Abstract
Despite the application of conventional therapies, the prognosis of advanced gastric cancer (GC) or gastroesophageal junction cancer (GEJC) is still poor. In recent years, immune checkpoint inhibitors (ICIs) have reshaped the paradigm of cancer therapy. Emerging evidence support the feasibility of programmed cell death-1 (PD-1) and its ligand (PD-L1) inhibition in chemo-refractory GC/GEJC. Nivolumab and pembrolizumab have initially been approved in Japan and United States, respectively for the third-line treatment of progressive GC or GEJC. In March 2020, nivolumab has also been licensed in China for treating advanced GC/GEJC who received ≥2 lines of systemic therapies. Current studies are moving forward to the first-line application or focusing on combination strategies, though data are insufficient and disputable. In this review, we summarize the recently reported and ongoing clinical trials in ICIs for advanced GC/GEJC. Molecular characteristics and clinical implications of different tumor subtypes are also reviewed. We further discuss the safety profile and biomarkers for predicting the response of ICIs, which has guiding values in clinical practice.
Keywords: Biomarker, gastric cancer, gastroesophageal junction cancer, immune checkpoint inhibitors, immunotherapy, safety
Introduction
Gastric cancer (GC) and gastroesophageal junction cancer (GEJC) represent major global health burden. GC is the fifth common cancer and the third leading cause of global cancer mortality, accounting for over 1.2 million cancer-related deaths worldwide in 2018 (1). GEJC, with incidence rate on the rise, is genetically close to GC and shares similar clinical presentations. Both have generally developed into metastatic disease when detected, as a consequence of nonspecific symptoms and inactive screening measures in China (2,3). Studies in the past have validated the clinical benefits of active chemotherapy agents in the treatment of patients with advanced GC or GEJC. The administration of platinum- and fluoropyrimidine-based regimen in the first-line setting and taxanes/irinotecan in the second-line setting have been recognized as the standard scheme (4-7). However, a wide range of patients with poor physical state are unfit for chemotherapy due to severe toxicities. For human epidermal growth factor receptor-2 (HER2) positive tumors, first-line anti-HER2 therapy is recommended, evidenced by a significant survival improvement triggered by trastuzumab (an anti-HER2 monoclonal antibody) in the ToGA trial (8). Second-line ramucirumab (an anti-angiogenic monoclonal antibody) in conjunction with conventional cytotoxic agents is also associated with a survival benefit (9,10). Despite the sequencing of these targeting agents, the prognosis of advanced GC/GEJC remains very poor with a median overall survival (OS) of 8−13 months (4,11). More disappointedly, targeting other molecular pathways including epidermal growth factor receptor (EGFR) or mesenchymal and epithelial transition (MET) receptor is futile, necessitating the development of new drugs (12-15).
Recently, immunotherapy has reshaped the management of cancers. Tumor cells are capable of evading host immune clearance via the downregulation of T-cell immune responses. This process is mediated by activation of immune checkpoints such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), PD-1 and PD-L1 (16-19). Blocking the PD-1/PD-L1 axis by immune checkpoint inhibitors (ICIs) has shown efficacy and safety in several solid cancers. It was not until 2017 when the anti-PD-1 antibodies nivolumab and pembrolizumab were licensed in Japan and United States, respectively, for patients with heavily treated, chemo-resistant GC/GEJC. In March 2020, nivolumab also gained approval in China for the treatment of advanced GC/GEJC who received ≥2 lines of systemic therapies. Presently, numerous new strategies are proposed for the realization of stronger and safer activities.
The review aims to summarize the current and evolving scenario of ICIs in advanced GC or GEJC. We further review the biomarkers, molecular subtyping and potential combination strategies to guide future therapies.
ICIs and clinical outcomes in advanced GC/GEJC
Monotherapy in second- or later-treatment line
The first exploratory study KEYNOTE-012 recruited 39 patients with recurrent or metastatic PD-L1 positive [defined as ≥1% staining of tumor cells and contiguous mononuclear cells, also known as combined positive score (CPS) ≥1] GCs who received pembrolizumab monotherapy (10 mg/kg every 2 weeks for up to 24 months) after progression on previous lines of therapies (20). Judged by central assessment, among 36 evaluable patients, 8 (22%) showed an objective response [all partial response (PR)], and median OS was 11.4 months. Among four patients with microsatellite instability high (MSI-H) tumors, two (50%) achieved an objective response.
Subsequently, a global, open-label, multicohort, phase II trial KEYNOTE-059 comprising of 3 cohorts initiated. In cohort 1, 259 previously treated GC or GEJC patients irrespective of PD-L1 status (positivity defined as CPS≥1) received pembrolizumab (200 mg every 3 weeks) (21). The overall response rate (ORR) and disease control rate (DCR) were 12% and 27%, respectively, and median OS was 5.6 months. The ORR was higher in PD-L1 positive (16%) vs. PD-L1 negative subgroup (6%), though complete response (CR) could be attained in both subgroups. Four out of 7 (57%) MSI-H patients demonstrated an objective response. Grade ≥3 treatment-related adverse effects (TRAEs) occurred in 18% of patients including 2 treatment-related death. Based on the promising results from this large early-phase trial, Food Drug Administration (FDA) approved pembrolizumab after ≥2 lines of therapy for GC/GEJC patients with PD-L1 overexpression.
The largest Asian study ATTRACTION-2 is a phase III trial evaluating the efficacy and safety of nivolumab monotherapy (3 mg/kg every 2 weeks) vs. placebo in 493 patients with advanced GC/GEJC who had received ≥2 chemotherapy regimens (22). The ORR and median OS were 11% vs. 0%, 5.3 vs. 4.1 months, respectively. Durable responses with nivolumab could be observed, evidenced by a sustaining separation of the OS curves. Grade 3−4 TRAEs occurred in 10% of nivolumab-treated patients. According to this result, nivolumab was granted approval in China for treating advanced GC/GEJC without biomarker selection in March 2020.
Novel anti-PD-1 agents are also explored in patients with chemo-refractory advanced GC/GEJC, including toripalimab (23) and SHR-1210 (24). Of note, superior response to toripalimab monotherapy was relevant with high tumor mutation burden (TMB). The safety and efficacy profile of both studies were comparable to that in KEYNOTE-059.
Despite these initial success, due to the phase III KEYNOTE-061, pembrolizumab failed to achieve an improvement in median OS (9.1 vs. 8.3 months) and median progression-free survival (PFS) (1.5 vs. 4.1 months) compared to paclitaxel in the second-line treatment of PD-L1 positive GC/GEJC (defined as CPS≥1) (25). Interestingly, pembrolizumab monotherapy resulted in much more durable responses than paclitaxel, evidenced by median duration of response (DOR) of 18.0 vs. 5.2 months. A separation of OS curve at the tail was noted (1-year survival rate: 40% for pembrolizumab and 26% for paclitaxel). Grade 3−5 TRAEs were 14% with pembrolizumab and 35% with paclitaxel, respectively. In apost hoc analysis, MSI-H, or PD-L1 CPS≥10 tumors were associated with higher response rate (26). These outcomes suggested the potential long-term benefits of ICIs and further underlined the necessity of biomarker selection.
The safety and efficacy of avelumab (an anti-PD-L1 monoclonal antibody) among patients with advanced GC/GEJC were also assessed in the phase Ib JAVELIN Solid Tumor trial (27). In this study, 150 eligible patients received avelumab (10 mg/kg every 2 weeks) in a first-line maintenance or second-line pattern. In the second-line cohort, the ORR was comparable to that of nivolumab and pembrolizumab (4/60, 6.7%). Grade ≥3 TRAEs occurred in 8.3% of patients. On this basis, the global, randomized phase III trial, GASTRIC-300 compared avelumab to the physician’s choice of chemotherapy (either paclitaxel or irinotecan) in 371 pretreated GC/GEJC patients (28). Disappointedly, avelumab did not improve OS, PFS and ORR compared to chemotherapy (median OS, 4.6 vs. 5.0 months; median PFS, 1.4 vs. 2.7 months; ORR, 2.2% vs. 4.3%) irrespective of PD-L1 status. Grade ≥3 TRAEs were rarer in the avelumab arm (9.2%) than in the chemotherapy arm (32%).
To summarize, these late-line results definitely affirmed the favorable efficacy and tolerability of anti-PD-1/PD-L1 therapies among certain individuals, which contributed to the license of nivolumab and pembrolizumab in Japan, United States and China. Though only a small portion of populations involved, ICIs single therapy indeed results in prolonged OS. It should be highlighted that durable response and long-term benefits could only be achieved by ICIs instead of chemotherapy, seen in KEYNOTE-061 and 3-year data of ATTRACTION-2 (29). Apparently, patient screening based on molecular biomarkers is necessary, for distinct treatment outcome achieved by different management.
Combination therapy in second- or later-treatment line
To expand potential beneficiaries, combination immunotherapy is being investigated. In the phase I/II trial CheckMate-032 (anti-PD-1 + anti-CTLA-4), three cohorts of GC/GEJC patients who progressed on one or more lines of chemotherapy were administrated with different doses of nivolumab and ipilimumab (N1I3: nivolumab 1 mg/kg + ipilimumab 3 mg/kg; N3I1: nivolumab 3 mg/kg + ipilimumab 1 mg/kg; N3: 3 mg/kg) (30). The ORRs were 12% in N3, 24% in N1I3 and 8% in N3I1, with a trend of higher ORRs in PD-L1 positive subgroups. Median DOR in the N1I3 and N3I1 subgroups were 6.9 months and 4.8 months, respectively. Grade ≥3 TRAEs were more common in N1I3 and N3I1 compared with N3. Recently, another phase Ib/II trial also evaluated the activity of anti-PD-L1 (durvalumab) plus anti-CTLA-4 (tremelimumab) therapies among patients with chemotherapy-refractory, metastatic or recurrent GC/GEJC (31). The second-line cohort was randomized 2:2:1 to D+T, D, or T. The third-line cohort received D+T. Besides, IFN-γ gene signature was evaluated as a predictive biomarker in a separate arm containing second- and third-line patients receiving D+T. The ORRs were 7.4%, 0%, 8.3%, 4.0% and 16% respectively, in second-line D+T, D, T, third-line D+T and D+T with positive IFN-γ signature. Grade 3−4 TRAEs occurred more frequently in second-line T and second- or third-line D+T. Based on these two studies, doublet immunotherapy seemed to present modest efficacy but fairly severe toxicities.
Many clinical studies focused on combining ICIs with anti-angiogenic agents. In a multi-cohort phase Ia/b trial I4T-MC-JVDJ, 29 patients with GC/GEJC who progressed on first- or second-line systemic therapy received the combination of ramucirumab (8 mg/kg) and durvalumab (750 mg) (32). Six out of 29 patients (21%) achieved a PR. Median PFS and median OS were 2.6 and 12.4 months, respectively. Notably, the ORRs for patients with positive (defined as ≥25% expression in tumor and immune cells) and negative PD-L1 status were 36% and 0%. Grade 3 TRAEs were noted in 10 (35%) patients and no grade 4−5 toxicities occurred. In the phase Ia/b multicohort study 14T-MC-JVDF/KEYNOTE-098, 41 previously treated GC/GEJC patients were recruited and received the doublet of ramucirumab and pembrolizumab (33). Among evaluable patients, 3 (7%) presented a PR. Median PFS and median OS were 2.5 and 5.9 months, respectively. Ten (24%) patients suffered grade 3−4 TRAEs. NivoRam was a phase I/II trial assessing the safety and efficacy of nivolumab plus ramucirumab in 46 patients with chemo-resistant GC (34). According to the preliminary result, the ORR and DCR were 27% and 62%, respectively. Grade 3−4 TRAEs took up 28% of total populations. As a result, despite the varying data, it is feasible to integrate immunotherapy and anti-angiogenic therapy together into comprehensive management of GC/GEJC.
Other evolving strategies also bring about inspirations. A phase I study evaluated SHR-1210 in conjunction with apatinib for advanced GC/GEJC patients who were refractory to prior line chemotherapy (35). The ORR and DCR were 17% and 78%. Median PFS and median OS were 2.9 and 11.4 months. In a phase I/II study MEDIOLA, 39 heavily-pretreated patients were administrated with olaparib (a poly-ADP-ribose polymerase inhibitor) followed by olaparib and durvalumab combinations (36). Impressively, in these two studies, several patients with early progressive disease (PD) showed a long survival, contributing to low PFS and high OS. In Japan, a phase I study was conducted to assess the safety and efficacy of ADX (a matrix metalloproteinase 9 inhibitor) alone or in combination with nivolumab in GC/GEJC patients who received prior chemotherapy (37). In the combination cohort, the ORR was impressively 40%. However, another phase II study failed to suggest survival superiority of ADX plus nivolumab compared to nivolumab alone in terms of PFS and OS (38).
For immunohistochemistry-assessed HER2 positive GC/GEJC patients who progressed on first-line trastuzumab, there remained a second-line salvage option. In a phase I/II study, margetuximab (an anti-HER2 Fc-optimized antibody) and pembrolizumab were simultaneously administrated to 66 eligible patients (39). The ORR and DCR were 41% and 72%. Of note, HER2 expression post trastuzumab was lost in 41% of patients detected via circulating tumor DNA, while those with HER2 retention obtained higher response rate, especially accompanying positive PD-L1 status. Twelve out of 66 (18%) patients developed grade≥3 TRAEs. This study demonstrated that HER2 retention and PD-L1 expression are important selection biomarkers for the second-line treatment of HER2 positive GC/GEJC.
In conclusion, late-line combination immunotherapies are partially effective but also notorious for intimidating toxicity. Anti-HER2 or anti-angiogenic agents might play a synergistic effect with ICIs, while these combinations need further validations. An unprecedented number of targeting agents including regorafenib, apatinib and relatlimab have become combination candidates. Whether other therapeutic targets, such as LAG-3, FGFR, TGF-β could be novel biological determinants and still need to be addressed. Incorporating ICIs with multiple evidence-based anti-cancer medications is pending results of future trials.
Monotherapy in first-line treatment
Pembrolizumab monotherapy (200 mg every 3 weeks) in the first-line setting was investigated in cohort 3 of the KEYNOTE-059 study (40). Among 31 patients with PD-L1 positive (defined as CPS≥1), HER2 negative GC/GEJC, the ORR was 26%, including 2 CRs. Median PFS and median OS were 3.3 and 20.7 months, respectively. Grade ≥3 TRAEs occurred in 23% of patients, including one treatment-related death from pneumonitis. Besides, in KEYNOTE-062, first-line pembrolizumab monotherapy yielded an ORR of 15%, with median PFS of 2.0 months and median OS of 10.6 months in PD-L1 positive populations (41). Pembrolizumab was superior to chemotherapy regarding OS in CPS≥10 subgroup (17.4 vs. 10.8 months). We noticed that most patients developed PD during an early period, while a small portion of treatment-sensitive patients indicated durable response to ICIs, contributing to poor PFS and disproportionately long OS.
Several studies also took interests in maintenance immunotherapy in patients without disease progression after first-line chemotherapy. CA184-162 was a phase II trial comparing the efficacy of maintenance ipilimumab with best supportive care (BSC) following first-line chemotherapy in patients with unresectable GC/GEJC (42). Among 114 enrolled patients, median OS were 12.7 months for ipilimumab and 12.1 months for BSC. Grade ≥3 TRAEs occurred in 23% with ipilimumab vs. 9% with BSC, respectively. This negative result negated the justification of ipilimumab maintenance therapy in GC/GEJC patients.
JAVELIN Solid Tumor trial expansion cohort evaluated the efficacy and safety of avelumab maintenance therapy (10 mg/kg every 2 weeks) in 90 patients with advanced GC/GEJC without progression following first-line chemotherapy (27). The DCR and ORR were 57% and 6.7%, respectively. Median PFS and median OS were 2.8 and 11.1 months, respectively. However, avelumab maintenance failed to improve survival compared to continuation of first-line chemotherapy in the phase III JAVELIN Gastric 100 (43). Among 499 patients, median OS was 10.4 vs. 10.9 months in avelumab arm vs. chemotherapy arm. The ORRs were 13% and 14% in two arms, respectively. Grade ≥3 TRAEs were 13% with avelumab and 33% with chemotherapy. Despite the unengaging results, we still captured that those with response from avelumab showed a long DOR and suffered less severe TRAEs in contrast to chemotherapy.
In summary, the DCR and ORR for first-line ICIs single therapy barely meet expectations. However, its activity presents an “all or none” pattern, for minor beneficiaries could obtain major benefits. Therefore, we once again highlight the necessity of patient selection on the basis of biomarkers (PD-L1 status, TMB, and so on) before initiating therapies. Besides, patients who progressed on ICIs were reported to be more sensitive to second-line chemotherapy and anti-angiogenic therapy (44), which elicits our rethinking of the best deliver order. If it makes sense, a few selected patients might benefit from first-line ICIs followed by later-line chemotherapies.
Combination therapy in first-line treatment
In the cohort 2 of KEYNOTE-059 study, 25 patients with advanced HER2 negative GC/GEJC received first-line pembrolizumab and 5-FU/cisplatin (40). The ORR was 60%, with higher response rate (69%) in PD-L1 positive tumors (defined as CPS≥1). Median OS was 13.8 months. The incidence of grade 3−4 TRAEs was 76%, much higher than that seen with pembrolizumab monotherapy (23%).
KEYNOTE-062 compared the safety and efficacy of pembrolizumab plus cisplatin/5-FU (P+C) to cisplatin/5-FU alone (C) in patients with PD-L1 positive, HER2 negative advanced GC/GEJG (41). P+C was not superior to C in OS (12.5 vs. 11.1 months), PFS (6.9 vs. 6.4 months) in CPS≥1 and OS (12.3 vs. 10.8 months) in CPS≥10 subgroups. Only MSI-H patients overwhelmingly benefited from combination immunotherapy. Grade 3−5 TRAEs were 73% and 69% in P+C and C respectively.
Several clinical trials are actively pursuing first-line chemotherapy combined with immunotherapy. In part one of ATTRACTION-4, the safety and efficacy of nivolumab combined with SOX/CapeOX for advanced or recurrent HER2 negative GC/GEJC were evaluated (45). The ORR and DCR were 57% and 81% with nivolumab plus SOX, 76% and 88% with nivolumab plus CapeOX, respectively. Grade ≥3 TRAEs occurred in 52% and 67% of patients, respectively. Part 2 of this study (phase III) is ongoing to compare nivolumab plus SOX/CapeOX vs. placebo plus SOX/CapeOX. KEYNOTE-659 was a phase II study investigating the efficacy and safety of SOX or cisplatin combined with pembrolizumab in patients with HER2 negative, PD-L1 positive GC or GEJC (46). Among 54 enrolled patients, the ORR and DCR were 72% and 96%, respectively. Median PFS was 9.4 months while median OS was not reached. Grade ≥3 TRAEs were reported in 31 patients (57%). In another phase I/II trial, enrolled patients were administrated with toripalimab, oxaliplatin and capecitabine (23). The ORR and DCR were 67% and 89%, respectively, and 39% of patients experienced grade≥3 TRAEs.
In the phase I trial JVDF, 28 patients with treatment naïve, late-stage GC/GEJC received the combination of pembrolizumab and ramucirumab (47). The ORR was 25%. Median PFS was 5.3 months and median OS has not been reached. Seventeen (61%) patients experienced grade 3 TRAEs.
In a phase II trial, patients with treatment naïve, HER2 positive esophagogastric adenocarcinoma irrespective of PD-L1 status received the combination of pembrolizumab, trastuzumab, oxaliplatin and capecitabine (48). Among 37 evaluable patients, 22 received 1 cycle of pembrolizumab and trastuzumab induction before initiation of chemotherapy. Preliminary results yielded an ORR of 81%. Median PFS was 14.2 months, which was uncorrelated with PD-L1 status. The promising results led to the initiation of the phase III KEYNOTE-811 trial.
Conclusively, first-line combination of immunotherapy and chemotherapy is associated with high efficiency and high toxicity. When compared to chemotherapy alone, though, combination immunotherapy presents heterogenous results. Whether different compatibility regimens play a role in the outcome remains undefined. We speculate that reasonable compatibility could better synergize with immunotherapy. Furthermore, a cautious evaluation of safety issues is indispensable.
Reported phase II/III studies are summarized in Table 1 . Ongoing studies are listed in Table 2 .
1. Summarized data from reported phase II/III clinical studies.
| Clinical trial name | Clinicaltrial.gov ID | Phase | Settings | Patients | Treatment | Efficacy results | Safety results |
| 1L, first-line; 2L, second-line; 3L, third-line; Mn, maintenance; GC, gastric cancer; GEJC, gastroesophageal junction cancer; PD-L1, programmed death ligand 1; HER2, human epidermal growth factor receptor 2; NIV, nivolumab; PEM, pembrolizumab; IPI, ipilimumab; CIS, cisplatin; 5-FU, 5-fluorouracil; CAPE, capecitabine; OX, oxaliplatin; ADX, andecaliximab; BSC, best supportive care; ORR, objective response rate; mPFS, median progression-free survival; mOS, median overall survival; TRAEs, treatment related adverse effects. | |||||||
| CheckMate-032 (30) | NCT01928394 | I/II | 2L or 3L+ | GC/GEJC (PD-L1 unselected): N1I3: 48 N3I1: 52 N3: 59 | N3: NIV 3 mg/kg, q2w; N1I3: NIV 1 mg/kg + IPI 3 mg/kg, q3w; N3I1: NIV 3 mg/kg + IPI 1 mg/kg, q3w | ORR: N1I3, 24%; N3I1, 8%; N3, 12%
mPFS: N1I3, 1.4 months; N3I1, 1.6 months; N3, 1.4 months mOS: N1I3, 6.9 months; N3I1, 4.8 months; N3, 6.2 months |
Grade 3−4 TRAEs: N1I3 47%; N3I1 27%; N3 17% |
| KEYNOTE-059 cohort 1 (21) | NCT02335411 | II | 3L+ | GC/GEJC (PD-L1 unselected): 259 | PEM 200 mg, q3w | ORR: 12% (PD-L1 + 16%; PD-L1− 6%)
mPFS: 2.0 months mOS: 5.5 months |
Grade ≥3 TRAEs: 18% |
| KEYNOTE-059 cohort 2 (40) | NCT02335411 | II | 1L | GC/GEJC (PD-L1 unselected, HER2−): 25 | PEM 200 mg, q3w + CIS + 5-FU or CAPE | ORR: 60% (PD-L1+ 73%; PD-L1− 38%)
mPFS: 6.6 months mOS: 13.8 months |
Grade ≥3 TRAEs: 76% |
| KEYNOTE-059 cohort 3 (40) | NCT02335411 | II | 1L | GC/GEJC (PD-L1+, HER2-): 31 | PEM 200 mg, q3w | ORR: 26%
mPFS: 3.3 months mOS: 20.7 months |
Grade ≥3 TRAEs: 23% |
| N/A cohort 1 (23) | NCT02915432 | I/II | 2L or 3L+ | GC/GEJC (PD-L1 unselected): 58 | Toripalimab 3 mg/kg, q2w | ORR: 12%
mPFS: 1.9 months mOS: 4.8 months |
Grade 3−4 TRAEs: 22% |
| N/A cohort 2 (23) | NCT02915432 | I/II | 1L | GC/GEJC (PD-L1 unselected): 18 | Toripalimab 360 mg, q3w + OX + CAPE | ORR: 67%
mPFS: 5.8 months mOS: not reached |
Grade ≥3 TRAEs: 39% |
| N/A (31) | NCT02340975 | I/II | 2L or 3L | GC/GEJC (PD-L1 unselected, HER2−): 2L-D+T: 27; 2L-D: 24; 2L-T: 12; 3L-D+T: 25; 2L/3L-D+T INF-γ+: 19 | 2L-D+T: durvalumab 20 mg/kg + tremelimumab 1 mg/kg, q4w
2L-D: durvalumab 10 mg/kg, q2w 2L-T: tremelimumab 10 mg/kg, q4w → q12w 3L-D+T: durvalumab 20 mg/kg + tremelimumab 1 mg/kg, q4w → durvalumab 10 mg/kg, q2w 2L/3L-D+T INF-γ+: durvalumab 20 mg/kg + tremelimumab 1 mg/kg, q4w → durvalumab 10 mg/kg, q2w |
ORR: 2L-D+T, 7.4%; 2L-D, 0%; 2L-T, 8.3%; 3L-D+T, 4%; 2L/3L-D+T INF-γ+, 16%
mPFS: 2L-D+T, 1.8 months; 2L-D, 1.6 months; 2L-T, 1.7 months; 3L-D+T, 1.8 months; 2L/3L-D+T INF-γ+, 1.8 months mOS: 2L-D+T, 9.2 months; 2L-D, 3.4 months; 2L-T, 7.7 months; 3L-D+T, 10.6 months; 2L/3L-D+T INF-γ+, 7.0 months |
Grade ≥3 TRAEs: 2L-D+T 17%; 2L-D 4%; 2L-T 42%; 3L-D+T 16%; 2L/3L-D+T INF-γ+ 11% |
| NivoRam (34) | NCT02999295 | I/II | 2L | GC (PD-L1 unselected): 46 | NIV 3mg/kg, q2w + ramucirumab 8 mg/kg, q2w | ORR: 27%
mPFS: 2.9 months mOS: 9.0 months |
Grade ≥3 TRAEs: 28% |
| MEDIOLA (36) | NCT02734004 | I/II | 2L or 3L+ | GC (PD-L1 unselected): 39 | Olaparib 300 mg → olaparib + durvalumab 1,500 mg, q4w | ORR: 10%
mPFS; not reached mOS: not reached |
Grade ≥3 TRAEs: 57% |
| N/A (38) | NCT02864381 | II | 2L or 3L+ | GC/GEJC (PD-L1 unselected): 144 | Arm1: ADX 800 mg + NIV 3 mg/kg, q2w
Arm2: NIV 3 mg/kg, q2w |
ORR: arm1, 11%; arm2, 7%
mPFS: arm1, 1.8 months; arm2, 7.2 months mOS: arm1, 1.9 months; arm2, 5.9 months |
Grade ≥3 TRAEs: not reported |
| N/A (39) | NCT02689284 | I/II | 2L | GC/GEJC (PD-L1 unselected, HER2+): 66 | Margetuximab 15 mg/kg + PEM 200 mg, q3w | ORR: 41%
mPFS: 5.5 months mOS: not reached |
Grade ≥3 TRAEs: 18% |
| CA184-162 (42) | NCT01585987 | II | 1L Mn | GC/GEJC (PD-L1 unselected): 143 | Arm1: IPI 10 mg/kg, q3w → 10 mg/kg, q12w
Arm2: BSC |
ORR: arm1, 1.8%; arm2, 7.0%
mPFS: arm1, 2.9 months; arm2, 4.9 months mOS: arm1, 12.7 months; arm2, 12.1 months |
Grade ≥3 TRAEs: Ipili 23%; BSC 9% |
| ATTRACTION-4 (45) | NCT02746796 | II | 1L | GC/GEJC (PD-L1 unselected, HER2−): 38 | Arm1: NIV 360 mg, q3w + S-1 + OX (SOX)
Arm2: NIV 360 mg, q3w + CAPE + OX (CapeOX) |
ORR: arm1, 57%; arm2, 77%
mPFS: arm1, 9.7 months; arm2, 10.6 months mOS: not reached |
Grade ≥3 TRAEs: arm1 57%; arm2 67% |
| KEYNOTE-659 (46) | NCT03382600 | II | 1L | GC/GEJC (PD-L1+, HER2−): 54 | Cohort1: PEM 200 mg, q3w + S-1 + OX (SOX) | ORR: 72%
mPFS: 9.4 months mOS: not reached |
Grade ≥3 TRAEs: 57% |
| N/A (48) | NCT03615326 | II | 1L | GC/GEJC (PD-L1 unselected, HER2+): 37 | PEM 200 mg, q3w + trastuzumab 6 mg/kg + OX + CAPE | ORR: 81%
mPFS: 14.2 months mOS: not reached |
Grade ≥3 TRAEs: not reported |
| ATTRACTION-2
(22) |
NCT02267343 | III | 3L+ | GC/GEJC (PD-L1 unselected): 493 | Arm1: NIV 3 mg/kg, q2w
Arm2: placebo |
ORR: arm1, 11%; arm2, 0%
mPFS: arm1, 1.6 months; arm2, 1.5 months mOS: arm1, 5.3 months; arm2, 4.1 months |
Grade 3−4 TRAEs: arm1 10%; arm2 4% |
| KEYNOTE-061
(25) |
NCT02370498 | III | 2L | GC/GEJC (PD-L1+): 395 | Arm1: PEM 200 mg, q3w
Arm2: paclitaxel |
ORR: arm1, 16%; arm2, 14%
mPFS: arm1, 1.5 months; arm2, 4.1 months mOS: arm1, 9.1 months; arm2, 8.3 months |
Grade 3−5 TRAEs: arm1 14%; arm2 35% |
| GASTRIC-300
(28) |
NCT02625623 | III | 3L | GC/GEJC (PD-L1+): 371 | Arm1: 10 mg/kg, q2w
Arm2: physician’s choice of chemotherapy |
ORR: arm1, 2.2%; arm2, 4.3%
mPFS; arm1, 1.4months; arm2, 2.7 months mOS: arm1, 4.6 months; arm2, 5.0 months |
Grade ≥3 TRAEs: arm1 9.2%; arm2 32% |
| GASTRIC-100
(43) |
NCT02625610 | III | 1L Mn | GC/GEJC (PD-L1 unselected, HER2−): 499 | Arm1: avelumab 10 mg/kg, q2w
Arm2: continued chemotherapy |
ORR: arm1, 13%; arm2, 14%
mPFS: not reported mOS: not reported |
Grade ≥3 TRAEs: arm1 13%; arm2 33% |
| KEYNOTE-062
(41) |
NCT02494583 | III | 1L | GC/GEJC (PD-L1+, HER2−): 763 | P: PEM 200 mg, q3w
P+C: PEM 200 mg, q3w + CIS + 5-FU or CAPE C: placebo + CIS + 5-FU or CAPE |
ORR: P, 15%; P+C, 49%; C, 37%
mPFS; P, 2.0 months; P+C, 6.9 months; C, 6.4 months mOS: P, 10.6 months; P+C, 12.5 months; C, 11.1 months |
Grade 3−5 TRAEs: P 17%; P+C 73%; C 69% |
2. Summarized data of ongoing trials.
| Clinical trial name | Clinicaltrial.gov ID | Phase | Settings | Drug treatment | Primary endpoints |
| 1L, first-line; 2L, second-line; 3L, third-line; ORR, overall response rate; DOR, duration of response; PFS, progression-free survival; OS, overall survival; BOR, best overall response; EFS, event-free survival; pCR, pathological complete response. | |||||
| MORPHEUS | NCT03193190, NCT03281369, NCT03555149 | I/II | 1L or 2L | Atezolizumab + bevacizumab, BL-8040, cobimetinib, imprime PGG, linagliptin, PEGPH20, RO6874281, selicrelumab | ORR |
| FRACTION-GC | NCT02935634 | II | All | Nivolumab + ipilimumab
Nivolumab + relatlimab Nivolumab + BMS-986205 |
ORR, DOR, PFS rate |
| INTEGA | NCT03409848 | III | 1L | Nivolumab + ipilimumab + trastuzumab
Nivolumab + mFOLFOX6 + trastuzumab |
PFS, OS |
| CA224-060 | NCT03662659 | II | 1L | Relatlimab + nivolumab + chemotherapy
Nivolumab + chemotherapy |
ORR |
| N/A | NCT02901301 | I/II | 1L | Pembrolizumab + trastuzumab + capecitabine + cisplatin | ORR |
| N/A | NCT02013154 | I | 2L+ | DKN-01 + pembrolizumab | Recommended dose |
| N/A | NCT01633970 | I | 2L+ | Atezolizumab + bevacizumab ± chemotherapy | Recommended dose |
| N/A | NCT01968109 | I/II | All | Relatlimab + nivolumab | ORR, DOR, PFS rate |
| N/A | NCT03126110 | I/II | 2L+ | INCAGN01876 + nivolumab + ipilimumab | ORR |
| N/A | NCT02954536 | II | 1L | Pembrolizumab + trastuzumab + capecitabine + cisplatin | PFS |
| N/A | NCT02830594 | I | All | Pembrolizumab + radiotherapy | T cell counts |
| N/A | NCT03122548 | II | 2L+ | Pembrolizumab + CRS-207 | ORR |
| N/A | NCT03342417 | I/II | 2L+ | Nivolumab + ipilimumab | ORR, BOR |
| N/A | UMIN000025947 | I/II | 2L | Paclitaxel + ramucirumab + nivolumab | 6-month PFS rate |
| KEYNOTE-585 | NCT03221426 | III | 1L | Pembrolizumab + cisplatin + capecitabine + 5-fluorouracil
Pembrolizumab + 5-fluorouracil + docetaxel + oxaliplatin + leucovorin |
OS, EFS, pCR rate |
| KEYNOTE-659 | NCT03382600 | II | 1L | Pembrolizumab + TS-1 + cisplatin/oxaliplatin | ORR |
| CheckMate-649 | NCT02872116 | III | 1L | Nivolumab + ipilimumab
Nivolumab + oxaliplatin + capecitabine Nivolumab + oxaliplatin + leucovorin + fluorouracil |
OS, PFS |
| ATTRACTION-4 | NCT02746796 | II/III | 1L | Nivolumab + oxaliplatin + S-1 + capecitabine | OS, PFS |
| KEYNOTE-811 | NCT03615326 | III | 1L | Pembrolizumab + trastuzumab + chemotherapy | PFS, OS |
Molecular subtyping of GC/GEJC and predictive biomarkers for immunotherapy
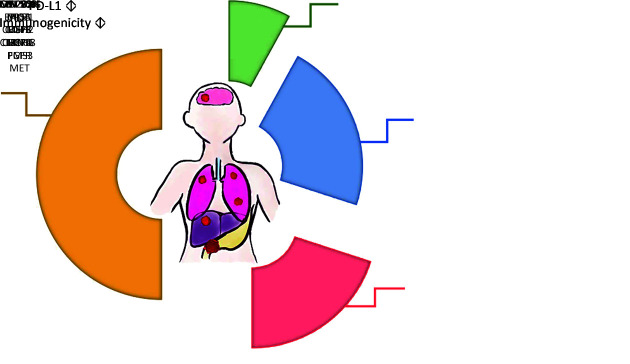
GC and GEJC are a group of genetically heterogenous diseases, with molecular characteristics varying across different subtypes, which might account for the discrepant prognosis observed in clinical studies. A classification system published in 2014 by the Cancer Genome Atlas Research Network has divided GC/GEJC into four subgroups based on molecular profiling (Figure 1 ): genome stable (GS); chromosomal instability (CIN); Epstein-Barr virus (EBV); and microsatellite instability (MSI) (49-51). GS tumors, taking up 20% of the total cases, are usually immune evasive and enrich for CDH1/RHOA or CLDN18-ARHGAP mutations. CIN tumors are the most common subtype (50% of all GC/GEJC patients) characterized by TP53 mutation and receptor tyrosine kinase amplifications. The third subtype, EBV associated GCs (8%) are featured with amplification of JAK2, CD274 and PDCD1LG2 which accounts for PD-L1/2 overexpression (52). MSI subtypes represent 22% of the total patients, while its incidence in late-stage populations is much fewer, reported by a large-scale analysis (53). These subsets of patients have defect mismatch repair (MMR) system due to inactivation of MMR genes (MLH1, MSH2, MSH6 and PMS2) or hypermethylation of the MLH1 promoter (54,55), leading to elevated replication errors and accumulated mutations in the microsatellite regions of DNA. MSI-H tumors are associated with high-degree CD8 positive T-cell infiltration and favorable response to anti-PD-1 therapy (56-58). This classification system proposed two GC/GEJC subtypes, EBV and MSI, as preferable candidates for anti-PD1/PD-L1 therapies. Actually, the predictive value on prognosis of a range of biomarkers, including but far more than MSI-H and EBV, is being actively investigated.
1. Four molecular subtypes of GC/GEJC proposed by the Cancer Genome Atlas Research Network. Each portion of the pie chart lists defect or mutated genes involved in certain subtype. PD-L1 expression level and tumor immunogenicity are also labeled. GC, gastric cancer; GEJC, gastroesophageal junction cancer; PD-L1, programmed cell death 1 ligand; EBV, Epstein-Barr virus; MSI, microsatellite instability; GS, genome stable; CIN, chromosomal instability.
PD-L1 is normally expressed by endothelial cells and a variety of inflammatory cells, constituting negative feedback of normal immune response. However, tumor cells and stroma cells stationed in the tumor microenvironment are able to upregulate PD-L1 to resist anti-tumor effect (19). Paradoxical observations regarding the relationship between PD-L1 status and responses to ICIs coexist in different clinical studies. In most studies, positive PD-L1 expression is predictive for a better response. In the cohort 1 and cohort 2 of KEYNOTE-059, the ORRs were 16% and 73% in PD-L1 positive subgroup vs. 6% and 38% in PD-L1 negative subgroup, respectively (21,40). PD-L1 also serves as a positive predictive biomarker for immunotherapy response in CheckMate-032, KEYNOTE-012, JAVELIN Solid Tumor Trial. In particular, higher PD-L1 expression was relevant with better responses. In KEYNOTE-061, the best treatment outcome by immunotherapy was reported in populations with high-level PD-L1 expression (CPS≥10) (25). Nevertheless, it should be noted that a few PD-L1 negative patients also generated durable responses, even CRs, from ICIs. Besides, there are also studies where PD-L1 was uncorrelated to clinical outcome. In ATTRACTION-2, nivolumab monotherapy demonstrated similar OS in PD-L1 positive and negative patients (5.2 months vs. 6.0 months) (22). In JAVELIN GASTRIC-300 and GASTRIC-100, no survival benefit compared to placebo was derived in either PD-L1 positive or PD-L1 negative populations (28,43). Several reasonable factors should be considered to interpret these controversial results. First, there was no standardized assay or methodology to evaluate PD-L1 expression (unifying antibodies, protocols or algorithms). Second, the heterogeneity of the assessed cells (tumor cells only, or tumor cells and immune cells) also led to the divergence. Third, PD-L1 expression may vary across different testing sites and shift over time, making a single detection less valid. Several retrospective analyses sustained CPS as a more useful method of determining PD-L1 expression vs. tumor proportion score (59,60). Tumor infiltrating immune cells, other than tumor cells, are undoubtedly critical regulators of immune response. CPS incorporates PD-L1 expression by both tumor cells and adjacent immune cells into an integrated value, thus reflecting a more comprehensive state of tumor microenvironment. In conclusion, PD-L1 alone is insufficient for stratifying patients. The optimized standards and clinical utility of PD-L1 testing still need to be defined in prospective trials.
It has been reported that MSI-H tumors are relevant with high mutation-associated neoantigens recharge, therefore facilitate the accumulation of tumor specific cytotoxic CD8 positive T lymphocytes (56). Overwhelming evidence support MSI-H status as a positive prognostic biomarker. On the basis of several landmark studies (20,61-63), pembrolizumab has been approved by FDA for treating MSI-H solid tumors in 2017. Although this subtype is merely comprised of approximately 5%−10% advanced GC, MSI-H tumors are particularly sensitive to ICIs. In KEYNOTE-059 cohort 1 and KEYNOTE-061, 4/7 (57%) and 7/15 (47%) MSI-H patients showed responses to pembrolizumab (21,25). According to a comprehensive molecular analysis of GC or GEJC patients who received salvage immunotherapy, a dramatic response rate of 86% was noted in the MSI-H subgroup (64). Despite multiple negative results from KEYNOTE-062 in the first-line treatment of GC or GEJC patients, the MSI-H subgroup receiving immunotherapy or combination immunotherapy still harvested the best prognosis (41). Similarly, in the second-line setting (KEYNOTE-061), MSI-H patients reported significantly higher ORR and lower TRAEs in pembrolizumab arm vs. chemotherapy arm. These results justified the earlier use of ICIs among patients with MSI-H advanced GC/GEJC. Several translational studies are underway to evaluate combination immunotherapy in patients with MSI-H gastrointestinal tumors.
EBV infection also serves as a predictor of response to immunotherapy. As reported, EBV associated tumors, including lymphomas and nasopharyngeal carcinomas, are susceptible to antibodies targeting PD-1/PD-L1 axis (65,66). EBV positive malignancies are characterized by robust immune cell infiltration and elevated PD-L1 and PD-L2 expression, which confer the potential activity of immunotherapy among these patients (67,68). A few small-scale studies have demonstrated the superior activity of ICIs in EBV positive GCs (69). In a phase II prospective study, among 61 metastatic GC patients, the ORR was 100% in the EBV positive subgroup, with all 6 patients achieving a PR after delivered with third-line pembrolizumab (64). Considering the low rates of EBV related GCs, a large-cohort clinical trial is hard, yet realizable in the future.
Helicobacter pylori (HP) is a key infectious factor for the development of GC. HP status could be a potential predictor to the response of ICIs. This anaerobic bacterium colonizes the gastric mucosa, induces chronic inflammation and subsequent malignant transformation. An upregulation of PD-1/PD-L1 signaling could be detected in HP positive GCs (70-72). However, it remains undefined whether the latent infection of HP is involved in immune escape or determines the prognosis. Further investigations are warranted to clarify the utility of HP in the prediction of immunotherapy response.
Other potential biomarkers are also proposed to refine patient selection. Tumor infiltrating lymphocytes are selected immune cells characterized by high tumor specificity. Previous studies have unveiled a robust relationship between a higher density of tumor-infiltrating lymphocytes with better response to ICIs (73). There are also data reflecting the predictive value of gene signatures, including IFN-γ gene signature or T-cell-inflamed gene signature (21,31). Both have shown to be associated with better prognosis, though mature and large-sample clinical studies are lacking. Patients with HER2 positive tumors also reserve potentials to immunotherapy. Preclinical research demonstrated that targeting HER2 could synergize with immunotherapy via enhanced antibody-dependent cell-mediated cytotoxicity (74). However, large clinical studies containing the correlation analysis of HER2 status and immunotherapy responses are warranted. Of note, intricate interactions between different markers have not been fully elucidated. For example, PD-L1 overexpression is closely associated with MSI-H, EBV or HP infection. High-density tumor-infiltrating lymphocytes could be compatible with both MSI-H or microsatellite stable status. In conclusion, these findings offer intriguing insights into the molecular mechanisms of GC/GEJC, though more fundamental or translational researches are eagerly awaited.
Safety profile of checkpoint inhibitors in patients with GC or GEJC
The common TRAEs derived from anti-PD-1/PD-L1 therapies include fatigue, nausea, diarrhea, anorexia, skin rash, anemia, thrombocytopenia, pruritus, liver function abnormality, hypothyroidism and pyrexia. According to KEYNOTE-059 and ATTRACTION-2, grade ≥3 TRAEs occurred in 5%−10% of GC/GEJC patients with ICIs monotherapy (21,22). Combination immunotherapy is less tolerated, with ≥70% incidences of grade ≥3 TRAEs occurred in patients treated with cytotoxic reagents and ICIs (21,41). In CheckMate-032, the combination of nivolumab 1 mg and ipilimumab 3 mg resulted in 47% rates of grade 3−5 TRAEs.
Besides, immune-related adverse effects (irAEs) affect the function of multiple organ system including gastrointestinal, skin, cardiovascular and endocrine system. Reportedly, the most frequent irAEs in GCs are interstitial lung disease, hyperthyroidism and colitis (21,22,75). Though adverse effects are generally manageable, severe toxicities compromise therapies and are even life-threatening. Interestingly, the occurrence of irAEs is reported to be associated with favorable treatment efficacy (76), while the mechanism has not been fully defined.
Hyperprogression, characterized by rapid growth of primary lesions or new lesions formation compared to pre-treatment, is a noxious effect caused by anti-PD-1 therapy. Approximately 10% of ICIs-treated GC patients develop hyperprogression, leading to a dismal prognosis (77,78). Currently, predictors of hyperprogression have not been elucidated, placing ICIs-treated patients (particularly in first-line pattern) at risk of rapid failure. However, recent research suggested that the proliferation of PD-1 positive regulatory T cells might be a signal noted in hyperprogression cases, enlightening us with a direction for future prevention (77).
Conclusively, ICIs introduce new safety challenges into the management of GC/GEJC patients. Balancing the safety and efficacy of ICIs is inevitable to maximize clinical benefits.
Conclusions and future directions
Advanced GC or GEJC are aggressive diseases with dim prognosis. ICIs, navigating future therapeutics, have shown promising activity in GC patients. KEYNOTE-059 and ATTRACTION-2 have contributed to the approval of ICIs in the third-line treatment of advanced GC/GEJC. In March 2020, nivolumab was also licensed in China for treating GC/GEJC patients who received ≥2 lines of systemic therapies. Conversely, when compared to chemotherapy, ICIs failed to suggest superiority in KEYNOTE-061 and KYTNOTE-062. Besides, avelumab maintenance also indicated disappointing results. A range of ongoing trials are underway for assessing combination strategies, especially in earlier settings. GC/GEJC are identified as heterogeneous diseases. Distinct genomic aberrations in each subtype serve as promising targets for precision therapy, which is the direction of future drug development. The utility of biomarkers including EBV, MSI-H, PD-L1 expression and HP infection has been preliminarily confirmed, while new predictors are also actively investigated to define beneficiaries. We are urging for a deeper understanding of the genomic and immunological traits of GC/GEJC. Potential interplay between anti-cancer medications and combination strategies are also future research directions.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2017YFC1308900).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Zheng R, Wang N, et al Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res. 2018;30:291–8. doi: 10.21147/j.issn.1000-9604.2018.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth EC, Verheij M, Allum W, et al Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 5.Shitara K, Ohtsu A Advances in systemic therapy for metastatic or advanced gastric cancer. J Natl Compr Canc Netw. 2016;14:1313–20. doi: 10.6004/jnccn.2016.0138. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, D’Amico TA, Almhanna K, et al Gastric Cancer, Version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:1286–312. doi: 10.6004/jnccn.2016.0137. [DOI] [PubMed] [Google Scholar]
- 7.National Health Commission of the People’s Republic of China Chinese guidelines for diagnosis and treatment of gastric cancer 2018 (English version) Chin J Cancer Res. 2019;31:707–37. doi: 10.21147/j.issn.1000-9604.2019.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bang Y, Van Cutsem E, Feyereislova A, et al Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs CS, Tomasek J, Yong CJ, et al Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–9. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 10.Wilke H, Muro K, Van Cutsem E, et al Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet. 2014;15:1224–35. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 11.Shah MA Update on metastatic gastric and esophageal cancers. J Clin Oncol. 2015;33:1760–9. doi: 10.1200/JCO.2014.60.1799. [DOI] [PubMed] [Google Scholar]
- 12.Lordick F, Kang Y, Chung H, et al Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–9. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 13.Waddell T, Chau I, Cunningham D, et al Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–9. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah MA, Bang Y, Lordick F, et al Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma. JAMA Oncol. 2017;3:620–7. doi: 10.1001/jamaoncol.2016.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catenacci DVT, Tebbutt NC, Davidenko I, et al Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1467–82. doi: 10.1016/S1470-2045(17)30566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardoll DM The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadzadeh M, Johnson LA, Heemskerk B, et al Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn GP, Bruce AT, Ikeda H, et al Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 19.Iwai Y, Ishida M, Tanaka Y, et al Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muro K, Chung HC, Shankaran V, et al Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–26. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs CS, Doi T, Jang RW, et al Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang Y, Boku N, Satoh T, et al Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–71. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Wei XL, Wang FH, et al Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol. 2019;30:1479–86. doi: 10.1093/annonc/mdz197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Mo H, Zhang W, et al Promising efficacy of SHR-1210, a novel anti-programmed cell death 1 antibody, in patients with advanced gastric and gastroesophageal junction cancer in China. Cancer. 2019;125:742–9. doi: 10.1002/cncr.31855. [DOI] [PubMed] [Google Scholar]
- 25.Shitara K, Özgüroğlu M, Bang Y, et al Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–33. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs CS, Ozguroglu M, Bang Y, et al Pembrolizumab (pembro) vs paclitaxel (PTX) for previously treated advanced gastric or gastroesophageal junction (G/GEJ) cancer: Phase 3 KEYNOTE-061 trial. J Clin Oncol. 2018;(15 suppl):4062. doi: 10.1200/JCO.2018.36.15_suppl.4062. [DOI] [Google Scholar]
- 27.Chung HC, Arkenau H, Lee J, et al Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: phase 1b results from the JAVELIN Solid Tumor trial. J Immunother Cancer. 2019;7:30. doi: 10.1186/s40425-019-0508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bang YJ, Ruiz EY, Van Cutsem E, et al Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052–60. doi: 10.1093/annonc/mdy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Kang Y, Satoh T, et al A phase III study of nivolumab (Nivo) in previously treated advanced gastric or gastric esophageal junction (G/GEJ) cancer (ATTRACTION-2): Three-year update data. J Clin Oncol. 2020;38(4 suppl):383. doi: 10.1200/JCO.2020.38.4_suppl.383. [DOI] [Google Scholar]
- 30.Janjigian YY, Bendell J, Calvo E, et al CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;28:2836–44. doi: 10.1200/JCO.2017.76.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly RJ, Lee J, Bang Y, et al Safety and efficacy of durvalumab and tremelimumab alone or in combination in patients with advanced gastric and gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2020;26:846–54. doi: 10.1158/1078-0432.CCR-19-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bang Y, Golan T, Lin C, et al Interim safety and clinical activity in patients (pts) with locally advanced and unresectable or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma from a multicohort phase I study of ramucirumab (R) plus durvalumab (D) J Clin Oncol. 2018;36(4 suppl):92. doi: 10.1200/JCO.2018.36.4_suppl.92. [DOI] [Google Scholar]
- 33.Herbst RS, Arkenau HT, Santana-Davila R, et al Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol. 2019;20:1109–23. doi: 10.1016/S1470-2045(19)30458-9. [DOI] [PubMed] [Google Scholar]
- 34.Hara H, Shoji H, Takahari D, et al Phase I/II study of ramucirumab plus nivolumab in patients in second-line treatment for advanced gastric adenocarcinoma (NivoRam study) J Clin Oncol. 2019;37(4 suppl):129. doi: 10.1200/JCO.2019.37.4_suppl.129. [DOI] [Google Scholar]
- 35.Xu J, Zhang Y, Jia R, et al Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019;25:515–23. doi: 10.1158/1078-0432.CCR-18-2484. [DOI] [PubMed] [Google Scholar]
- 36.Bang Y, Kaufman B, Geva R, et al An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): Results in patients with relapsed gastric cancer. J Clin Oncol. 2019;37:140. doi: 10.1200/JCO.2019.37.4_suppl.140. [DOI] [Google Scholar]
- 37.Yamaguchi K, Satoh T, Muro K, et al Phase 1b study of andecaliximab (GS-5745, ADX) as monotherapy and in combination with nivolumab (nivo) in Japanese subjects with gastric or GEJ adenocarcinoma. J Clin Oncol. 2019;37(4 suppl):137. doi: 10.1200/JCO.2019.37.4_suppl.137. [DOI] [Google Scholar]
- 38.Shah MA, Metges J, Cunningham D, et al A phase II, open-label, randomized study to evaluate the efficacy and safety of andecaliximab combined with nivolumab versus nivolumab alone in subjects with unresectable or recurrent gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2019;37(4 suppl):75. doi: 10.1200/JCO.2019.37.4_suppl.75. [DOI] [Google Scholar]
- 39.Catenacci DVT, Lim KH, Uronis HE, et al Antitumor activity of margetuximab (M) plus pembrolizumab (P) in patients (pts) with advanced HER2+ (IHC3+) gastric carcinoma (GC) J Clin Oncol. 2019;37(4 suppl):65. doi: 10.1200/JCO.2019.37.4_suppl.65. [DOI] [Google Scholar]
- 40.Bang Y, Kang Y, Catenacci DV, et al Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22:828–37. doi: 10.1007/s10120-018-00909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabernero J, Van Cutsem E, Bang Y, et al Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study. J Clin Oncol. 2019;37(18 suppl):A4007. doi: 10.1200/JCO.2019.37.18_suppl.LBA4007. [DOI] [Google Scholar]
- 42.Bang Y, Cho JY, Kim YH, et al Efficacy of sequential ipilimumab monotherapy versus best supportive care for unresectable locally advanced/metastatic gastric or gastroesophageal junction cancer. Clin Cancer Res. 2017;23:5671–8. doi: 10.1158/1078-0432.CCR-17-0025. [DOI] [PubMed] [Google Scholar]
- 43.Moehler MH, Dvorkin M, Ozguroglu M, et al Results of the JAVELIN Gastric 100 phase 3 trial: avelumab maintenance following first-line (1L) chemotherapy (CTx) vs continuation of CTx for HER2− advanced gastric or gastroesophageal junction cancer (GC/GEJC) J Clin Oncol. 2020;38(4 suppl):278. doi: 10.1200/JCO.2020.38.4_suppl.278. [DOI] [Google Scholar]
- 44.Fonkoua LAK, Chakrabarti S, Sonbol MB, et al Enhanced efficacy of anti-VEGFR2/taxane therapy after progression on immune checkpoint inhibition (ICI) in patients (pts) with metastatic gastroesophageal adenocarcinoma (mGEA) J Clin Oncol. 2020;38(4 suppl):362. doi: 10.1200/JCO.2020.38.4_suppl.362. [DOI] [Google Scholar]
- 45.Boku N, Ryu MH, Kato K, et al Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4) Ann Oncol. 2019;30:250–8. doi: 10.1093/annonc/mdy540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasui H, Kawazoe A, Yamaguchi K, et al S-1+oxaliplatin with pembrolizumab for advanced gastric cancer: The cohort 1 in a phase IIb KEYNOTE-659 study. J Clin Oncol. 2020;38(4 suppl):382. doi: 10.1200/JCO.2020.38.4_suppl.382. [DOI] [Google Scholar]
- 47.Chau I, Penel N, Arkenau H, et al Safety and antitumor activity of ramucirumab plus pembrolizumab in treatment naïve advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: Preliminary results from a multi-disease phase I study (JVDF) J Clin Oncol. 2018;36(4 suppl):101. doi: 10.1200/JCO.2018.36.4_suppl.101. [DOI] [Google Scholar]
- 48.Janjigian YY, Maron S, Chou JF, et al 817P First-line pembrolizumab (P), trastuzumab (T), capecitabine (C) and oxaliplatin (O) in HER2-positive metastatic esophagogastric adenocarcinoma. Ann Oncol. 2019;30:z143–z247. doi: 10.1093/annonc/mdz247.143. [DOI] [Google Scholar]
- 49.Sohn BH, Hwang J, Jang H, et al Clinical significance of four molecular subtypes of gastric cancer identified by the Cancer Genome Atlas Project. Clin Cancer Res. 2017;23:4441–9. doi: 10.1158/1078-0432.CCR-16-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maron SB, Luke JJ, Hovey R, et al Identification of T-cell-inflamed gastric adenocarcinoma in The Cancer Genome Atlas (TCGA) J Clin Oncol. 2017;35(7 suppl):16. doi: 10.1200/JCO.2017.35.7_suppl.16. [DOI] [Google Scholar]
- 51.Adam J. Bass, Vesteinn Thorsson, Ilya Shmulevich, et al Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma C, Patel K, Singhi AD, et al Programmed death-ligand 1 expression is common in gastric cancer associated with Epstein-Barr virus or microsatellite instability. Am J Surg Pathol. 2016;40:1496–506. doi: 10.1097/PAS.0000000000000698. [DOI] [PubMed] [Google Scholar]
- 53.Smyth EC, Wotherspoon A, Peckitt C, et al Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the medical research council adjuvant gastric infusional chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3:1197–203. doi: 10.1001/jamaoncol.2016.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayakawa Y, Sethi N, Sepulveda AR, et al Oesophageal adenocarcinoma and gastric cancer: should we mind the gap? Nat Rev Cancer. 2016;16:305–18. doi: 10.1038/nrc.2016.24. [DOI] [PubMed] [Google Scholar]
- 55.Cortes-Ciriano I, Lee S, Park W, et al A molecular portrait of microsatellite instability across multiple cancers. Nat Commun. 2017;8:15180. doi: 10.1038/ncomms15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le DT, Uram JN, Wang H, et al PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le DT, Durham JN, Smith KN, et al Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Le DT, Uram JN, Wang H, et al PD-1 blockade in mismatch repair deficient non-colorectal gastrointestinal cancers. J Clin Oncol. 2016;34(4 suppl):195. doi: 10.1200/jco.2016.34.4_suppl.195. [DOI] [Google Scholar]
- 59.Kulangara K, Zhang N, Corigliano E, et al Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143:330–7. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 60.Yamashita K, Iwatsuki M, Harada K, et al Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer. 2019;23:95–104. doi: 10.1007/s10120-019-00999-9. [DOI] [PubMed] [Google Scholar]
- 61.Marabelle A, Le DT, Ascierto PA, et al Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le DT, Kim TW, Van Cutsem E, et al Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38:11–19. doi: 10.1200/JCO.19.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pietrantonio F, Miceli R, Raimondi A, et al Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37:3392–400. doi: 10.1200/JCO.19.01124. [DOI] [PubMed] [Google Scholar]
- 64.Kim ST, Cristescu R, Bass AJ, et al Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–58. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 65.Ansell SM, Lesokhin AM, Borrello I, et al PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Specenier P Nivolumab in squamous cell carcinoma of the head and neck. Expert Rev Anticancer Ther. 2018;18:409–20. doi: 10.1080/14737140.2018.1456337. [DOI] [PubMed] [Google Scholar]
- 67.Sasaki S, Nishikawa J, Sakai K, et al EBV-associated gastric cancer evades T-cell immunity by PD-1/PD-L1 interactions. Gastric Cancer. 2019;22:486–96. doi: 10.1007/s10120-018-0880-4. [DOI] [PubMed] [Google Scholar]
- 68.De Rosa S, Sahnane N, Tibiletti MG, et al EBV+ and MSI gastric cancers harbor high PD-L1/PD-1 expression and high cd8+ intratumoral lymphocytes . Cancers. 2018;10:102. doi: 10.3390/cancers10040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie T, Liu Y, Zhang Z, et al Positive status of Epstein-Barr virus as a biomarker for gastric cancer immunotherapy: a prospective observational study. J Immunother. 2020;43:139–44. doi: 10.1097/CJI.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu B, Chen L, Liu L, et al T-cell-mediated tumor immune surveillance and expression of B7 co-inhibitory molecules in cancers of the upper gastrointestinal tract. Immunol Res. 2011;50:269–75. doi: 10.1007/s12026-011-8227-9. [DOI] [PubMed] [Google Scholar]
- 71.Holokai L, Chakrabarti J, Broda T, et al Increased programmed death-ligand 1 is an early epithelial cell response to Helicobacter pylori infection . PLoS Pathog. 2019;15:e1007468. doi: 10.1371/journal.ppat.1007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen B, Qian A, Lao J, et al Relationship between Helicobacter pyloriand expression of programmed death-1 and its ligand in gastric intraepithelial neoplasia and early-stage gastric cancer . Cancer Manag Res. 2019;11:3909–19. doi: 10.2147/CMAR.S203035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fridman WH, Pagès F, Sautès-Fridman C, et al The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 74.Stagg J, Loi S, Divisekera U, et al Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci USA. 2011;108:7142–7. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michot JM, Bigenwald C, Champiat S, et al Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 76.Das S, Johnson DB Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamada T, Togashi Y, Tay C, et al PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer . Proc Natl Acad Sci USA. 2019;116:9999–10008. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ji Z, Peng Z, Gong J, et al Hyperprogression after immunotherapy in patients with malignant tumors of digestive system. BMC Cancer. 2019;19:705. doi: 10.1186/s12885-019-5921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]