Abstract
Background
Problem solving therapy (PST) and “Engage”, a reward-exposure” based therapy, are important treatment options for late-life depression, given modest efficacy of antidepressants in this disorder. Abnormal function of the reward and default mode networks has been observed during depressive episodes. This study examined whether resting state functional connectivity (rsFC) of reward and DMN circuitries is associated with treatment outcomes.
Methods
Thirty-two older adults with major depression (mean age = 72.7) were randomized to 9-weeks of either PST or “Engage”. We assessed rsFC at baseline and Week 6. We placed seeds in three a priori regions of interest: subgenual cingulate (sgACC), dorsal anterior cingulate cortex (dACC), and nucleus accumbens (NAcc). Outcome measures included the Hamilton Depression Rating Scale (HAMD) and the Behavioral Activation for Depression Scale (BADS).
Results
In both PST and “Engage”, higher rsFC between the sgACC and middle temporal gyrus at baseline was associated with greater improvement in depression severity (HAMD). Preliminary findings suggested that in “Engage” treated participants, lower rsFC between the dACC and DMPFC at baseline was associated with HAM-D improvement. Finally, in Engage only, increased rsFC from baseline to Week 6 between NAcc and Superior Parietal Cortex was associated with increased BADS scores.
Conclusion
The results suggest that patients who present with higher rsFC between the sgACC and a structure within the DMN may benefit from behavioral psychotherapies for late life depression. ‘Engage’ may lead to increased rsFC within the reward system reflecting a reconditioning of the reward systems by reward exposure.
Depressed individuals often have abnormalities in the reward systems and the default mode network (DMN),1 behaviorally expressed as a negative view of the self and the world and reduced anticipation and response to rewards. DMN and reward systems abnormalities are particulary pronounced in late-life depression, because aging preferentially affects these systems.2–8
Abnormal reward related behaviors, including reduced responsiveness to reward, reward anticipation, and effort valuation, have been reported in late-life depression.9–11 The dorsal anterior cingulate cortex12 and the nucleus accumbens (NAcc)13,14 – two key nodes of the reward system – participate in reward anticipation and response. Depressed adults show reduced activation of the dACC during reward anticipation.15 Older adults with major depression have lower resting state functional connectivity (rsFC) of the dACC compared to non-depressed individuals,16 and this abnormality predicts persistence of depressive symptoms.2 Depressed apathetic older adults exhibit reduced functional connectivity of the NAcc compard to depressed non-apathetic patients.3
During depression, there is increased rsFC of the DMN that is associated with negative self-referential thoughts, rumination, and negativity bias.17,18 Specifically, depressed individuals show increased rsFC of the subgenual anterior cingulate cortex (sgACC) with other DMN regions.19,20 Abnormal rsFC of the sgACC is associated with overall depression severity,19 negative self-referential thoughts, and affect dysregulation.21 Further, the sgACC may be especially important in late-life depression.2,22 Depressed older adults exhibit sgACC hyperactivation during affective processing, which is associated with greater white matter hyperintensity burden.23
Only few studies have examined neuroimaging correlates of response to psychotherapy for depression. These studies suggest that greater cortical volume of the dACC and the sgACC24, as well as higher rsFC between the sgACC and DMN nodes prior to treatment 25 predict superior response to cognitive behavioral therapy (CBT). A preliminary study showed that CBT may increase rsFC between the amygdala and fronto-parietal regions in depressed adults.26
Problem Solving Therapy (PST) and ‘Engage’ psychotherapy are effective in the treatment of late-life major depression.27–31 PST imparts problem solving skills aimed to increase the individuals mastery of the environment and reduce the experience of adversity. ‘Engage’ is a novel neurobiologically-informed therapy whose principal intervention is ‘reward exposure’ (i.e. engagement in meaningful, rewarding activities), aimed to reactivate the reward systems. When needed, Engage treats barriers to reward exposure (i.e. negativity bias, apathy, and affect dysregulation) so that engagement in rewarding activities can procede unimpeded.32 To our knowledge, this is the first study to investigate the relationship between rsFC of the reward systems and the DMN, and response to psychotherapies for late-life depression.
The aims of the current study were to investigate whether: a) baseline rsFC between the sgACC, NAcc and/or dACC and other networks is associated with change in depressive symptoms and with behavioral activation; and b) early change (weeks 0–6) of rsFC between these nodes and other networks is associated with change in depression severity and behavioral activation over the course of treatment. We hypothesized that baseline rsFC of the sgACC, NAcc, and/or dACC with other networks is associated with reduction in depression severity in both psycotherapies. We also hypothesized that rsFC of the NAcc, a central reward system node, is associated with reduced depression severity and increased behavioral activation for participants receiving ‘Engage’ therapy, but not PST. Because ‘Engage’ is designed to improve reward sensitivity in late-life depression, while PST is thought to improve depression through other mechanisms. These hypotheses were tested in a sample of 32 depressed older participants who were randomized to 9-weeks of either PST or ‘Engage’ as part of a larger two-site randomized controlled trial. The participants in the MRI study were recruited from the Cornell site.
Methods
Participants
Participants were recruited by the Weill Cornell Institute of Geriatric Psychiatry through local newspaper and radio advertisements as well as referral by their outpatient providers. Patients were recruited to participate in a psychotherapy RCT for late-life depression and randomly assigned to 9 weekly sessions of either ‘Engage’ psychotherapy or Problem-Solving Therapy. Of the patients enrolled in the psychotherpay trial at the Weill Cornell site (N = 138), 32 patients met elligibility and consented to an MRI scan. Scans were conducted at baseline and at week 6 of treatment. Inclusion criteria were: (a) aged 60 or older; (b) diagnosed with unipolar major depression without psychotic features using the Diagnostic and Statistical Manual of Medical Disorders 4th Edition (DSM-IV) and the Structured Clinical Interview for DSM Disorders;33 (c) A score of ≥24 on the Mini-Mental State Examination;34 (d) not on antidepressant medication or on a stable dose; (e) capacity to consent. Exclusion criteria were: (a) active suicidal ideation, inclusing intent and/or plan; (b) history or presence of psychiatric disorders other than major depression or generalized anxiety disorder; (c) use of psychotropic drugs or cholinesterase inhibitors othan than a mild dose of bezodiazepines or a stable dose of an antidepressant; (d) currently receiving psychotherapy; (e) acute or severe medical illness (e.g., delirium, metastatic cancer, decompensated cardiac, liver, or kidney failure, major surgery, or stroke; (f) presence of a neurological disease such as Parkinson’s disease, primary or secondary seizure disorders, intracranial tumors, mutiple sclorosis, or severe head trauma; (g) inability to speak English; (h) corrected visual acuity < 20/70 or color blindness; (i) contraindications for MRI scanning, including a cardiac pacemaker, metallic objects or implants in the body or head, and claustrophobia. The study was approved by the Weill Cornell Medicine and the Nathan Kline Institute for Psychiatric Research Instituional Review Boards, and participants received compensation for their time.
Measures
All measures were administered by trained and reliable raters who received ongoing supervision from PhD-level clinical psychologists. Diagnosis was determined by agreement of two clinicians after review of the participants’ psychiatric history and Structured Clinical Interview for DSM Disorders. Severity of depression was measured using the 24-item Hamilton Depression Rating Scale (HAMD; Hamilton, 1960) and behavioral activation was measured using the 25-item Behavioral Activation for Depression Scale (BADS; Kanter, Mulick, Busch, Berlin, & Martell, 2007), administered at baseline, Weeks 2, 4, 6, and 9. Following the results of a previously published study,30 we eliminated item 22 from the BADS scale (‘my work/schoolwork suffered because I was not as active as I needed to be’) as it was not relevant to most patients in this sample.
Treatments
‘Engage’ is a streamlined, stepped psychotherapy for mid- late-life depression based on the view that late life depression is associated dysfunctions in the reward system. Its principal intervention is “reward exposure” – i.e. encouraging patients to engage in (i.e. expose themselves to) rewarding activities between sessions in order to facilitate activation of the reward system.32 In patients with negativity bias, apathy, or inadequate emotional regulation interfering with “reward exposure”, ‘Engage’ offers additional interventions so that “reward exposure” proceeds unimpeded.
Problem-Solving Therapy (PST)
PST is a skills-based psychotherapy model which has been shown to reduce late-life depression severity.27,28,31 The first five weeks of therapy focused on training in a 5-step problem-solving model – teaching patients to set goals, develop ways to reach them, formulate action plans and assess their progress towards goals. The remaining sessions focus on enhancing the learned skills. The last two sessions focused on creating a relapse prevention plan.
Data Analytic Plan
Statistical Data Analysis
We investigated change over time in HAMD and BADS using mixed-effects linear models. Analysis of clinical change was conducted using lme4 package in R37. The models included subject-specific random intercept and fixed effects for time. Treatment was initially included in the model (main and interaction effects) but was removed due to non-significance of the effects.
MRI Data Acquisition
MRI data were acquired on the 3T Siemens TIM Trio scanner at the Center for Biomedical Imaging and Neuromodulation (C-BIN) of the Nathan Kline Institute for Psychiatric Research. As part of the MRI scan procedure, structural and functional images were acquired with a 32-channel head coil. Anatomical images were acquired with a high resolution T1-weighted MPRAGE for co-registration of functional data (192 slices; interleaved acquisition; no gap; TR = 2500ms; TE = 3.5ms; voxel size = 1mm isovoxel; FoV = 256mm; flip angle = 8°). Resting state images (awake, eyes closed) were acquired with a T2-weighted echoplanar BOLD sequence (150 volumes; 34 slices; interleaved acquisition; no gap; TR = 2500ms, TE = 30ms, voxel size = 3mm isovoxel; FOV = 216mm; flip angle = 80º). Acquisition time for the resting state sequence was 6 minutes, 20 seconds. Wakefulness throughout the resting state scan was confirmed by the MR technician. None of the participants reported falling asleep.
MRI preprocessing
MRI data were preprocessed with the FMRIB Software Library (FSL; version 6.00; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/; Smith et al., 2004) and the Analysis of Functional Neuroimages (AFNI) software package (version XXX; https://afni.nimh.nih.gov/; Cox, 1996). First, anatomical images were transformed to standard MNI space using affine transformation to align anatomical images to the anterior and posterior commissures of the standard MNI-152 template brain, and nonlinear transformation to warp the participant’s anatomical image to the same template.40,41 Rigid-body motion correction was first performed on rsFC images. Next, affine co-registration was performed on motion corrected resting-state functional images, aligning these images to the participant’s anterior/posterior commissure-aligned anatomical image. The subsequent image was nonlinearly normalized to the MNI-152 template brain using FSL’s FNIRT program. Preprocessing of normalized functional images included temporal shifting, spatial smoothing with a 6mm full width-half maximum Gaussian kernel, and brain extraction. Motion- and physiologically-derived noise was addressed first via ICA-AROMA, which uses independent component analysis automatically regress out motion-related and physiologically-derived artifacts.42 A final denoising step in AFNI modelled the following: linear trends, 12 variables corresponding to the demeaned and first temporal derivatives of the translational and directional motion traces outputted from rigid-body motion correction, fast ANATICOR regression to account for local cardiovascular and respiratory noise in white matter, and the first three principal components isolated from a lateral ventricle mask, and a low frequency bandpass filter (0.01 – 0.1 Hz). Preprocessed images were then used to perform further connectivity analyses. We did not remove any participants from analysis due to motion parameters. Mean framewise displacement for baseline was 0.196 mm and for week 6 was 0.199.
Region of Interest (ROI) Selection
Three a priori regions of interest (ROIs) were selected based on existing literature on reward functions and depression (see introduction) and were utilized as seed regions in our rsFC analyses: subgenual cingulate (sgACC), dorsal anterior cingulate cortex (dACC), and nucleus accumbens (Nacc). We generated the ROIs from the Harvard-Oxford cortical and subcortical structural atlases.43 We constrained the cortical and striatal ROIs further by masking the Harvard-Oxford parcels using resting state functional networks of interest (i.e. default mode (DMN) and reward networks). In particular, these ROIs were created by masking the clusters extracted from the Harvard-Oxford atlases by the corresponding networks in the 17-network Cortical Parcellation by Yeo et al.44 and the 17-network Striatal Parcellation by Choi et al.45
Whole-Brain ROI-to-voxel resting-state functional connectivity (rsFC) analysis
For each resting-state run, we used a general linear model for each ROI’s mean time-series independently, modeling the whole brain parametric mask corresponding to the whole-brain rsFC of each ROI. The resultant contrasts of parameter estimates were then used to generate higher level group statistics.
Higher-level statistics
Two group-level baseline mixed-effects general linear models were performed to assess whether pre-treatment rsFC was significantly associated with response to psychotherapy. First, we examined the association between baseline rsFC and percentage of improvement on the HAMD from baseline to Week 9. Second, we examined the associations between rsFC at baseline and BADS scores at Week 9, while controlling for baseline BADS scores. Second, two group-level, repeated-measures, mixed-effects general linear models were performed for each ROI to examine the association between change in rsFC in Weeks 0–6 and clinical outcomes (change BADS scores and HAMD percentage of improvement in Weeks 0–9). In all models we accounted for age and gender.
We implemented cluster-based inference using Gaussian Random Field Theory with a height z-score > 3.2 and a Bonferroni-corrected cluster-p < 0.0125 (two-tailed) to correct for the number of ROIs analyzed. For all analyses, we first examined the associations in the combined sample (both PST and ‘Engage’) and then separately at each treatment condition by exploring the two-way interaction effect.
Results
Our sample consisted of 32 patients (16 received ‘Engage’; 16 received PST) who completed an rsFC scan at baseline. Out of this sample, 23 patients completed an additional scan at Week 6 (see Table 1 for demographics and clinical characteristics). All patients in this sample completed treatment and participated at baseline and week 9 assessments.
Table 1.
Demographics and Baseline Clinical Characteristics
| Engage N=16 | PST N=16 | P value | |
|---|---|---|---|
| Age | 70.4 (7.43) | 74.3 (8.54) | 0.174 |
| Gender | 0.433 | ||
| Male | 3 (18.8%) | 6 (37.5%) | |
| Female | 13 (81.2%) | 10 (62.5%) | |
| Ethnicity | 1.00 | ||
| Non-Hispanic | 16 (100%) | 15 (93.8%) | |
| Hispanic | 0 (00%) | 1 (6.25%) | |
| HAMD | 22.9 (4.29) | 22.2 (4.45) | 0.659 |
| BADS | 74.9 (17.8) | 75.2 (19.5) | 0.963 |
Note. Numeric variables are presented with summary statistics of mean and standard deviation as well as test statistic from independent two-sample t-test (df=30). Categorical variables are presented with summary statistics of frequency and percentage as well as test statistic from Fisher’s exact test. HAMD = Hamilton Depression Scale; BADS = Behavioral Activation for Depression Scale
Change in Depression Severity and Behavioral Activation over time
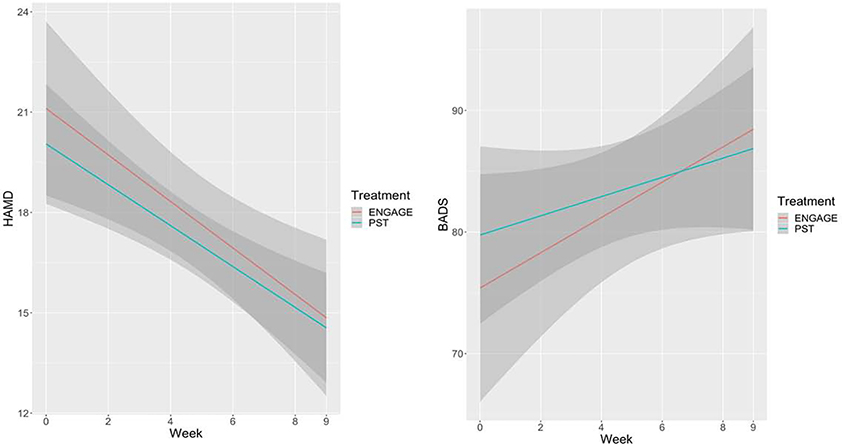
A mixed-effects model showed that depression severity (HAM-D) improved over the course of 9 weeks of ‘Engage’ or PST (Total Sample; F(5,145) = 14.30; p < .0001). BADS scores also increased over the course of treatment (F(5,145) = 5.24; p < .0001). The main effects of treatment and of time by treatment interaction were not significant in both models (Figure 1).
Figure 1.
Trajectories of change in means of depression severity (HAMD; left) and behavioral activation (BADS; right) over 9 weeks of Engage and PST. Linear Regression lines with 95% Confidence Interval.
Resting State Functional Connectivity (rsFC) at Baseline and Change in Depression All Subjects
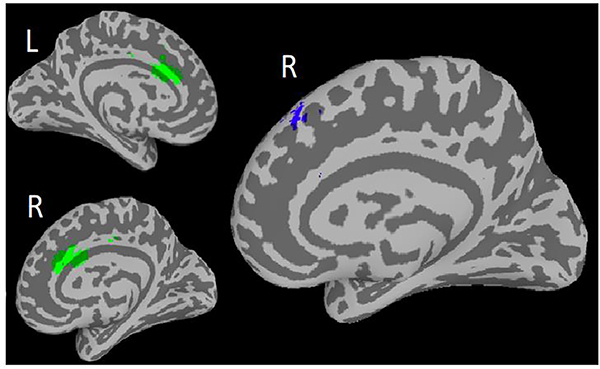
In the combined sample (PST and ‘Engage’), higher rsFC between the sgACC and the middle temporal gyrus (MTG) at baseline was associated with greater improvement in depression severity (195 voxels; Cluster-p = <0.0001, Peak z-score = 4.29; see Table 2; Figure 3). This association remained significant after applying Bonferroni correction.
Table 2.
MNI Coordinates for significant regions of functional connectivity and clinical outcomes (depression severity and behavioral activation)
| ROI | Hemisphere | Region | BA | Cluster-p | MNI | |||
|---|---|---|---|---|---|---|---|---|
| Peak voxel | x | y | z | |||||
| Combined Sample | ||||||||
| SgACC* | L | Temporal Pole, Middle Frontal Gyrus | 21, 38 | <.001 | 4.43 | −56 | 10 | −22 |
| Engage Only | ||||||||
| dACC | R | DMPFC | .02 | 3.79 | 6 | 38 | 41 | |
| NAcc | L | Superior Parietal Lobe | 7 | .02 | 3.92 | −17 | −52 | 63 |
Note. All findings indicate correlation between baseline functional connectivity and depression improvement, with the exception of NAcc-Superior Parietal Lobe finding which indicates correlation between baseline-Week 6 change in functional connectivity and change in behavioral activation scores.
survived Bonferroni correction for multiple ROIs; ROI = Region of Interest; L = left; R = Right; BA = Brodmann Area; DMPFC = Dorsal Medial Prefrontal Cortex; SgACC = Subgenual Anterior Cingulate Cortex; dACC = Dorsal Interior Cingulate Cortex; NAcc = Nucleus Accumbens
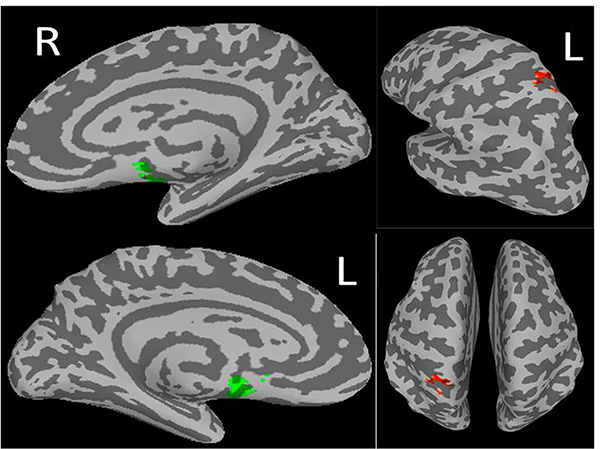
Figure 3.
Preliminary Finding 1: In ‘Engage’, but not PST: Lower functional connectivity between the dorsal anterior cingulate cortex (dACC; Left) and Dorsal Medial Prefrontal Cortex (DMPFC) associated with greater improvement in depression severity.
Preliminary Findings
‘Engage’ Group Only
We found two significant results at the cluster level which did not survive the Bonferroni correction of p=0.0167 for multiple comparisons for the three ROI’s.
Association between baseline rsFC and treatment outcome
Lower rsFC between the dACC and dorsomedial prefrontal cortex (DMPFC) (107 voxels, cluster-p = 0.02, peak z-score = 3.79; Table 2; Figure 3); was associated with greater improvement in depression severity over the course of treatment. We did not find significant associations in the PST group.
Association between change in rsFC from baseline to Week 6 and treatment outcome
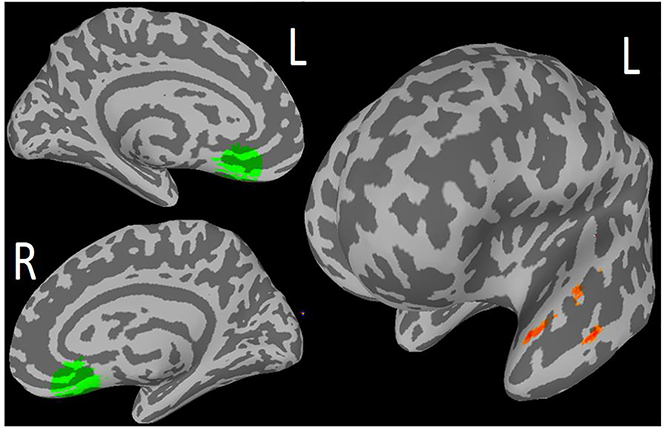
Increase in rsFC from baseline to Week 6 between the NAcc and the Superior Parietal Cortex was associated with increase in behavioral activation (BADS scores; 97 voxels, cluster-p = 0.022, peak z-score = 3.92; Table 2; Figure 4). We did not find significant associations in the PST group.
Figure 4.
Preliminary Finding 2: In ‘Engage’, but not PST: Increase in resting state functional connectivity between seed nucleus accumbens (NAcc; right) and Superior Parietal Cortex (SPC; left) associated with increase in behavioral activation
Discussion
The principal finding of this study is that higher rsFC between the sgACC and the middle temporal gyrus (MTG) at baseline was associated with greater reduction in depression severity in both PST and ‘Engage’. The MTG is a node of the default mode network (DMN), which underlies regulation of negative self-referential thinking in major depression.46,47 Functional and structural abnormalities in this region are common in major depression in adults48 and older adults.49,50 Hyperactivation of sgACC is associated with depression severity19 and depression-related negative thought processes and affect.21 Our finding suggests that depressed older adults with high rsFC between the sgACC and a node of the DMN are good candidates for behavioral psychotherapies for late life depression. Our finding is consistent with findings in young and middle-aged individuals with depression showing that high connectivity of sgACC-DMN was associated with superior response to CBT but poor response to pharmacotherapy.25
Preliminary findings also suggest that lower rsFC between the dACC and dorsomedial prefrontal cortex (DMPFC) was associated with greater improvement in depression over the course of ‘Engage’, but not PST. The dACC, a node of the cognitive control network, and the DMPFC, a node of the DMN, both underlie processes of directed attention and emotion regulation in depression.1,51 Activation of the dACC-DMPFC underlies the conscious threat appraisal and emotional processing, including catastrophizing and worrying.52,53 A meta-analysis suggests that psychotherapy for anxiety and depression in adults may reduce rsFC within the DMPFC.54 ‘Engage’ is aimed to improve abnormalities in these regions through interventions focused on emotion regulation.32 Our finding suggests that lower rsFC of the dACC/DMPFC at baseline may facilitate response to ‘Engage’.
Our preliminary findings also suggest that increase from baseline to Week 6 in rsFC between the NAcc and the Superior Partial Cortex (SPC) was associated with increase in behavioral activation over the course of treatment. The SPC is involved in visuospatial attention and attention shifting,55 while the NAcc is a core node responsible for reward processing, which is a core impairment in major depression.56 Our results show that increase in rsFC between reward processing circuitry and regions responsible for directed attention is associated with greater response to ‘Engage’. This preliminary finding is consistent with the conceptual premise of ‘Engage’, a treatment aimed to reactivate and recondition the reward systems through systematic reward exposure.32 It also parallels our previous observation that ‘Engage’ increases engagement in rewarding and pleasant activities, which is followed by reduction of late life depression severity. 30,57,58
Our findings should be considered in the context of the study’s limitations. First, our sample is small and findings that did not meet correction for multiple comparisons require replication. Sample size decreased further for the second scan, further reducing power for analyses that included mid-treatment scan. While all eligible participants recruited at the Cornell site were invited to participate in the MRI study, we cannot rule out potential self-selection effects that may have contributed to certain individuals’ decision to participate in MRI scans. Despite these limitations, given the limited knowledge on neurobiological predictors of response in psychotherapies for late life depression, our findings may be used to generate focused hypotheses for larger studies. Our study did not include a post-treatment scan since our a-priori aim was to investigate early change in rsFC in PST and ‘Engage’. Future studies can expand this line of inquiry to later treatment phases and examine trajectories of change in rsFC and clinical outcomes in psychotherapy. The length of the resting state scan was relatively short by today’s standards, which may have had a negative impact on our ability to detect relationships between rsFC and response to psychotherapy.
In conclusion, our study suggests that patients who present with higher rsFC between the sgACC and a structure within the DMN may benefit from behavioral psychotherapies for late life depression. ‘Engage’ may lead to increased rsFC within the reward system reflecting a reconditioning of the reward systems by reward exposure. This is the first study to investigate the association between abnormalities in the DMN and reward network and response to psychotherapies for late life depression. Our results parallel the observation that targeting the sgACC via dorsolateral prefrontal transcranial magnetic stimulation (TMS) is associated with greater antidepressant responses in treatment-resistant depression in adults.59–61 While our results are preliminary, they suggest that identifying specific behavioral and neural network targets can guide the development of streamlined, efficacious psychotherapy interventions.
Figure 2.
Primary Finding: In ‘Engage’ and PST: Higher baseline resting state functional connectivity between seed Subgenual Anterior Cingulate Cortex (sgACC; right) and Middle Temporal Gyrus (MTG; left) associated with depression improvement over 9 weeks of treatment
Highlights.
What is the primary question addressed by this study? The study investigated whether there is an association between resting state functional connectivity (rsFC) of the reward and default mode network (DMN) and outcomes of Problem Solving Therapy (PST) and “Engage” Psychotherapy for late life depression.
What is the main finding of this study? We found that in both PST and “Engage”, higher rsFC between DMN nodes at baseline was associated with greater improvement in depression severity. Additionally, in “Engage” treated participants, lower rsFC between the dACC and DMPFC at baseline was associated with depression improvement. Finally, in “Engage” only, early increase between NAcc and Superior Parietal Cortex was associated with increased behavioral activation.
What is the meaning of the finding? Depressed older adults who present with higher functional connectivity between DMN nodes may benefit from behavioral psychotherapies. Additionally, “Engage”, a reward-exposure based therapy, may facilitate increase in rsFC of the reward system, and lead to increase in behavioral activation.
Acknowledgements
We would like to thank Lindsay L. Arader, Merete Chaplin, Eve Root, Emily M. Parker, Jamie Glass, and Chloe J. Blau for their assistance in data collection of this project.
Conflicts of Interest and Source of Funding: This research was funded by National Institute of Mental Health grants P50 MH113838, R01 MH102252; T32 MH019132. Dr. Alexopoulos serves on the Eisai Advisory Board and Otsuka Speakers Bureau. He also served on the Speakers Bureaus of Allergan and Takeda-Lundbeck and Janssen Advisory Board. All other authors report no conflicts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139(1):56–65. doi: 10.1016/J.JAD.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexopoulos GS, Hoptman MJ, Yuen G, et al. Functional connectivity in apathy of late-life depression: A preliminary study. J Affect Disord. 2013;149(1–3):398–405. doi: 10.1016/j.jad.2012.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dombrovski AY, Szanto K, Clark L, et al. Corticostriatothalamic reward prediction error signals and executive control in late-life depression. Psychol Med. 2015;45:1413–1424. doi: 10.1017/S0033291714002517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sexton CE, Allan CL, Le Masurier M, et al. Magnetic resonance imaging in late-life depression: Multimodal examination of network disruption. Arch Gen Psychiatry. 2012;69(7):680–689. doi: 10.1001/archgenpsychiatry.2011.1862 [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Serotonin transporter polymorphisms, microstructural white matter abnormalities and remission of geriatric depression. J Affect Disord. 2009;119(1–3):132–141. doi: 10.1016/J.JAD.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae JN, MacFall JR, Krishnan KRR, Payne ME, Steffens DC, Taylor WD. Dorsolateral Prefrontal Cortex and Anterior Cingulate Cortex White Matter Alterations in Late-Life Depression. Biol Psychiatry. 2006;60(12):1356–1363. doi: 10.1016/j.biopsych.2006.03.052 [DOI] [PubMed] [Google Scholar]
- 8.Andreescu C, Butters MA, Begley A, et al. Gray matter changes in late life depression - A structural MRI analysis. Neuropsychopharmacology. 2008;33(11):2566–2572. doi: 10.1038/sj.npp.1301655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. J Psychiatr Res. 2008;43(1):76–87. doi: 10.1016/J.JPSYCHIRES.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pizzagalli DA. Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10(1):393–423. doi: 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bush G, Vogt BA, Holmes J, et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc Natl Acad Sci. 2002;99(1):523–528. doi: 10.1073/pnas.012470999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14(9):609–625. doi: 10.1038/nrn3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuber GD, Sparta DR, Stamatakis AM, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011. doi: 10.1038/nature10194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smoski MJ, Felder J, Bizzell J, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118(1–3):69–78. doi: 10.1016/j.jad.2009.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aizenstein HJ, Butters MA, Wu M, et al. Altered functioning of the executive control circuit in late-life depression: Episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17(1):30–42. doi: 10.1097/JGP.0b013e31817b60af [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry. 2015;78(4):224–230. doi: 10.1016/j.biopsych.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herwig U, Kaffenberger T, Jäncke L, Brühl AB. Self-related awareness and emotion regulation. Neuroimage. 2010;50(2):734–741. doi: 10.1016/j.neuroimage.2009.12.089 [DOI] [PubMed] [Google Scholar]
- 19.Greicius MD, Flores BH, Menon V, et al. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/J.BIOPSYCH.2006.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nejad AB, Fossati P, Lemogne C. Self-Referential Processing, Rumination, and Cortical Midline Structures in Major Depression. Front Hum Neurosci. 2013;7:666. doi: 10.3389/fnhum.2013.00666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eyre HA, Yang H, Leaver AM, et al. Altered resting-state functional connectivity in late-life depression: A cross-sectional study. J Affect Disord. 2016;189:126–133. doi: 10.1016/j.jad.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aizenstein HJ, Andreescu C, Edelman KL, et al. fMRI Correlates of White Matter Hyperintensities in Late-Life Depression. Am J Psychiatry. 2011;168(10):1075–1082. doi: 10.1176/appi.ajp.2011.10060853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambataro F, Doerig N, Hänggi J, et al. Anterior cingulate volume predicts response to psychotherapy and functional connectivity with the inferior parietal cortex in major depressive disorder. Eur Neuropsychopharmacol. 2018;28(1):138–148. doi: 10.1016/j.euroneuro.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 25.Dunlop BW, Rajendra JK, Craighead WE, et al. Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am J Psychiatry. 2017;174(6):533–545. doi: 10.1176/appi.ajp.2016.16050518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shou H, Yang Z, Satterthwaite TD, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. NeuroImage Clin. 2017;14:464–470. doi: 10.1016/j.nicl.2017.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexopoulos GS, Raue PJ, Kiosses DN, et al. Problem-Solving Therapy and Supportive Therapy in older adults with major depression and executive dysfunction. Arch Gen Psychiatry. 2011;68(1):33. doi: 10.1001/archgenpsychiatry.2010.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Areán PA, Raue P, Mackin RS, Kanellopoulos D, McCulloch C, Alexopoulos GS. Problem-Solving Therapy and Supportive Therapy in older adults with major depression and executive dysfunction. Am J Psychiatry. 2010;167(11):1391–1398. doi: 10.1176/appi.ajp.2010.09091327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexopoulos GS, Raue PJ, Kiosses DN, Seirup JK, Banerjee S, Arean PA. Comparing engage with PST in late-life major depression: A preliminary report. Am J Geriatr Psychiatry. 2015;23(5):506–513. doi: 10.1016/j.jagp.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexopoulos GS, Raue PJ, Gunning F, et al. “Engage” therapy: Behavioral activation and improvement of late-life major depression. Am J Geriatr Psychiatry. 2016;24(4):320–326. doi: 10.1016/J.JAGP.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexopoulos GS, Raue P, Areán P. Problem-Solving Therapy versus Supportive Therapy in geriatric major depression with executive dysfunction. Am J Geriatr Psychiatry. 2003;11(1):46–52. doi: 10.1097/00019442-200301000-00007 [DOI] [PubMed] [Google Scholar]
- 32.Alexopoulos GS, Areán PA. A model for streamlining psychotherapy in the RDoC era: the example of “Engage”. Mol Psychiatry. 2014;19(1):14–19. doi: 10.1038/mp.2013.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical interview for DSM-IV Axis I disorders. New York: Biometrics Res Dep New York State Psychiatr Institute. 1999. doi: 10.1093/molbev/msp224 [DOI] [Google Scholar]
- 34.Folstein M, Folstein S, McHugh P. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 35.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanter JW, Mulick PS, Busch AM, Berlin KS, Martell CR. The Behavioral Activation for Depression Scale (BADS): Psychometric Properties and Factor Structure. J Psychopathol Behav Assess. 2007;29(3):191–202. doi: 10.1007/s10862-006-9038-5 [DOI] [Google Scholar]
- 37.Bates D, Mächler M, Zurich E, Bolker BM, Walker SC. Fitting Linear Mixed-Effects Models Using Lme4. https://arxiv.org/pdf/1406.5823.pdf. Accessed November 14, 2018.
- 38.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/J.NEUROIMAGE.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 39.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/CBMR.1996.0014 [DOI] [PubMed] [Google Scholar]
- 40.Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K. The WU-Minn Human Connectome Project: An overview. Neuroimage. 2013;80:62–79. doi: 10.1016/J.NEUROIMAGE.2013.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glasser MF, Sotiropoulos SN, Wilson JA, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/J.NEUROIMAGE.2013.04.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064 [DOI] [PubMed] [Google Scholar]
- 43.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/J.NEUROIMAGE.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 44.Thomas Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi EY, Yeo BTT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108(8):2242–2263. doi: 10.1152/jn.00270.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunlop BW, Mayberg HS. Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin Neurosci. 2014;16(4):479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma C, Ding J, Li J, et al. Resting-state functional connectivity bias of middle temporal gyrus and caudate with altered gray matter volume in major depression. Fan Y, ed. PLoS One. 2012;7(9):e45263. doi: 10.1371/journal.pone.0045263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoptman MJ, Gunning-Dixon FM, Murphy CF, et al. Blood pressure and white matter integrity in geriatric depression. J Affect Disord. 2009;115(1–2):171–176. doi: 10.1016/j.jad.2008.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Respino M, Jaywant A, Kuceyeski A, et al. The impact of white matter hyperintensities on the structural connectome in late-life depression: Relationship to executive functions. NeuroImage Clin. 2019;23. doi: 10.1016/j.nicl.2019.101852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16(11):693–700. doi: 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- 52.Maier S, Szalkowski A, Kamphausen S, et al. Clarifying the role of the rostral dmPFC/dACC in fear/anxiety: learning, appraisal or expression? Bruce A, ed. PLoS One. 2012;7(11):e50120. doi: 10.1371/journal.pone.0050120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalisch R, Gerlicher AMV. Making a mountain out of a molehill: On the role of the rostral dorsal anterior cingulate and dorsomedial prefrontal cortex in conscious threat appraisal, catastrophizing, and worrying. Neurosci Biobehav Rev. 2014;42:1–8. doi: 10.1016/j.neubiorev.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 54.Messina I, Sambin M, Palmieri A, Viviani R. Neural Correlates of Psychotherapy in Anxiety and Depression: A Meta-Analysis. Mechelli A, ed. PLoS One. 2013;8(9):e74657. doi: 10.1371/journal.pone.0074657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alnæs D, Sneve MH, Richard G, et al. Functional connectivity indicates differential roles for the intraparietal sulcus and the superior parietal lobule in multiple object tracking. Neuroimage. 2015;123:129–137. doi: 10.1016/j.neuroimage.2015.08.029 [DOI] [PubMed] [Google Scholar]
- 56.Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural Responses to Monetary Incentives in Major Depression. Biol Psychiatry. 2008;63(7):686–692. doi: 10.1016/J.BIOPSYCH.2007.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alexopoulos GS, O’Neil R, Banerjee S, et al. “Engage” therapy: Prediction of change of late-life major depression. J Affect Disord. 2017;221:192–197. doi: 10.1016/j.jad.2017.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solomonov N, Bress JN, Anne Sirey JA, et al. Engagement in socially and interpersonally rewarding activities as a predictor of outcome in ‘Engage’ behavioral activation therapy for late-life depression. Am J Geriatr Psychiatry. 2019;27(6):571–578. doi: 10.1016/j.jagp.2018.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weigand A, Horn A, Caballero R, et al. Prospective Validation That Subgenual Connectivity Predicts Antidepressant Efficacy of Transcranial Magnetic Stimulation Sites. Biol Psychiatry. 2018;84(1):28–37. doi: 10.1016/j.biopsych.2017.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liston C, Chen AC, Zebley BD, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76(7):517–526. doi: 10.1016/j.biopsych.2014.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72(7):595–603. doi: 10.1016/j.biopsych.2012.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]