Abstract
Objective
The accuracy of colposcopy-guided biopsy is key to the success of colposcopic triage in cervical cancer screening programs. However, there is no widely adopted biopsy guideline up to date. Our study aimed to determine whether multi-quadrants biopsy improves the yield of cervical lesions.
Methods
Eleven population-based cervical cancer screening studies were conducted in China. Cytology, high-risk human papillomavirus (hrHPV) testing and visual inspection were performed for primary screening. Females positive on one or more tests were referred for colposcopy and biopsy. The proportion of detected cervical intraepithelial neoplasia (CIN)2+ and yields by quadrant lesion-targeted biopsy or 4-quadrant random biopsy were compared.
Results
Among 4,923 females included, 1,606 had quadrant lesion-targeted biopsy, and 3,317 had 4-quadrant random biopsy. The cumulative CIN2+ yield increased from 0.10 for only one quadrant-targeted biopsy to 0.21, 0.34, and 0.58 for at most two, three and four quadrants targeted biopsies. Among hrHPV positive females with high-grade squamous intraepithelial lesion (HSIL)+ cytology, the cumulative CIN2+ yield of a second targeted biopsy in another quadrant was significantly increased (P<0.05). Among hrHPV-negative females, the yield of 4-quadrant random biopsies was 0.005, and the yield by lesion-targeted biopsies was 0.017. For hrHPV positive females who had 4-quadrant random biopsy, the additional CIN2+ yield for HSIL+, low-grade squamous intraepithelial lesion (LSIL) cytology, or abnormal visual inspection via acetic acid and Lugol’s iodine (VIA/VILI) were 0.46, 0.11, 0.14.
Conclusions
A 4-quadrant random biopsy is recommended only for hrHPV positive females with HSIL cytology, and is acceptable if hrHPV positive with LSIL cytology or with abnormal VIA/VILI. Our findings add evidences for an objective and practical biopsy standard to guide colposcopy in cervical cancer screening programs in low- and middle-income countries.
Keywords: Cervical cancer and precancerous lesion, colposcopy, low- and middle-income countries, multi-quadrant biopsy, screening
Introduction
Cervical cancer ranks fourth for both incidence and mortality worldwide among females, especially in low- and middle-income countries (1) where effective preventive interventions are inaccessible for most women (2). It has been shown that high-quality screening program with adequate coverage can reduce cervical cancer incidence and mortality (3-5). Definitive diagnosis of cervical intraepithelial neoplasia (CIN) or cancer is obtained through colposcopy with biopsy followed by histopathology examination, which makes colposcopy the central link between primary screening and therapy decisions. In the era of high-risk human papillomavirus (hrHPV) screening, the population referred to colposcopy under different screening algorithms combining HPV testing, cytology, or visual inspection, would be different (6).
The performance of colposcopy depends on the experience of the performers, the referral indications, and other factors (7). The accuracy and reproducibility among different clinicians, and the concordance between colposcopy and histopathology confirmed CIN varies greatly (8,9). In low- and middle-income countries, the lack of experienced personnel for colposcopy may lead to failure of a screening program. To address the limitations of colposcopic directed biopsy, some have recommended additional random biopsies from normal-appearing area (10,11). Some have reported additional biopsies could increase the sensitivity of colposcopic guided biopsies (12). Studies in the U.S. and European countries recommended to take additional biopsies when multiple lesions are present, whereas untargeted biopsies from normal appearing areas added detection of very little disease (13,14).
China is a populous country with a large and increasing number of cervical cancer but unsatisfactory 5-year survival (15). The government is organizing a nationwide screening system to curb the trend; however, the lack of adequate trained local doctors remains a problem. An objective criterion for biopsy would improve the performance. Only very few studies addressed the performance of multi-quadrant biopsy in resource-limited settings. Our study aims at evaluating the performance on detecting high-grade CINs with colposcopic lesion-targeted biopsies by quadrant and random biopsy under negative colposcopy among Chinese females.
Materials and methods
Population
During the year 1999 and 2008, 11 population-based cervical cancer screening studies were conducted in China by Cancer Hospital, Chinese Academy of Medical Sciences (CHCAMS), including Shanxi Province Cervical Cancer Screening Study (SPOCCS) I (1999), SPOCCS II (2001−2002), SPOCCS III (2006−2007), International Agency for Research on Cancer (IARC) study, FastHPV trial, Prevalence survey and HC2 trial. Study procedures and methodology have been described extensively elsewhere (16), as well as in Supplementary Table S1. The screened females were 17−65 years old, not pregnant currently, had no history of pelvic surgery or irradiation. The screening protocols conformed to the provisions of the Declaration of Helsinki. The Ethics Committee of CHCAMS approved all studies prior to commencing. Written informed consent was obtained.
S1. Characteristics of pooled studies.
| Study name | Study year | No. of Screened | Age
(year) |
Screening tests | Follow-up procedure | Histology location and review |
| SPOCCS, Shanxi Province Cervical Cancer Screening Study; IARC, International Agency for Research on Cancer; HC2, hybrid capture 2; LBC, liquid-based cytology; VIA, visual inspection via acetic acid; ECC, endocervical curettage; CICAMS, Cancer Institute, Chinese Academy of Medical Sciences; AFB, ampersand’s fluorescent biomolecular markers; HPV, human papillomavirus; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells, favor high grade; LSIL, low-grade squamous intraepithelial lesion; VILI, visual inspection via Lugol’s iodine. | ||||||
| SPOCCS I | 1999 | 1,997 | 35−45 | HC2 (self, physician), fluorescence test, LBC, VIA, colposcopy | All females received 4-quadrant biopsies and ECC under colposcopy | CICAMS; Blinded international review |
| SPOCCS II | 2001−
2002 |
8,497 | 35−50 | HC2 (self, physician), LBC, VIA, AFB | Positive VIA, self- or physician-test for high-risk HPV, or an abnormal AFB, or a positive Pap test (ASCUS or worse): 4-quadrant biopsies and ECC | CICAMS |
| SPOCCS III−
Shanxi |
2006 | 884 | 16−54 | HC2 (self, physician), LBC, VIA | 1) Positive VIA or positive self-HC2: colposcopy and directed biopsy, ECC if necessary; 2) positive physician-collected HC2 or ≥ASC-H on LBC: colposcopy and 4-quadrant biopsies, ECC if necessary | CICAMS; Blinded international review |
| SPOCCS III−
Beijing |
2006 | 795 | 16−54 | HC2, LBC, VIA | 1) Positive VIA: colposcopy and directed biopsy, ECC if necessary; 2) Positive physician-collected HC2 and ASC-US on LBC or ≥ASC-H: colposcopy and 4-quadrant biopsies, ECC if necessary | Peking University People’s Hospital; Blinded international and CICAMS review |
| SPOCCS III−
Xinjiang |
2006 | 883 | 16−54 | HC2 (self, physician), LBC, VIA | Same as SPOCCS III−Shanxi | CICAMS; Blinded international review |
| SPOCCS III−
Henan |
2006 | 879 | 16−54 | HC2 (self, physician), LBC, VIA | Same as SPOCCS III−Shanxi | CICAMS; Blinded international review |
| SPOCCS III−
Shanghai |
2007 | 774 | 16−54 | HC2, LBC, VIA | 1) Positive VIA: colposcopy and directed biopsy, ECC if necessary; 2) Positive physician-collected HC2 or ≥ASC-H: colposcopy and 4-quadrant biopsies, ECC if necessary | Shanghai; Blinded international and CICAMS review |
| IARC−
Shanxi |
2004 | 745 | 15−59 | HC2 fluorescence test, LBC, VIA, VILI, colposcopy | 1) Positive colposcopy or fluorescence test: directed biopsy, and ECC if necessary; 2) Negative colposcopy, but HC2 positive and ASC-US, or ≥LSIL on LBC: Follow up one year later | Yangchen County Cancer Hospital; Blinded CICAMS review |
|
Fast
HPV trial |
2007 | 818 | 30−50 | HC2, careHPVTM, LBC, VIA, VILI | 1) Either VIA/VILI or careHPVTM was positive: colposcopy and directed biopsy, and ECC if necessary; 2) VIA, VILI and careHPVTM were negative, or colposcopy was negative, but HC2 positive or ≥LSIL on cytology: 4-quadrant biopsies, and ECC if necessary | CICAMS |
| Prevalence
Survey, Jiangsu |
2008 | 316 | 18−25 | HC2, LBC, VIA | 1) Positive VIA: colposcopy and directed biopsy, and ECC if necessary; 2) Negative VIA but ≥ASC-H on LBC: colposcopy and 4-quadrant biopsies, and ECC if necessary; 3) Negative VIA but HC2 positive and ≤ASC-US on LBC: colposcopy and directed biopsy, and ECC if necessary | CICAMS |
| HC2
trial |
2008 | 1,059 | 30−59 | HC2, LBC, VIA, VILI | 1) Positive VIA/VILI: directed biopsy, and ECC if necessary; 2) Negative VIA/VILI but HC2 positive and ASC-US, or ≥ASC-H on LBC: colposcopy and 4-quadrant biopsies, and ECC if necessary; 3) Either HC2 positive or ASC-US on LBC: directed biopsy, and ECC if necessary | CICAMS |
Primary screening
Liquid-based cytology, hrHPV testing and visual inspection via acetic acid and Lugol’s iodine (VIA/VILI) were performed for primary screening. Cytology was reported according to the 2001 Bethesda nomenclature, including negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), atypical squamous cells, favor high grade (ASC-H), atypical glandular cells (AGC) and squamous cell carcinoma (SCC). Self-sampling and/or physician-sampled hybrid capture 2 (HC2) test were performed for hrHPV detection. During VIA, 5% acetic acid was applied to the surface of the cervix and aceto-white changes on the cervix were recorded; Lugol’s iodine was applied if necessary.
Colposcopy and biopsy protocol
All females included in SPOCCS I underwent colposcopy, 4-quadrant biopsy and endocervical curettage (ECC). In SPOCCS II, females with any positive primary screening were referred to colposcopy. In SPOCCS III, females with positive primary screening results were referred to colposcopy, except for ASC-US cytology but negative HPV. In IARC study, FastHPV trial, prevalence survey and HC2 trial, colposcopy-guided directed biopsy and 4-quadrant biopsies were required. ECC was performed if necessary. Specific results of primary screening tests were masked from colposcopists. In the colposcopy examination, 5% of acetic acid was applied on the cervix. The surface of the cervix was divided by perpendicular lines drawn from 12- to 6- o’clock and from 3- to 9-o’clock. The four cervical quadrants were labeled clockwise with the first quadrant located on the right upper. The severity of colposcopic findings per quadrant was recorded, including LSIL, HSIL or cancer (17). Females received lesion-targeted biopsy in most severe suspicious lesion areas and random biopsy in other normal-appearing quadrants at the squamocolumnar junction around 2-, 4-, 8-, or 10-o’clock. All random biopsy was performed with a bronchoscopy biopsy instrument that has 2 mm jaws to prevent severe pain, bleeding, etc. All lesion-targeted biopsies were performed with regular biopsy forceps. The cytology and histopathology samples have been blindly reviewed by international or CHCAMS pathology experts.
Statistical analysis
Participants with complete data of screening results were included for analysis. It was defined that a female had quadrant lesion-targeted biopsy if visible lesions in one or more quadrants were biopsied under abnormal colposcopic impression; otherwise, it was defined as 4-quadrant random biopsy. If more than one lesion-targeted biopsy were recorded in one quadrant, the most severe finding of the colposcopy and histopathology findings were included in the dataset. CIN2+ was set as the endpoint due to the clinical practice for treatment in China. The data were stratified by biopsy type (quadrant lesion-targeted biopsy, or 4-quadrant random biopsy), cytology result (NILM, ASC-US, LSIL, HSIL+: ASC-H, HSIL, SCC), hrHPV status (negative or positive), VIA/VILI results (normal or abnormal), colposcopy diagnosis (normal, LSIL, HSIL or cancer), and age (≤34, 35−39, 40−44, ≥45 years).
Females with histopathology confirmed CIN2+ under abnormal colposcopic impression were stratified into four groups based on the maximum number of quadrants targeted biopsies needed to detect at least one quadrant of CIN2+, ranking order by the severity of colposcopy impression per quadrant. When fewer than four quadrant biopsies were taken in some cases, the maximum number of biopsied needed ranking by colposcopy severity could not be directly measured. Since the most majority of females included (93.9%) have had 4-quadrant biopsy irrespective of the colposcopic findings, ordinal logistic regression models were used to impute the histopathology findings for the non-biopsied quadrant, adjusting for colposcopy impression per quadrant, cytology, HPV infection, VIA/VILI and age. Proportion of cases was the percentage of females found to have CIN2+ cases stratified by the maximum number of lesion-targeted biopsy needed or additional random biopsy among all females eventually diagnosed as CIN2+ (CIN2+ detected by ECC alone was excluded). The yield was the percentage of CIN2+ cases among females with quadrant-targeted biopsies or 4-quadrant random biopsy in each stratum. The average number of random biopsy needed per additional CIN2+ cases was calculated for both normal and abnormal colposcopic impression females. Chi-square test was performed to compare proportions. Chi-square trend test was used to detect the yield trend. The data analysis was performed with IBM SPSS Statistics (Version 23.0; IBM Corp., New York, USA). Two-sided P<0.05 was considered statistically significant.
Results
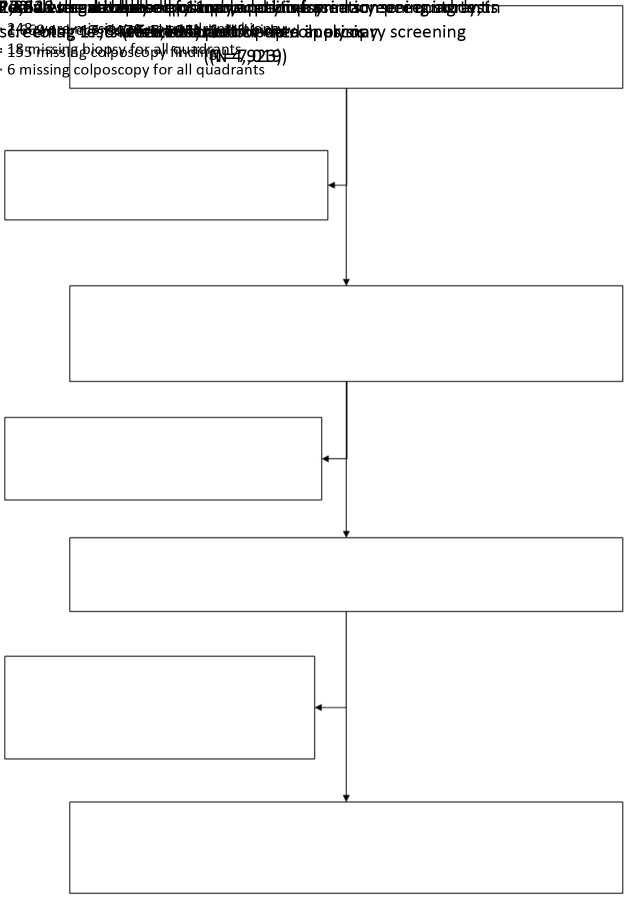
Among 17,647 females participated in primary screening, 7,019 females were positive on one or more primary screening tests, and 5,195 were referred to colposcopy successfully. Among them, 272 were excluded for no quadrants biopsy or missing data, and 4,923 females having data of all primary screening results and quadrant record of colposcopy and biopsy were included (Figure 1). Among them, 1,606 females (32.6%) had at least one quadrant lesion-targeted biopsy, 3,317 females (67.4%) had 4-quadrant random biopsy. The median age was 40 (range: 19−59) years. Of the 4,923 patients, 600 (12.2%) were CIN1, 266 (5.4%) were CIN2, 264 (5.4%) were CIN3 and 39 (0.8%) were cancer. No additional CIN2+ per quadrant was estimated by the regression models for the quadrant not biopsied. The CIN2+ prevalence of the screening population was 3.2%. The 24 CIN2+ cases found by ECC alone will not be further discussed here. The CIN2+ yield increased with age, worse cytology, positive hrHPV status, abnormal VIA/VILI, and worse colposcopy impression as shown inTable 1.
1. Flowchart of data included.
1. Clinical characteristics of study population by biopsy types*.
| Variables | Abnormal colposcopy impression and up
to 4 targeted biopsies (N=1,606) |
Normal colposcopy impression and 4-quadrant
random biopsies (N=3,317) |
|||||
| Normal/CIN1
[n (%)] |
CIN2+ [n (%)] | χ2, P | Normal/CIN1
[n (%)] |
CIN2+ [n (%)] | χ2, P | ||
| *, 24 cases of CIN2+ found by endocervical curettage alone, 8 of them had lesion targeted biopsy and 16 of them had 4-quadrant random biopsy; **, 5 cases of unsatisfactory cytology were not listed; CIN, cervical intraepithelial neoplasia; NILM, negative for intraepithelial lesion or malignancy; ASC-US, atypical squamous cells of undetermined significance; AGC, atypical glandular cells; ASC-H, atypical squamous cells, favor high grade; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous cell carcinoma; HPV, human papillomavirus; VIA/VILI, visual inspection via acetic acid and Lugol’s iodine. | |||||||
| Age (year) | 32.74, <0.001 | 15.53, 0.001 | |||||
| ≤34 | 178 (87.7) | 25 (12.3) | 178 (92.2) | 15 (7.8) | |||
| 35−39 | 473 (79.8) | 120 (20.2) | 1,282 (96.2) | 50 (3.8) | |||
| 40−44 | 382 (75.6) | 123 (24.4) | 999 (94.6) | 57 (5.4) | |||
| ≥45 | 205 (67.2) | 100 (32.8) | 681 (92.5) | 55 (7.5) | |||
| Cytology** | 633.65, <0.001 | 690.59, <0.001 | |||||
| NILM | 720 (96.1) | 29 (3.9) | 1,877 (98.8) | 23 (1.2) | |||
| ASC-US | 234 (87.3) | 34 (12.7) | 721 (96.9) | 23 (3.1) | |||
| AGC | 6 (100) | 0 (0) | 15 (83.3) | 3 (16.7) | |||
| ASC-H | 5 (71.4) | 2 (28.6) | 7 (77.8) | 2 (22.2) | |||
| LSIL | 183 (72.3) | 70 (27.7) | 406 (90.8) | 41 (9.2) | |||
| HSIL | 85 (27.5) | 224 (72.5) | 109 (55.3) | 88 (44.7) | |||
| SCC | 0 (0) | 9 (100) | 1 (50.0) | 1 (50.0) | |||
| HPV | 176.71, <0.001 | 31.92, <0.001 | |||||
| Negative | 475 (98.3) | 8 (1.7) | 572 (99.5) | 3 (0.5) | |||
| Positive | 763 (67.9) | 360 (32.1) | 2,568 (93.7) | 174 (6.3) | |||
| VIA/VILI | 61.98, <0.001 | 9.35, 0.009 | |||||
| Normal | 510 (81.5) | 116 (18.5) | 2,738 (94.9) | 148 (5.1) | |||
| Abnormal | 728 (74.3) | 252 (25.7) | 402 (93.3) | 29 (6.7) | |||
| Colposcopy | 205.52, <0.001 | − | |||||
| Normal | 0 (0) | 0 (0) | 3,140 (94.7) | 177 (5.3) | |||
| LSIL | 1,096 (83.9) | 211 (16.1) | 0 (0) | 0 (0) | |||
| HSIL | 137 (51.7) | 128 (48.3) | 0 (0) | 0 (0) | |||
| Cancer | 5 (14.7) | 29 (85.3) | 0 (0) | 0 (0) | |||
Quadrant lesion-targeted biopsy
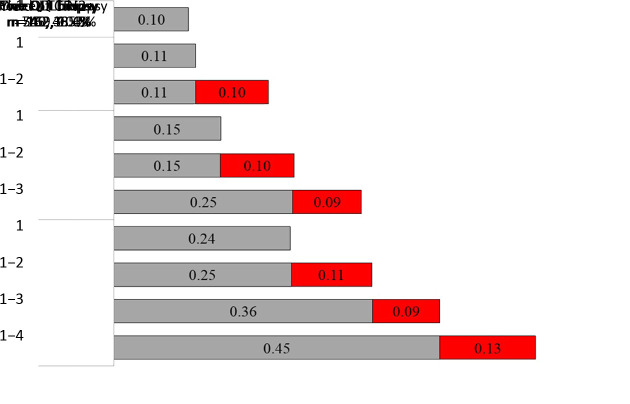
Among 1,606 females had lesion-targeted biopsies, the yield was 0.23 (368/1,606). In Figure 2, females were stratified by the number of quadrant lesion-targeted biopsies. The CIN2+ yield of the first quadrant-targeted biopsy increased from 0.10 in females with only one quadrant-targeted biopsy to 0.24 in females with four. The incremental yield of each additional quadrant lesion-targeted biopsy in another quadrant ranged from 0.09 to 0.13. The incremental yield of a second quadrant lesion-targeted biopsy for females having had two, three and four were 0.10, 0.10 and 0.11 (χ2=0.34, P=0.844). The incremental yield of a third biopsy for females who had three and four quadrants lesion-targeted biopsy were 0.09. The incremental yield of a fourth quadrant lesion-targeted biopsy was 0.13.
2. Yield of CIN2+ by number of QLT biopsy needed. Females have had lesion-targeted biopsy were grouped by the number of QLT biopsy. The bars show CIN2+ yield by the number of quadrant-targeted biopsy needed. Red bars are additional yield of each additional lesion-targeted biopsy ranked by severity of the colposcopic impression of each quadrant. CIN, cervical intraepithelial neoplasia; QLT, quadrant lesion-targeted.
There were 1,454 females have had less than four quadrants of colposcopic suspicious lesions. The CIN2+ yield by quadrant-targeted biopsy was 0.13 (94/745) for one, 0.23 (126/540) for two, 0.36 (60/169) for three and 0.58 (88/152) for four quadrant-targeted biopsies. Among them, 30 CIN2+ cases were found by additional random biopsy on normal-appearing quadrant, of which 17 females had one quadrant-targeted biopsy, 11 females had two and 2 females had three. The additional yield was 0.02 (30/1,454). The 30 CIN2+ cases were all hrHPV-positive, 93.3% (28/30) were older than 35 years, 66.7% (20/30) were cytological LSIL+, 90.0% (27/30) were LSIL impression under colposcopy, and the CIN2+ lesion of 86.7% (26/30) cases were within one quadrant.
Table 2 shows the stratified cumulative proportion of cases and yield of CIN2+ by the first quadrant-targeted biopsy ranked by the severity of colposcopic finding, or, at most two, three, four quadrant-targeted biopsies needed, and additional random biopsies stratified. The cumulative proportion for only one, at most two, three and four lesion-targeted biopsy were 54.3% [95% confidence interval (95% CI): 49.2%, 59.4%], 78.3% (95% CI: 73.8%, 82.2%), 86.4% (95% CI: 82.5%, 89.5%) and 91.8% (95% CI: 88.6%, 94.2%) among all CIN2+ cases. The overall increasing proportion of cases for each additional quadrant-targeted biopsy needed showed significant difference, the first to the second (χ2=47.09, P<0.001), the second to the third biopsy (χ2=8.41, P=0.004) and third to the fourth (χ2=5.61, P=0.018). Except for hrHPV negative or age ≤34 years old, the increasing proportion of cases from the first to the second biopsy showed statistical significance in all strata. In HSIL+ cytology, hrHPV positive, VIA abnormal, colposcopy LSIL, the increasing proportion of cases from the second to the third biopsy also showed statistical significance. The highest cumulative CIN2+ yield and the additional random biopsy yield were found in HSIL+ cytology (0.69, 0.05).
2. Proportion of detected cases and yield of CIN2+ by biopsies numbers among females with abnormal colposcopy impression.
| Clinical diagnosis | Biopsy needed* | Lesion-targeted biopsy | Additional random biopsy | |||||||||
| CIN2+ (n) | Cumulative proportion (%) | 95% CI (%) | Cumulative CIN2+ yield | CIN2+ (n) | Cumulative proportion (%) | 95% CI (%) | Cumulative CIN2+ yield | |||||
| *, “Biopsy needed” describes that to detect one quadrant of CIN2+, the maximum number of lesion-targeted biopsy needed by quadrant, ranking order by the severity of colposcopy impression; **, 5 cases of unsatisfactory cytology and 6 cases of AGC were not listed here. HSIL+ including ASC-H, HSIL, and SCC; CIN, cervical intraepithelial neoplasia; NILM, negative for intraepithelial lesion or malignancy; ASC-US, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; HPV, human papillomavirus; VIA/VILI, visual inspection via acetic acid and Lugol’s iodine; 95% CI, 95% confidence interval; ASC-H, atypical squamous cells, favor high grade; SCC, squamous cell carcinoma; AGC, atypical glandular cells. | ||||||||||||
| ALL (N=1,606) | 1 | 200 | 54.3 | 49.2 | 59.4 | 0.12 | 17 | 4.6 | 2.9 | 7.3 | 0.01 | |
| 1−2 | 288 | 78.3 | 73.8 | 82.2 | 0.18 | 28 | 7.6 | 5.3 | 10.8 | 0.02 | ||
| 1−3 | 318 | 86.4 | 82.5 | 89.5 | 0.20 | 30 | 8.2 | 5.8 | 11.4 | 0.02 | ||
| 1−4 | 338 | 91.8 | 88.6 | 94.2 | 0.21 | |||||||
| Cytology** | ||||||||||||
| NILM (N=749) | 1 | 15 | 51.7 | 34.4 | 68.6 | 0.02 | 1 | 3.4 | 0.6 | 17.2 | 0 | |
| 1−2 | 24 | 82.8 | 65.5 | 92.4 | 0.03 | 3 | 10.3 | 3.6 | 26.4 | 0 | ||
| 1−3 | 26 | 89.7 | 73.6 | 96.4 | 0.03 | 3 | 10.3 | 3.6 | 26.4 | 0 | ||
| 1−4 | 26 | 89.7 | 73.6 | 96.4 | 0.03 | |||||||
| ASC-US (N=268) | 1 | 13 | 38.2 | 23.9 | 55.0 | 0.05 | 5 | 14.7 | 6.5 | 30.1 | 0.02 | |
| 1−2 | 23 | 67.6 | 50.8 | 80.9 | 0.09 | 7 | 20.6 | 10.4 | 36.8 | 0.03 | ||
| 1−3 | 25 | 73.5 | 56.9 | 85.4 | 0.09 | 7 | 20.6 | 10.4 | 36.8 | 0.03 | ||
| 1−4 | 27 | 79.4 | 63.2 | 89.7 | 0.10 | |||||||
| LSIL (N=253) | 1 | 41 | 58.6 | 46.9 | 69.4 | 0.16 | 2 | 2.9 | 0.8 | 9.8 | 0.01 | |
| 1−2 | 55 | 78.6 | 67.6 | 86.6 | 0.22 | 4 | 5.7 | 2.2 | 13.8 | 0.02 | ||
| 1−3 | 58 | 82.9 | 72.4 | 89.9 | 0.23 | 4 | 5.7 | 2.2 | 13.8 | 0.02 | ||
| 1−4 | 66 | 94.3 | 86.2 | 97.8 | 0.26 | |||||||
| HSIL+ (N=325) | 1 | 131 | 56.2 | 49.8 | 62.4 | 0.41 | 9 | 3.9 | 2.0 | 7.2 | 0.03 | |
| 1−2 | 186 | 79.8 | 74.2 | 84.5 | 0.58 | 14 | 6.0 | 3.6 | 9.8 | 0.04 | ||
| 1−3 | 209 | 89.7 | 85.1 | 93.0 | 0.66 | 16 | 6.9 | 4.3 | 10.9 | 0.05 | ||
| 1−4 | 219 | 94.0 | 90.2 | 96.4 | 0.69 | |||||||
| HPV | ||||||||||||
| HPV− (N=483) | 1 | 8 | 100 | 67.6 | 100 | 0.02 | 0 | 0 | 0 | 0 | 0 | |
| 1−2 | 8 | 100 | 67.6 | 100 | 0.02 | 0 | 0 | 0 | 0 | 0 | ||
| 1−3 | 8 | 100 | 67.6 | 100 | 0.02 | 0 | 0 | 0 | 0 | 0 | ||
| 1−4 | 8 | 100 | 67.6 | 100 | 0.02 | |||||||
| HPV+ (N=1,123) | 1 | 192 | 53.6 | 48.2 | 58.4 | 0.17 | 17 | 4.7 | 3.0 | 7.4 | 0.02 | |
| 1−2 | 280 | 77.8 | 73.2 | 81.8 | 0.25 | 28 | 7.8 | 5.4 | 11.0 | 0.02 | ||
| 1−3 | 310 | 85.8 | 82.2 | 89.3 | 0.28 | 30 | 8.3 | 5.9 | 11.7 | 0.03 | ||
| 1−4 | 330 | 91.7 | 88.4 | 94.1 | 0.29 | |||||||
| VIA/VILI | ||||||||||||
| Normal (N=626) | 1 | 62 | 53.4 | 44.4 | 62.3 | 0.10 | 7 | 6.0 | 3.0 | 11.9 | 0.01 | |
| 1−2 | 88 | 75.9 | 67.3 | 82.7 | 0.14 | 14 | 12.1 | 7.3 | 19.2 | 0.02 | ||
| 1−3 | 97 | 83.6 | 75.8 | 89.3 | 0.15 | 14 | 12.1 | 7.3 | 19.2 | 0.02 | ||
| 1−4 | 102 | 87.9 | 80.8 | 92.7 | 0.16 | |||||||
| Abnormal
(N=980) |
1 | 138 | 54.8 | 48.6 | 60.8 | 0.14 | 10 | 4.0 | 1.6 | 6.4 | 0.01 | |
| 1−2 | 200 | 79.4 | 74.0 | 83.9 | 0.20 | 14 | 5.6 | 2.7 | 8.4 | 0.01 | ||
| 1−3 | 221 | 87.7 | 83.1 | 91.2 | 0.23 | 16 | 6.3 | 3.3 | 9.4 | 0.02 | ||
| 1−4 | 236 | 93.7 | 89.9 | 96.1 | 0.24 | |||||||
| Colposcopy | ||||||||||||
| LSIL (N=1,307) | 1 | 103 | 48.8 | 42.2 | 55.5 | 0.08 | 16 | 7.6 | 3.9 | 10.1 | 0.01 | |
| 1−2 | 154 | 73.0 | 66.6 | 78.5 | 0.12 | 25 | 11.8 | 6.8 | 14.2 | 0.02 | ||
| 1−3 | 176 | 83.4 | 77.8 | 87.8 | 0.13 | 27 | 12.8 | 7.5 | 15.1 | 0.02 | ||
| 1−4 | 184 | 87.2 | 82.0 | 91.1 | 0.14 | |||||||
| HSIL+ (N=299) | 1 | 97 | 61.8 | 54.0 | 69.0 | 0.32 | 1 | 0.6 | 0.1 | 3.5 | 0 | |
| 1−2 | 134 | 85.4 | 79.0 | 90.0 | 0.45 | 3 | 1.9 | 0.7 | 5.5 | 0.01 | ||
| 1−3 | 142 | 90.4 | 84.8 | 94.1 | 0.47 | 3 | 1.9 | 0.7 | 5.5 | 0.01 | ||
| 1−4 | 154 | 98.1 | 94.5 | 99.4 | 0.52 | |||||||
| Age (year) | ||||||||||||
| ≤34 (N=203) | 1 | 14 | 56.0 | 37.1 | 73.3 | 0.07 | 0 | 0 | 0 | 0 | 0 | |
| 1−2 | 19 | 76.0 | 56.6 | 88.5 | 0.09 | 2 | 8.0 | 2.2 | 25.0 | 0.01 | ||
| 1−3 | 22 | 88.0 | 70.1 | 95.8 | 0.11 | 2 | 8.0 | 2.2 | 25.0 | 0.01 | ||
| 1−4 | 23 | 92.0 | 75.0 | 97.8 | 0.11 | |||||||
| 35−39 (N=593) | 1 | 55 | 45.8 | 37.2 | 54.7 | 0.09 | 3 | 2.5 | 0.9 | 7.1 | 0.01 | |
| 1−2 | 95 | 79.2 | 71.1 | 85.5 | 0.16 | 5 | 4.2 | 1.8 | 9.4 | 0.01 | ||
| 1−3 | 105 | 87.5 | 80.4 | 92.3 | 0.18 | 6 | 5.0 | 2.3 | 10.5 | 0.01 | ||
| 1−4 | 114 | 95.0 | 89.5 | 97.7 | 0.19 | |||||||
| 40−44 (N=505) | 1 | 72 | 58.5 | 49.7 | 66.9 | 0.14 | 11 | 8.9 | 5.1 | 15.3 | 0.02 | |
| 1−2 | 92 | 74.8 | 66.5 | 81.6 | 0.18 | 15 | 12.2 | 7.5 | 19.2 | 0.03 | ||
| 1−3 | 103 | 83.7 | 76.2 | 89.2 | 0.20 | 15 | 12.2 | 7.5 | 19.2 | 0.03 | ||
| 1−4 | 108 | 87.8 | 80.9 | 92.5 | 0.21 | |||||||
| ≥45 (N=305) | 1 | 59 | 59.0 | 49.2 | 68.1 | 0.19 | 3 | 3.0 | 1.0 | 8.5 | 0.01 | |
| 1−2 | 82 | 82.0 | 73.3 | 88.3 | 0.27 | 6 | 6.0 | 2.8 | 12.5 | 0.02 | ||
| 1−3 | 88 | 88.0 | 80.2 | 93.0 | 0.29 | 7 | 7.0 | 3.4 | 13.8 | 0.02 | ||
| 1−4 | 93 | 93.0 | 86.3 | 96.6 | 0.30 | |||||||
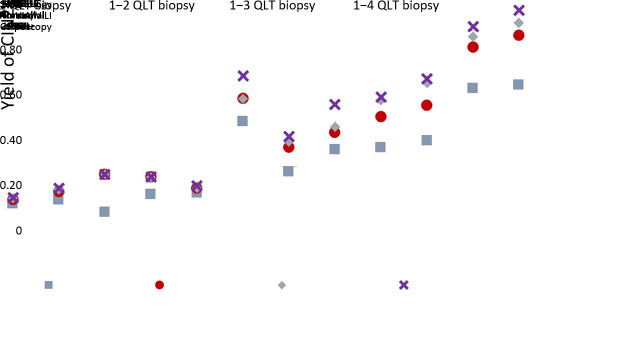
The stratified cumulative CIN2+ yield combining cytology, VIA/VILI and colposcopy for only one, at most two, three, or four lesion-targeted biopsies among hrHPV positive females were shown in Figure 3. The yields were very low in hrHPV positive females with NILM or ASC-US cytology with LSIL colposcopy impression. In LSIL cytology, the cumulative CIN2+ yield for only one, at most two, three, or four quadrant lesion-targeted biopsies were not significantly different in normal VIA/VILI and LSIL colposcopy, but was nearly doubled in abnormal VIA/VILI for the fourth vs. the first biopsy (0.33 vs. 0.18, χ2=5.28, P=0.021). Interestingly, 10 hrHPV positive females with LSIL cytology were VIA/VILI normal in primary screening, however were HSIL+ under colposcopy. The CIN2+ yield of the 10 females was high as 0.60, with 66.67% (4/6) could be detected by the first lesion-targeted biopsy. In HSIL+ cytology, the cumulative yield was significantly increased from the first to the third quadrant-targeted biopsy if the VIA/VILI was normal and colposcopy was LSIL (0.28 vs. 0.51, χ2=8.37, P=0.004). There was significant increased yield from the first to the second quadrant-targeted biopsy in abnormal VIAVILI with LSIL (0.31 vs. 0.47, χ2=5.27, P=0.022) or HSIL+ colposcopy (0.56 vs. 0.78, χ2=11.95, P=0.001).
3. Yield of CIN2+ by quadrant-targeted biopsy in hrHPV infected females stratified by cytology, VIA and colposcopy results. CIN, cervical intraepithelial neoplasia; hrHPV, high-risk human papillomavirus; QLT, quadrant lesion-targeted; VIA/VILI, visual inspection via acetic acid and Lugol’s iodine; ASC-US, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion.
4-quadrants random biopsy
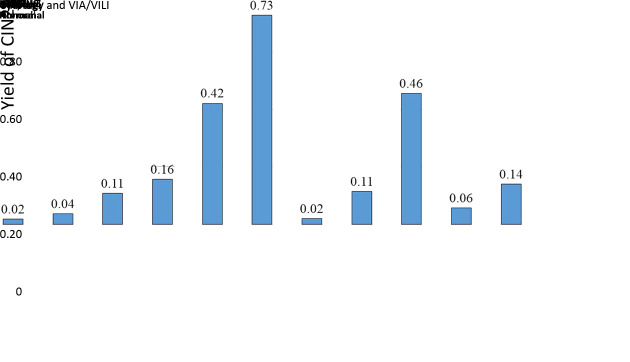
A total of 3,317 females had 4-quadrant random biopsy and 177 were found to be CIN2+, the yield was 0.05. The yield was low in hrHPV negative females, which was 0.005 (3/575). For females infected with hrHPV, the yield of CIN2+ stratified by cytology and VIA/VILI was shown in Figure 4. The highest CIN2+ yield was 0.73 among hrHPV positive females with HSIL+ cytology and abnormal VIA/VILI. For hrHPV positive females, the difference between yields of LSIL and HSIL+ cytology showed statistical significance (0.11 vs. 0.46, χ2=81.16, P<0.001), normal and abnormal VIA/VILI (0.06vs. 0.14, χ2=21.85, P<0.001). Eighty-eight CIN2+ cases were found in 193 females with hrHPV and HSIL+ cytology but normal colposcopy, that is to say, almost every 2−3 females would be detected as CIN2+ by 4-quadrant random biopsy in this population. Less than 8−9 females should have 4-quadrant random biopsy to detect one more CIN2+ cases among hrHPV positive females with abnormal primary VIA/VILI (7.2, 208/29), or LSIL cytology (8.7, 348/40). In hrHPV positive females with HSIL+ cytology, the CIN2+ yield of 4-quadrant random biopsy was the same as four quadrants targeted biopsies (16/22, 0.73vs. 164/211, 0.78; χ2=0.283, P=0.595) if the primary VIA/VILI was abnormal, and regardless of the severity of colposcopy findings. No adverse events such as severe pain, bleeding requiring additional interventions, cervical infections and complications requiring hospitalization occurred in our study.
4. Yield of CIN2+ by random biopsy in hrHPV infected females with normal colposcopic impression, stratified by cytology and VIA/VILI. CIN, cervical intraepithelial neoplasia; hrHPV, high-risk human papillomavirus; VIA/VILI, visual inspection via acetic acid and Lugol’s iodine; ASC-US, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion.
Discussion
The main findings of our study proved that multi-quadrants biopsy increases the accuracy of colposcopy and the CIN2+ yield, especially in some subgroups, such as positive hrHPV and high-grade cytological findings. Females testing positive on cervical cancer screening are referred for colposcopy triage and lesion-targeted biopsy from abnormal-looking areas for diagnosis of cervical neoplasia. The efficiency of detecting high-grade CIN and cervical cancer by colposcopic directed biopsy has been a significant concern for decades and colposcopy with a single biopsy can miss high-grade CIN. It has been well documented that colposcopic assessment and biopsy directing practices are less reproducible and could miss a substantial proportion of prevalent high-grade CIN (12,18,19). A recent study in China reported that among females having LSIL+ cytology but negative biopsy finding at baseline, 20% of the called-back population were histopathological confirmed as high-grade CIN or cervical intraepithelial cancer at the follow-up punch biopsy, cone biopsy, or hysterectomy specimen (20). These observations have led to the practice of taking multiple targeted biopsies from colposcopic abnormal areas and taking random biopsies from normal looking areas to improve the detection rate of high-grade CIN. It has been shown that multiple quadrant lesion-directed biopsies during colposcopy increased detection of high-grade CIN (13,19,21-23) and our results are consistent with this observation. The incremental benefit of taking multiple quadrant biopsies in improving the diagnosis of high-grade CIN is clear in our study. With the rigorous study design and large sample size, our findings provided evidence for colposcopy-guided biopsy that may be helpful for the current clinical practice.
The number and size of visible lesions under colposcopy are closely related to the severity of the lesion (7,24), which is reasonable for the increased yield of the first quadrant lesion-targeted biopsy along with the number of targeted biopsies. Nearly two-fold increase in CIN2+ yield for the first quadrant biopsy was found for females having targeted biopsies in four quadrants than females had only one quadrant-targeted biopsy. The highest increment of the second biopsy was found in females having four quadrant-targeted biopsies. These results are similar to Wentzensen’s study (13) that reported a three-fold (12%−34%) increase in yield from females with one directed biopsy to four directed biopsies. Besides, the greatest increase in yield was also found in the second biopsy in their study. However, stratified by the number of lesion-targeted biopsies, the incremental yield of each additionally targeted biopsy ranges between 0.09−0.13 whereas they reported decreasing increments for each additional biopsy in the third and fourth targeted biopsy (13). A possible explanation could be the different biopsy protocols. In their study, multiple lesion-directed biopsies were taken at a lower threshold for abnormality, possibly explaining the lower yield from subsequent non-directed biopsies (13). However, the most severe suspicious areas in each quadrant were biopsied in our studies. The incremental CIN2+ yield would be affected by the size, number and the distribution of the optical lesions. It also explains that 80.0% (1,285/1,606) of females in our study had less than three targeted biopsies and 42.0% in theirs.
Several studies have reported a higher yield of CIN2+ by additional biopsies. Zuchna et al. (21) reported that two biopsies achieved a highly significant improvement in the agreement between punch biopsy and cone specimen compared with one biopsy, and suggested three or more biopsies may improve sensitivity. Stoler et al. (19) found taking more than one biopsy was significantly associated with fewer missed CIN3+ cases. A Japanese study concluded that two targeted biopsies should be taken, and random biopsies at normal appearing quadrants are recommended for low-grade cytology (22). A study in Thailand concluded that multiple biopsies increased the sensitivity of CIN2+ detection (23). In our study, in addition to the first targeted biopsy, a second one could significantly improve the cumulative sensitivity of lesion-targeted biopsy by nearly 25%. Besides, a third targeted biopsy could increase the sensitivity by nearly 10%, suggesting that a third targeted biopsy also should be performed.
The primary screening results are pivotal indications for biopsy under colposcopy. In our study, the cumulative CIN2+ yield of quadrant-targeted biopsy increased gradually from only one to at most four targeted biopsies in different primary screening and colposcopy findings strata. The highest CIN2+ yield was found in hrHPV positive females with abnormal VIA/VILI, HSIL+ cytology, and HSIL+ colposcopy. It is worth noting that the CIN2+ yield between normal and abnormal colposcopy findings in these females was not significantly different. It has been reported a significantly less mean average epithelial thickness of CIN2 or CIN3 between normal colposcopy impression and low-, high-grade CIN or suspected cancer colposcopic impression (25), that a false negative colposcopic impression is sometimes secondary to the relative thinness of some CIN2−3 lesions (25). It also may be partly attributed to the misclassification of benign changes under colposcopy.
Strengths of our study include the large sample size with rigorous methodology, high quality of diagnostic assurance. In our study, females with one or more positive primary screening results referred to colposcopy, the majority of them (93.9%) have had four quadrants biopsies regardless of the colposcopic findings per quadrant, which could minimize the verification bias. Since different cervical cancer screening practices were recommended worldwide according to the accessibility to health resources, the screening method for different countries and population varies significantly. In China, cytology, visual inspection, and hrHPV testing were recommended for primary screening. Our data presented the accuracy of multi-quadrants biopsy under different combinations of primary screening, which were practical for different population-based screening programs.
A major limitation is the limited information of hrHPV testing. Basu et al. found significantly higher detection rate of CIN2+ in females with intermediate and high viral load and recommended multiple punch biopsies in this population, even if colposcopy is normal (26). HPV 16/18 positive females have a much higher risk of developing high-grade CINs (27,28) and could be detected in most cervical cancer tissues (29,30). It is recommended that females with negative cytology but positive HPV16/18 should be referred for colposcopy (31). Another limitation is that random biopsy was taken at fixed location on the cervix. Several studies reported there might be a topographical pattern of CIN on the cervix, which may result in underestimating the CIN2+ yield of biopsy at normal-appearing areas (32-34). Possible harms of taking multiple biopsies are discomfort and increased the cost of pathology processing and evaluation. In our study, taking additional quadrant-targeted biopsies with sharp high-quality biopsy forceps and random biopsies using bronchoscopy biopsy instrument was well tolerated. No adverse events occurred in our study. It has been shown that multiple biopsies did not increase the risk of subsequent HPV infections (35).
Conclusions
The CIN2+ yield could be improved by each additional targeted biopsy in different quadrants under abnormal colposcopic impression. A 4-quadrant random biopsy under the normal colposcopic impression is recommended for positive hrHPV and HSIL+ cytology, and is acceptable if hrHPV positive with LSIL cytology or abnormal VIA/VILI.
Acknowledgements
We thank all collaborators of the original screening studies, including Dr. Jerome L. Belinson from the Department of Obstetrics and Gynecology, the Cleveland Clinic Foundation, Dr. Robert G. Pretorius from the Department of Obstetrics and Gynecology, Southern California Permanente Medical Group. We thank Dr. Nicolas Wentzensen from the National Cancer Institution for his review of the original manuscript. We thank the field doctors and the participants, as well as the International Agency for Research on Cancer, and the Cleveland Clinic for their generous support. This work was supported by the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (No. 2016-I2M-1-019); and the National Natural Science Foundation of China (No. 81322040).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Bruni L, Diaz M, Barrionuevo-Rosas L, et al Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4:e453–63. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 3.Ronco G, Dillner J, Elfström KM, et al Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 5.Morris E, Roett MA Genital cancers in women: cervical cancer. FP Essent. 2015;438:18–23. [PubMed] [Google Scholar]
- 6.Schiffman M, Wentzensen N Issues in optimising and standardising the accuracy and utility of the colposcopic examination in the HPV era. Ecancermedicalscience. 2015;9:530. doi: 10.3332/ecancer.2015.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdul-Karim FW, Yang B Cytologic-histologic discrepancies in pathology of the uterine cervix: Analysis of the clinical and pathologic factors. Adv Anat Pathol. 2017;24:304–9. doi: 10.1097/PAP.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 8.Bifulco G, De Rosa N, Lavitola G, et al A prospective randomized study on limits of colposcopy and histology: the skill of colposcopist and colposcopy-guided biopsy in diagnosis of cervical intraepithelial lesions. Infect Agent Cancer. 2015;10:47. doi: 10.1186/s13027-015-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh I, Mittal S, Banerjee D, et al Study of accuracy of colposcopy in VIA and HPV detection‐based cervical cancer screening program. Aust N Z J Obstet Gynaecol. 2014;54:570–5. doi: 10.1111/ajo.12282. [DOI] [PubMed] [Google Scholar]
- 10.Hu SY, Zhang WH, Li SM, et al Pooled analysis on the necessity of random 4-quadrant cervical biopsies and endocervical curettage in women with positive screening but negative colposcopy. Medicine (Baltimore) 2017;96:e6689. doi: 10.1097/MD.0000000000006689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baasland I, Hagen B, Vogt C, et al Colposcopy and additive diagnostic value of biopsies from colposcopy-negative areas to detect cervical dysplasia. Acta Obstet Gynecol Scand. 2016;95:1258–63. doi: 10.1111/aogs.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gage JC, Hanson VW, Abbey K, et al Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264–72. doi: 10.1097/01.AOG.0000220505.18525.85. [DOI] [PubMed] [Google Scholar]
- 13.Wentzensen N, Walker JL, Gold MA, et al Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33:83–9. doi: 10.1200/JCO.2014.55.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Marel J, van Baars R, Rodriguez A, et al The increased detection of cervical intraepithelial neoplasia when using a second biopsy at colposcopy. Gynecol Oncol. 2014;135:201–7. doi: 10.1016/j.ygyno.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 15.Ginsburg O, Bray F, Coleman MP, et al The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017;389:847–60. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao FH, Lin MJ, Chen F, et al Performance of high-risk human papillomavirus DNA testing as a primary screen for cervical cancer: a pooled analysis of individual patient data from 17 population-based studies from China. Lancet Oncol. 2010;11:1160–71. doi: 10.1016/S1470-2045(10)70256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sellors J, Sankaranarayanan R. Colposcopy and treatment of cervical intraepithelial neoplasia. Geneva: WHO, 2003.
- 18.Massad LS, Jeronimo J, Schiffman M, et al Interobserver agreement in the assessment of components of colposcopic grading. Obstet Gynecol. 2008;111:1279–84. doi: 10.1097/AOG.0b013e31816baed1. [DOI] [PubMed] [Google Scholar]
- 19.Stoler MH, Vichnin MD, Ferenczy A, et al The accuracy of colposcopic biopsy: analyses from the placebo arm of the Gardasil clinical trials. Int J Cancer. 2011;128:1354–62. doi: 10.1002/ijc.25470. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Xiao JN, Tao X, et al Consistency of diagnosis between cervical cytology and colposcopic biopsy diagnosis. Zhonghua Bing Li Xue Za Zhi. 2018;47:444–8. doi: 10.3760/cma.j.issn.0529-5807.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Zuchna C, Hager M, Tringler B, et al Diagnostic accuracy of guided cervical biopsies: a prospective multicenter study comparing the histopathology of simultaneous biopsy and cone specimen. Am J Obstet Gynecol. 2010;203:321.e1–6. doi: 10.1016/j.ajog.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura Y, Matsumoto K, Satoh T, et al Optimizing biopsy procedures during colposcopy for women with abnormal cervical cancer screening results: a multicenter prospective study. Int J Clin Oncol. 2015;20:579–85. doi: 10.1007/s10147-014-0739-6. [DOI] [PubMed] [Google Scholar]
- 23.Vallapapan A, Chandeying N, Srijaipracharoen S, et al The role of random cervical biopsies in addition to colposcopy-directed biopsies in detection of CIN2. J Obstet Gynaecol. 2019;39:184–9. doi: 10.1080/01443615.2018.1474186. [DOI] [PubMed] [Google Scholar]
- 24.Qian XY, You ZX, Cao QW, et al Analysis of the missed diagnosis of invasive carcinoma under the microscope in HSIL diagnosed by colposcopy-guided biopsy and related influencing factors. Zhonghua Fu Chan Ke Za Zhi. 2018;53:613–9. doi: 10.3760/cma.j.issn.0529-567x.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Yang B, Pretorius RG, Belinson JL, et al False negative colposcopy is associated with thinner cervical intraepithelial neoplasia 2 and 3. Gynecol Oncol. 2008;110:32–6. doi: 10.1016/j.ygyno.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Basu P, Muwonge R, Mittal S, et al Implications of semi-quantitative HPV viral load estimation by Hybrid capture 2 in colposcopy practice. J Medical Screen. 2016;23:104–10. doi: 10.1177/0969141315606483. [DOI] [PubMed] [Google Scholar]
- 27.Kjær SK, Frederiksen K, Munk C, et al Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102:1478–88. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright TC, Jr., Stoler MH, Sharma A, et al. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol. 2011;136:578–86. doi: 10.1309/AJCPTUS5EXAS6DKZ. [DOI] [PubMed] [Google Scholar]
- 29.de Sanjose S, Quint WG, Alemany L, et al Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Zhang X, Molijn A, et al Human papillomavirus type-distribution in cervical cancer in China: the importance of HPV 16 and 18. Cancer Causes Control. 2009;20:1705–13. doi: 10.1007/s10552-009-9422-z. [DOI] [PubMed] [Google Scholar]
- 31.Saslow D, Solomon D, Lawson HW, et al American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–72. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allard JE, Rodriguez M, Rocca M, et al Biopsy site selection during colposcopy and distribution of cervical intraepithelial neoplasia. J Low Genit Tract Dis. 2005;9:36–9. doi: 10.1097/00128360-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 33.He G, Li H, Lin H, et al Topographical distribution pattern of cervical intraepithelial neoplasia across the cervix. J Int Med Res. 2012;40:1897–903. doi: 10.1177/030006051204000530. [DOI] [PubMed] [Google Scholar]
- 34.Zhao YQ, Chang IJ, Zhao FH, et al Distribution of cervical intraepithelial neoplasia on the cervix in Chinese women: pooled analysis of 19 population based screening studies. BMC Cancer. 2015;15:485. doi: 10.1186/s12885-015-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castle PE, Wentzensen N, Wheeler CM, et al Effect of the number of biopsies on the subsequent acquisition of new human papillomavirus infections. Obstet Gynecol. 2009;114:1057–62. doi: 10.1097/AOG.0b013e3181bb5632. [DOI] [PubMed] [Google Scholar]