Abstract
Olfactory dysfunction is recognized in neurodevelopmental disorders and may serve as an early indicator of global dysfunction. The present meta-analysis measures olfaction effect sizes in attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorders (ASD), and obsessive-compulsive disorder (OCD). Meta-analysis included 320 ADHD, 346 ASD, and 208 OCD individuals as compared to 910 controls. Olfactory performance deficits were small-to-moderate and heterogeneous (d = −0.42, 95% CI = −0.59 < δ < −0.25). Meta-analytic results indicate that olfactory dysfunction is evident in individuals with ASD and OCD, with small-to-negligible effects in ADHD. These findings imply olfactory dysfunction is related to clinical phenotype in ASD and OCD, but not ADHD, and warrant inclusion in clinical assessment and evaluation of certain neurodevelopmental disorders.
Keywords: Autism spectrum disorders, attention-deficit/hyperactivity disorder, obsessive-compulsive disorder, meta-analysis, olfaction
INTRODUCTION
Atypical sensory processing is common in autism spectrum disorders (ASDs) with more than 90% of children with ASD experiencing hyper- or hyposensitivity in one or more sensory domains. (Boudjarane, Grandgeorge, Marianowski, Misery, & Lemonnier, 2017; Marco, Hinkley, Hill, & Nagarajan, 2011). As such, sensory processing issues are included in the diagnostic criteria of the DSM-5 (Lee et al., 2013), and have been found to contribute to social, cognitive and behavioral problems (Marco et al., 2011). Clinical assessment typically focuses on the senses of touch, vision and audition, while smell and taste are largely ignored. This is surprising given the impact that environmental sensory stimuli have on measures quality of life in individuals with ASD, and the historical perception that proximal senses were particularly at risk and most likely to indicate developmental immaturity (Ayres & Tickle, 1980; Baranek, Foster, & Berkson, 1997). For example, it is likely that disturbances of smell and taste have significant influence upon the development of food preferences, which have been shown to be atypical in ASD (Luisier et al., 2015). Moreover, the olfactory sensory neural pathway includes numerous brain regions implicated in ASD and elucidation of the ASD-related alterations in this pathway could lead to a better understanding of underlying ASD pathophysiology.
Poor olfactory ability is documented in ASD (Larsson, Tirado, & Wiens, 2017; Marco et al., 2011; Tonacci et al., 2017) across a variety of domains (Schecklmann et al., 2013), but appears most prominent in odor identification (i.e., identifying that a certain smell is “lemon”). Other neurodevelopmental disorders also experience poor olfactory ability (Atanasova et al., 2008; Burón & Bulbena, 2013; Croy & Hummel, 2017; Islam et al., 2015; Kamath et al., 2014; Kazour et al., 2017; Kohli, Soler, Nguyen, Muus, & Schlosser, 2016), including disorders that frequently co-occur with ASD (Doshi-Velez, Ge, & Kohane, 2014), such as attention-deficit/hyperactivity disorder (ADHD; Schecklmann et al., 2013) and obsessive-compulsive disorder (OCD;Schecklmann et al., 2013). Impairments in olfactory functioning in these neurodevelopmental conditions are likely due to alterations at multiple levels of the olfactory system (e.g., peripheral and central). It is also likely that olfactory deficits are not merely specific exemplars of diffuse cognitive impairment. Rather they are the consequence, at least in part, of prominent structural and functional abnormalities of the central (brain) and peripheral (nasal, oral) olfactory system (Bennetto, Kuschner, & Hyman, 2007; Karsz et al., 2008; Moberg & Turetsky, 2003; Turetsky, Hahn, Borgmann-Winter, & Moberg, 2009). In essence, it appears that olfactory dysfunction represents an important functional deficit reported in – and risk factor for – several related neurodevelopmental conditions.
The extant literature suggests both common and separable deficits in olfactory function between ADHD, ASD, and OCD. However, the psychophysical literature examining olfactory dysfunction across these disorders has yet to be analyzed quantitatively. A comprehensive meta-analysis of olfactory dysfunction in ASD, ADHD, and OCD could help characterize olfactory deficits and phenotypic heterogeneity in these disorders while advancing hypotheses about the specific neurodevelopmental disruptions that underlie olfactory impairments.
The present meta-analysis summarizes patterns of deficits in olfactory function in ASD, ADHD, and OCD relative to typically developing (TD) peers. This analysis concentrates on five olfactory domains: odor detection threshold sensitivity, identification, discrimination, hedonics (i.e., subjective pleasantness ratings), and subjective ratings of odor intensity (Eibenstein et al., 2005). We hypothesized significant overall olfactory deficits across these three disorders, with common dysfunction in odor identification, while observing separable deficits in other olfactory domains. Specifically, we hypothesized that individuals with ASD will show greater deficits in pleasantness ratings (e.g. hedonics) as compared to OCD and ADHD.
METHODS
Literature Search Strategy
The report and extraction of relevant articles and data followed the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) reporting standards (Stroup et al., 2000). The search strategy included an initial broad search in PubMed, MEDLINE, PsychINFO, and Google Scholar databases from inception to July 31, 2018 using the following search criteria for Attention Deficit Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), and Obsessive-Compulsive Disorder (OCD), respectively. Literature for relevant articles in ADHD was searched using (“attention deficit disorder” OR “attention deficit hyperactivity disorder” OR “ADD” OR “ADHD”) AND (“olfactory” OR “olfaction” OR “smell”). Literature within ASD was searched using (“autism” OR “autism spectrum disorder” OR “ASD” OR “Asperger syndrome”) AND (“olfactory” OR “olfaction” OR “smell”). The OCD literature was searched using (“obsessive compulsive disorder” OR “obsessive compulsive” OR “OCD”) AND (“olfactory” OR “olfaction” OR “smell”). The search was limited to articles that enrolled human subjects. Additionally, a thorough manual review of articles was performed utilizing cross-references from identified original articles and reviews. Studies eligible for inclusion used performance-based measures of olfactory functioning, which provided statistical information that permitted meta-analytic methods to be used. Excluded articles are listed in the Supplemental Material.
Data Extraction
Studies included in the meta-analysis met the following criteria: 1) standard or experimental tasks of olfactory function in patients with ASD, ADHD, or OCD, 2) age-matched comparison group of typical, unrelated participants with no history of ASD, ADHD, or OCD, and 3) data or statistical information for calculating effect sizes (Figure 1). Based on these criteria, three authors (AJDC, JMJ, KLV) initially reviewed each potential study for inclusion and the primary and senior authors (DRR, PJM) reviewed articles and data entry for accuracy. After passing this stage, relevant data were extracted for meta-analytic analysis, including data on tests of olfactory function, clinical criteria, demographic information, percentage of sample taking psychotropic medication, and intellectual performance scores, if provided. Consensus of both senior authors was needed to exclude an article. A complete list of excluded articles is presented in Supplement A.
Figure 1.
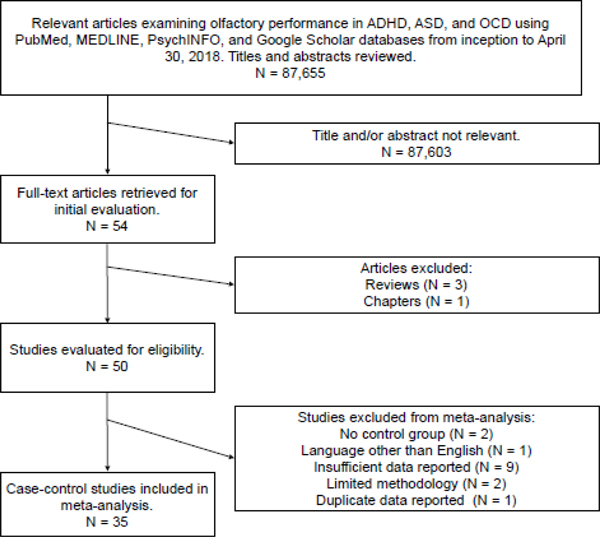
Flowchart of literature search in the present meta-analysis.
Seventeen ASD publications reported comparative results of psychophysical olfactory testing, totaling 33 effects (Table 1). Seven (n=7) additional ASD articles were excluded for: (a) absence of a typical comparison group (N = 1); (b) insufficient reporting of olfactory data (N = 4); (c) limited olfactory methodology (N = 1); and (d) duplication of olfactory data (N = 1).
Table 1.
Olfaction Studies in Attention-Deficit/Hyperactivity Disorder (ADHD), Autism Spectrum Disorders (ASD), and Obsessive-Compulsive Disorder (OCD)
| First Author | Year | Domain | Modality | Patient | Control | ||
|---|---|---|---|---|---|---|---|
| N (% M) | MAge | N (% M) | MAge | ||||
| ADHD (k = 19) | |||||||
| Ghanizadeh | 2012 | Identification | Lab-made | 50 (70.00) | 10.70 | 50 (70.00) | 10.70 |
| Detection | PEA | ||||||
| Karsz | 2008 | Identification | UPSIT | 44 (79.55) | 12.16 | 44 (79.55) | 12.23 |
| Lorenzen | 2016 | Detection | PEA | 18 (100.00) | 10.00 | 17 (100.00) | 10.50 |
| Intensity | SAM | ||||||
| Hedonics | SAM | ||||||
| Murphy | 2001 | Identification | UPSIT | 105 (75.23) | 21.10 | 64 (68.75) | 21.20 |
| Romanos | 2008 | Identification | SS | 20 (50.00) | 9.83 | 20 (50.00) | 10.17 |
| Discrimination | SS | ||||||
| Detection | SS | ||||||
| Schecklmann | 2011a | Identification | SS | 27 (74.07) | 12.67 | 22 (36.36) | 12.42 |
| Discrimination | SS | ||||||
| Detection | SS | ||||||
| Schecklmann | 2011b | Identification | SS | 29 (51.72) | 28.20 | 29 (51.72) | 27.80 |
| Discrimination | SS | ||||||
| Detection | SS | ||||||
| Vučinić | 2016 | Identification | SS | 24 (91.67) | 11.57 | 26 (88.46) | 11.59 |
| Discrimination | SS | ||||||
| Weiland | 2011 | Detection | SS | 12 (0.00) | 41.00 | 12 (0.00) | 32.00 |
| ASD (k = 39) | |||||||
| Addo | 2017 | Identification | SS | 16 (18.75) | 38.20 | 14 (21.43) | 42.07 |
| Detection | SS | ||||||
| Intensity | SS | ||||||
| Hedonics | SS | ||||||
| Ashwin | 2014 | Detection | AST | 17 (100.00) | 37.90 | 17 (100.00) | 27.20 |
| Assumpção | 2007 | Identification | Lab-made | 21 (100.00) | NR | 21 (100.00) | NR |
| Bennetto | 2007 | Identification | SS | 21 (80.95) | 14.35 | 27 (74.07) | 14.48 |
| Brewer | 2008 | Identification | UPSIT | 15 (80.00) | 6.43 | 15 (80.00) | 7.12 |
| Dudova | 2011 | Identification | SS | 35 (88.57) | 10.80 | 35 (80.00) | 10.40 |
| Detection | SS | ||||||
| Fadda | 2017 | Identification | SS | 15 (86.67) | 19.00 | 15 (80.00) | 21.70 |
| Discriminatio | SS | ||||||
| Detection | SS | ||||||
| Galle a | 2013 | Identification | UPSIT | 10 (100.00) | 25.50 | 11 (100.00) | 22.00 |
| Identification | UPSIT | 9 (100.00) | 25.44 | 11 (100.00) | 22.00 | ||
| Detection | PEA | 5 (100.00) | 24.80 | 5 (100.00) | 21.40 | ||
| Detection | PEA | 5 (100.00) | 22.20 | 5 (100.00) | 21.40 | ||
| Detection | n-Butanol | 5 (100.00) | 24.80 | 5 (100.00) | 21.40 | ||
| Detection | n-Butanol | 5 (100.00) | 22.20 | 5 (100.00) | 21.40 | ||
| Discrimination | Lab-made | 10 (100.00) | 23.50 | 5 (100.00) | 21.40 | ||
| Hedonics | Lab-made | 10 (100.00) | 23.50 | 5 (100.00) | 21.40 | ||
| Intensity | Lab-made | 10 (100.00) | 23.50 | 5 (100.00) | 21.40 | ||
| Hrdlicka | 2011 | Hedonics | SS | 35 (88.57) | 10.80 | 35 (80.00) | 10.40 |
| Kumazike | 2016 | Detection | IA | 23 (73.91) | 13.20 | 20 (70.00) | 12.50 |
| Detection | AC | ||||||
| May | 2011 | Identification | UPSIT | 9 (88.89) | 6.28 | 9 (77.78) | 7.10 |
| Muratori | 2017 | Identification | SS | 20 (100.00) | 10.90 | 20 (100.00) | 11.30 |
| Discrimination | SS | ||||||
| Detection | SS | ||||||
| Parma | 2014 | Identification | UPSIT | 20 (50.00) | 13.20 | 20 (50.00) | 13.40 |
| Rosenkrantz | 2015 | Hedonics | NA | 18 (94.44) | 7.00 | 18 (94.44) | 6.65 |
| Hedonics | NA | ||||||
| Suzuki | 2003 | Identification | UPSIT | 12 (100.00) | 32.90 | 12 (100.00) | 30.80 |
| Detection | n-Butanol | ||||||
| Tavassoli | 2012 | Detection | SS | 38 (52.63) | 35.90 | 42 (52.38) | 28.80 |
| Wicker | 2016 | Identification | Lab-made | 15 (73.33) | 26.30 | 15 (73.33) | 27.80 |
| Detection | Lab-made | ||||||
| Hedonics | Lab-made | ||||||
| Intensity | Lab-made | ||||||
| OCD (k = 17) | |||||||
| Barnett | 1999 | Identification | UPSIT | 20 (40.00) | 37.65 | 23 (43.48) | 37.34 |
| Berlin | 2017 | Identification | UPSIT | 15 (53.33) | 34.07 | 15 (53.33) | 32.67 |
| Intensity | LMS | ||||||
| Hedonics | LHS | ||||||
| Bersani | 2013 | Identification | BSIT | 25 (24.00) | 36.44 | 21 (47.62) | 36.85 |
| Dittrich | 2010 | Identification | Lab-made | 55 (30.91) | 40.70 | 80 (47.50) | 37.40 |
| Intensity | Lab-made | ||||||
| Hedonics | Lab-made | ||||||
| Fenger | 2005 | Identification | BSIT | 15 (46.67) | 39.00 | 17 (47.06) | 32.80 |
| Gross-Isseroff | 1994 | Detection | IA | 14 (28.57) | 34.86 | 14 (28.57) | 36.21 |
| Hermesh | 1999 | Detection | IA | 16 (18.75) | 33.40 | 16 (18.75) | 33.40 |
| Segalàs | 2011 | Identification | SS | 29 (51.72) | 35.50 | 29 (48.28) | 36.70 |
| Discrimination | SS | ||||||
| Detection | SS | ||||||
| Segalàs | 2014 | Identification | SS | 19 (57.89) | 30.84 | 19 (52.63) | 27.95 |
| Discrimination | SS | ||||||
| Detection | SS | ||||||
Note. Seventy-five studies (k = 75) included in the present meta-analysis and descriptive statistics of study samples. Age is reported in years. % M = Proportion of males in sample. M = Mean. NR = Not reported. PEA = Phenylethyl alcohol. UPSIT = University of Pennsylvania Smell Identification Test (Doty, Shaman, & Dann, 1984). SAM = Self-Assessment Manikin (Bradley & Lang, 1994). SS = Sniffin’ Sticks (Hummel et al., 2007). AST = Alcohol Sniff Test (Davidson & Murphy, 1997). LMS = Labeled Magnitude Scale (Green et al., 1996). LHS = Labeled Hedonic Scale (LHS). IA = Isoamyl acetate. AC = Allyl coproate. NA = Nasal cannula. BSIT = Brief Smell Identification Test (Doty, Marcus, & Lee, 1996).
Galle et al. (2013) was a multiple experiment study involving two different samples of three separate cohorts of patients with ASD, patients with Asperger syndrome, and typically developing control subjects.
Nine (n=9) ADHD publications reported comparative results of psychophysical olfactory testing, totaling 19 effects (Table 1). Three (n=3) additional ADHD articles were excluded for: (a) absence of a typical comparison group (N = 1); (b) article published in a language other than English (N = 1); and (c) insufficient data reported (N = 1).
Nine OCD publications reported comparative results of psychophysical olfactory testing, totaling 17 effects (Table 1). Three (n=3) additional OCD articles were excluded for insufficient reporting of olfactory data.
Statistical Analyses
Meta-analysis was completed across all three disorders to determine overall effects, followed by within-disorder analyses. All analyses were carried out using Comprehensive Meta-Analysis version 2.2.064 (Biostat, 2005) using standard random-effects models. Olfactory scores between patients (ASD, ADHD, OCD) and healthy comparison subjects were standardized using Cohen’s d (effect size; calculated as the difference between the two raw mean scores divided by the pooled standard deviation (SD). When means and SDs were not available, Cohen’s d was calculated from reported univariate F-tests, t-statistics, or p-values. Confidence intervals (CI) for each effect are reported. In order to control for differences in sample size during effect size computation, studies were weighted according to their inverse variance estimates. Prior convention has classified effect sizes as small (d = 0.2), medium (d = 0.5), or large (d ≥ 0.8) based on these methods (Cohen, 1988). Negative values index poorer performance on olfactory measures patients relative to controls. Homogeneity of effect size (dependent measure) across studies was assessed using the Cochran Q-statistic (Hedges & Olkin, 1985).
It is possible that studies with large effects are overrepresented in the extant literature. The specific concern is that studies with relatively large effects are more likely to be published than studies with small effects for the same question. This may lead to a publication bias in the literature that can then influence the results of a meta-analysis. Thus, publication bias was evaluated using several convergent methods: a) assessing the symmetry of a funnel plot: an asymmetric scatterplot of effect size vs. study precision is indicative of publication bias; (b) adjusted rank-correlation (e.g. Spearman) test according to the methods of Begg and Mazumdar (Begg & Mazumdar, 1994), Egger et al. (Egger, Smith, Schneider, & Minder, 1997), and Duval and Tweedy (Duval & Tweedie, 2000): a high correlation between the effect size and corresponding sampling variances indicates publication bias; and (c) a fail-safe file drawer analysis, a probability-based metric used to determine the number of null studies needed to invalidate the reported effect, was performed to examine the effect of null results on effect size (Rosenthal, 1979). Finally, to address the potential of certain studies being outliers, a trim-and-fill method was used to adjust average effect size to account for publication bias where appropriate (Duval & Tweedie, 2000).
Moderator Variables and Meta-regression
When there is substantial unaccounted heterogeneity in the outcome of interest across studies, additional investigation of the potential influence of other characteristics (e.g. age, sex, IQ) of the included studies on the outcome is warranted. Meta-regression is used to investigate these potential moderating variables. Moderator analysis of olfactory domain was undertaken across the entire sample of studies, which included: odor (a) detection threshold sensitivity, (b) discrimination, (c) identification, (d) intensity, and (e) hedonics. Within each diagnostic group, the following demographic and clinical moderator variables were evaluated in follow-up analysis with meta-regression: (a) age, (b) sex, (c) full-scale intellectual quotient (FSIQ) score (ADHD and ASD only), (d) the percentage of participants receiving psychotropic medication (ADHD and OCD only) and (e) clinical symptom ratings (OCD only). Additional demographic and clinical characteristics, including ethnicity, years of education, smoking status, depression and anxiety, and laterality of olfactory test presentation were indexed for each study, however, these data were insufficiently reported to be included in formal analyses.
RESULTS
Overall Meta-analysis
Relative to all TD subjects, analysis of effect sizes across olfactory domains for the combined ADHD, ASD, and OCD sample revealed effect sizes in the small to moderate range of magnitude (k = 75, d = −0.42, 95% CI = −0.59 < δ < −0.25) that were significantly heterogeneous (QB[74] = 407.64, p < 0.001).
Publication Bias
Analysis for the presence of possible response bias revealed a symmetric funnel plot and nonsignificant Begg (p = 0.63) and Egger (p = 0.52) tests. Consistent with the latter statistics, calculation of a fail-safe N revealed that 2,199 “null” studies would need to be located and incorporated into the analysis to negate the observed effect. As such, these findings indicate that the current meta-analytic data accurately represent the extant literature concerning olfactory function in patients with ADHD, ASD, and OCD.
Moderator Analysis and Meta-regression
Diagnosis.
Analysis revealed significant heterogeneity among effect sizes between diagnoses (QB[2] = 6.66, p = 0.04; Figure 2). The effects of ASD (d = −0.42, 95% CI = −0.69 < δ < −0.18, p = 0.001) and OCD (d = −0.76, 95% CI = −1.12 < δ < −0.41, p < 0.001) on olfactory performance were significant, however ADHD (d = −0.12, 95% CI = −0.46 < δ < 0.21, p= 0.48) was not. As the effect of diagnosis on olfactory performance was heterogeneous, the effect of each diagnosis on olfactory function was considered independently (see below).
Figure 2.
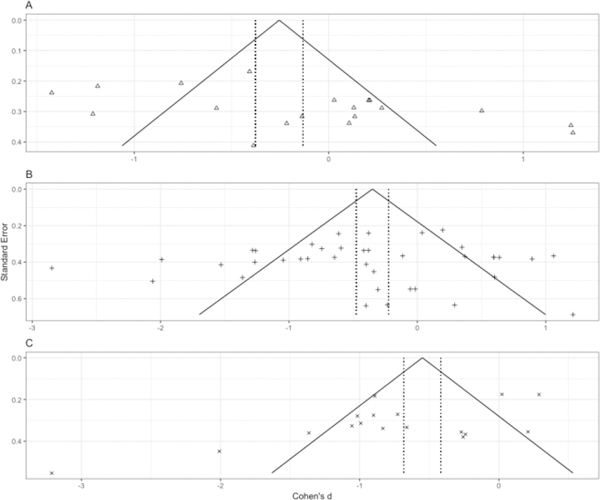
Funnel plots of effect size (Cohen’s d) by standard error in the (a) ADHD, (b) ASD, and (c) OCD literatures.
Olfactory Domain.
Analysis revealed homogeneity among effect sizes across olfactory performance domains (QB[4] = 3.78, p = 0.44; Supplemental Figure 1). Given that the diagnosis differences noted above and inconsistent test types used in the literature could influence the overall effects within domain, we performed an exploratory analysis of each olfactory domain across all three neurodevelopmental disorders. Odor identification (d = −0.62, 95% CI = −0.91 < δ < −0.34) was significantly more impaired (p < .001) than odor detection threshold sensitivity (d = −0.24, 95% CI = −0.55 < δ < 0.08), odor discrimination (d = −0.36, 95% CI = −0.83 < δ < 0.11), odor hedonic ratings (d = −0.45, 95% CI = −0.95 < δ < 0.05), and odor intensity ratings (d = −0.21, 95% CI = −0.83 < δ < 0.41). Contrasts between all other olfactory domains was similar. Follow-up moderator analyses of the effect of diagnosis on odor identification revealed homogeneous performance deficits (QB[2] = 0.53, p = 0.77) between ADHD (d = −0.49, 95% CI = −0.95 < δ < −0.02), ASD (d = −0.64, 95% CI = −1.02 < δ < −0.27), and OCD (d = −0.73, 95% CI = −1.21 < δ < −0.25). These statistics indicate that odor identification performance is consistently impaired across disorders.
ASD Meta-analysis
Across domains, there was a moderate (Figure 3; k = 39, d = −0.42, 95% CI = −0.68 < δ < −0.16) and heterogeneous (QB[38] = 181.74, p < 0.001) effect of ASD on olfactory performance relative to TD participants.
Figure 3.
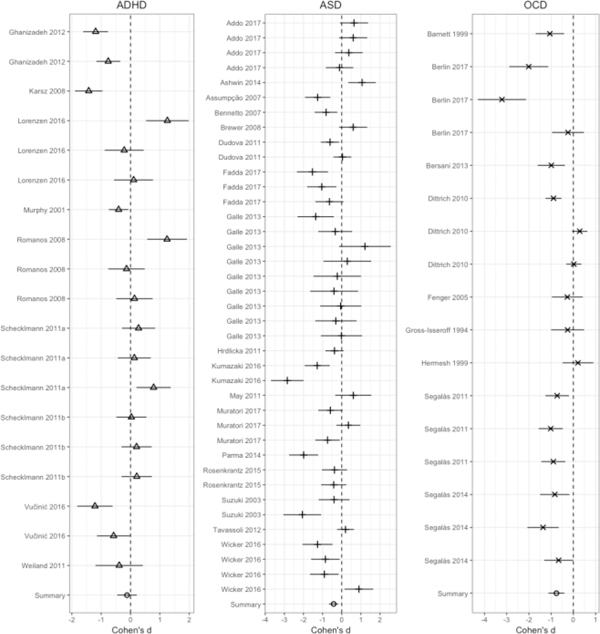
Average effect size (Cohen’s d) of diagnosis on olfactory performance independent of olfactory performance domain with 95% confidence intervals.
Publication Bias in ASD
Analysis for the presence of possible response bias revealed a symmetric funnel plot (Figure 2) and nonsignificant Begg (p = .63) and Egger (p = .62) tests. Consistent with the latter statistics, calculation of a fail-safe N revealed that 427 “null” studies would need to be located and incorporated into the analysis to negate the observed effect.
Moderator Analysis and Meta-regression in ASD
Olfactory Domain.
Analysis revealed homogeneity among effect sizes (Figure 4; QB[4] = 2.99, p = 0.56). The effect size for odor identification was moderate (d = −0.65, 95% CI = −1.11 < δ < −0.19) and larger than odor intensity (d = 0.27, 95% CI = −0.70 < δ < 1.24), odor detection threshold sensitivity (d = −0.39, 95% CI = −0.86 < δ < 0.08), odor discrimination (d = −0.40, 95% CI = −1.24 < δ < 0.43), and odor hedonics (d = −0.34, 95% CI = −1.00 < δ < 0.33).
Figure 4.
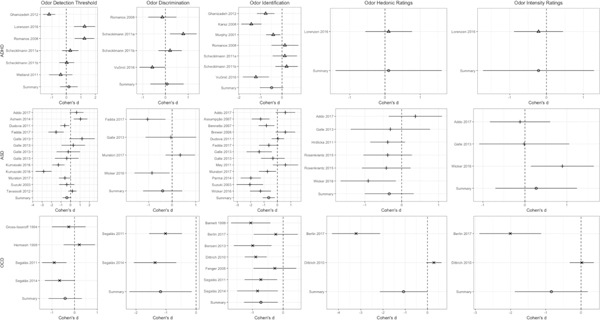
Effect size (Cohen’s d) by olfactory performance domain within ADHD, ASD, and OCD with 95% confidence intervals.
Meta-regression of demographic and clinical variables in ASD.
Among individuals with ASD, younger age, being male, and having lower FSIQ was associated with greater olfactory deficit compared to TD controls (Supplemental Figure 2).
ADHD Meta-analysis
Across domains, there was a small (Figure 3; k = 19, d = −0.12, 95% CI = −0.45 < δ < 0.20) and heterogeneous (QB[18] = 123.26, p < 0.001) effect of ADHD on olfactory performance relative to TD subjects.
Publication Bias in ADHD
Response bias analyses revealed a symmetric funnel plot (Figure 2) and nonsignificant Begg test (p = 0.14), but a significant Egger test (p = 0.029). Subsequent Duval and Tweedie trim-and-fill adjustment trimmed four studies and revealed a moderate effect of ADHD on olfactory performance (d = −0.40, 95% CI = −0.75 < δ < 0.05). Calculation of a fail-safe N revealed that 19 “null” studies would need to be located and incorporated into the analysis to negate the observed effect.
Moderator Analysis and Meta-regression in ADHD
Olfactory Domain.
Analysis revealed homogeneity among effect sizes (Figure 4; QB[4] = 2.94, p = 0.57) in ADHD. The effect size for odor identification (d = −0.48, 95% CI = −1.03 < δ < 0.06) was moderate in size, but did not differ from odor intensity ratings (d = −0.22, 95% CI = −1.73 < δ < 1.29), odor detection threshold sensitivity (d = 0.17, 95% CI = −0.44 < δ < 0.78), 3) odor discrimination (d = 0.07, 95% CI = −0.67 < δ < 0.81) or odor hedonic ratings (d = 0.10, 95% CI = −1.40 < δ < 1.61).
Meta-regression of demographic and clinical variables in ADHD.
In the existing ADHD literature, being male, having lower FSIQ, and use of psychotropic medication was related to greater olfactory impairment. (Supplemental Figure 2). Age was not a significant moderator of effect size within the ADHD literature (p = .10).
OCD Meta-analysis
Relative to TD subjects, analysis of effect sizes across olfactory domains for the OCD sample revealed a moderate effect (Figure 3; k = 17, d = −0.75, 95% CI = −1.09 < δ < −0.42) that was significantly heterogeneous (QB[16] = 92.40, p < 0.001).
Publication Bias in OCD
Analysis for the presence of possible response bias revealed a symmetric funnel plot (Figure 3) and nonsignificant Begg test (p = 0.27), but a significant Egger test (p = 0.012). A follow-up Duval and Tweedie trim-and-fill method trimmed zero studies and did not adjust the effect size estimate (d = −0.75, 95% CI = −1.09 < δ < −0.42). Calculation of a fail-safe N test revealed that 382 “null” studies would need to be located and incorporated into the analysis to negate the observed effect.
Moderator Analysis and Meta-regression in OCD
Olfactory Domain.
Analysis revealed homogeneity among effect sizes (Figure 4; QB[4] = 1.85, p = 0.76). Significant differences between TD subjects and OCD participants were found in odor hedonic ratings (d = −1.08, 95% CI = −2.15 < δ < −0.01), odor identification (d = −0.73, 95% CI = −1.28 < δ < −0.18), and odor discrimination (d = −1.18, 95% CI = −2.22 < δ < −0.15). There were no significant differences in odor intensity ratings (d = −0.84, 95% CI = −1.88 < δ < 0.19) and odor detection (d = − 0.42, 95% CI = −1.16 < δ < 0.32).
Meta-regression of demographic and clinical variables in OCD.
Within the OCD literature, patients with OCD who were younger, male, had more severe symptoms on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS), and taking psychotropic medications demonstrated greater olfactory impairment (Supplemental Figure 2).
DISCUSSION
The present results support our overall hypothesis that olfactory dysfunction is evident across ASD, OCD and ADHD and that deficits in olfactory identification are the most robust. However, individuals with ASD did not show deficits in olfactory hedonics as we predicted, yet those with OCD did. More specifically, olfactory deficits in ASD are moderate and homogenous across olfactory domains, with the largest deficit in olfactory identification. In contrast, olfactory deficits in OCD are moderate-to-large and heterogenous, with specific deficits in identification, hedonics, and discrimination evident. Olfactory deficits in ADHD were small and heterogeneous. Younger age, male sex, lower FSIQ, higher symptom level in OCD, and more pharmacological intervention were factors that significantly altered the effect size of olfactory dysfunction across neurodevelopmental disorders. These findings imply olfactory dysfunction is related to demographic and clinical aspects of ASD and OCD, and may play a part in their clinical manifestations and prognosis.
Many individuals with ASD exhibit behavioral responses associated with sensory processing difficulties, such as sensory hyperactivity, hypo-reactivity and sensory seeking (Baker, Lane, Angley, & Young, 2008; Baranek, Boyd, Poe, David, & Watson, 2007; Lane, Molloy, & Bishop, 2014; Rogers & Ozonoff, 2005; Tomchek & Dunn, 2007). Although sensory features are included in DSM-5 criteria for ASD, olfaction (and gustation) are rarely assessed in practice. Smells are prominent environmental and appetitive stimuli, so understanding olfactory dysfunction in ASD could have direct clinical implications that shed light on the underlying causes of behavioral hyperactivity, hypo-reactivity and sensory-seeking behaviors. For example, it is possible that modulating the olfactory environment could influence social or emotional functioning in some individuals with ASD (e.g., For example smells have been used as a positive reinforcer to improve cognition in ASD (Hrdlicka et al., 2011). On the other hand, intolerance of strong smells may force families of ASD patients to avoid certain settings (e.g., restaurants, bowling alleys, movie theatres). Children’s sensory abnormalities therefore contribute significantly to caregiver burden and social isolation (Koenig & Kinnealey, 2008). Finally, deeper understanding of olfaction in ASD could improve our understanding of aberrant food-related behaviors. The development of food preferences occurs in early childhood (Harris, 2008) during the time when signs of ASD begin to emerge. More attention to olfactory function could shed light on the underlying causes of individual differences in food preferences, avoidance behaviors, and selectivity (Luisier et al., 2015). Broadly speaking, improved understanding of specific sensory differences, including olfaction, may help elucidate behavioral subgroups or phenotypes of children with ASD.
The results reported here are consistent with prior literature on olfactory sensitivities in OCD. Individuals with OCD have a high incidence of sensory complaints (50–80%), often referred to as “sensory phenomena” (da Silva Prado et al., 2008; Miguel et al., 2000). These phenomena consist of mental sensations and bodily sensations (Ben-Sasson & Podoly, 2017). Bodily sensations include a range of physical sensitivities, including a strong aversion to certain smells, particularly smells associated with food. It is likely that some of these bodily sensations are related to alterations in olfactory discrimination and hedonics in OCD – as reported here. However, targeted research is warranted to further probe these interactions.
In line with the significant heterogeneity that characterizes ASD, ADHD, and OCD, differences in olfactory function across domains in these clinical populations are influenced by demographic and clinical aspects of the neurodevelopmental samples under study. Studies of younger individuals and studies with higher proportions of males show greater deficits in olfactory function. Taken together, these findings indicate age and sex are relevant demographic moderators of olfactory performance in ASD, ADHD, and OCD. Of the clinical factors investigated, FSIQ, symptom ratings, and pharmacological intervention were also important moderators of olfactory dysfunction. Higher FSIQ scores were associated with better olfactory performance in ADHD and ASD, which is consistent with prior studies suggesting greater intellectual ability is associated with improved olfactory performance (Danthiir, Roberts, Pallier, & Stankov, 2001; Hedner, Larsson, Arnold, Zucco, & Hummel, 2010; Larsson, Nilsson, Olofsson, & Nordin, 2004). Higher OCD symptom ratings were associated lower olfactory performance in OCD, illustrating that olfactory performance deficits may become increasingly pronounced in OCD as symptoms worsen. Overall, these associations implicate higher general ability level as a buffer against olfactory dysfunction in ASD and ADHD, while olfactory dysfunction may be related to the clinical presentation of OCD.
The present findings imply that aspects of both the central and peripheral olfactory system may be abnormal in neurodevelopmental disorders. In fact, the olfactory bulbs have been shown to be atypical in various clinical subgroups (Eslinger, Damasio, & Van Hoesen, 1982). Prior studies demonstrated deviations in olfactory bulbar volume in ADHD (Lorenzen et al., 2016) while others have hypothesized that olfactory bulbs may be dysmorphic in ASD (Brang & Ramachandran, 2010). Early functional and structural neuroimaging work has demonstrated that tertiary olfactory cortical regions, such as the orbitofrontal cortex, piriform cortex, and amygdala, are affected in ADHD and OCD (Berlin et al., 2017; Lorenzen et al., 2016; Segalàs et al., 2014). While it is tempting to speculate on the origins of olfactory deficits in neurodevelopmental conditions, the literature is in need of high-quality morphopmetric studies of the olfactory system to better understand the neural underpinnings of behavioral dysfunction.
Despite novel contributions, the present meta-analysis should be considered in context of its limitations. Primarily, the meta-analysis included studies using differing performance measures of olfactory function, which may have made their synthesis less precise. Additionally, a significant number of studies included participants referred from outpatient clinics. As such, the effects observed may have been larger than would be observed in a community sample. Thirdly, there were few effects available in some disorders (e.g. ADHD) and for specific olfactory domains, particularly odor intensity and hedonics. Therefore, findings in these disorders and domains should be considered tentative and future studies are warranted. Fourth, moderator and meta-regression analyses were performed only on those studies where relevant data was reported. Several studies did not report data that may be relevant to olfactory dysfunction observed in the meta-analysis including duration of illness, symptom ratings, psychotropic medication, years of education, and smoking status. Of additional interest, olfactory dysfunction in ASD, ADHD, and OCD may be informed by clinical genetic and epidemiological studies indicating that these disorders share common genetic causes (Gratten, Wray, Keller, & Visscher, 2014; Lee et al., 2013; Lionel et al., 2013) and phenotypic variance in the general population (Doshi-Velez et al., 2014; Kessler et al., 2006; Larson, Russ, Kahn, & Halfon, 2011; Ruscio, Stein, Chiu, & Kessler, 2010). Moreover, molecular genetic studies indicate that specific polymorphisms of catecholaminergic receptor genes involved in the reuptake of dopamine (Azzam & Mathews, 2003; Hamilton et al., 2013; Li, Sham, Owen, & He, 2006; Pooley, Fineberg, & Harrison, 2007), acetylcholine (Anand et al., 2011; Taylor, 2013; Wallis et al., 2009), and γ–aminobutyric acid (GABA; (Coghlan et al., 2012; Edden, Crocetti, Zhu, Gilbert, & Mostofsky, 2012; Richter et al., 2012) are represented in all three of these conditions. Since catecholamines are instrumental in olfaction (Doty, 2017), dysfunction in catecholaminergic neurotransmission may be a common mechanism that causes olfactory performance deficits in ASD, ADHD, and OCD. As such, future studies should explore olfactory performance in relation to the genetic underpinnings of these complex disorders. Finally, the findings reported here may also be due in part to the interaction of sensory and cognitive impairments found across neurodevelopmental disorders (Demetriou et al., 2018; Pievsky & McGrath, 2017; Snyder, Kaiser, Warren, & Heller, 2015). Odor identification performance relies on broader cognitive functions including executive functioning, processing speed, and verbal and visuospatial abilities (Hedner et al., 2010; Larsson et al., 2004). Researchers should continue to investigate olfactory function in neurodevelopmental disorders such as ASD, ADHD, and OCD with comparable methodologies and report sufficient data so that study outcomes may be synthesized.
In conclusion, the present meta-analytic review is the first to summarize findings in ADHD and OCD and updates a recent meta-analysis of ASD (Larsson et al., 2017). The results of this meta-analysis demonstrate clear olfactory dysfunction in ASD and OCD, and suggest that olfactory performance should continue to be explored in ADHD. Investigations of olfactory dysfunction in ASD, ADHD, and OCD, including underlying genetic and neurobiological mechanisms, is a fruitful direction for future research.
Supplementary Material
ACKNOWLEDGEMENTS
Funding Sources: This work was supported by K01 MH102609, R21 MH108895, a Young Investigator Award from the Brain and Behavior Foundation, and the University Research Foundation of the University of Pennsylvania.
REFERENCES
- Anand R, Amici SA, Ponath G, Robson JI, Nasir M, & McKay SB (2011). Nicotinic acetylcholine receptor alterations in autism spectrum disorders–biomarkers and therapeutic targets. In Autism-A Neurodevelopmental Journey from Genes to Behaviour: Intech. [Google Scholar]
- Atanasova B, Graux J, El Hage W, Hommet C, Camus V, & Belzung C (2008). Olfaction: a potential cognitive marker of psychiatric disorders. Neuroscience and Biobehavioral Reviews, 32(7), 1315–1325. [DOI] [PubMed] [Google Scholar]
- Ayres AJ, & Tickle LS (1980). Hyper-responsivity to touch and vestibular stimuli as a predictor of positive response to sensory integration procedures by autistic children. American Journal of Occupational Therapy, 34(6), 375–381. [DOI] [PubMed] [Google Scholar]
- Azzam A, & Mathews CA (2003). Meta‐analysis of the association between the catecholamine‐O‐methyl‐transferase gene and obsessive‐compulsive disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 123(1), 64–69. [DOI] [PubMed] [Google Scholar]
- Baker AE, Lane A, Angley MT, & Young RL (2008). The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: A pilot study. Journal of Autism and Developmental Disorders, 38(5), 867–875. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Boyd BA, Poe MD, David FJ, & Watson LR (2007). Hyperresponsive sensory patterns in young children with autism, developmental delay, and typical development. American Journal on Mental Retardation, 112(4), 233–245. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Foster LG, & Berkson G (1997). Tactile defensiveness and stereotyped behaviors. American Journal of Occupational Therapy, 51(2), 91–95. [DOI] [PubMed] [Google Scholar]
- Begg CB, & Mazumdar M (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 1088–1101. [PubMed] [Google Scholar]
- Ben-Sasson A, & Podoly TY (2017). Sensory over responsivity and obsessive compulsive symptoms: a cluster analysis. Comprehensive Psychiatry, 73, 151–159. [DOI] [PubMed] [Google Scholar]
- Bennetto L, Kuschner ES, & Hyman SL (2007). Olfaction and taste processing in autism. Biological Psychiatry, 62(9), 1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin HA, Stern ER, Ng J, Zhang S, Rosenthal D, Turetzky R, . . . Goodman W (2017). Altered olfactory processing and increased insula activity in patients with obsessive-compulsive disorder: An fMRI study. Psychiatry Research: Neuroimaging, 262, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudjarane MA, Grandgeorge M, Marianowski R, Misery L, & Lemonnier É (2017). Perception of odors and tastes in autism spectrum disorders: a systematic review of assessments. Autism Research, 10(6), 1045–1057. [DOI] [PubMed] [Google Scholar]
- Brang D, & Ramachandran V (2010). Olfactory bulb dysgenesis, mirror neuron system dysfunction, and autonomic dysregulation as the neural basis for autism. Medical Hypotheses, 74(5), 919–921. [DOI] [PubMed] [Google Scholar]
- Burón E, & Bulbena A (2013). Olfaction in affective and anxiety disorders: a review of the literature. Psychopathology, 46(2), 63–74. [DOI] [PubMed] [Google Scholar]
- Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, & Nutt DJ (2012). GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neuroscience and Biobehavioral Reviews, 36(9), 2044–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, New Jersey: Erlbaum. [Google Scholar]
- Croy I, & Hummel T (2017). Olfaction as a marker for depression. Journal of Neurology, 264(4), 631–638. [DOI] [PubMed] [Google Scholar]
- da Silva Prado H, do Rosario MC, Lee J, Hounie AG, Shavitt RG, & Miguel EC (2008). Sensory phenomena in obsessive-compulsive disorder and tic disorders: a review of the literature. CNS spectrums, 13(5), 425–432. [DOI] [PubMed] [Google Scholar]
- Danthiir V, Roberts RD, Pallier G, & Stankov L (2001). What the nose knows: Olfaction and cognitive abilities. Intelligence, 29(4), 337–361. [Google Scholar]
- Demetriou E, Lampit A, Quintana D, Naismith S, Song Y, Pye J, . . . Guastella A (2018). Autism spectrum disorders: a meta-analysis of executive function. Molecular Psychiatry, 23(5), 1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi-Velez F, Ge Y, & Kohane I (2014). Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics, 133(1), e54–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL (2017). Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? The Lancet Neurology, 16(6), 478–488. [DOI] [PubMed] [Google Scholar]
- Duval S, & Tweedie R (2000). Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics, 56(2), 455–463. [DOI] [PubMed] [Google Scholar]
- Edden RA, Crocetti D, Zhu H, Gilbert DL, & Mostofsky SH (2012). Reduced GABA concentration in attention-deficit/hyperactivity disorder. Archives of General Psychiatry, 69(7), 750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, & Minder C (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibenstein A, Fioretti A, Lena C, Rosati N, Amabile G, & Fusetti M (2005). Modern psychophysical tests to assess olfactory function. Neurological Sciences, 26(3), 147–155. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR, & Van Hoesen GW (1982). Olfactory dysfunction in man: anatomical and behavioral aspects. Brain and Cognition, 1(3), 259–285. [DOI] [PubMed] [Google Scholar]
- Gratten J, Wray NR, Keller MC, & Visscher PM (2014). Large-scale genomics unveils the genetic architecture of psychiatric disorders. Nature Neuroscience, 17(6), 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PJ, Campbell NG, Sharma S, Erreger K, Hansen FH, Saunders C, . . . Boerwinkle E (2013). De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Molecular Psychiatry, 18(12), 1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G (2008). Development of taste and food preferences in children. Current Opinion in Clinical Nutrition and Metabolic Care, 11(3), 315–319. [DOI] [PubMed] [Google Scholar]
- Hedges LV, & Olkin I (1985). Statistical methods for meta-analysis: Academic press. [Google Scholar]
- Hedner M, Larsson M, Arnold N, Zucco GM, & Hummel T (2010). Cognitive factors in odor detection, odor discrimination, and odor identification tasks. Journal of Clinical and Experimental Neuropsychology, 32(10), 1062–1067. [DOI] [PubMed] [Google Scholar]
- Hrdlicka M, Vodicka J, Havlovicova M, Urbanek T, Blatny M, & Dudova I (2011). Brief report: significant differences in perceived odor pleasantness found in children with ASD. Journal of Autism and Developmental Disorders, 41(4), 524–527. [DOI] [PubMed] [Google Scholar]
- Islam MA, Fagundo AB, Arcelus J, Agüera Z, Jiménez-Murcia S, Fernández-Real JM, . . . Frühbeck G (2015). Olfaction in eating disorders and abnormal eating behavior: a systematic review. Frontiers in Psychology, 6, 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Turetsky BI, Calkins ME, Kohler CG, Conroy CG, Borgmann-Winter K, . . . Moberg PJ (2014). Olfactory processing in schizophrenia, non-ill first-degree family members, and young people at-risk for psychosis. The World Journal of Biological Psychiatry, 15(3), 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsz FR, Vance A, Anderson VA, Brann PG, Wood SJ, Pantelis C, & Brewer WJ (2008). Olfactory impairments in child attention-deficit/hyperactivity disorder. The Journal of clinical psychiatry, 69(9), 1462–1468. [DOI] [PubMed] [Google Scholar]
- Kazour F, Richa S, Desmidt T, Lemaire M, Atanasova B, & El Hage W (2017). Olfactory and gustatory functions in bipolar disorders: A systematic review. Neuroscience and Biobehavioral Reviews, 80, 69–79. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, . . . Secnik K (2006). The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. American Journal of Psychiatry, 163(4), 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig K, & Kinnealey M (2008). Research brief: Sensory, motor, and communication challenges for persons with autism spectrum disorders. Sensory Integration Special Interest Section Quarterly Newsletter, 31(2), 3–4. [Google Scholar]
- Kohli P, Soler ZM, Nguyen SA, Muus JS, & Schlosser RJ (2016). The association between olfaction and depression: a systematic review. Chemical Senses, 41(6), 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane AE, Molloy CA, & Bishop SL (2014). Classification of Children With A utism S pectrum D isorder by Sensory Subtype: A Case for Sensory‐Based Phenotypes. Autism Research, 7(3), 322–333. [DOI] [PubMed] [Google Scholar]
- Larson K, Russ SA, Kahn RS, & Halfon N (2011). Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics, peds. 2010–0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M, Nilsson L-G, Olofsson JK, & Nordin S (2004). Demographic and cognitive predictors of cued odor identification: evidence from a population-based study. Chemical Senses, 29(6), 547–554. [DOI] [PubMed] [Google Scholar]
- Larsson M, Tirado C, & Wiens S (2017). A Meta-Analysis of Odor Thresholds and Odor Identification in Autism Spectrum Disorders. Frontiers in Psychology, 8, 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, . . . Witte JS (2013). Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature Genetics, 45(9), 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Sham PC, Owen MJ, & He L (2006). Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Human Molecular Genetics, 15(14), 2276–2284. [DOI] [PubMed] [Google Scholar]
- Lionel AC, Tammimies K, Vaags AK, Rosenfeld JA, Ahn JW, Merico D, . . . Carter MT (2013). Disruption of the ASTN2/TRIM32 locus at 9q33. 1 is a risk factor in males for autism spectrum disorders, ADHD and other neurodevelopmental phenotypes. Human Molecular Genetics, 23(10), 2752–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen A, Scholz-Hehn D, Wiesner CD, Wolff S, Bergmann TO, van Eimeren T, . . . Prehn-Kristensen A (2016). Chemosensory processing in children with attention-deficit/hyperactivity disorder. Journal of Psychiatric Research, 76, 121–127. [DOI] [PubMed] [Google Scholar]
- Luisier A-C, Petitpierre G, Ferdenzi C, Clerc Bérod A, Giboreau A, Rouby C, & Bensafi M (2015). Odor perception in children with autism spectrum disorder and its relationship to food neophobia. Frontiers in Psychology, 6, 1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EJ, Hinkley L, Hill SS, & Nagarajan SS (2011). Sensory processing in autism: a review of neurophysiologic findings. Pediatric Research, 69(5 Pt 2), 48R–54R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel EC, Prado H, Rauch S, Coffey B, Baer L, Savage C, . . . Leckman J (2000). Sensory phenomena in obsessive-compulsive disorder and Tourette’s disorder. The Journal of clinical psychiatry, 61(2), 150–156; quiz 157. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, & Turetsky BI (2003). Scent of a disorder: olfactory functioning in schizophrenia. Current psychiatry reports, 5(4), 311–319. [DOI] [PubMed] [Google Scholar]
- Pievsky MA, & McGrath RE (2017). The neurocognitive profile of attention-deficit/hyperactivity disorder: a review of meta-analyses. Archives of Clinical Neuropsychology, 33(2), 143–157. [DOI] [PubMed] [Google Scholar]
- Pooley E, Fineberg N, & Harrison P (2007). The met 158 allele of catechol-O-methyltransferase (COMT) is associated with obsessive-compulsive disorder in men: case–control study and meta-analysis. Molecular Psychiatry, 12(6), 556. [DOI] [PubMed] [Google Scholar]
- Richter MA, De Jesus DR, Hoppenbrouwers S, Daigle M, Deluce J, Ravindran LN, . . . Daskalakis ZJ (2012). Evidence for cortical inhibitory and excitatory dysfunction in obsessive compulsive disorder. Neuropsychopharmacology, 37(5), 1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, & Ozonoff S (2005). Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry, 46(12), 1255–1268. [DOI] [PubMed] [Google Scholar]
- Rosenthal R (1979). The file drawer problem and tolerance for null results. Psychological Bulletin, 86(3), 638. [Google Scholar]
- Ruscio A, Stein DJ, Chiu WT, & Kessler RC (2010). The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Molecular Psychiatry, 15(1), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M, Schwenck C, Taurines R, Freitag C, Warnke A, Gerlach M, & Romanos M (2013). A systematic review on olfaction in child and adolescent psychiatric disorders. Journal of Neural Transmission, 120(1), 121–130. [DOI] [PubMed] [Google Scholar]
- Segalàs C, Alonso P, Orbegozo A, Real E, Subirà M, López-Solà C, . . . Pujol J (2014). Brain structural imaging correlates of olfactory dysfunction in obsessive–compulsive disorder. European Archives of Psychiatry and Clinical Neuroscience, 264(3), 225–233. [DOI] [PubMed] [Google Scholar]
- Snyder HR, Kaiser RH, Warren SL, & Heller W (2015). Obsessive-compulsive disorder is associated with broad impairments in executive function: A meta-analysis. Clinical Psychological Science, 3(2), 301–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, . . . Thacker SB (2000). Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA, 283(15), 2008–2012. [DOI] [PubMed] [Google Scholar]
- Taylor S (2013). Molecular genetics of obsessive–compulsive disorder: a comprehensive meta-analysis of genetic association studies. Molecular Psychiatry, 18(7), 799. [DOI] [PubMed] [Google Scholar]
- Tomchek SD, & Dunn W (2007). Sensory processing in children with and without autism: a comparative study using the short sensory profile. American Journal of Occupational Therapy, 61(2), 190–200. [DOI] [PubMed] [Google Scholar]
- Tonacci A, Billeci L, Tartarisco G, Ruta L, Muratori F, Pioggia G, & Gangemi S (2017). Olfaction in autism spectrum disorders: A systematic review. Child Neuropsychology, 23(1), 1–25. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Hahn C-G, Borgmann-Winter K, & Moberg PJ (2009). Scents and nonsense: olfactory dysfunction in schizophrenia. Schizophrenia Bulletin, 35(6), 1117–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis D, Arcos-Burgos M, Jain M, Castellanos FX, Palacio JD, Pineda D, . . . Berg K (2009). Polymorphisms in the neural nicotinic acetylcholine receptor α4 subunit (CHRNA4) are associated with ADHD in a genetic isolate. ADHD Attention Deficit and Hyperactivity Disorders, 1(1), 19–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


