Abstract
Objective:
The aims of this study were to identify a subset of children with autism spectrum disorder (ASD) and co-occurring symptoms of psychopathology, and to evaluate associations between this subgroup and biological sex and amygdala volume.
Method:
Participants included 420 children (ASD: 91 girls, 209 boys; typically developing controls: 57 girls, 63 boys). Latent profile analysis was used to identify ASD subgroups based on symptoms of psychopathology, adaptive functioning, cognitive development, and autism severity. Differences in the proportions of girls and boys across subgroups were evaluated. Magnetic resonance imaging scans were acquired (346 children); amygdala volumes were evaluated in relation to subgroups and problem behavior scores.
Results:
Three ASD subgroups were identified. One group was characterized by high levels of psychopathology and moderate impairment on other measures (High Psychopathology Moderate Impairments [HPMI], comprising 27% of the sample). The other two subgroups had lower symptoms of psychopathology but were differentiated by high and low levels of impairment on other measures. A higher proportion of girls were classified into the HPMI subgroup (40% of girls versus 22% of boys). Relative to controls, amygdala volumes were enlarged only in the HPMI subgroup. There was a positive association between right amygdala volume and internalizing behaviors in girls but not in boys with ASD.
Conclusion:
A higher proportion of girls with ASD faced greater challenges with psychopathology, suggesting a need for closer evaluation and potentially earlier intervention to help improve outcomes. Amygdala enlargement was associated with co-occurring symptoms of psychopathology, and sex-specific correlations with symptoms were observed.
Keywords: autism spectrum disorder, psychopathology, girls, amygdala, MRI
Autism spectrum disorder (ASD) is heterogeneous, and multiple etiologies and subtypes exist.1–3 One approach to parsing heterogeneity in ASD is to identify clinically meaningful subgroups based on behavioral profiles and underlying etiologies. In this study, our goal was to investigate heterogeneity in psychopathology, biological sex, and amygdala volume in a cohort of preschool-aged children with ASD.
Biological sex is one source of heterogeneity in ASD, with female individuals being diagnosed less frequently than male individuals at a roughly 1 to 4 ratio.4,5 Recent efforts have begun to elucidate differences between boys and girls with ASD, both in behavioral6 as well as in neural characteristics.7,8 Co-occurring psychiatric conditions, including affective and anxiety disorders, attention-deficit/hyperactivity disorder (ADHD), and oppositional defiant disorder (ODD), also contribute to the heterogeneity of ASD, and occur at higher rates in individuals with ASD than in the general population.9 Across childhood and adolescence, an estimated 70% to 80% of individuals with ASD have at least one co-occurring psychiatric condition, and approximately 40% have 2 or more.10,11 There is evidence suggesting that female adolescents and adults with ASD have higher rates of mood disorders12 and internalizing problems13 than male counterparts. Less is known about what proportion of very young children exhibit co-occurring symptoms of psychopathology, whether there are sex differences in symptom presentation, and what the underlying neural mechanisms might be.
The amygdala, which plays an important role in emotion regulation and threat detection, is widely implicated in ASD as well as other psychiatric disorders, such as anxiety and depression.14,15 Amygdala enlargement is reported in many,16–19 but not all, studies of ASD, and heterogeneity is often cited as a reason for inconsistent findings.20 There is evidence that older children and adolescents with ASD with and without co-occurring anxiety disorders have distinct patterns of amygdala development.21 The relationship between amygdala enlargement and co-occurring symptoms of psychopathology has not yet been evaluated in young children with ASD. We predicted that amygdala enlargement would be most pronounced in a subset of children who also exhibited high rates of psychopathology. In addition, based on previous findings of more extreme amygdala enlargement in female children with ASD,17 we also predicted possible sex differences in either amygdala volume or in associations between amygdala volume and symptoms of psychopathology.
The current study used a person-centered approach to identify distinct patterns of psychopathology, adaptive functioning, cognitive development, and autism severity data collected on a large sample of preschool-aged children with ASD. We first derived subgroups of ASD children with similar patterns of symptoms across the variables examined using latent profile analysis (LPA). LPA is a latent variable approach for clustering individuals into more homogeneous subgroups using multiple continuous aspects of behavior. In this framework, the multiplicity of outcomes is handled by summarizing observations into latent constructs. A key assumption of LPA is that inter-item correlations are accounted for solely by class membership (conditional independence).
We then explored sex differences in the proportion of male and female participants in each subgroup. We predicted that there would be a subgroup of children with high levels of psychopathology accompanied by deficits in other areas and that a higher proportion of girls than boys with ASD would be classified into this subgroup. We also explored amygdala volume in relation to subgroups and symptoms of psychopathology. Age-matched TD controls served as a reference to the ASD group.
METHOD
Participants
Participants were enrolled in the University of California (UC) Davis MIND Institute Autism Phenome Project or Girls with Autism Imaging of Neurodevelopment Study, and included 300 children with ASD (91 girls, 209 boys) and 120 TD controls (57 girls, 63 boys). Children were 2–3.5 years of age at study entry (mean age 36.3 months). Studies were approved by the UC Davis Institutional Review Board. Informed consent was obtained from each participant’s parent or guardian. Parents completed a demographic form that included parental level of education and race/ethnicity. All participants were native English speakers, ambulatory, and had no suspected vision or hearing problems, known genetic disorders (including Fragile X), or other neurological conditions.
Assessments
Diagnostic evaluation for ASD was carried out using the Autism Diagnostic Interview–Revised and the Autism Diagnostic Observation Schedule (ADOS)–Generic or ADOS-222–24 by licensed clinical psychologists trained to research standards. The ADOS-2 provides a diagnostic cutoff and a calibrated severity score (CSS) to compare autism severity across different modules.25,26 TD children were screened using the Social Communication Questionnaire27 and excluded for scores greater than the clinical cutoff (≥11) or if they had first-degree relatives with ASD.
Developmental ability was assessed using the Mullen Scales of Early Learning (MSEL).28 TD children were excluded if developmental scores were two or more SDs below normative means on any MSEL subscale. Developmental quotients (DQ) were calculated as the average of the age-equivalent subscale scores divided by the chronological age and multiplied by 100.
Symptoms of psychopathology were measured using the parent-report preschool form of the Child Behavior Checklist (CBCL 1.5–5).29 The CBCL includes scales developed to reflect disorders catalogued in the DSM; these DSM-oriented scales yield age-normed t scores representing symptom severity.29,30 Four of the five CBCL DSM-oriented scales were included in current analyses, including Depressive Problems, Anxiety Problems, Attention-Deficit/ Hyperactivity Problems, and Oppositional Defiant Problems. The DSM-oriented Autism Problems scale was not used because data from this scale were redundant with other measures of autism symptom severity (ADOS CSS). The Internalizing and Externalizing Problems T scores were used to investigate associations with amygdala volume.
Adaptive behavior encompasses skills important for independence during daily activities. Parents reported adaptive behavior using the Vineland Adaptive Behavior Scales–II Parent/Caregiver Rating Form (VABS-II).31 The VABS-II measures adaptive skills across four domains: Communication, Daily Living, Socialization, and Motor Skills, yielding age-referenced standard scores.
Imaging
Magnetic resonance imaging (MRI) scans were acquired during natural nocturnal sleep32 in all TD participants and a subset of ASD participants (73 girls, 153 boys) at the UC Davis Imaging Research Center on a 3T Siemens Trio whole-body MRI system using an eight-channel head coil. A 3-dimensional T1-weighted magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence (TR 2,170 milliseconds; TE 4.86 milliseconds; matrix 256 × 256; 192 slices in the sagittal direction; 1.0-mm isotropic voxels) was acquired. To control for distortion associated with changes in hardware and software over time, a calibration phantom (ADNI MAGPHAM, The Phantom Laboratory) was scanned at the end of each MRI session. Distortion correction was implemented for each participant’s MPRAGE (Image Owl, Inc, Greenwich, NY; http://www.imageowl.com/).
Of the 74 children with ASD for whom a useable MRI scan was not acquired (18 girls, 56 boys), 29 children (7 girls, 22 boys) were unable to sleep through the MRI scan, 26 (8 girls, 18 boys) declined to attempt the MRI scan, and 7 (2 girls, 5 boys) had images acquired that did not pass quality control measures (ie, poor segmentation quality due to motion artifact or lack of accompanying phantom image for distortion correction). An additional 12 children (1 girl, 11 boys) were excluded because sequences were acquired using a different sequence protocol. Children with ASD in the MRI subsample had slightly higher mean DQ than children without an MRI scan (64.2 versus 58.6, p = .049), but there were no differences in ADOS CSS, CBCL DSM-oriented scores, or VABS subscale scores.
The distortion corrected, anonymized, and defaced MRIs were uploaded to MRICloud (https://mricloud.org)33 and segmented into anatomically defined regions using a multi-atlas approach in the fully automated MRICloud T1-Segmentation pipeline version 7A, which is based on the Diffeomorphic Multi-Atlas Likelihood Fusion algorithm.34 For each participant, an age-specific atlas set was used, based on age in years at the time of the scan. For 2- and 3- year-olds, the multi-atlas sets (each comprising 13 pediatric atlases) were optimized using data from our site at each age group, and are available on MRI Cloud (UC Davis 2 Years and UC Davis 3 Years). For children 4 years of age and older, the Pediatric 4–8 Years multi-atlas (based on 10 pediatric atlases) from the Johns Hopkins University inventory was used. The whole-brain segmentation output for each participant was downloaded and visually inspected for segmentation quality. Total hemispheric volumes and volumes for right and left amygdala were extracted. Total hemispheric volumes were summed for a total cerebral volume (TCV) measurement. To assess reliability of amygdala segmentations, we compared 107 amygdala volumes from the current study to manually segmented amygdala volumes from a previous study that includes a subset of male participants in the current study.18 Intraclass correlation coefficients were high (0.86 and 0.81 for left and right amygdala volumes).
Statistical Approach
Prior to analysis, distributions of CBCL DSM-oriented scales, VABS subscales, DQ, and ADOS CSS were examined. Whereas VABS and DQ were normally distributed, the CBCL DSM-oriented scales and ADOS-CSS exhibited a large number of observations clustered at the lower limits (ie, 50 for CBCL and 4 for ADOS) of the distribution. Thus, they were treated as censored normal variables in the LPA models.
Two-, three-, four-, five-, and six-class LPA models were run and compared using both statistical goodness-of-fit criteria and interpretability, taking into account whether the classes captured clinically meaningful features. We selected the optimal number of groups using the most parsimonious model that still provided good relative fit using a combination of statistical goodness-of-fit criteria, which included the Bayesian information criterion (BIC), Akaike information criterion (AIC), entropy, and Lo–Mendell–Rubin (LMR) and parametric bootstrapped likelihood ratio test (PBLRT).35,36 Smaller values of AIC and BIC indicate better fit and entropy values closer to 1 indicate better classification quality of individuals by each model. The likelihood ratio tests compare the fit of the specified class solution to models with one less class, and a significant p value indicates that the specified model should be preferred. The local maximum problem was addressed by using a large number of starting points (up to 500) to replicate each model.
Each LPA model provides two important pieces of information: it identifies the number of latent subgroups within the overall sample, and it estimates posterior probabilities for each participant’s assignment to a latent subgroup. For descriptive analyses, the highest posterior probability from the best-fitting model was used to assign each child to the most likely subgroup. For subsequent analyses using latent subgroup membership (ie, examination of sex differences and amygdala associations, described below), multiple pseudo-class draws were used to reduce bias by accounting for the uncertainty in class assignments.37 Children were randomly classified into latent classes 100 times based on their distribution of posterior probabilities from the best-fitting model. The subsequent analyses were performed 100 times (ie, for each draw), and results were combined across draws using standard methods for multiple imputation for missing data.38 χ2 tests were used to examine sex differences across groups. LPA was performed in Mplus version 8.39 All other analyses were implemented using SAS Version 9.4 (SAS Institute Inc, Cary, NC). All tests were two-sided, with α = 0.05.
MRI Analyses
A general linear model framework was used to investigate group differences in amygdala volume, as well as associations between CBCL Internalizing and Externalizing T scores and amygdala volume, while accounting for the effect of TCV, age, and sex. Separate models were fit for right and left amygdala volumes. A first set of models was fit to test for differences between all ASD and TD children, after adjusting for TCV, age, and sex. A second set of models included fixed effects to test for differences between each of the LPA groups and the TD group, after adjusting for TCV, age, and sex. A third set of models was used to explore associations between amygdala volume and CBCL internalizing and externalizing T scores. This third set of models included terms for TCV, CBCL scores, sex, and the interaction between sex and CBCL scores. The interaction allowed for evaluation of sex differences in the association of amygdala volume with CBCL scores.
RESULTS
Participant characteristics and scores on diagnostic and behavioral assessments are detailed in Table 1. Scores for typically developing controls are provided for reference. Information on race/ethnicity and parental education is provided in Table S1, available online.
TABLE 1.
Participant Characteristics
| ASD-F | ASD-M | TD-F | TD-M | |
|---|---|---|---|---|
| Participants, n | 91 | 209 | 57 | 63 |
| Age, moa | 37.5 (6.3) [25–60] | 36.1 (6.0) [24–56] | 36.7 (7.1) [25–52] | 34.7 (6.0) [24–53] |
| DQb | 63.5 (21.8) [22–129] | 62.5 (21.1) [27–137] | 107.6 (12.2) [74–134] | 105.3 (12.7) [79–135] |
| VQ/NVQb | 56.9 (27.1)/70.0 (19.1) | 55.1 (25.7)/69.9 (18.7) | 107.2 (15.4)/107.6 (12.9) | 106.6 (12.4)/103.8 (15.8) |
| ADOS-CSSc | 7.5 (1.7) [4–10] | 7.7 (1.7) [4–10] | – | – |
| CBCL DSM-Oriented Scalesd | ||||
| Depressive problems | 64.7 (10.3) [50–98] | 61.6 (8.6) [50–84] | 52.8 (4.4) [50–70] | 54.0 (6.0) [50–77] |
| Anxiety problems | 59.2 (10.0) [50–87] | 56.4 (9.3) [50–96] | 51.7 (3.8) [50–67] | 52.3 (4.7) [50–79] |
| Attention-deficit/hyperactivity problems | 61.1 (8.7) [50–76] | 59.1 (7.2) [50–76] | 51.9 (4.2) [50–76] | 52.9 (445) [50–71] |
| Oppositional defiant problems | 57.0 (8.9) [50–80] | 57.9 (8.7) [50–80] | 52.9 (4.3) [50–70] | 53.5 (5.2) [50–77] |
| VABS Subscalese | ||||
| Daily living skills | 75.3 (12.9) [51–109] | 79.0 (12.5) [53–113] | 109.7 (11.7) [87–145] | 108.3 (12.7) [79–143] |
| Communication | 70.8 (16.4) [42–113] | 73.1 (16.1) [33–116] | 111.5 (12.4) [81–142] | 110.7 (13.5) [61–143] |
| Socialization | 71.2 (11.2) [41–103] | 74.8 (11.7) [49–114] | 111.8 (12.8) [79–148] | 111.9 (12.9) [81–152] |
| Motor skills | 83.2 (13.6) [59–121] | 87.8 (12.7) [51–131] | 107.4 (12.0) [88–140] | 105.2 (14.2) [67–146] |
Note: Data are summarized as mean (SD) [range]. ADOS-CSS = Autism Diagnostic Observation Scale–Calibrated Severity Score; ASD-F = female participant with ASD; ASD-M = male participant with ASD; CBCL = Child Behavior Checklist; DQ = Developmental Quotient; TD-F = typically developing female participant; TD-M = typically developing male participant; VABS = Vineland Adaptive Behavior Scales.
Overall test, p = .052. Post hoc comparisons (using Tukey–Kramer correction) revealed that ASD female participants were older than TD male participants (p < .01); no other pairwise comparison was significant.
Overall test, p < .001. Post hoc comparisons revealed that typically developing (TD) controls had higher DQ, VQ, and NVDQ than ASD (p < .001), but there were no significant differences between male and female participants within each diagnostic group.
ADOS was not administered to TD controls. Male and female participants with ASD did not differ.
Overall test, p < .001, post-hoc comparisons revealed TD controls had lower scores on all scales relative to ASD p < .001. TD males and females did not differ on any scales. ASD females had higher scores than ASD males on the Depressive Problems scale (p = .001), Anxiety Problems scale (p = .008), and ADHD Problems scale (p = .001).
Overall test, p < .001. Post hoc comparisons revealed that TD controls had higher scores on all scales relative to ASD (p < .001). TD male and female participants did not differ on any scales. ASD female participants had lower scores on the motor skills subscale (p = .007) than ASD male participants, but did not differ on any other scale.
LPA Subgroups in ASD
Fit indices for two-class to six-class solutions are summarized in Table 2. They provided a mixed picture of the optimal number of classes. BIC and AIC indices never increased with added classes, PBLRT continued to decrease in models up to six classes, whereas the LMR likelihood ratio test suggested that a three-class solution was optimal (three-class was better than two-class, and four-class was not better than three-class). All models provided similar classification quality (entropy ranging from 0.82 to 0.85). In latent profile analyses, AIC and BIC may not increase with additional parameters, but the resulting models may have additional classes that are not meaningful. For example, in both five- and six-class models, one class with a lower level of impairments was differentiated into two classes that were not meaningfully different. Thus, the three-class solution was chosen as optimal because it was the most parsimonious model that still provided adequate fit.
TABLE 2.
Model Fit Statistics for Latent Profile Analysis (LPA) Models With Two to Six Classes
| No. of Classes | AICa | BICa | aBICa | LMRT p Value | PBLRT p Value | Entropy | Class Proportion Based on the Estimated Model | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||||
| Two | 20567 | 20682 | 20584 | < .001 | < .001 | 0.84 | 0.51 | 0.49 | – | – | – | – |
| Three | 20413 | 20568 | 20436 | .02 | < .001 | 0.82 | 0.40 | 0.32 | 0.28 | – | – | – |
| Four | 20318 | 20514 | 20346 | .25 | < .001 | 0.84 | 0.11 | 0.32 | 0.37 | 0.19 | – | – |
| Five | 20239 | 20476 | 20273 | .12 | < .001 | 0.85 | 0.10 | 0.28 | 0.20 | 0.29 | 0.12 | – |
| Six | 20183 | 20460 | 20222 | .57 | < .001 | 0.84 | 0.08 | 0.11 | 0.27 | 0.20 | 0.18 | 0.15 |
Note: Small p values of the LMRT and PBLRT tests indicate that the model with a greater number of classes fits the data better than the previous model. Entropy closer to 1 indicates that the children are well categorized into classes. The final model is shown in boldface type for emphasis. aBIC = sample size–adjusted Bayesian information criterion; AIC = Akaike information criterion; ASD = autism spectrum disorder; BIC = Bayesian information criterion; LMRT = Lo–Mendell–Rubin adjusted likelihood ratio test; PBLRT = parametric bootstrapped likelihood ratio test.
Lower numbers indicate more optimal model fit.
Based on the pattern of symptom severity across all measures, the three classes were named high psychopathology moderate impairments (HPMI), low psychopathology higher impairments (LPHI), and low psychopathology lower impairments (LPLI). Only one class, the HPMI class, had estimated scores in the borderline (65–69) to clinical (≥70) range on the CBCL DSM scales. Estimated scores for VABS subscales and DQ were moderately low and low ranges (≤85) but intermediate to the 2 other classes. The other 2 classes had CBCL DSM estimated scores in the normal range (≤64) but were differentiated based on the other measures. The LPHI class had the lowest estimated scores on VABS and DQ and the most severe ADOS CSS scores. The LPLI class had the highest estimated scores on VABS, with scores primarily in the adequate (86–100), and the highest DQ out of the 3 classes (although still in the moderately low range). Figure 1A illustrates the profiles for the three-class solution in greater detail.
FIGURE 1. Characterization of Autism Spectrum Disorder (ASD) Subgroups.
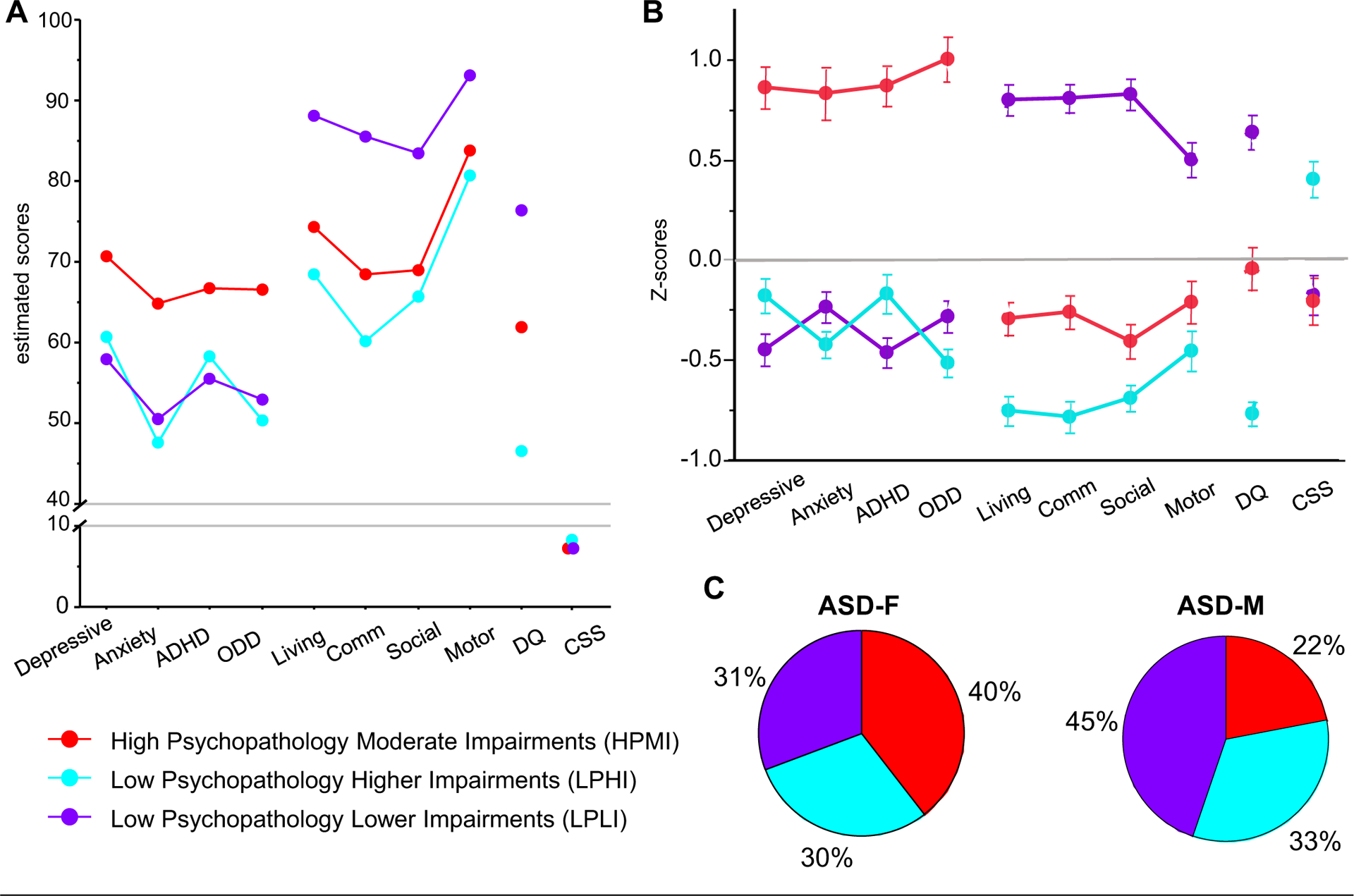
Note: (A) Estimated means for latent profile subgroups. (B) Average z scores and standard errors (SE) for each subgroup. The z scores were calculated based on means and standard deviations for all ASD participants. Averages and SE for each subgroup were calculated after generating 100 data sets using pseudo-draws to assign group membership and pooling the results. (C) Sex differences in the proportion of male and female participants in each latent class subgroup. A higher proportion of girls are classified into the HPMI (red) group than boys. ASD = autism spectrum disorder; Depressive = Child Behavior Checklist (CBCL) Depressive Problems; Anxiety = CBCL Anxiety Problems; ADHD = CBCL attention-deficit/hyperactivity problems; ODD = CBCL Oppositional Defiant Problems; Living = Vineland Adaptive Behavior Scales (VABS) Daily Living Skills; Comm = VABS Communication Skills; Social = VABS Socialization Skills; Motor = VABS Motor Skills; DQ = Mullen Scales of Early Learning (MSEL) Developmental Quotient; CSS = Autism Diagnostic Observation Schedule (ADOS) Calibrated Severity Score; ASD-F = female participant with ASD; ASD-M = male participant with ASD.
The highest posterior probability was used to assign each child to 1 of the 3 groups; 82 (27%) were assigned to the HPMI group, 97 (32%) to the LPHI group, and 121 (40%) to the LPLI group (average assignment probabilities for the groups were 89.5%, 90.3%, and 94.2% respectively). Figure 1B depicts averaged z scores and standard errors for each of the measures and each of the subgroups. Scores are depicted separately for girls and boys in Table S2, available online.
Sex Differences in LPA Subgroups in ASD
Next, we examined the sex distribution across the three LPA subgroups. Using the highest probability assignment, of the 91 female participants with ASD, 36 (40%) were classified into the HPMI group, 27 (30%) in the LPHI group, and 28 (31%) in the LPLI group. Of the 209 male participants with ASD, 46 (22%) were classified into the HPMI group, 70 (33%) in the LPHI group, and 93 (45%) in the LPLI group. Participant sex was significantly different across class membership (χ2 = 10.4, p = .006). depicted in Figure 1C, a much As higher proportion of girls, almost twice the proportion of boys, were in the HPMI group. This pattern of sex differences remained significant when 100 pseudo class draws were used to account for uncertainty in class assignment (p = .02).
Amygdala Findings
Amygdala and total cerebral volumes by diagnostic group and sex are summarized in Table S3, available online. After accounting for age, TCV, and sex, there was an overall diagnostic group difference between ASD and TD controls in right amygdala (estimated difference = 29.56 mm3, p = .04), but not in the left amygdala (estimated difference = −1.54 mm3, p = .91). Sex-by-diagnosis interactions were not significant (both p > .74).
Next, each LPA subgroup (HPMI: 25 girls, 32 boys; LPHI: 24 girls, 48 boys; LPLI: 24 girls, 73 boys) was compared to TD controls (Figure 2A) as well as to each other in analyses that accounted for uncertainty in class assignment (using 100 draws) and adjusting for age, TCV, and sex. Only the children in the HPMI group had significantly larger right amygdala volume (estimated difference = 46.62 mm3, p = .02) (Table 3, Figure 2A). Sex-by LPA group interactions were not significant (all p > .26). The LPHI and LPLI groups did not differ from TD controls (Table 3). Among ASD subgroups, the HPMI had larger volume than both LPHI and LPLI groups (estimated differences = 20.54 mm3 and 27.92 mm3, respectively) but these differences did not reach statistical significance (both p > .20). The difference between the LPHI and LPLI groups was modest (−7.38 mm3, p = .74).
FIGURE 2. Amygdala Volume and Autism Spectrum Disorder (ASD) Subgroups.
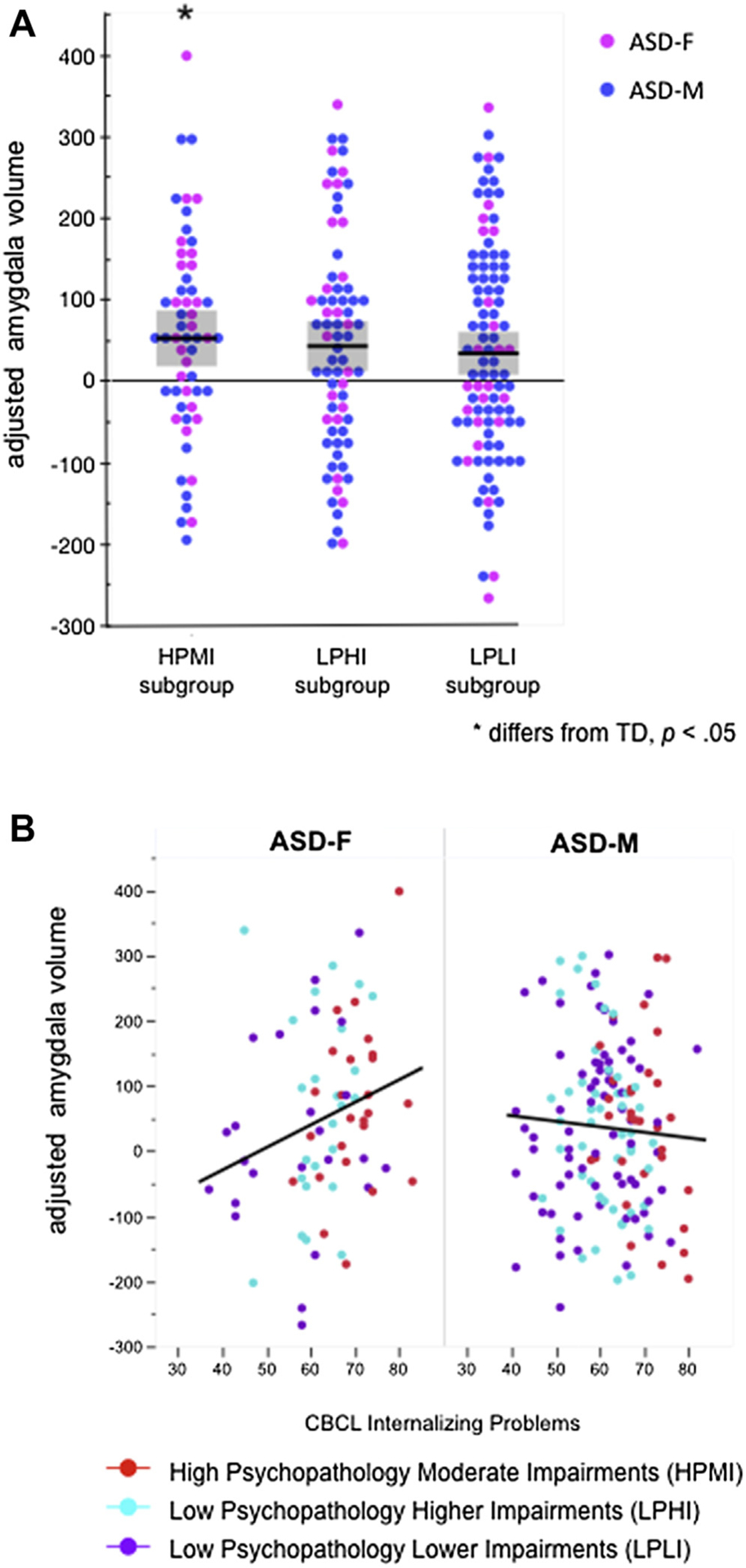
Note: (A) Right amygdala volumes (adjusted for sex, age, and total cerebral volume) by latent profile subgroups. Only the HPMI group differs from TD controls. (B) CBCL internalizing problems score is associated with right amygdala volume in female participants with ASD but not in male participants. Colors represent LPA group assignment. Amygdala volumes were adjusted by generating residuals for all participants using the estimates obtained from a regression model (using sex, age, and total cerebral volume as predictors) for the control group only. Each residual represents the deviation for each participant’s observed amygdala volume from what would be expected of a control participant with the same age, sex, and total cerebral volume. ASD = autism spectrum disorder; HPMI = High Psychopathology Moderate Impairments; LPLI = Low Psychopathology Lower Impairments; LPHI = Low Psychopathology Higher Impairments; LPA = latent profile analysis; TD = typically developing; CBCL = Child Behavior Checklist; ASD-F = female participant with ASD; ASD-M = male participant with ASD.
TABLE 3.
Parameter Estimates (Standard Errors) for the General Linear Models Predicting Right and Left Amygdala Volume (mm3)
| Model Term | Right Amygdala | Left Amygdala | ||
|---|---|---|---|---|
| Estimate (SE) | P | Estimate (SE) | P | |
| Intercept | 1661.45 (13.17) | < .001 | 1534.29 (12.73) | < .001 |
| Difference between HPMI and TD groups, mm3 | 46.62 (19.71) | .02 | 12.01 (18.66) | .52 |
| Difference between LPHI and TD groups, mm3 | 26.08 (20.62) | .21 | −1.20 (19.43) | .95 |
| Difference between LPLI and TD groups, mm3 | 18.70 (17.58) | .29 | −10.76 (17.87) | .55 |
| Age, mo | 2.65 (1.09) | .02 | 2.10 (1.06) | .047 |
| Female sex | −47.17 (15.07) | .002 | −54.13 (14.68) | < .001 |
| Total cerebral volume, for 100 mm3 | 0.12 (0.01) | < .001 | 0.11 (0.01) | < .001 |
Note: To account for the uncertainty in class assignments, we used 100 pseudo-class draws to randomly classify children into latent classes 100 times based on their distribution of posterior probabilities from the best fitting model. We subsequently performed the general linear model analysis 100 times (ie, for each draw) and results were combined across draws using standard methods for multiple imputation for missing data. Age and total cerebral volume were centered at the mean in the TD group. Because of centering, the intercept can be interpreted as the average amygdala volume for a TD male child with average age and average total cerebral volume. HPMI = high psychopathology moderate impairments; LPHI = low psychopathology higher impairments; LPLI = low psychopathology lower impairments; SE = standard error; TD = typically developing.
Next, associations between CBCL Internalizing and Externalizing Problems scores and amygdala volume were evaluated across all children with ASD. As depicted in Figure 2B, there was a significant interaction between sex and CBCL Internalizing score (p = .01), reflected in a strong association between right amygdala volume and CBCL Internalizing scores in female participants (estimate = 3.72 mm3, p = .01, ie, an estimated increase in volume of 3.72 mm3 for each unit increase in score) but not in male participants (estimate −1.01 mm3, p = .38). The pattern was similar for associations between right amygdala volume and CBCL Externalizing scores (girls: estimate = 3.03 mm3, p = .01, estimate = 0.94 mm3, boys: p = .34), although the interaction between sex and CBCL externalizing score did not reach statistical significance (p = .18).
DISCUSSION
Our results indicate that female children with ASD were significantly more likely than males to exhibit co-occurring emotional and behavioral problems associated with psychopathology. Relative to age-matched TD controls, amygdala enlargement was most prominent in the subset of children with ASD who showed more severe symptoms of psychopathology, and amygdala volume was associated with severity of internalizing problem behaviors in girls, but not in boys with ASD.
Identifying differences between boys and girls with ASD has been an area of increased interest. Existing evidence suggests that female adults and adolescents with ASD have higher rates of mood disorders12 and internalizing problems.13 In children with ASD, there is evidence for greater emotional problems in girls, but more externalizing problems in boys,40 including higher rates of ADHD and ODD in boys.41 Our results suggest that sex differences in psychopathology begin to manifest as early as 2 to 4 years of age in children with ASD, and that preschool-aged girls have higher symptoms of psychopathology across both internalizing and externalizing domains. One interpretation of these findings is that associated symptoms of psychopathology are more closely related to autism in girls than in boys.
By using a latent profile analysis, we were able to investigate how patterns of impairments cluster on measures of psychopathology, adaptive functioning, cognitive ability, and autism severity. We identified three distinct subgroups, and there were sex differences in group membership. One subgroup, comprising 27% of children with ASD in our sample, was distinguished based on having scores within the clinical range on measures of problem behaviors related to depression, anxiety, ADHD, and ODD. This subgroup had moderate impairments on the other measures of adaptive functioning and cognitive development but did not have the most impaired autism severity scores. More than one-third of girls with ASD (40%) versus less than one-fourth of boys (22%) were classified into this HPMI subgroup.
We originally hypothesized that children with the highest symptoms of psychopathology would also exhibit high levels of impairment on measures of adaptive functioning. Although children in the HPMI group did have moderately high levels of impairment on adaptive functioning and cognitive development, our results indicate that there was a separate subgroup, comprising 32% of the total ASD sample, with low symptoms of psychopathology but significantly more severe impairments on adaptive functioning and cognitive development. This subgroup was also distinguished by very high autism severity scores. Male and female participants were more evenly represented in this LPHI subgroup, with 30% of girls and 33% of boys classified into this subgroup. A third subgroup, comprising 40% of the ASD sample, had relatively low impairment on all measures (ie, low scores on measures of psychopathology and higher adaptive functioning and cognitive abilities), and 45% of boys versus 31% of girls were classified into this LPLI group.
We also evaluated amygdala enlargement, first across all children with ASD relative to TD controls, as many previous studies have done, and then within each subgroup of children with ASD relative to TD controls. Consistent with previous studies of young children,16–19 including a prior study from our group using a subset of males in the current study, we found enlargement in right amygdala volume in the group average for all children with ASD. In the current study, we also compared each of the ASD subgroups to TD controls and observed that right amygdala enlargement was most pronounced in HPMI group. The other subgroups did not differ significantly from TD controls, suggesting that amygdala enlargement was differentiated more by symptoms of psychopathology than the other measures of adaptive functioning, cognitive ability, and autism severity.
These results are consistent with a previous study that found differences in amygdala morphology in children and adolescents with ASD based on whether co-occurring anxiety disorders were present,21 although that study found reduced right amygdala volume in relation to co-occurring anxiety disorders, whereas we observed right amygdala enlargement in the HPMI group. This discrepancy in the directionality of the findings may be explained by the different ages of the samples: our study focused on 2- to 4-year-old children, whereas the previous study focused on older children and adolescents. Recent human postmortem evidence suggests that individuals with autism initially have excess numbers of mature neurons in the amygdala during early childhood, followed by a marked decline beginning in adolescence.42 Longitudinal studies that span early to middle childhood and adolescence will be instrumental in determining whether symptoms of psychopathology at 2 to 4 years of age persist. Additionally, longitudinal studies are needed to test whether associations between amygdala volume and symptoms of psychopathology change over the course of development and can predict clinical presentations of affective and anxiety disorders, ADHD, and ODD at later ages.
We also evaluated sex differences in amygdala enlargement in ASD. Across all children with ASD, we did not observe any differences in the pattern of enlargement across male and female children with ASD. Thus, with a larger sample of female children with ASD, we did not replicate the earlier findings of more extreme amygdala enlargement in girls with ASD.17 We also did not observe any sex-by-subgroup interactions in our comparison of each LPA subgroup relative to TD controls, suggesting that amygdala volume did not differ by sex within each LPA group. We did, however, observe sex-specific associations between amygdala volume and internalizing problem behaviors on the CBCL. Larger amygdala volumes were associated with more severe scores in girls, but not in boys with ASD. Thus, whereas the pattern of amygdala enlargement did not differ across male and female children with ASD, the associations with behavioral symptoms did.
Although the precise implications of this finding remain unclear, it is interesting to consider that within typical development, there is evidence from human imaging studies for sex differences in amygdala function related to emotional processing and fear detection, sometimes in the absence of overt behavioral differences (reviewed by Whittle et al.43) and a different developmental trajectory of amygdala growth, with girls reaching peak amygdala volume about 1 year earlier than boys.44,45 In addition, recent evidence from animal studies have found sex differences in fear extinction,46 which is heavily dependent on amygdala function, and suggest a potential role for estradiol in explaining female vulnerability to anxiety and mood disorders.47 As we learn more about sex differences, both in typical development as well as in ASD, it will also be important to consider how sex differences in the neural circuitry related to psychopathology interact with ASD. Evidence from the current study suggests that the amygdala may play a more circumscribed role related to symptoms of psychopathology in female children with ASD.
There are several strengths and limitations to consider regarding the current study. Strengths include a relatively large sample size of girls with ASD, particularly for a neuroimaging study. It is also important to evaluate young children, close in time to the age of diagnosis, prior to intensive behavioral and pharmacological interventions likely altering neural circuitry and possibly masking the true underlying neural basis of ASD. One important limitation to the study is that the measures of psychopathology and adaptive functioning used in this study are based on parent- report, which could be biased by differences in implicit gender-based expectations or a reflection of greater symptom camouflaging in girls.48 Moreover, longitudinal stability of the CBCL DSM-oriented scales, in particular, has not yet been empirically demonstrated in preschool-aged children with ASD. Longitudinal follow-up of these subgroups will be necessary to determine whether subgroups identified in early childhood are meaningful in identifying risk for later psychopathology. Another potential limitation is that all children enrolled in the study had a clinical diagnosis of ASD based on current gold standard diagnostic instruments. Although this was necessary in order to define our ASD group, recent evidence suggests there may be diagnostic gender bias resulting in fewer girls being clinically diagnosed with ASD,49 and girls who do have a clinical diagnosis of ASD often have additional problem behaviors or cognitive difficulties.50 With the current study design, it is impossible to determine whether a subset of girls exists who would meet criteria for a clinical diagnosis but do not have high levels of co-occurring psychopathology. Additional population-based studies and/or development of sex-specific clinical assessments will be critical toward addressing this question. Finally, the LPA assumption of conditional independence implies that there is no variation within classes. Factor mixture models (FMM) would allow for an analysis that overcomes this assumption. Our sample size did not allow the use of FMM, but future larger studies in ASD could use FMM to replicate the present findings and to permit characterization of symptom severity differences between class members.
In conclusion, we used a data-driven approach to tease apart heterogeneity related to biological sex, co-occurring psychopathology symptoms, and amygdala volume in 2-to 4-year-old children with ASD. Our results suggest that a higher proportion of female children with ASD have clinically significant symptoms of psychopathology. We also found that amygdala enlargement is not present in all children with ASD but, rather, only in the subset with the most prominent psychopathology. Earlier detection of co-occurring psychopathology and better understanding of underlying neural mechanisms of these symptoms could lead to interventions and treatments to improve outcomes in later childhood and adolescence.
Supplementary Material
Acknowledgments
Funding for this study was provided by the National Institute of Mental Health (R01MH104438 [CWN], R01MH103284 [MS], R01MH103371 [DGA]) and the University of California (UC) Davis MIND Institute. This project was also supported by the MIND Institute Intellectual and Developmental Disabilities Research Center (U54HD079125) and the Autism Center of Excellence (P50 HD093079) awarded by the National Institute of Child Health and Development (NICHD). J.L. and V.R. were supported by the MIND Institute Autism Research Training Program (T32MH073124) and L.L. was supported by the University of California President’s Postdoctoral Fellowship.
The authors would like to thank the families and children who participated in the GAIN and APP studies. They also thank Cory Coleman, BS and Natasha Sharma, BS, both of UC Davis, for their technical assistance, and the entire research study staff.
Footnotes
Disclosure:
Dr. Amaral has served on the Scientific Advisory Boards of Stemina Biomarkers Discovery, Inc. and Axial Therapeutics. Dr. Solomon has received research grant funding from the National Institutes of Health. Dr. Ozonoff has received research grant funding from the National Institutes of Health and Autism Speaks; travel reimbursement and honoraria for editorial activities from Autism Speaks, the Autism Science Foundation, and Wiley; and book royalties from Guilford Press and American Psychiatric Press, Inc. Dr. Young has received research funding from Autism Science Foundation. Drs. Nordahl, Iosif, Heath, Lee, Libero, Reinhardt, Winder-Patel, Rogers, and Ms. Hechtman report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Christine Wu Nordahl, University of California at Davis School of Medicine, Sacramento, CA.; MIND Institute, Sacramento, CA.
Ana-Maria Iosif, University of California at Davis School of Medicine, Sacramento, CA..
Gregory S. Young, University of California at Davis School of Medicine, Sacramento, CA.; MIND Institute, Sacramento, CA.
Alexa Hechtman, University of California at Davis School of Medicine, Sacramento, CA.; MIND Institute, Sacramento, CA.
Brianna Heath, University of California at Davis School of Medicine, Sacramento, CA.; MIND Institute, Sacramento, CA.
Joshua K. Lee, University of California at Davis School of Medicine, Sacramento, CA.; MIND Institute, Sacramento, CA.
Lauren Libero, University of California at Davis School of Medicine, Sacramento, CA.; California Department of Developmental Services, Sacramento, CA. MIND Institute, Sacramento, CA, University of California at Davis School of Medicine, Sacramento, CA.
Vanessa P. Reinhardt, University of California at Davis School of Medicine, Sacramento, CA.; Peel Children’s Center, Ontario, Canada. MIND Institute, Sacramento CA, University of California at Davis School of Medicine, Sacramento, CA.
Breanna Winder-Patel, University of California at Davis School of Medicine, Sacramento, CA..
David G. Amaral, University of California at Davis School of Medicine, Sacramento, CA.; MIND Institute, Sacramento, CA.
Sally Rogers, University of California at Davis School of Medicine, Sacramento, CA.; MIND Institute, Sacramento, CA.
Marjorie Solomon, University of California at Davis School of Medicine, Sacramento, CA.; MIND Institute, Sacramento, CA.
Sally Ozonoff, University of California at Davis School of Medicine, Sacramento, CA.; MIND Institute, Sacramento, CA.
REFERENCES
- 1.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. [DOI] [PubMed] [Google Scholar]
- 2.Lenroot RK, Yeung Pk. Heterogeneity within autism spectrum disorders: what have we learned from neuroimaging studies? Front Hum Neurosci. 2013;7:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Prevalence of autism spectrum disorderseAutism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012; 61:1–19. [PubMed] [Google Scholar]
- 5.CDC. Prevalence of autism spectrum disorder among children aged 8 yearseAutism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- 6.Kirkovski M, Enticott PG, Fitzgerald PB. A review of the role of female gender in autism spectrum disorders. J Autism Dev Disord. 2013;43:2584–2603. [DOI] [PubMed] [Google Scholar]
- 7.Lai MC, Lombardo MV, Suckling J, et al. Biological sex affects the neurobiology of autism. Brain. 2013;136:2799–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaer M, Kochalka J, Padmanabhan A, Supekar K, Menon V. Sex differences in cortical volume and gyrification in autism. Mol Autism. 2015;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen TE, Mazefsky CA, Vasa RA, Lerner MD. Co-occurring psychiatric conditions in autism spectrum disorder. Int Rev Psychiatry. 2018;30:40–61. [DOI] [PubMed] [Google Scholar]
- 10.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47: 921–929. [DOI] [PubMed] [Google Scholar]
- 11.Houghton R, Ong RC, Bolognani F. Psychiatric comorbidities and use of psychotropic medications in people with autism spectrum disorder in the United States. Autism Res. 2017;10:2037–2047. [DOI] [PubMed] [Google Scholar]
- 12.Kreiser NL, White SW. ASD Traits and co-occurring psychopathology: the moderating role of gender. J Autism Dev Disord. 2015;45:3932–3938. [DOI] [PubMed] [Google Scholar]
- 13.Solomon M, Miller M, Taylor SL, Hinshaw SP, Carter CS. Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. J Autism Dev Disord. 2012;42:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nordahl CW, Schumann CM. Early variations in amygdala development may signal divergent behavioral outcomes. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019; 4:3–4. [DOI] [PubMed] [Google Scholar]
- 15.Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49:745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sparks BF, Friedman SD, Shaw DW, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. [DOI] [PubMed] [Google Scholar]
- 17.Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66:942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordahl CW, Scholz R, Yang X, et al. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: a longitudinal study. Arch Gen Psychiatry. 2012;69:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellani M, Calderoni S, Muratori F, Brambilla P. Brain anatomy of autism spectrum disorders II. Focus on amygdala. Epidemiol Psychiatr Sci. 2013;22:309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrington JD, Maddox BB, Connor, et al. Amygdala volume differences in autism spectrum disorder are related to anxiety. J Autism Dev Disord. 1234;47: 3682–3691. [DOI] [PubMed] [Google Scholar]
- 22.Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 23.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule, Second Edition Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- 24.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. [DOI] [PubMed] [Google Scholar]
- 25.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39:693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esler AN, Bal VH, Guthrie W, Wetherby A, Weismer SE, Lord C. The Autism Diagnostic Observation Schedule, Toddler Module: standardized severity scores. J Autism Dev Disord. 2015;45:2704–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutter M, Bailey AJ, Lord C. Social Communication Questionnaire (SCQ). Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 28.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service, Inc; 1995. [Google Scholar]
- 29.Achenbach TM, Rescorla LA. Manual for the ASEBA preschool forms & profiles. Burlington, VT: University of Vermont Research Center for Children, Youth, and Families; 2000. [Google Scholar]
- 30.Achenbach T DSM-Oriented Guide for the Achenbach System of Empirically Based Assessment (ASEBA). Burlington, VT: University of Vermont Research Center for Children, Youth, and Families; 2014. [Google Scholar]
- 31.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales, Second Edition Minneapolis, MN: Pearson Assessments; 2006. [Google Scholar]
- 32.Nordahl CW, Simon TJ, Zierhut C, Solomon M, Rogers SJ, Amaral DG. Brief report: methods for acquiring structural MRI data in very young children with autism without the use of sedation. J Autism Dev Disord. 2008;38:1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori S, Wu D, Ceritoglu C, et al. MRICloud: delivering high-throughput mri neuroinformatics as cloud-based software as a service. Comput Sci Eng. 2016; 18:21–35. [Google Scholar]
- 34.Tang X, Crocetti D, Kutten K, et al. Segmentation of brain magnetic resonance images based on multi-atlas likelihood fusion: testing using data with a broad range of anatomical and photometric profiles. Front Neurosci. 2015;9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- 36.Nylund KL, Asparouhov T, Muth en BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equat Model. 2007;14:535–569. [Google Scholar]
- 37.Bandeen-Roche K, Miglioretti DL, Zeger SL, Rathouz PJ. Latent variable regression for multiple discrete outcomes. J Am Stat Assoc. 1997;92:1375–1386. [Google Scholar]
- 38.Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons Inc; 1987. [Google Scholar]
- 39.Muthen LK, Muthen BO. Statistical analysis with latent variables user’s guide. Los Angeles, CA: Muthen & Muthen; 2017. [Google Scholar]
- 40.Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D. Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. J Autism Dev Disord. 2012;42:1304–1313. [DOI] [PubMed] [Google Scholar]
- 41.Salazar F, Baird G, Chandler S, et al. Co-occurring psychiatric disorders in preschool and elementary school-aged children with autism spectrum disorder. J Autism Dev Disord. 2015;45:2283–2294. [DOI] [PubMed] [Google Scholar]
- 42.Avino TA, Barger N, Vargas MV, et al. Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc Natl Acad Sci. 2018;115: 3710–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whittle S, Yücel M, Yap MBH, Allen NB. Sex differences in the neural correlates of emotion: evidence from neuroimaging. Biol Psychol. 2011;87:319–333. [DOI] [PubMed] [Google Scholar]
- 44.Uematsu A, Matsui M, Tanaka C, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7: e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ. 2012;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruene TM, Roberts E, Thomas V, Ronzio A, Shansky RM. Archival report: sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol Psychiatry. 2015;78:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cover KK, Maeng LY, Lebr on-Milad K, Milad MR. Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Transl Psychiatry. 2014;4 e422–e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratto AB, Kenworthy L, Yerys BE, et al. What about the girls? Sex-based differences in autistic traits and adaptive skills. J Autism Dev Disord. 2018;48:1698–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56:466–474. [DOI] [PubMed] [Google Scholar]
- 50.Dworzynski K, Ronald A, Bolton P, Happe F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J Am Acad Child Adolesc Psychiatry. 2012;51:788–797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


