the 10% of patients that were node positive, nearly all (almost 95%) were N1mi or N1. Welsh et al have also designed a prediction rule to help guide clinicians in their decision to omit SLNB in older women 20. In a similar cohort to ours, they created a model which predicted patients to either have >10% or <10% chance of nodal positivity which fared similarly to a simple clinical rule. For example, patients with Grade 1, T1mi – T1c cancers or Grade 2, T1mi – T1b cancers had a nodal positivity incidence of 7.5% (versus 22.7% for those not meeting these criteria). However, the defined threshold (10%) may not be clinically meaningful. In the context of our existing knowledge about the impact of positive axillary nodes on survival in breast cancer, our data should reassure clinicians that omission of axillary surgery in elderly patients is safe, independent of T1 substage (T1a, T1b, or T1c) or grade. In addition to the prospective studies specifically evaluating omission of axillary surgery in elderly patients, the CALGB 9343 study omitted axillary surgery in 60% of patients (half of which were randomized to no adjuvant radiation) with no difference breast-cancer specific or overall survival with 10 years of follow up 5. In the landmark ACOSOG Z0011 trial, patients with ≤2 positive axillary nodes after SLNB were randomized to no further axillary surgery or ALND. Twenty-seven percent of patients randomized to completion ALND had additional positive lymph nodes in Z0011 and we can assume that a similar number of patients randomized to no further axillary surgery also had additional positive nodes. However, the omission of additional axillary surgery did not result in differences in overall survival or local recurrence 21 despite few patients receiving specific axillary radiation 22.
Retrospective studies show an association between axillary surgery and overall survival in elderly patients and proponents have argued this is perhaps due to additional adjuvant therapies received based on nodal status. However, the lack of disease-specific survival information suggests that these studies have significant selection bias, where women with overall better health and functional status were more likely to undergo surgery and survive (irrespective of their treatment choice) 7,8. A review of four randomized trials which included both younger and older women showed that overall survival was worse for older patients due to causes other than breast cancer and that older women are more likely to die from treatment associated morbidity than younger patients 23. Our data demonstrate that among women with node positive disease, older women were much less likely to receive adjuvant chemotherapy (but not radiation or endocrine therapy) than younger patients. While some of these patients may not have been offered adjuvant chemotherapy at all if axillary surgery was omitted, nodal status is clearly not the only factor being used to guide adjuvant treatment decisions in older women, otherwise we expect adjuvant chemotherapy use to be equivalent across age groups. Clinicians may already be moving away from using nodal stage to inform the use of chemotherapy in this patient population, further evidence that axillary staging is unnecessary in this patient population. It is notable that in patients over 70 who underwent axillary surgery, the finding of a positive node was associated with an increased rate of adjuvant radiation recommendation. However, omission of axillary surgery was associated with a 40% reduction in recommendation for adjuvant radiation compared to those who underwent axillary surgery and were node negative, suggesting that for some, adjuvant radiation recommendations are not predicated on nodal status but instead determined prior to pursuing surgical nodal staging, similar to the design of the CALGB 9343 study.
We recognize several limitations of this study. First, our data on nodal positivity is only representative of those patients who underwent axillary surgery, and axillary surgery was not randomly performed. However, this selection bias likely overestimates the actual proportion of women who are node positive, especially in older patients (in whom a significant number did not have axillary surgery). Notably, a significant number of pathologically node negative patients in all age cohorts underwent axillary lymph node dissection, suggesting either deviations from standard of care treatment and/or important clinical information not captured in our database. Additionally, we are unable to accurately assess patients’ functional status and life expectancies from this database, which could be important factors in surgical and adjuvant therapy decision-making as chronological age and biological age are not always equivalent. Patient preference may also be an important determinant of treatment that is absent from our data. Finally, participation in NCDB is voluntary, and these results may not generalize to all cancer treatment centers.
In conclusion, we found that nodal positivity declines with age in women with T1, ER+, clinically node negative breast cancer who underwent axillary surgery, and that among the small minority of older women with positive nodes, the vast majority are N1mi or N1. Additionally, the majority of node positive women over 70 are not recommended to have adjuvant chemotherapy. Among older adults, a multi-disciplinary assessment of their life expectancy, goals, and expected tolerance of chemotherapy should be assessed before embarking upon axillary surgery. Moreover, unless nodal positivity status is needed to inform their treatment, surgery should be avoided. Geriatric assessment can help in these decisions and provide information on function not caputured in the routine history and physical examination24. In addition life-expectancy can be accurately calculated from validated online caclualtors.25 Even in patients with a relatively long life expectancy with early stage ER+ breast cancer in whom chemotherapy might be well-tolerated, it is not clear that a SLNB is the only way to inform our decisions about adjuvant therapy. The biology of these patients is different than their younger counterparts and perhaps, assuming chemotherapy aligns with their goals and life expectancy, a measure of biologic behavior, such as recurrence score (Oncotype™26 or other genetic predictors such as Mammaprint27 or Prosigna28), could prove to be equally as useful.
Supplementary Material
Acknowledgments
Funding Statement: No external funding was used to support the preparation of this manuscript.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest related to this manuscript.
References
- 1.The Society of Surgical Oncology Encourages Doctors, Patients to Question Specific Commonly-used Tests and Treatments as part of Choosing Wisely Campaign. https://www.choosingwisely.org/the-society-of-surgical-oncology-joins-choosing-wisely-campaign/. Accessed April 29, 2019.
- 2.Martelli G, Miceli R, Daidone MG, et al. Axillary dissection versus no axillary dissection in elderly patients with breast cancer and no palpable axillary nodes: results after 15 years of follow-up. Ann Surg Oncol. 2011;18(1):125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Breast Cancer Study G, Rudenstam CM, Zahrieh D, et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group Trial 10–93. J Clin Oncol. 2006;24(3):337–344. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347(8):567–575. [DOI] [PubMed] [Google Scholar]
- 5.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):971–977. [DOI] [PubMed] [Google Scholar]
- 6.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chagpar AB, Hatzis C, Pusztai L, et al. Association of LN Evaluation with Survival in Women Aged 70 Years or Older With Clinically Node-Negative Hormone Receptor Positive Breast Cancer. Ann Surg Oncol. 2017;24(10):3073–3081. [DOI] [PubMed] [Google Scholar]
- 8.Tamirisa N, Thomas SM, Fayanju OM, et al. Axillary Nodal Evaluation in Elderly Breast Cancer Patients: Potential Effects on Treatment Decisions and Survival. Ann Surg Oncol. 2018;25(10):2890–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boughey JC, Haffty BG, Habermann EB, Hoskin TL, Goetz MP. Has the Time Come to Stop Surgical Staging of the Axilla for All Women Age 70 Years or Older with Hormone Receptor-Positive Breast Cancer? Ann Surg Oncol. 2017;24(3):614–617. [DOI] [PubMed] [Google Scholar]
- 10.Downs-Canner S, Zabor EC, Wind T, et al. Radiation Therapy After Breast-Conserving Surgery in Women 70 Years of Age and Older: How Wisely Do We Choose? Ann Surg Oncol. 2019;26(4):969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 12.Chung A, Gangi A, Amersi F, Zhang X, Giuliano A. Not Performing a Sentinel Node Biopsy for Older Patients With Early-Stage Invasive Breast Cancer. JAMA Surg. 2015;150(7):683–684. [DOI] [PubMed] [Google Scholar]
- 13.Poodt IGM, Schipper RJ, Vugts G, et al. The rationale for and long-term outcome of incomplete axillary staging in elderly women with primary breast cancer. Eur J Surg Oncol. 2018;44(11):1714–1719. [DOI] [PubMed] [Google Scholar]
- 14.Caywood J, Gray RJ, Hentz J, Pockaj BA. Older age independently predicts a lower risk of sentinel lymph node metastasis in breast cancer. Ann Surg Oncol. 2005;12(12):1061–1065. [DOI] [PubMed] [Google Scholar]
- 15.Wildiers H, Van Calster B, van de Poll-Franse LV, et al. Relationship between age and axillary lymph node involvement in women with breast cancer. J Clin Oncol. 2009;27(18):2931–2937. [DOI] [PubMed] [Google Scholar]
- 16.Bello DM, Russell C, McCullough D, Tierno M, Morrow M. Lymph Node Status in Breast Cancer Does Not Predict Tumor Biology. Ann Surg Oncol. 2018;25(10):2884–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibraheem AF, Press DJ, Olopade OI, Huo D. Community clinical practice patterns and mortality in patients with intermediate oncotype DX recurrence scores: Who benefits from chemotherapy? Cancer. 2019;125(2):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petkov VI, Miller DP, Howlader N, et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPJ Breast Cancer. 2016;2:16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herr D, Wischnewsky M, Joukhadar R, et al. Does chemotherapy improve survival in patients with nodal positive luminal A breast cancer? A retrospective Multicenter Study. PLoS One. 2019;14(7):e0218434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh JL, Hoskin TL, Day CN, Habermann EB, Goetz MP, Boughey JC. Predicting Nodal Positivity in Women 70 Years of Age and Older with Hormone Receptor-Positive Breast Cancer to Aid Incorporation of a Society of Surgical Oncology Choosing Wisely Guideline into Clinical Practice. Ann Surg Oncol. 2017;24(10):2881–2888. [DOI] [PubMed] [Google Scholar]
- 21.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagsi R, Chadha M, Moni J, et al. Radiation field design in the ACOSOG Z0011 (Alliance) Trial. J Clin Oncol. 2014;32(32):3600–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293(9):1073–1081. [DOI] [PubMed] [Google Scholar]
- 24.Jolly TA, Deal AM, Nyrop KA, et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist. 2015;20(4):379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suemoto CK, Ueda P, Beltran-Sanchez H, et al. Development and Validation of a 10-Year Mortality Prediction Model: Meta-Analysis of Individual Participant Data From Five Cohorts of Older Adults in Developed and Developing Countries. J Gerontol A Biol Sci Med Sci. 2017;72(3):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2018;379(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016;375(8):717–729. [DOI] [PubMed] [Google Scholar]
- 28.Wallden B, Storhoff J, Nielsen T, et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med Genomics. 2015;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.