Abstract
Background
Human papillomavirus (HPV)-positive oropharyngeal cancer (HPV-OPC) is distinct from HPV-unassociated head and neck cancer. However, whether risk factors for HPV-positive oropharyngeal and nonoropharyngeal cancer are the same is unclear.
Methods
Incident cases of HPV-positive head and neck cancer and matched non-cancer controls were enrolled in a multi-institutional, prospective study examining risk factors, biomarkers, and survival.
Results
HPV-nonOPC (n=20) were more likely to be ever smokers than controls (n=80, OR 3.49, 95%CI 1.11–10.9) and HPV-OPC (n=185, OR 3.28, 95%CI 1.10–10.2). Compared with HPV-OPC, HPV-nonOPC were less likely to have had over 3 oral sexual partners (OR 0.29, 95%CI 0.06–0.9), more likely to have multimorbidity (OR 3.30, 95%CI 1.04–10.5), and less likely to have antibodies to HPV16 E6 (90% vs. 28%, OR 0.05, 95%CI 0.02–0.2). HPV-nonOPC had worse 4-year OS (77% vs. 96%, p=0.001) and RFS (69% vs. 94%, p<0.001) than HPV-OPC.
Conclusions
HPV-positive nonoropharyngeal cancers are distinct from HPV-OPC.
Keywords: HPV, Head and Neck Cancer, Oropharyngeal Cancer, Biomarkers, Survival
Introduction
Human papillomavirus-associated oropharyngeal squamous cell carcinoma (HPV-OPC) is considered a distinct disease entity from HPV-negative head and neck cancer.1 HPV transcripts are also present in2 and appear to play a causative role3 in a small proportion (2–10%)2 of nonoropharyngeal (HPV-nonOPC) head and neck cancers. Although the improved prognosis conferred by HPV-positive tumor status appears to be limited to the oropharynx where the majority of North American cases are HPV-positive,4,5 the prognostic role of p16 (INK4A) overexpression in nonoropharyngeal cancers has been variable.4–9 It is unclear whether HPV-positive tumors have a unique phenotype in nonoropharyngeal sites.
Risk factor profiles for head and neck squamous cell cancer (HNSCC) have been previously explored by HPV tumor status, although few of the HPV-positive cases described were non-oropharyngeal.10 Sexual exposure has been shown to be strongly associated with a diagnosis of HPV-positive head and neck cancer, when the oropharynx is predominantly involved.10,11 In contrast, tobacco and alcohol use are strongly associated with cases without exposure to HPV11 or at nonoropharyngeal sites where HPV tumor status was not examined.12,13 Furthermore, HPV-OPC patients tend to be younger, are more likely to be male and white14 and have a lower comorbidity index15 compared to non-oropharyngeal HNSCC.
In light of the significant shifts in HNSCC epidemiology16 and staging1 due to HPV, there remains interest in characterizing HPV-positive nonoropharyngeal (HPV-nonOPC) cancers. Whether risk factor and HPV biomarker profiles resemble those of HPV-negative cancers or HPV-OPC may give context for how to conceptualize the role of HPV in nonoropharyngeal cancers.
Materials and Methods
Study Participants
Participants were enrolled between 2013 and 2018 in a multicenter, case-control study of squamous cell carcinomas (SCCs) called the Papillomavirus Role in Oral Cancer Viral Etiology study (PROVE). Enrollment occurred at three NCCN-designated Comprehensive Cancer Centers: The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital (JHH, Baltimore, MD), University of California San Francisco Helen Diller Family Comprehensive Cancer Center (UCSF, San Francisco, CA) and Tisch Cancer Institute at Icahn School of Medicine at Mount Sinai (New York, NY). Case eligibility criteria included incident head and neck SCCs, and no prior radiation or head and neck cancer. Controls were adult patients attending otolaryngology clinics for chief complaints that did not include cancer. Written consent was given by all enrollees, and institutional review boards at each site approved the study.
Data Collection
At enrollment, participants completed a survey and gave a pre-treatment blood sample. For cases, a tumor sample was obtained and medical record abstraction was performed for tumor site, tumor and nodal stage using American Joint Committee on Cancer (AJCC) 7th edition,17 vital and recurrence status, and for missing smoking data. The computer-assisted survey included questions on demographics, behavioral risk factors, comorbidities and health status. Data were stored using RedCap (Vanderbilt University, Nashville TN).
Tumor testing
Centralized tumor HPV testing was performed prospectively at Johns Hopkins and interpreted by two head and neck pathologists (W.H.W. and L.M.R.). P16 immunohistochemistry (MTM Laboratories, Heidelberg, Germany) was performed on each tumor, with p16 expression considered positive if ≥70% strong and diffuse nuclear and cytoplasmic staining pattern was observed.18,19 In addition, in situ hybridization (ISH) was performed for HPV16 E6/E7 RNA ISH (RNAscope®, Advanced Cell Diagnostics, Hayward, CA). In cases that were p16-positive but HPV16 RNA ISH-negative, additional testing was done using a E6/E7 RNA ISH probe (RNAscope, Advanced Cell Diagnostics) that included 18 high-risk HPV types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82).20 Cases were thus defined as HPV-related if they were HPV16 RNA ISH positive, or HPV HR probe E6/E7 RNA ISH positive.
A head and neck pathologist (LR) reviewed all available histologic sections and classified the morphology of each case.
Human papillomavirus serology
Sera were tested for antibodies to HPV major capsid protein (L1) and early proteins (oncoproteins E6 and E7) from oncogenic HPV types 16, 18, 31, 33, 35, 45, 52, and 58 and non-oncogenic HPV types 6 and 11. Regulatory proteins E1 and E2 were tested from HPV types 16 and 18. Testing was performed at the German Cancer Research Center (DKFZ, Heidelberg, Germany) using multiplex serology, an antibody detection method based on glutathione S-transferase (GST) capture ELISA, in combination with fluorescent bead-based technology, in a procedure that has been previously described.21 Median fluorescence intensity (MFI) values were dichotomized as antibody positive or negative, using standardized cutoff values.22,23
Analysis
Analysis was restricted to cases that were HPV-positive; that is, where transcriptional activity of high-risk HPV types was detected in tumor by in situ hybridization (ISH). The analytic population represented 90% of 205 enrolled oropharyngeal cancer cases, 18% of 34 larynx, 9% of 111 oral cavity, and all 3 nasopharyngeal cancers.
HPV-nonOPC cases were each matched with four unique controls on the basis of sex, age (within 5 years), and race (white non-Hispanic, non-white). For the case-control analysis, conditional logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CI) for odds of a diagnosis of HPV-nonOPC cancer. For the case-case and serum biomarker analysis, logistic regression models were used to estimate ORs and 95% CIs for a diagnosis of HPV-nonOPC compared with HPV-OPC. Due to known differences in HPV serology by sex and age,24,25 seroprevalence ORs were adjusted for these variables. Chi-squared tests were used to compare categorical variables including tumor characteristics and demographics. The effect of tumor anatomic site on survival was evaluated with Kaplan-Meier, log-rank, and Cox proportional hazards methods. Patients were censored at death, administrative censoring, or loss to follow-up. Associations were considered statistically significant where two-sided p-value was less than 0.05. STATA version 15.1 (College Station, TX) was used for analysis.
Results
Risk factors in HPV-nonOPC compared to controls
There were 20 HPV-nonOPC, including oral cavity (10, 50%), larynx (6, 30%), nasopharynx (3, 15%), and hypopharynx (1, 5%). The majority of cases were men (172, 84%) and white (175, 85%), with overall median age of 59 years (interquartile range 53–65). Compared to age-, sex-, and race-matched controls, HPV-nonOPC were similar in terms of income and education. A significantly lower proportion of HPV-nonOPC were married or living with a partner than controls (Supplemental Table 1).
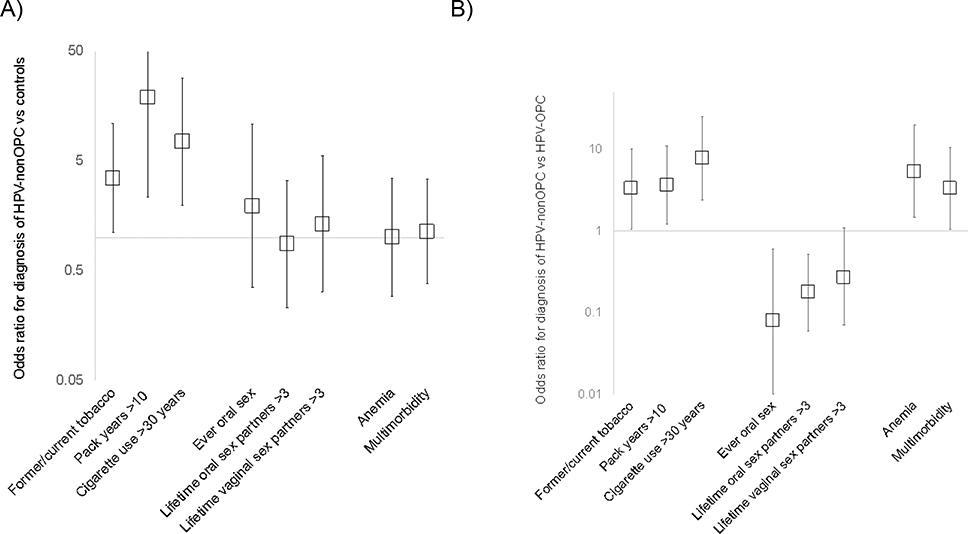
HPV-nonOPC cases and controls were similar by history of tonsillectomy, oral hygiene, obesity, sexual exposure, or prevalent comorbidities (Table 1, Supplemental Table 2, Figure 1). Several measures of tobacco use were significantly associated with the diagnosis of HPV-nonOPC cancer. These included increased odds of HPV-nonOPC among ever tobacco users (OR 3.49, 95%CI 1.11–10.9), those with greater than 10 pack-years of cigarette smoking (OR 18.88, 95%CI 2.34–152.6), or greater than 30 years of cigarette use (OR 7.49, 95%CI 1.97–28.4).
Table 1.
Risk factors for human papillomavirus (HPV) -positive nonoropharyngeal (HPV-nonOPC) compared with matched controls, and with HPV-oropharyngeal cancer (HPV-OPC).
| HPV-nonOPC N=20 | Matched controls N=80 | HPV-nonOPC cases vs. controls | HPV-OPC N=185 | HPV-nonOPC vs. HPV-OPC | |
|---|---|---|---|---|---|
| N (%) | N (%) | OR (95% CI) | N (%) | OR (95% CI) | |
| History of tonsillectomy | 3 (50) | 26 (42) | 2.05 (0.19–22.0) | 42 (45) | 1.24 (0.24–6.5) |
| Excellent/very good oral hygiene | 11 (69) | 40 (53) | 2.04 (0.57–7.4) | 97 (57) | 1.67 (0.56–5.0) |
| Obese | 3 (19) | 19 (26) | 0.64 (0.17–2.5) | 44 (26) | 0.67 (0.18–2.5) |
| Sexual exposure | |||||
| Age at sexual debut <18 | 9 (60) | 25 (35) | 2.79 (0.89–8.7) | 109 (65) | 0.80 (0.27–2.4) |
| Ever oral sex | 13 (87) | 60 (81) | 2.79 (0.35–10.8) | 166 (99) | 0.08 (0.01–0.6) |
| >3 lifetime oral sex partners | 7 (47) | 35 (47) | 0.88 (0.23–3.3) | 126 (75) | 0.29 (0.06–0.9) |
| >3 lifetime vaginal sex partners | 10 (77) | 42 (63) | 1.34 (0.32–5.5) | 148 (93) | 0.27 (0.10–1.1) |
| Substance use | |||||
| Ever tobacco use | 16 (80) | 40 (51) | 3.49 (1.11–10.9) | 100 (55) | 3.28 (1.10–10.2) |
| >10 pack years | 9 (60) | 5 (11) | 18.88 (2.34–152.6) | 43 (29) | 3.66 (1.23–10.9) |
| >30 year cigarette use | 9 (64) | 7 (12) | 7.49 (1.97–28.4) | 25 (19) | 7.78 (2.40–25.2) |
| Alcohol use ≥15 days/month | 9 (45) | 38 (48) | 0.91 (0.34–2.4) | 68 (37) | 1.41 (0.56–3.6) |
| Regular marijuana use | 5 (50) | 21 (49) | 0.40 (0.06–2.7) | 47 (36) | 1.79 (0.49–6.5) |
| >10 pot years | 1 (14) | 8 (18) | 0.78 (0.07–8.9) | 28 (36) | 0.30 (0.03–2.6) |
| Ever cocaine/heroin/ methamphetamine use |
4 (29) | 15 (21) | 1.40 (0.39–5.0) | 56 (34) | 0.79 (0.24–2.6) |
Figure 1.
Odds ratios for the diagnosis of HPV-nonOPC versus (vs) A) noncancer controls and B) HPV-OPC. Squares represent odds ratios. Vertical lines represent 95% confidence intervals, and horizontal lines represent null associations.
HPV, human papillomavirus; nonOPC, nonoropharyngeal; OP, oropharyngeal; vs, versus
Risk factors in HPV-nonOPC compared to HPV-OPC
In case-case comparison of HPV-nonOPC and HPV-OPC, HPV-nonOPC were more likely to be female and less likely to be married or living with a partner (Supplemental Table 1). In addition, HPV-nonOPC were significantly more likely to be ever tobacco users (OR 3.28, 95%CI 1.10–10.2), to have smoked more than 10 pack-years (OR 3.66, 95%CI 1.23–10.9), and to have used cigarettes for more than 30 years (OR 7.78, 95%CI 2.40–25.2, Table 1, Figure 1). As anatomic subgroups were different by sex and marital status, measures of tobacco exposure were examined in multivariate analysis controlling for these factors. Although HPV-nonOPC cases compared with HPV-OPC were not significantly associated with ever tobacco use after adjustment for sex and marital status (aOR 3.09, 95%CI 0.83–11.5), this group remained significantly associated with greater than 10 pack-years of smoking history (aOR 3.66, 95%CI 1.03–12.9) and over 30 years of cigarette use (aOR 5.97, 95%CI 1.46–24.4) after the same adjustment. A sensitivity analysis including only survey-reported smoking exposure showed similar results.
There were notable differences in oral sexual behavior between HPV-nonOPC and HPV-OPC cases, although both had HPV-positive cancer. HPV-nonOPC cases were significantly less likely to have had oral sex (OR 0.08, 95%CI 0.01–0.6) and to have had >3 lifetime oral (OR 0.29, 95%CI 0.06–0.9) sexual partners compared with HPV-OPC cases (Table 1, Figure 1).
Prevalent comorbidities were then examined. Multimorbidity was defined as the presence of two or more chronic conditions. HPV-nonOPC were more likely to have a history of anemia (OR 5.37, 95%CI 1.46–19.7) and multimorbidity (OR 3.30, 95%CI 1.04–10.5) compared with HPV-OPC; however, these differences were not independent of sex and marital status (aORanemia 2.95, 95%CI 0.69–12.7; aORmultimorbidity 2.60, 95%CI 0.77–8.8, Supplemental Table 2, Figure 1).
Tumor histology and HPV status
Although the predominant type of both HPV-nonOPC (75%) and HPV-OPC (91%) evaluable cases were found to be non-keratinizing, HPV-nonOPC were significantly less likely to be non-keratinizing (p=0.03). Out of the total HPV positive tumors, eight (4%) tumors were of the keratinizing histologic subtype, and HPV-nonOPC cases were more likely to be keratinizing than HPV-OPC (15% vs. 3%, p=0.008).
All HPV-nonOPC and 98% of HPV-OPC tumors were p16-positive (Supplemental Table 3). Notably, the proportion of HPV16 positive cases as detected by RNA and/or DNA in situ hybridization was significantly lower for nonoropharyngeal (70%) compared with oropharyngeal (91%) cases (p=0.005).
Serum biomarkers
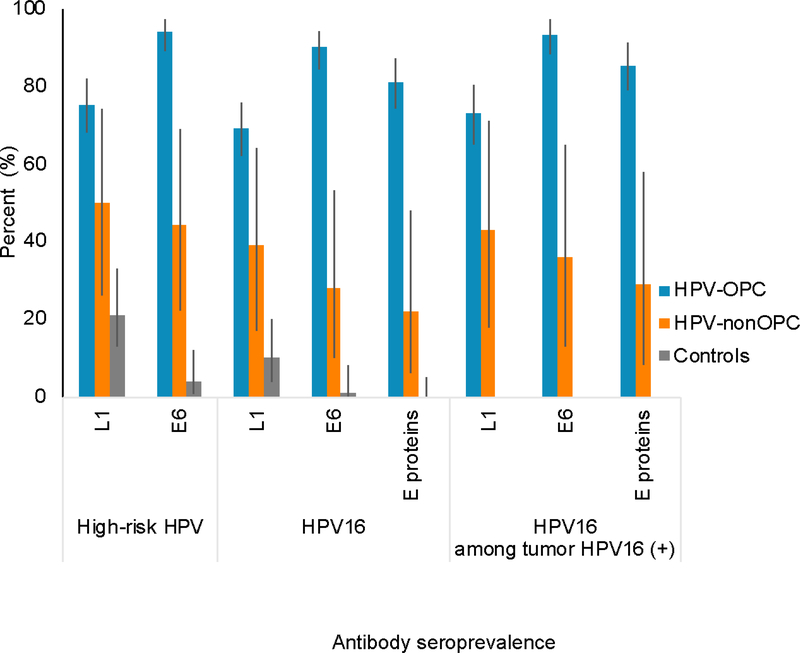
Next, differences in serum biomarker prevalence were explored as an indirect marker of HPV viral exposure. Compared with controls, HPV-nonOPC cases (70% of which were HPV16 tumor positive) were significantly more likely to be seropositive to any high-risk HPV type L1 (50% vs. 21%, OR 4.22, 95%CI 1.36–13.1) viral capsid protein, and high-risk HPV E6 (44% vs. 4%, OR 22.86, 95%CI 4.55–114.7) oncoproteins (Figure 2). Similar findings were observed when HPV16 specific antibodies were examined. HPV-nonOPC cases and controls were found to have similarly low seroprevalence of E6 and E7 proteins of all other HPV types examined (Supplemental Table 4).
Figure 2.
Antibody seroprevalence among HPV-positive oropharyngeal (HPV-OPC) cases, HPV-positive nonoropharyngeal (HPV-nonOPC) cases, and matched noncancer controls. Bars represent 95% confidence interval. High risk denotes any one of HPV types 16, 18, 31, 33, 35, 45, 52, 58. “E proteins” are defined as seropositive for any three of E1, E2, E6, E7 for HPV type 16.
Next, prevalence of HPV serum biomarkers was compared by site grouping. Sera from HPV-nonOPC participants had a significantly lower prevalence of antibodies to high-risk HPV16 E6 proteins overall (28% vs. 90%, OR 0.05, 95%CI 0.02–0.2) compared to HPV-OPC. This finding persisted when comparisons were restricted to participants with HPV16-positive tumors (36% vs. 93%, OR 0.05, 95%CI 0.01–0.2). HPV-nonOPC cases had a lower seroprevalence of antibodies to E6 and E7 oncoproteins of most other high-risk HPV types examined compared with HPV-OPC (Supplemental Table 4). Non-oropharyngeal cases positive for HPV type 16 were not limited to sites adjacent to the oropharynx.
Overall and recurrence-free survival
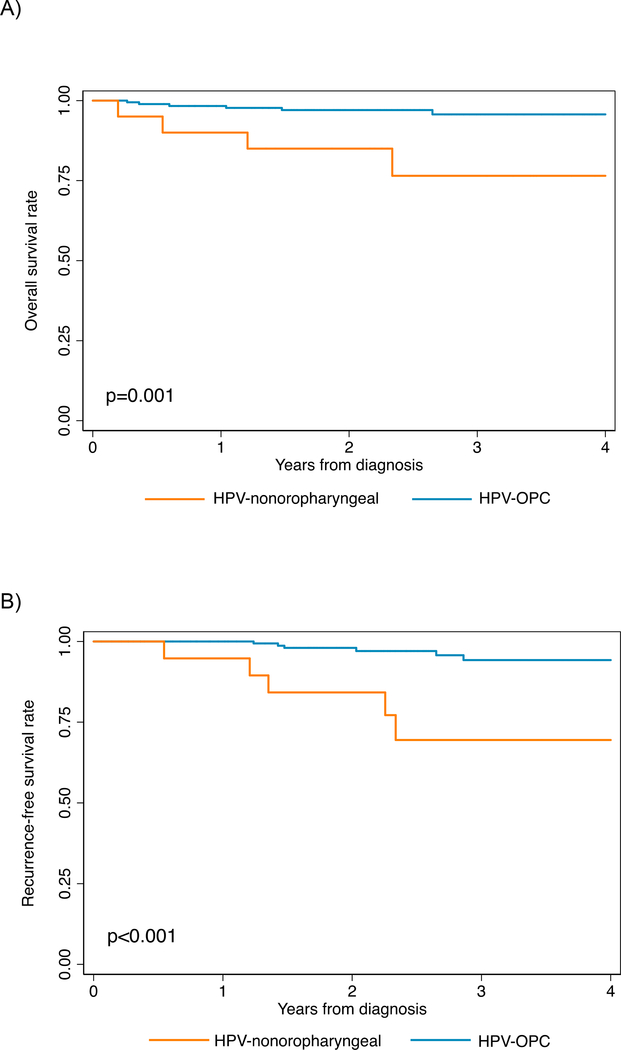
Median follow up time was 2.2 years (IQR 1.6–3.6). Ten deaths occurred, four among the 20 HPV-nonOPC (20%) and six deaths among the 181 HPV-oropharyngeal (4%, p=0.001) for whom survival data was available. Overall survival at four years was significantly worse for HPV-nonOPC (77% vs. 96%, p=0.001, Figure 3A), with a hazard ratio of 6.12 (95% CI=1.73–21.7). The poorer survival among HPV-nonOPC remained significant after controlling for tobacco use and age (aHR=4.05, 95%CI 1.10–14.9). Similar results were found when adjusting for pack years (results not shown).
Figure 3.
Overall survival (panel A) and recurrence-free survival (panel B), stratified by HPV-positive nonoropharyngeal (HPV-nonOPC) and HPV-positive oropharyngeal (HPV-OPC) primary tumor sites.
A. Overall survival was 80% for HPV-nonOPC at 4 years compared with 96% for HPV-OPC
B. Progression-free survival was 69% vs. 94% for HPV-nonOPC and HPV-OPC, respectively, at 4 years.
Eleven recurrences were observed, 5 among the 19 HPV-non-oropharyngeal and 6 among the 171 HPV-OPC (26% vs. 4%, p=0.004) with recurrence data available. Similar to overall survival, recurrence-free survival was significantly worse among HPV-nonOPC (69% vs. 94%, p<0.001; HR 7.25, 95%CI 2.21–23.8) among HPV-nonOPC independent of tobacco use and age (aHR 7.61, 95%CI 2.05–28.2, Supplemental Table 5).
An additional analysis comparing HPV-nonOPC to 135 HPV-negative nonoropharyngeal cases enrolled in the larger PROVE cohort revealed similar overall and recurrence-free survival by HPV tumor status conferred by either HPV ISH or p16 IHC (data not shown).
Discussion
This is the first study to our knowledge designed to examine risk factors of patients with HPV-positive nonoropharyngeal (HPV-nonOPC) compared with HPV-positive oropharyngeal (HPV-OPC) squamous cell cancers. In this prospective, case-control and case-case comparison, nonoropharyngeal cancers with confirmed HPV oncogene expression were found to be distinct from HPV-positive oropharyngeal cases in terms of behavior, biomarkers, comorbidity, and prognosis. In many ways, HPV-nonOPC were found to resemble HPV-negative HNSCC. Our findings support consideration of this relatively rare entity as different from HPV-OPC, and align with current guidelines that recommend against routine testing of p16 and HPV in nonoropharyngeal sites,19 outside the clinical trial setting.
Lifetime sexual exposure and younger age of oral sexual debut have previously been shown to be associated with oral HPV infection in population-level data.26 Landmark case-control data have also demonstrated that lifetime sexual exposure is associated with oropharyngeal cancer,11 and with HPV- HNSCC when comprised largely of oropharyngeal cancers.10 Sexual exposure is a surrogate for HPV exposure and although not previously examined, was expected to be a risk factor for HPV-nonOPC. Surprisingly, we found similar sexual exposure among HPV-nonOPC cases and controls, and significantly lower oral sexual exposure among HPV-nonOPC than among HPV-OPC. In this way, HPV-nonOPC cancers resemble HPV-negative subsets in existing data.10
Tobacco is a well-known risk factor for HNSCC27 and for oral HPV infection.28 Some reports have found an additive or synergistic role of tobacco and HPV exposure in HPV-HNSCC,29 whereas others found no interaction.30,31 Herein, tobacco exposure was significantly associated with HPV-nonOPC, similar to HPV-negative HNSCC in existing literature.10 Consistent with our findings, a higher prevalence of tobacco use among HPV-nonOPC compared with HPV-OPC cancers has been reported elsewhere.7,32
We found that the prevalence of anemia and multimorbidity was significantly higher among HPV-nonOPC compared with HPV-OPC patients. These results again suggest similarity between HPV-nonOPC and HPV-negative HNSCC. Prognostic studies describe worse performance status14,33 and higher rates of anemia33 among those with HPV-unassociated HNSCC. In addition, large database analyses have demonstrated higher comorbidity indices in nonoropharyngeal compared with oropharyngeal34 and in HPV-negative compared to HPV-positive cancers.15
Alhough HPV-nonOPC behavioral risk factors and comorbidities resemble HPV-negative HNSCC rather than HPV-OPC, biological characteristics examined tell a different story. HPV-nonOPC demonstrate a biological similarity to HPV-OPC in that both entities exhibit a clear association with HPV exposure in contrast to controls (Figure 2). Despite this, we demonstrate a weaker strength of the association with HPV among HPV-nonOPC compared to HPV-OPC cases. First, HPV-nonOPC tumors are less likely than HPV-OPC to harbor HPV16, although HPV16 is associated with most HPV-OPC.35 Second, HPV-nonOPC are significantly less likely to be non-keratinizing than HPV-OPC, which is typically non-keratinizing.36 In contrast, HPV-unassociated laryngeal, hypopharyngeal and oral cavity SCC is classically keratinizing.37 Despite a significantly lower proportion of non-keratinizing tumors among HPV-nonOPC, however, the predominant histological subtype among both HPV-positive entities was still non-keratinizing, consistent with their shared viral etiology. Third, HPV-nonOPC patients had a lower seroprevalence of antibodies to oncogenic proteins specific to most high-risk HPV types examined when compared with HPV-OPC patients, among whom these antibodies were near ubiquitous. This is consistent with the previously reported low sensitivity of HPV serology for HPV-driven tumors outside the oropharynx.38
Although not explored in the present study, there are a few potential reasons for the lower seroprevalence among HPV-nonOPC. There may be differences in immune response or different mechanisms of immune tolerance among lymphoid and other epithelial sites. This is consistent with the greater local immune infiltrate in HPV-OPC compared to HPV-nonOPC tumors,3 with the caveat that infiltrates do not necessarily represent antigen-specific cells. In addition, the lower seroprevalence may be a function of lower overall HPV exposure resulting in less frequent seropersistence. The differences in seroprevalence provide clues to how these tumors may respond to treatment. Both HPV oncoproteins and mutations represent cancer neoantigens that can render a tumor susceptible to immune recognition. It has been proposed that in pre-malignant lesions that ultimately progress and transform to cancer, an immune suppressive microenvironment is generated which blocks these responses from eliminating the tumor cells.39 These mechanisms are known to differ across cancer types and could underlie the differences in behavior of HPV-nonOPC versus HPV-OPC.
Our behavioral and biomarker results suggest that while HPV is implicated in oncogenesis in HPV-HNSCC, a second hit of field cancerization is more often required in nonoropharyngeal sites. Indeed, over 80% of the HPV-nonOPC patients included herein were smokers compared to less than half of the HPV-OPC patients. This hypothesis implies that possibly a greater number of oncogenic mutations may be present in HPV-nonOPC than HPV-OPC cancers. The biological role of HPV outside the oropharynx, and how HPV infection may interact with tobacco exposure in tumorigenesis, presently remains unclear.
Our study represents a valuable addition to the current understanding of the significance of HPV outside the oropharynx, which has been called into question for two reasons. First, the prevalence of HPV in nonoropharyngeal sites is low. 40 Although not well understood, this is thought to be partly due to oral mucosal epithelium as a barrier to HPV infection compared with the reticulated endothelium lining the palatine and lingual tonsillar crypts.41 Second, although the prognostic significance of p16 overexpression in nonoropharyngeal sites has been previously examined with discordant results, there does not appear to be a survival benefit conferred by HPV.42 We observed a significantly worse survival among HPV-nonOPC compared with HPV-OPC cases, which again supports consideration of these entities as distinct. The prognostic difference may be partially explained by tobacco exposure, however, the persistence of a survival difference among those with tobacco exposure implies that there are other etiologic factors.
We present findings from a multi-institutional, prospective case-control study with centralized biomarker testing. We acknowledge the limited sample size due to low numbers of HPV-nonOPC cancers among the HNSCC in our study, consistent with global prevalence rates.40 This limited our to perform a survival analysis stratified by tumor or nodal stage, and may have limited our detection of additional risk factor differences. In addition, behavioral surveys may be subject to recall bias and it is possible exposures were reported differently by cases and controls.
In conclusion, patients with HPV-positive nonoropharyngeal cancer have a risk factor profile that resembles HPV-negative HNSCC attributed mainly to tobacco exposure. Although the predominant morphology is similar among HPV-nonOPC and HPV-OPC, we found key differences in HPV biomarker prevalence, and significantly worse survival for HPV-nonOPC. These findings are important for understanding the population at risk for HPV-positive HNSCC and generating hypotheses regarding biological differences by anatomic location. Furthermore, our results support consideration of HPV tumor status as a distinguishing clinical and prognostic feature primarily among head and neck cancers in the oropharynx.
Supplementary Material
Acknowledgments
Funding: NIDCR (P50DE019032, R35DE026631, 5T32DC000027-29)
References
- 1.Amin MB, Edge SB, Greene FL. AJCC Cancer Staging Manual, 8th Ed. New York: Springer; 2017. [Google Scholar]
- 2.D’Souza G, Westra WH, Wang SJ, et al. Differences in the Prevalence of Human Papillomavirus (HPV) in Head and Neck Squamous Cell Cancers by Sex, Race, Anatomic Tumor Site, and HPV Detection Method. JAMA Oncology. 2017;3(2):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakravarthy A, Henderson S, Thirdborough SM, et al. Human Papillomavirus Drives Tumor Development Throughout the Head and Neck : Improved Prognosis Is Associated With an Immune Response Largely Restricted to the Oropharynx. J Clin Oncol. 2016;34:4132–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung CH, Zhang Q, Kong CS, et al. p16 Protein Expression and Human Papillomavirus Status As Prognostic Biomarkers of Nonoropharyngeal Head and Neck Squamous Cell Carcinoma. J Clin Oncol. 2014;32(35):3930–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakhry C, Westra WH, Wang SJ, et al. The Prognostic Role of Sex, Race, and Human Papillomavirus in Oropharyngeal and Nonoropharyngeal Head and Neck Squamous Cell Cancer. Cancer. 2017;123(9):1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lassen P, Primdahl H, Johansen J, et al. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113(3):310–316. [DOI] [PubMed] [Google Scholar]
- 7.Salazar CR, Smith RV, Garg MK, et al. Human Papillomavirus-Associated Head and Neck Squamous Cell Carcinoma Survival : A Comparison by Tumor Site and Initial Treatment. Head Neck Pathol. 2014;8(1):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant AK, Sojourner EJ, Vitzthum LK, et al. Prognostic Role of p16 in Nonoropharyngeal Head and Neck Cancer. J Natl Cancer Inst. 2018;110(12):1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fakhry C, Ferris RL. P16 as a Prognostic Biomarker for Nonoropharyngeal Squamous Cell Cancers: Avatar or Mirage? J Natl Cancer Inst. 2018;110(12):1290–1291. [DOI] [PubMed] [Google Scholar]
- 10.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. [DOI] [PubMed] [Google Scholar]
- 11.D’Souza G, Pawlita M, Fakhry C, et al. Case–control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19): 1944–1956. [DOI] [PubMed] [Google Scholar]
- 12.Heck JE, Berthiller J, Vaccarella S, et al. Sexual behaviours and the risk of head and neck cancers : a pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int J Epidemiol. 2010;39(1):166–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlstrom KR, Li G, Tortolero-Luna G, Wei Q, and Sturgis EM. Differences in history of sexual behavior between patients with oropharyngeal squamous cell carcinoma and patients with squamous cell carcinoma at other head and neck sites. Head Neck. 2011;33(6):847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. [DOI] [PubMed] [Google Scholar]
- 15.Stenmark MH, Shumway D, Guo C, et al. Influence of human papillomavirus on the clinical presentation of oropharyngeal carcinoma in the United States. Laryngoscope. 2017;127(10):2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A. AJCC cancer staging manual, 7th edition. New York: Springer; 2010. [Google Scholar]
- 18.Fakhry C, Lacchetti C, Rooper LM, et al. Human Papillomavirus Testing in Head and Neck Carcinomas: ASCO Clinical Practice Guideline Endorsement of the College of American Pathologists Guideline. J Clin Oncol. 2018;36(31):3152–3161. [DOI] [PubMed] [Google Scholar]
- 19.Lewis J, Beadle B, Bishop JA. Human Papillomavirus Testing in Head and Neck Carcinomas: Guideline From the College of American Pathologists. Arch Pathol Lab Med. 2018;142(5):559–597. [DOI] [PubMed] [Google Scholar]
- 20.Bishop Ja, Ma X-J, Wang H, et al. Detection of Transcriptionally Active High-risk HPV in Patients With Head and Neck Squamous Cell Carcinoma as Visualized by a Novel E6/E7 mRNA In Situ Hybridization Method. Am J Surg Pathol. 2012;36(12):1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione S-transferase fusion proteins. Clin Chem. 2005;51(10):1845–1853. [DOI] [PubMed] [Google Scholar]
- 22.Clifford GM, Shin HR, Oh JK, et al. Serologic response to oncogenic human papillomavirus types in male and female university students in Busan, South Korea. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1874–1879. [DOI] [PubMed] [Google Scholar]
- 23.Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of Human Papillomavirus Antibodies and Risk of Subsequent Head and Neck Cancer. J Clin Oncol. 2013;31(21):2708–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreimer AR, Alberg AJ, Viscidi R, Gillison ML. Gender Differences in Sexual Biomarkers and Behaviors Associated with Human Papillomavirus-16, −18, and −33 Seroprevalence. Sex Transm Dis. 2004;31(4):247–256. [DOI] [PubMed] [Google Scholar]
- 25.Windon MJ, Waterboer T, Hillel AT, et al. Sex differences in hpv immunity among adults without cancer. Hum Vaccin Immunother. 2019;15(7):1935–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillison ML, Broutian T, Pickard RKL, et al. Prevalence of Oral HPV Infection in the United States, 2009–2010. JAMA. 2012;307(7):693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sturgis EM, Wei Q, Spitz MR. Descriptive Epidemiology and Risk Factors for Head and Neck Cancer. Semin Oncol. 2004;31(6):726–733. [DOI] [PubMed] [Google Scholar]
- 28.D’Souza G, McNeel TS, Fakhry C. Understanding personal risk of oropharyngeal cancer: Risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann Oncol. 2017;28(12):3065–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha P, Logan H, Mendenhall W. Human Papillomavirus, Smoking, and Head and Neck Cancer Am J Otolaryngol. 2012;33(1):130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrero R, Castellsagu X, Pawlita M, et al. Human Papillomavirus and Oral Cancer : The International Agency for Research on Cancer Multicenter Study. J Natl Cancer Inst. 2003;95(23):1772–1783. [DOI] [PubMed] [Google Scholar]
- 31.Applebaum KM, Furniss CS, Zeka A, et al. Lack of Association of Alcohol and Tobacco with HPV16-Associated Head and Neck Cancer. J Natl Cancer Inst. 2007;99(23):1801–1810. [DOI] [PubMed] [Google Scholar]
- 32.Park J, McPike V, Kambhampati S, et al. Positivity Rates in Oropharyngeal and Nonoropharyngeal Head and Neck Cancer in the VA. Fed Pract. 2018;35:44–47. [PMC free article] [PubMed] [Google Scholar]
- 33.Ang KK, Harris J, Wheeler R, et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eytan DF, Blackford AL, Eisele DW, Fakhry C. Prevalence of Comorbidities and Effect on Survival in Survivors of Human Papillomavirus – Related and Human Papillomavirus – Unrelated Head and Neck Cancer in the United States. Cancer. 2019;125(2):249–260. [DOI] [PubMed] [Google Scholar]
- 35.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human Papillomavirus Types in Head and Neck Squamous Cell Carcinomas Worldwide: A Systematic Review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. [DOI] [PubMed] [Google Scholar]
- 36.El-Mofty SK. Human papillomavirus-related head and neck squamous cell carcinoma variants. Semin Diagn Pathol. 2015;32(1):23–31. [DOI] [PubMed] [Google Scholar]
- 37.Lewis JS Jr.,, Ukpo OC, Ma XJ, et al. Transcriptionally-active high-risk human papillomavirus is rare in oral cavity and laryngeal/hypopharyngeal squamous cell carcinomas--a tissue microarray study utilizing E6/E7 mRNA in situ hybridization. Histopathology. 2012;60(6):982–991. [DOI] [PubMed] [Google Scholar]
- 38.Holzinger D, Wichmann G, Baboci L, et al. Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma. Int J Cancer. 2017;140(12):2748–2757. [DOI] [PubMed] [Google Scholar]
- 39.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73(6):1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gelwan E, Malm I-J, Khararjian A. Nonuniform Distribution of High-risk Human Papillomavirus in Squamous Cell Carcinomas of the Oropharynx: Rethinking the Anatomic Boundaries of Oral and Oropharyngeal. Am J Surg Pathol. 2017;41(12):1722–1728. [DOI] [PubMed] [Google Scholar]
- 42.Vitzthum LK, Mell LK. The role of p16 as a biomarker in nonoropharyngeal head and neck cancer. Oncotarget. 2018;9(70):33247–33248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.