Abstract
OBJECTIVES:
To identify the association between cording and breast cancer-related lymphedema (BCRL); describe time course, location, symptoms and functional impairments.
METHODS:
1,181 patients were prospectively screened for BCRL after breast cancer (BC) surgery, including patient-reported outcome measures (4,193) and perometric arm volume measurements (BCRL defined as relative or weight-adjusted volume change [RVC or WAC] ≥10% ≥3 months postoperatively).
RESULTS:
374/1181 patients (31.7%) reported cording first a median of 4.5 months postoperatively, and were more likely to: have body mass index <30 kg/m2; be <55 years of age; have had mastectomy, axillary lymph node dissection, regional lymph node radiation, neoadjuvant chemotherapy (all p<0.001), or RVC/WAC≥ 10% (p=0.002). Patients who reported cording had 2.4 times the odds of developing BCRL compared to those who did not (OR, 2.40 95% CI 1.40–4.11, p=0.002), and most frequently reported these symptoms: tenderness (61.2%), aching (60.7%) and firmness/tightness (59.8%). On multivariable analysis, cording was significantly correlated with functional difficulty for 17 actions.
CONCLUSIONS:
Patients frequently present with cording, potentially months after BC surgery. Risk factors for and symptoms of cording are identified, and treatment is recommended. Patients reporting cording are at higher risk of BCRL, therefore cording should be incorporated into BCRL risk stratification.
Keywords: lymphedema, cording, axillary web syndrome, screening, breast cancer
INTRODUCTION:
Cording, also known as axillary web syndrome or Mondor’s disease,[1–6] is a painful condition that may occur after breast cancer (BC) treatment, causing significant morbidity. Cording is characterized by pain in the axilla radiating down the arm, sometimes across the antecubital space to the root of the thumb.[2] Diagnosis is clinical; patients have an obvious cord or web of tissue on and limited shoulder range of motion into abduction.[2] Incidence of cording in the literature varies significantly, from 6% in Moscovitz et al’s 2001 study[2] to 48.3% in more recent studies.[1,3,7–10]
While some studies have found that cording occurs soon after axillary surgery and is self-limiting,[2,3,7] others report later occurrence or recurrence.[3,11,12] Lower body mass index (BMI)[3,12] or younger age at BC diagnosis,[3,11] axillary lymph node dissection (ALND),[1,11–13] more advanced disease,[13] and numbness in the arm after intercostobrachial nerve injury[1] have been implicated as risk factors for cording. However, many of these studies are small series or study cohorts not reflecting current BC treatment.
The pathophysiology of cording has historically remained equivocal as it is difficult to image.[10] More recently, thrombosis and dilation in lymphatic and venous channels has been implicated,[2,14] and there is general agreement that cording is consistent with lymphatic origin.[14,15] This begs the question: are patients who develop cording at higher risk of breast cancer-related lymphedema (BCRL) than those who do not develop cording? Four studies have examined this question; three found cording was not associated with BCRL.[11,12,16] However, none of these studies incorporated baseline limb volume, leading to BCRL misdiagnosis.[17] In contrast, O’Toole et al. found that cording was associated with arm volume increase ≥5%; a ≥10% threshold, more frequently used for BCRL diagnosis, was not used given a low number of events.[11]
Although cording causes impaired range of motion, few studies describe functional limitations associated with cording. One study found that function was significantly more impaired in patients with cording compared to those without.[11] Another small study found that although patients with and without cording exhibited improved function from two to twelve weeks postoperatively, those with cording had worsening function at 18 months compared to those without cording (p=0.05).[12]
Due to limitations in current literature, it is imperative to better define cording association with BCRL, its risk factors, time course, and impact on the patient in order to better inform patient care after BC treatment.
METHODS:
Patient Population
The cohort included 1,181 patients who underwent BC surgery and were prospectively screened for BCRL at Massachusetts General Hospital between 2009 and 2019. All patients filled out a patient-reported outcome measure (PROM) and underwent arm volume measurement with perometry at each screening visit. The protocol for lymphedema screening has been previously published[18] and approved by the Institutional Review Board (Clinicaltrials.gov identification number NCT01521741, IRB 2008P000540). Verbal consent of all participants was obtained in accordance with institutional policy. The study was performed in accordance with the Declaration of Helsinki.
Patient-Reported Outcome Measures
The PROM in this study is a hybrid of four validated outcome measures: The Lymphedema and Breast Cancer Questionnaire; the Disabilities of the Arm, Shoulder and Hand; the Arm Activity Survey for Breast Cancer Survivors; and the Functional Assessment of Cancer Therapy[19–23]. The survey includes questions regarding symptoms (e.g. change in arm size, heaviness), arm function (e.g. pulling on a tight shirt), and post-operative exercises. Patients were asked “Do you see or feel a thin cord or string in any of the following areas: In your armpit that extends into the inside of your upper arm; across the inside of your elbow; along your forearm or wrist; under your breast extending toward your abdomen?” Patients could select multiple locations, and patients responding yes to any of these questions were defined as having cording. Patients received the following questions regarding postoperative stretching exercises prescribed by their surgical team: “Did you receive specific instructions on postoperative exercises?” and “Did you do any of the postoperative exercises for your affected arm within the first few months after surgery?”
Arm Volume Quantification
Postoperative arm volume changes are calculated for patients undergoing unilateral BC surgery using the relative volume change (RVC) formula[24]: , where A1 and A2 and, U1 and U2 are arm volumes of the affected and unaffected arms at preoperative baseline and follow-up respectively[24]. The weight-adjusted change formula (WAC) is used for patients who undergo bilateral breast surgery and therefore lack a contralateral control arm[25]: , where A1 and A2, are arm volumes of the affected arm W1 and W2 are body weight at preoperative baseline and follow-up, respectively[25]. BCRL is defined as an RVC or WAC ≥10% occurring more than three months after surgery[26].
Statistical Methods:
Patient demographics and BC treatment variables were obtained by medical record review, and chi-square tests were used to assess the relationship between these treatment variables and cording report (Table 1) and between cording report and the development of BCRL (Table 2). Wilcoxon signed-rank tests were also used to compared medians of certain variables between patients who did and did not develop BCRL (Table 2). Multivariable logistic regression was used to elucidate which BCRL-related symptoms are most strongly correlated with cording report. Chi-square tests were used to compare incidence of symptom reports in the groups that reported vs. did not report cording. Multivariable logistic regression was used to determine if cording is correlated with impaired arm function (Table 3), as well as if cording is correlated with a lack of post-operative exercise. A plot of cumulative incidence of cording over time (Figure 1) was produced.
Table 1.
Demographics and Incidence of Cording Stratified by Risk Factor
| All Patients (n=1,181) N (%) |
Cording (n=374) N (%) |
No Cording (n=807) N (%) |
p-value | |
|---|---|---|---|---|
| RVC/WAC >= 10% | ||||
| Yes | 129 (10.9%) | 57 (15.2%) | 72 (8.9%) | 0.002 |
| No | 1052 (89.1%) | 317 (84.8%) | 735 (91.1%) | |
| BMI at diagnosis | ||||
| >= 30 | 308 (26.2%) | 73 (19.5%) | 235 (29.3%) | <0.001 |
| < 30 | 868 (73.8%) | 301 (80.5%) | 567 (70.7%) | |
| Unknown | [5] | -- | [5] | |
| Age at diagnosis | ||||
| >= 55 | 569 (48.2%) | 144 (38.5%) | 425 (52.7%) | <0.001 |
| < 55 | 612 (51.8%) | 230 (61.5%) | 382 (47.3%) | |
| Type of surgery | ||||
| Mastectomy | 414 (35.1%) | 159 (42.5%) | 255 (31.6%) | <0.001 |
| Lumpectomy | 767 (64.9%) | 215 (57.5%) | 552 (68.4%) | |
| Reconstruction complication | ||||
| Yes | 59 (5.0%) | 22 (5.9%) | 37 (4.6%) | 0.427 |
| No | 1119 (95.0%) | 352 (94.1%) | 767 (95.4%) | |
| Unknown | [3] | -- | [3] | |
| Axillary surgery | ||||
| ALND | 223 (18.9%) | 142 (38.0%) | 81 (10.0%) | <0.001 |
| SLNB | 835 (70.7%) | 209 (55.9%) | 626 (77.6%) | |
| None | 123 (10.4%) | 23 (6.1%) | 100 (12.4%) | |
| Tumor quadrant | ||||
| Upper outer | 416 (35.2%) | 145 (38.8%) | 271 (33.6%) | |
| Upper inner | 126 (10.7%) | 35 (9.4%) | 91 (11.3%) | |
| Lower outer | 71 (6.0%) | 30 (8.0%) | 41 (5.1%) | 0.039 |
| Lower inner | 56 (4.7%) | 11 (2.9%) | 45 (5.6%) | |
| Central | 46 (3.9%) | 12 (3.2%) | 34 (4.2%) | |
| Multiple quadrant | 235 (19.9%) | 68 (18.2%) | 167 (20.7%) | |
| NA | 231 (19.6%) | 73 (19.5%) | 158 (19.6%) | |
| Radiation therapy | ||||
| RLNR | 278 (23.5%) | 151 (40.4%) | 127 (15.7%) | <0.001 |
| No RLNR | 903 (76.5%) | 223 (59.6%) | 680 (84.3%) | |
| Chemotherapy | ||||
| Neoadjuvant +/− adjuvant | 148 (12.5%) | 76 (20.3%) | 72 (8.9%) | <0.001 |
| Adjuvant only | 327 (27.7%) | 140 (37.4%) | 187 (23.2%) | |
| None | 706 (59.8%) | 158 (42.2%) | 548 (67.9%) |
Where RVC=relative volume change, WAC=weight adjusted change, BMI=body mass index, ALND=axillary node dissection, SLNB=sentinel lymph node biopsy, RLNR=regional lymph node radiation
Table 2.
Cording report and BCRL development
| All | BCRL (RVC≥10%) | No BCRL (RVC <10%) | p-value | |
|---|---|---|---|---|
| Surveys | 4,193 | 621 | 3,572 | -- |
| Patients | 1,181 | 129 | 1,052 | -- |
| Average number of surveys per patient (mean) | 3.6 | 4.8 | 3.4 | -- |
| Median follow up (months post surgery) | 24.3 | 33.6 | 23.9 | <0.001 |
| Surveys that report cording | 867/1181 (20.7%) | 204/621 (32.9%) | 663/3572 (18.6%) | <0.001 |
| Patients that reported cording | 374/1181 (31.7%) | 57/129 (44.2%) | 317/1052 (30.1%) | <0.001 |
| Time of first cording report* | 4.5 (0.3, 195.2) | 4.1 (0.3, 60.6) | 4.8 (0.3, 195.2) | 0.443 |
| Time of any cording report* | 12.4 (0.3, 195.2) | 15.4 (0.3, 99.0) | 11.8 (0.3, 195.2) | 0.006 |
| Time of last cording report* | 13.2 (0.3, 195.2) | 14.3 (0.5, 99.0) | 12.4 (0.3, 195.2) | 0.010 |
| RVC/WAC at any cording report (%, median) | 1.0 (−17.5, 25.3) | 7.1 (−17.5, 25.3) | −0.2 (−13.1, 9.8) | <0.001 |
| Time of BCRL onset* | -- | 17.7 (3.0, 96.5) | -- | -- |
| Reported cording before/at BCRL onset | -- | 53/57 (95.0%) | -- | -- |
| Reported cording after BCRL onset | -- | 35/57 (61.4%) | -- | -- |
Median value shown with (range), (months after surgery).
Where RVC=relative volume change, WAC=weight adjusted change, BCRL=breast cancer-related lymphedema
Table 3. Cording and loss of optimal arm functionality.
Risk factor is cording, outcome is difficulty in doing the following tasks
| Univariate Analysis | Multivariable Analysis* | |||
|---|---|---|---|---|
| Outcome: It is difficult to… | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Place something on a shelf above my head | 2.82 (2.19, 3.63) | <0.001 | 2.11 (1.60, 2.78) | <0.001 |
| Drive a car for more than 30 minutes | 2.75 (1.68, 4.50) | <0.001 | 2.48 (1.46, 4.24) | <0.001 |
| Pull a tight shirt on over my head | 2.89 (2.23, 3.76) | <0.001 | 2.34 (1.76, 3.11) | <0.001 |
| Unscrew a tight lid on a jar | 2.04 (1.59, 2.62) | <0.001 | 1.72 (1.32, 2.25) | <0.001 |
| Put on a bra that clips in the back | 2.06 (1.54, 2.76) | <0.001 | 1.80 (1.31, 2.46) | <0.001 |
| Carry a 10 lb. grocery bag for 10 minutes | 2.04 (1.59, 2.62) | <0.001 | 1.72 (1.32, 2.25) | <0.001 |
| Sleep on my affected side for more than 10 minutes | 2.07 (1.60, 2.67) | <0.001 | 1.68 (1.27, 2.21) | <0.001 |
| Pull wet clothes out of the washing machine | 2.50 (1.87, 3.36) | <0.001 | 2.15 (1.56, 2.95) | <0.001 |
| Push a heavy piece of furniture | 2.07 (1.57, 2.73) | <0.001 | 1.66 (1.24, 2.23) | <0.001 |
| Dry my back with a towel | 2.51 (1.86, 3.39) | <0.001 | 1.85 (1.33, 2.58) | <0.001 |
| Chop potatoes into small cubes | 2.43 (1.58, 3.75) | <0.001 | 1.99 (1.24, 3.19) | 0.004 |
| Push open a heavy door | 2.13 (1.65, 2.76) | <0.001 | 1.76 (1.33, 2.32) | <0.001 |
| Clean a window above shoulder height | 2.52 (1.95, 3.25) | <0.001 | 2.12 (1.61, 2.79) | <0.001 |
| Vacuum, iron, or scrub for more than 5–30 minutes | 2.22 (1.71, 2.89) | <0.001 | 1.81 (1.36, 2.40) | <0.001 |
| Catch a falling object with my affected hand | 2.09 (1.45, 3.02) | <0.001 | 1.68 (1.13, 2.49) | 0.011 |
| Carry a 10 lb. bag over my shoulder | 1.88 (1.46, 2.41) | <0.001 | 1.63 (1.25, 2.13) | <0.001 |
| Type on a keyboard for 20 minutes | 2.01 (1.41, 2.89) | <0.001 | 1.72 (1.17, 2.53) | 0.006 |
Each outcome was run separately, controlling for ALND, RLNR and type of surgery
Where OR = odds ratio, CI = confidence interval
Figure 1.
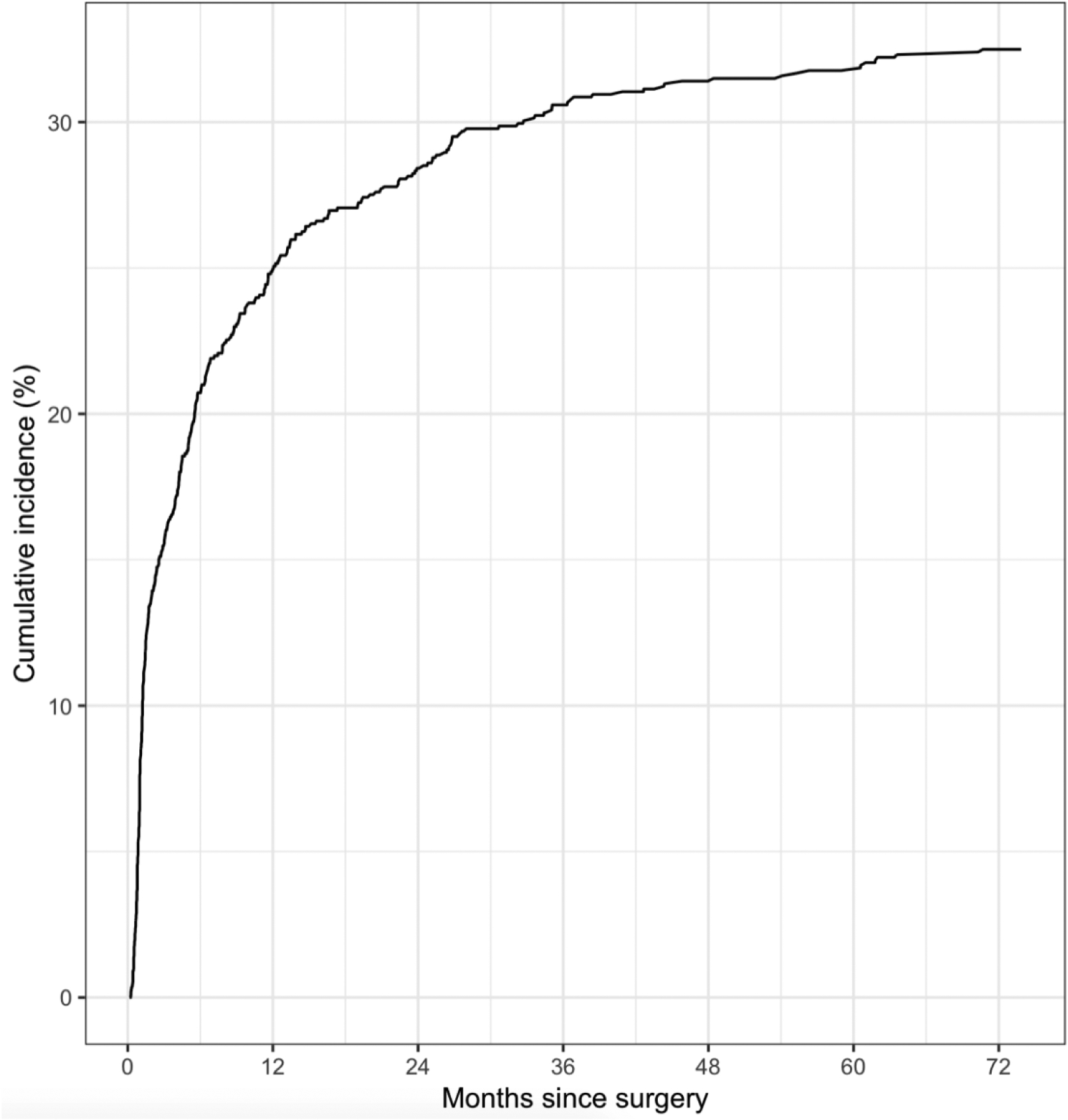
Cumulative Incidence of Cording Report
A multivariable regression model was developed with BCRL as the dependent variable and cording as the main independent variable of interest (Table 4). Cases were excluded from the analysis if cording status was reported after BCRL onset. A graph of cumulative incidence stratified by cording status was produced for the BCRL outcome (Figure 2). Using graphical and numerical methods derived from cumulative sums of martingale residuals over follow-up time and covariate values for checking the adequacy of a Cox proportional hazards regression model, the cording variable was found to violate the proportional hazards assumption. In the interest of simplifying interpretation of the results, logistic regression was performed instead of time-stratified Cox proportional hazards regression. Potential covariates, including age at diagnosis, BMI at baseline, type of nodal surgery (none, ALND, or sentinel lymph node biopsy [SLNB]), number lymph nodes sampled, surgery type (lumpectomy, mastectomy without reconstruction, or mastectomy with reconstruction), reconstruction complication leading to secondary intervention, regional lymph node radiation (RLNR), and chemotherapy (neoadjuvant ± adjuvant, adjuvant only, or none) were assessed using unadjusted logistic regression models for the BCRL outcome. All potential covariates were censored if the outcome occurred before the date of the covariate. Any significant potential covariates in the unadjusted analyses were included in the subsequent multivariable model. Hypothesis tests for all statistical tests were two-sided, with the significance threshold set to 0.05. The frequency of BCRL events that occurred before cording was reported descriptively. Analyses were performed using R version 1.2.1335 and SAS/STAT version 9.4. In terms of data sharing, research data are not shared for this manuscript.
Table 4.
Multivariable Logistic Regression Model Results for BCRL Outcome
| Variable | Odds Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Cording | 2.40 | 1.40–4.11 | 0.002 |
| BMI at diagnosis | 1.03 | 0.99–1.07 | 0.208 |
| Axillary surgery | |||
| ALND vs. SLNB | 3.50 | 1.32–9.27 | 0.012 |
| None vs. SLNB | 2.37 | 1.02–5.48 | 0.044 |
| Number of lymph nodes sampled | 1.02 | 0.97–1.07 | 0.425 |
| Type of surgery | |||
| Mastectomy without reconstruction vs. Lumpectomy | 1.30 | 0.70–2.41 | 0.414 |
| Mastectomy with reconstruction vs. Lumpectomy | 1.10 | 0.41–2.96 | 0.846 |
| Reconstruction complication | 2.46 | 1.07–5.67 | 0.034 |
| Radiation therapy (RLNR) | 0.80 | 0.39–1.66 | 0.554 |
| Chemotherapy | |||
| Adjuvant (no neoadjuvant) vs. No chemotherapy | 1.09 | 0.56–2.14 | 0.800 |
| Neoadjuvant +/− adjuvant vs. No chemotherapy | 1.12 | 0.51–2.50 | 0.775 |
Where BMI=body mass index, ALND=axillary node dissection, SLNB=sentinel lymph node biopsy, RLNR=regional lymph node radiation
Figure 2:
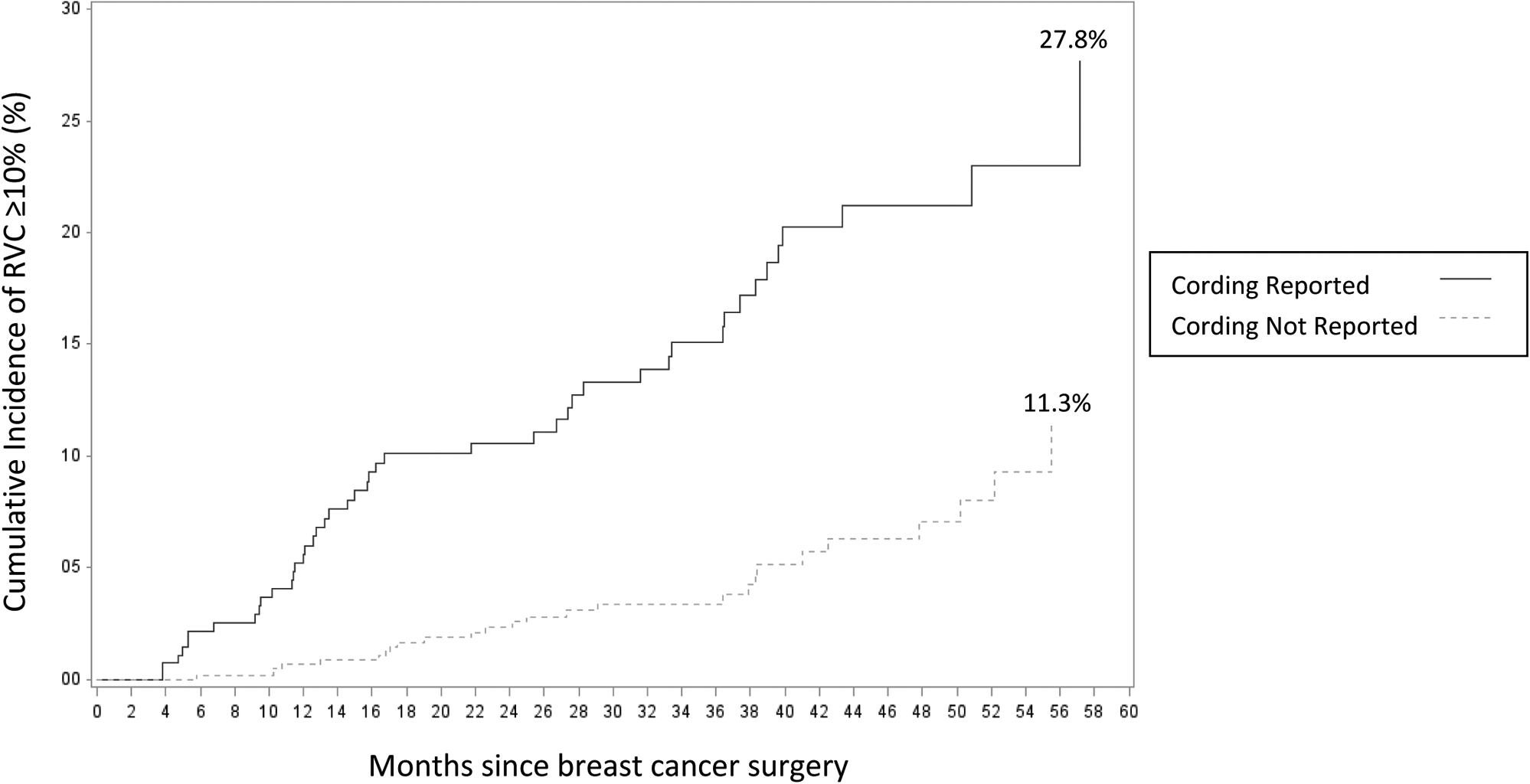
Cumulative Incidence of RVC ≥10% among patients reporting and not reporting cording
RESULTS:
Data was analyzed for 1,181 patients who completed 4,193 surveys (mean 3.6 per patient), over a median follow-up time of 24.3 months postoperatively. Demographic and treatment-related characteristics included: 308/1181 patients (26.2%) had BMI ≥ 30, 569/1181 (48.2%) were ≥ 55 years of age at diagnosis, 414/1181 (35.1%) and 767/1181 (64.9%) had mastectomy and lumpectomy respectively, 223/1181 (18.9%) and 835/1181 (70.7%) underwent ALND and SLNB respectively, 278/1181 (23.5%) underwent RLNR, and 148/1181 (12.5%) underwent neoadjuvant chemotherapy (Table 1).
Of the cohort, 374/1181 patients (31.7%) reported cording: 313/374 patients (83.7%) reported cording in the axilla into the inside of the upper arm, 59/374 (15.8%) across the inside of the elbow, 41/374 (11.0%) along the forearm or wrist, and 83/374 (22.2%) under the breast extending towards the abdomen.
Figure 1 depicts cumulative incidence of cording over time. Patients who reported cording were more likely to have a BMI <30 kg/m2; to be <55 years of age at time of BC diagnosis; to have had mastectomy, ALND, RLNR or neoadjuvant chemotherapy (all p<0.001), or RVC/WAC ≥ 10% at any point (p=0.002). The location of the tumor differed significantly between patients who reported and did not report cording (p=0.039). Secondary reintervention for reconstruction complications was not significantly associated with cording (p=0.427) (Table 1).
BCRL and Cording Timing:
Of the cohort, 129/1181 patients (10.9%) developed BCRL (RVC or WAC ≥ 10%) during follow-up, with a median onset of 17.7 months post-surgery (range 3.0–96.5 months). Patients with BCRL were more likely to report cording than those without BCRL (44.2% vs 30.1%, p<0.001). The 129 patients who developed BCRL completed 204 surveys (mean of 4.8 per patient) and 57/129 (44.2%) reported cording at some point post-operatively, with median time of first and last cording report at 4.1 months (range 0.3, 60.6) and 14.3 months (0.5, 99.0) post-operatively, respectively. While there was no significant difference in the timing of first cording report between patients with and without BCRL (median 4.1 months vs. 4.8 months, p=0.443), there was a significant difference in the timing of last cording report (median 14.3 months vs. 12.4 months, p=0.010) (Table 2). Figure 2 illustrates 5-year cumulative incidence of progression to RVC ≥10% among patients who reported and did not report cording (27.8% and 11.3%, respectively).
Of the 57 patients who reported cording and developed BCRL, 53/57 (95.0%) reported cording before or at BCRL onset at a median RVC/WAC of 7.1% (range −17.5%, 25.3%). Of the 374 patients who reported cording, 276/374 (73.8%) filled out at least one survey after the one in which they first reported cording; 174/374 (63.0%) patients reported no cording on a subsequent survey. There was a median of 10.2 months between first cording report and first report of no cording (range 0.9, 55.9 months).
Cording and Symptoms:
We identified symptoms reported at the time of first cording report not reported in the survey immediately prior. To be included in this analysis, patients had to have completed ≥one survey prior to that in which they first reported cording (n=104). At least 50% of patients reported the following new symptoms at cording onset: increase in arm size (70.0%), sleeve tightness (60.0%), ring tightness (63.67%), sleeve cuff tightness (100%), heaviness (61.5%), swelling (77.8%), firmness/tightness (61.5%) and stiffness (75.0%). Less than 50% of patients reported the following symptoms as new at cording onset: tenderness (41.7%), numbness (42.1%), aching (47.8%), pain (20.0%).
Patients who reported cording reported the following symptoms with higher frequency than patients who did not report cording: increase in arm size, sleeve tightness, ring tightness, increase in shoulder size, sleeve cuff tightness, heaviness, swelling, firmness/tightness, tenderness, numbness, stiffness, aching and pain (all p<0.001). There was no difference in frequency of reporting increase in neck size between groups (p=0.065). The symptoms reported most frequently by patients reporting cording were tenderness (61.2%), aching (60.7%) and firmness/tightness (59.8%) (all p<0.001).
Cording and Arm Function
For patients who reported cording, arm functionality was assessed in surveys completed at or after the date of first cording report. For patients who never reported cording, arm functionality was assessed from all surveys. On multivariable analysis, cording was positively correlated (all OR > 1.66) with difficulty in completing the following 17 actions: 1) placing something on a shelf above, 2) driving a car for > 30 minutes, 3) pulling a tight shirt on over one’s head, 4) unscrewing a tight lid on a jar, 5) putting on a bra that clips in the back, 6) carrying a 10 pound grocery bag for 10 minutes, 7) sleeping on the affected side for more than 10 minutes, 8) pulling wet clothes out of the washing machine, 9) pushing a heavy piece of furniture, 10) drying one’s back with a towel, 11) pushing open a heavy door, 12) cleaning a window above shoulder height, 13) performing household chores 14) carrying a 10 pound bag over one’s shoulder (all p<0.001), 15) catching a falling object with the affected hand (p=0.011), 16) chopping potatoes into small cubes (p=0.004), and 17) typing on a keyboard for 20 minutes (p=0.006) (Table 3).
Cording and Postoperative Exercises:
Patients were asked the following questions: “Did you receive specific instructions on postoperative exercises?” and “Did you do any of the postoperative exercises for your affected arm within the first few months after surgery?” We analyzed each question separately and looked at the correlation between responding “No” to either question and reporting cording at any point. When controlling for age, BMI, nodal surgery, mastectomy v. lumpectomy, total number of lymph nodes sampled and RLNR on multivariable analysis, not receiving instructions on postoperative exercises and not doing any of the postoperative exercises significantly increased risk of reporting cording (OR 1.79, 95% CI 1.17 – 2.74, p=0.008; OR 1.98, 95% CI 1.18 – 2.27, p < 0.001 respectively).
Cording and BCRL Risk:
Controlling for BMI at baseline, type of nodal surgery, number of lymph nodes sampled, surgery type, reconstruction complication, RLNR, and chemotherapy, compared to those who did not report cording, those reporting cording had 2.4 times the odds of developing BCRL (OR, 2.40, 95% CI 1.40–4.11, p=0.002). Type of nodal surgery (ALND vs. SLNB: OR 3.50, 95% CI 1.32–9.27, p=0.012; None vs. SLNB: OR 2.37, 95% CI 1.02–5.48, p=0.044) and intervention for reconstruction complication (OR 2.46, 95% CI 1.07–5.67, p=0.034) were also significantly associated with developing BCRL (Table 4).
DISCUSSION:
Cording incidence in this cohort (31.7%), is high, consistent with previous studies.[3,7–9] Most patients who report cording do so within 24 months postoperatively, with the incidence curve rising steeply up to 12 months; although incidence rises slowly through the sixth year postoperatively. Cording is not always an immediate and short-lived postoperative phenomenon as evidenced by patients’ last cording report at a median of 13.2 months postoperatively and 10.2 months after first cording report. Providers need be aware that cording incidence is high, it may develop and/or regress late after surgery, and it may persist for months to years.
Most patients reported cording in the axilla, consistent with previous findings.[1–4,6] However, many patients (22.2%) reported cording under the breast, an often-overlooked etiology of pain in the inframammary region. This cording can be elicited on clinical examination when the patient is in standing in slight thoracic extension and shoulder flexion, by elevating the breast. This can be successfully treated.
Our findings were consistent with several cording risk factors, including BMI<30, < 55 years of age, mastectomy and ALND. Additional risk factors identified included RLNR and neoadjuvant chemotherapy. Providers should be aware of the impact of these treatments on cording.
Although there was no difference in when patients with and without BCRL first reported cording, time of last cording report was later in patients with BCRL. We hypothesize that patients with venous and lymphatic occlusion caused by cording in addition to BCRL may be at risk of prolonged cording which does not self-limit as traditionally thought.
Many patients reported cording after reaching RVC ≥5% (i.e. with edema in their arm). It makes sense that most patients with BCRL reported cording prior to BCRL onset (93.0%), given median time to first cording report was early postoperatively (4.1 months), and median time to onset of BCRL was 17.7 months. However, this could also reflect that patients with cording are at higher risk of developing BCRL than those without cording. This study, which is the first to integrate preoperative baseline arm volume measurements in a large, prospectively followed longitudinal cohort, establishes cording report as a risk factor for BCRL. Accordingly, cording should be incorporated into BCRL risk stratification for patients treated for BC.
Some symptoms reported by those with cording may reflect arm edema, such as ring tightness and heaviness. Others may be more reflective of cording, such as firmness/tightness, tenderness, stiffness or aching. Firmness/tightness, tenderness, and aching were the most frequently reported symptoms in those reporting cording, were new symptoms for over 40% of the patients on first cording onset, and were reported more frequently in those reporting cording. Providers should be aware that although these symptoms may be secondary to surgery or radiation, they may also reflect cording.
Cording report was correlated with difficulty with all examined functional tasks in this cohort. These patients experience significant functional deficits which should be incorporated into goals for treatment of cording.
Finally, patients who reported they did not receive instructions for or they did not complete postoperative exercises were more likely to report cording. It appears provision of postoperative range of motion exercises and personalized instruction may decrease risk of cording.
Study Limitations:
BCRL screening visits are completed during oncology follow-up visits; cording or BCRL may also occur between visits. Cording and receiving and completing postoperative exercise were determined through self-report. Quantification of exercise prescription and compliance may aid in improved postoperative instruction for patients after BC surgery. Finally, impairments in functional tasks analyzed are likely interrelated.
CONCLUSIONS:
Incidence of cording after BC treatment is high, and patients who have BMI<30 or are < 55 years of age at time of BC diagnosis, undergo mastectomy, ALND, RLNR or neoadjuvant chemotherapy are at risk for cording.[3,7–10] It occurs in the postoperative period for some, but may occur later, preceding or conjunction with early BCRL. Cording persists for some patients. More research is recommended to identify which patients present with cording late after surgery, have cording that persists or recurs.
Although most patients report cording in the axilla or arm,[1–4,6] cording in the inframammary region is a common etiology of pain after BC treatment,[6] and these patients should be referred for treatment.
Patients with cording are likely to report firmness/tightness, tenderness and aching and to experience significant functional impairments. Function should be incorporated into treatment goals for these patients.
It is possible that gentle range of motion exercises postoperatively may be associated with decreased risk of cording; however, more research is required.
Patients reporting cording are at a higher risk of developing BCRL; therefore, cording should be incorporated into BCRL risk stratification.
Patients treated for BC should be educated about cording, its risk factors, symptoms, effect on function, time course and treatment. Patients with cording should be referred for treatment to prevent the morbidity associated with cording and its effect on function and quality of life.
Synopsis:
Cording is a frequent sequela of breast cancer surgery, and affected 31.7% of this study cohort (n=1,181). Body mass index <30 kg/m2; age <55 years at diagnosis; mastectomy, lymph node dissection, regional lymph node radiation, and neoadjuvant chemotherapy were associated with cording (p<0.001). Cording presented 2.4 times the odds of BCRL (95% CI 1.40–4.11, p=0.002).
Funding Sources:
The project was supported by Award Number R01CA139118 (AG Taghian) and Award Number P50CA08393 (AG Taghian) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This program is supported by the Adele McKinnon Research Fund for Breast Cancer-Related Lymphedema (AG Taghian), the Heinz Family Foundation (AG Taghian), and the Olayan-Xefos Family Fund for Breast Cancer Research (AG Taghian).
Footnotes
Data Availability Statement:
Research data are not shared.
Disclosures:
Alphonse Taghian is on the Scientific Advisory Board of Puretech Health and a previous Consultant in VisionRT. AGT has been loaned equipment from ImpediMed for use in investigator-initiated clinical trials. Cheryl Brunelle is on the Scientific Advisory Board of Puretech Health. These associations are unrelated to this manuscript. For the remaining authors none were declared.
References
- 1.Bergmann A, Mendes VV, De Almeida Dias R et al. Incidence and risk factors for axillary web syndrome after breast cancer surgery. Breast Cancer Research and Treatment 2012; 131: 987–92. [DOI] [PubMed] [Google Scholar]
- 2.Moskovitz AH, Anderson BO, Yeung RS et al. Axillary web syndrome after axillary dissection. American Journal of Surgery 2001; 181: 434–9. [DOI] [PubMed] [Google Scholar]
- 3.Torres Lacomba M, Mayoral Del Moral O, Coperias Zazo JL et al. Axillary web syndrome after axillary dissection in breast cancer: A prospective study. Breast Cancer Research and Treatment 2009; 117: 625–30. [DOI] [PubMed] [Google Scholar]
- 4.Yeung WM, McPhail SM, Kuys SS. A systematic review of axillary web syndrome (AWS). Journal of Cancer Survivorship 2015; 9: 576–98. [DOI] [PubMed] [Google Scholar]
- 5.Leduc O; Sichere M; Moreau A; Rigolet J; Tinlot A; Dare S; Wilputte F; Strapart J; Parijs T; Clement A; Snoeck T; Pastouret T; Leduc A Axillary Web Syndrome: Nature and Localization. Lymphology 2009; 42: 176–81. [PubMed] [Google Scholar]
- 6.Amano M, Shimizu T. Mondor’s disease: A review of the literature. Internal Medicine 2018; 57: 2607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leidenius M, Leppänen E, Krogerus L, Von Smitten K. Motion restriction and axillary web syndrome after sentinel node biopsy and axillary clearance in breast cancer. The American Journal of Surgery 2003; 185: 127–30. [DOI] [PubMed] [Google Scholar]
- 8.Wariss BR, Costa RM, Pereira ACPR et al. Axillary web syndrome is not a risk factor for lymphoedema after 10 years of follow-up. Supportive Care in Cancer 2017; 25: 465–70. [DOI] [PubMed] [Google Scholar]
- 9.Koehler LA, Blaes AH, Haddad TC et al. Movement, Function, Pain, and Postoperative Edema in Axillary Web Syndrome. Physical Therapy 2015; 95: 1345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koehler LA; Hunter DW ;Haddad TC; Blaes AH; Hirsch AT; Ludewig P. Characterizing axillary web syndrome: ultrasonic efficacy. Lymphology 2014; 47: 156–63. [PMC free article] [PubMed] [Google Scholar]
- 11.O’Toole J, Miller CL, Specht MC et al. Cording following treatment for breast cancer. Breast Cancer Research and Treatment 2013; 140: 105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koehler LA; Hunter DW; Blaes AH; Haddad T Function, Shoulder Motion, Pain, and Lymphedema in Breast Cancer With and Without Axillary Web Syndrome: An 18-Month Follow-Up. Physical Therapy 2018; 98: 518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furlan CMCJRDSS LO. Vascular Alterations in Axillary and Brachial Vessels in Patients with Axillary Web Syndrome After Breast Cancer Surgery. Lymphatic Research and Biology 2018; 16: 287–93. [DOI] [PubMed] [Google Scholar]
- 14.Rashtak S, Gamble GL, Gibson LE, Pittelkow MR. From furuncle to axillary web syndrome: Shedding light on histopathology and pathogenesis. Dermatology 2012; 224: 110–4. [DOI] [PubMed] [Google Scholar]
- 15.Leduc O; Fumiere E; Banse S; Vandervorst C; Clement A; Parijs T; Wilputte F; Maquerlot F; Ezquer Echandia M; Tinlot A; Leduc A Identification and description of the axillary web syndrome (AWS) by clinical signs, MRI and US imaging. Lymphology 2014; 47: 164–76. [PubMed] [Google Scholar]
- 16.Huang HLHYLYCTCYC. The upper-limb volumetric changes in breast cancer survivors wiht axillary web syndrome. European Journal of Cancer Care 2017; 26. [DOI] [PubMed] [Google Scholar]
- 17.Sun F, Skolny MN, Swaroop MN et al. The need for preoperative baseline arm measurement to accurately quantify breast cancer-related lymphedema. Breast Cancer Research and Treatment 2016; 157: 229–40. [DOI] [PubMed] [Google Scholar]
- 18.Brunelle C, Skolny M, Ferguson C et al. Establishing and sustaining a prospective screening program for breast cancer-related lymphedema at the Massachusetts General Hospital: Lessons Learned. Journal of personalized medicine 2015; 5: 153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nursing research 2003; 52: 370–9. [DOI] [PubMed] [Google Scholar]
- 20.Armer JM; Whitman M The problem of lymphedema following breast cancer treatment: Prevalence, symptoms and self-management. Lymphology 2002; 35 (Suppl): 153–9.12570324 [Google Scholar]
- 21.Gummesson C; Atroshi I; Ekdahl C The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: Longitudinal construct validity and measuring self-rated health change aftersurgery. BMC Musculoskelet Disord200AD. [DOI] [PMC free article] [PubMed]
- 22.Brady MJ, Cella DF, Mo F et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1997; 15: 974–86. [DOI] [PubMed] [Google Scholar]
- 23.Lee T, Kilbreath S, Sullivan G, Refshauge K. The development of an arm activity survey for breast cancer survivors using the protection motivation theory. BMC Cancer 2007; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ancukiewicz M, Russell TA, Otoole J et al. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. International Journal of Radiation Oncology Biology Physics 2011; 79: 1436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller CL, Specht MC, Horick N et al. A novel, validated method to quantify breast cancer-related lymphedema (BCRL) following bilateral breast surgery. Lymphology 2013; 46: 64–74. [PubMed] [Google Scholar]
- 26.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphatic Research and Biology 2005; 3: 208–17. [DOI] [PubMed] [Google Scholar]


