Abstract
Postural tachycardia syndrome (POTS) is a chronic form of orthostatic intolerance associated with cognitive dysfunction. We hypothesized executive function and attention is impaired in POTS during active standing. Eighty-seven POTS participants and 39 healthy controls of similar age, sex, and education level completed executive function (Stroop word-color) and attention (CogState Identification) tests in supine and standing postures in a cross-sectional study. POTS participants had lower executive function (t-score: 48± 11 vs. 55±10 control; p=0.009) and worse attention (reaction speed: 2.78±0.11 vs. 2.69±0.06 control; p<0.001) during standing. These data provide new evidence that active standing impairs attention and executive functioning in POTS.
Keywords: Postural tachycardia syndrome (POTS), cognition, brain fog, orthostatic intolerance
INTRODUCTION
Postural Tachycardia Syndrome (POTS) is a chronic form of orthostatic intolerance that has a strong female predominance (4-5:1), primarily affecting women of childbearing age.(Arnold et al., 2018) POTS is defined by an increase in heart rate from supine of at least 30 beats/minute in adults (>40 beats/minute for age 12-19) within 10 minutes of standing or head-up tilt and associated chronic orthostatic symptoms including fatigue, lightheadedness, nausea, and palpitations.(Sheldon et al., 2015) In addition, many patients with POTS experience subjective cognitive complaints or “brain fog” with reports ranging from 43% to 96% in the literature (Boris et al., 2018; Karas et al., 2000; McDonald et al., 2014; Ross et al., 2013; Shaw et al., 2019; Tsai Owens et al., 2019). The underlying nature and impact of orthostatic stress on cognition in POTS, however, is not fully understood. Most previous studies have reported normal cognition in POTS in the supine posture with impairment in measures of attention, executive function, and working memory in the seated posture or with head-up tilt.(Anderson et al., 2014; Arnold et al., 2015; Ocon et al., 2012; Stewart et al., 2015; Stewart et al., 2012; Tsai Owens et al., 2019) No published studies to date have examined cognition in POTS during active standing when patients are most symptomatic in daily life. In this study, we hypothesized that cognitive domains of executive function and attention are impaired in POTS compared to healthy participants during active standing.
METHODS
The Penn State Hershey Medical Center Institutional Review Board approved all protocols. A convenience sample of POTS participants (n=87) was recruited from the 2018 Dysautonomia International Conference, which included 24 adolescents (age range: 14-19 years) and 63 adults (age range: 20-59 years). POTS participants were enrolled if they were between 13 and 60 years of age, previously diagnosed with POTS, able to stand unassisted, and able and willing to provide informed consent or assent. POTS participants self-reported a previous diagnosis by a medical professional and remained on any prescribed treatments during the study protocol. Healthy controls (n=39, age range: 13-57 years) were recruited from the Penn State Health Milton S. Hershey Medical Center and the surrounding community. Healthy controls were matched to POTS participants based on age, sex, and educational level, were free of chronic illness, and able and willing to provide informed consent or assent. POTS and healthy participants were excluded if they were less than 13 or greater than 60 years of age, unable to give or withdraw informed consent, prisoners, or unable to stand. Participants 18 years and older provided written informed consent, and participants under 18 years old signed an assent form and a parent signed the consent form. Participants completed a demographic survey in REDCap that included current medications.
Blood pressure and heart rate were measured following 1, 3, and 5 minutes in both the supine and standing postures. Blood pressure and heart rate values were measured after 5 minutes in each posture are reported, to minimize potential hemodynamic and symptomatic carryover effects from the previous posture. Following blood pressure measurements, participants completed cognitive tests for 5 minutes in the supine and active standing postures. There were no differences in testing duration between groups or between postures within groups. The order of testing was randomized and counterbalanced to control for potential postural and learning effects; half of participants performed cognitive tests supine first, and then standing, and half of participants completed cognitive testing standing first, then supine.
The cognitive tests included the Stroop word-color test of executive function and the CogState Identification Task of visual attention. For the Stroop test, participants are asked to name the color of ink a word is printed in, when the word itself is a different color name.(Jensen et al., 1966) This test takes ~3 minutes to complete. The number of words correctly identified is transformed into a t-score based on age, gender, and educational level. The mean t-score is 50, with an individual t-score within ±1 SD of the mean (40-60) representing normal functioning. An individual t-score less than 40 indicates clinically meaningful impairment. The CogState Identification task is a computerized test that measures attention using a choice reaction time paradigm.(Maruff et al., 2009) In this task, a playing card is presented on the screen face down, and when the card flips over, the participant must decide if the color of the card is red or not. This test takes ~2 minutes to complete. The primary outcome measure is the mean of log10-transformed reaction time for correct responses, with higher values indicating worse performance. The normative data range for the age groups in this study is 2.66-2.71. We used these tests as: (1) they are well established in the literature to assess executive function and attention; (2) they can be accomplished within a short time period, so that POTS participants could finish testing during standing; (3) they are relatively immune to learning effects due to multiple versions or computerized paradigms; and (4) previous studies have shown impaired executive function and attention in POTS in seated and standing postures using these tasks (Anderson et al., 2014; Arnold et al., 2015).
Authors AJM, TS, KMB, and ACA performed the study procedures, and thus were not blinded to testing order. All investigators, however, were blinded to testing results, including author MF who analyzed and entered data. Data are shown as mean ± SD unless otherwise noted and were analyzed by either GraphPad Prism (Version 7) or SPSS (Version 26). Cognitive outcomes were analyzed by two-factor ANOVA to examine main effects of group (POTS, control) and posture (supine, standing) and their interaction with post-hoc Tukey’s tests. Demographic data were compared between POTS and healthy participants using Mann Whitney U tests. The proportions of POTS versus healthy participants that were female, had orthostatic tachycardia, and had mild cognitive impairment were compared using Chi-square tests. To determine potential clinical predictors for cognitive impairment, a general linear model was used with cognitive test scores defined as the dependent measure, participant group as a fixed factor, and hemodynamic and demographic variables as covariates.
RESULTS
Demographic data and orthostatic vital signs after 5 minutes in each posture are shown in Table 1. POTS participants and healthy controls had similar age, sex, education level, and systolic blood pressure. Supine and standing diastolic blood pressure and heart rate were higher in POTS compared to controls (p<0.05, Table 1). Since treatments were maintained during the study, most POTS participants did not exhibit orthostatic tachycardia >30 beats/minute. Common medications taken by POTS participants during the study included: β-blockers (49%), Vitamin B (30%), Fludrocortisone (29%), Midodrine (28%), IV saline (26%), Benzodiazepines (16%), Ivabradine (14%), Steroids (13%), Pyridostigmine (12%), and Opioids (5%). If participants wore compression garments, they were asked to remove them prior to the study visit.
Table 1.
Demographic Data and Orthostatic Vital Signs
| POTS (n = 87) |
Control (n = 39) |
P-value | |
|---|---|---|---|
| Age, median years (IQR) | 27 (19 - 37) | 24 (23 - 29) | 0.699 |
| Female, n (%) | 82 (94%) | 36 (92%) | 0.400 |
| Education Level, years | 15 ± 3 | 16 ± 2 | 0.110 |
| Systolic Blood Pressure (mmHg) | |||
| Supine | 114 ± 12 | 110 ± 10 | 0.087 |
| Standing | 115 ± 14 | 115 ± 11 | 0.836 |
| Standing-Supine | 2 ± 10 | 4 ± 7 | 0.141 |
| Diastolic Blood Pressure (mmHg) | |||
| Supine | 74 ± 10 | 69 ± 7 | 0.004* |
| Standing | 86 ± 11 | 74 ± 6 | >0.001* |
| Standing-Supine | 13 ± 7 | 5 ± 5 | >0.001* |
| Heart Rate (beats/min) | |||
| Supine | 77 ± 14 | 71 ± 12 | 0.035* |
| Standing | 91 ± 17 | 83 ± 12 | 0.0* |
| Standing-Supine | 15 ± 10 | 13 ± 7 | 0.188 |
| Heart Rate change > 30 beats/min | |||
| n (%) | 9 (10) | 0 (0) | >0.001* |
Blood pressure and heart rate values obtained after 5 minutes in the supine and standing postures are reported. IQR, interquartile range. Data are shown as mean±SD unless otherwise noted.
P < 0.05
The cognitive testing data are shown in Figure 1. The Stroop word-color t-scores of executive function were on average lower in POTS versus healthy participants regardless of posture (Pgroup= 0.001, Pposture= 0.891, PInt=0,502) Post-hoc analysis revealed that Stroop word-color t-scores were significantly lower in POTS versus controls in standing (POTS: 48±1, controls: 55±2, p=0.007) but not supine (POTS: 49±1, controls: 54±1, p=0.095, Figure 1A) positions. Most POTS participants had executive function scores within the normative range (t-score 40-60). A greater proportion of POTS participants, however, had scores consistent with clinically meaningful impairment in executive function (t-score <40) in both supine (POTS: 24%, Controls: 8%, p<0.001) and standing (POTS: 25%, Controls: 3%, p<0.001, Figure 1A) postures. Importantly, executive function remained impaired in POTS compared with healthy controls even after removal of t-scores <40 (Pgroup= 0.009, Pposture= 0.190, PInt= 0.446). Given that this study included both adolescent and adult participants, we used a general linear model to assess the potential contribution of age to impairments in executive function in POTS. We found that after adjustment for age, supine and standing executive function remained impaired in POTS (p=0.017 and p=0.002, respectively).
Figure 1. Executive function and attention are impaired in POTS during standing.
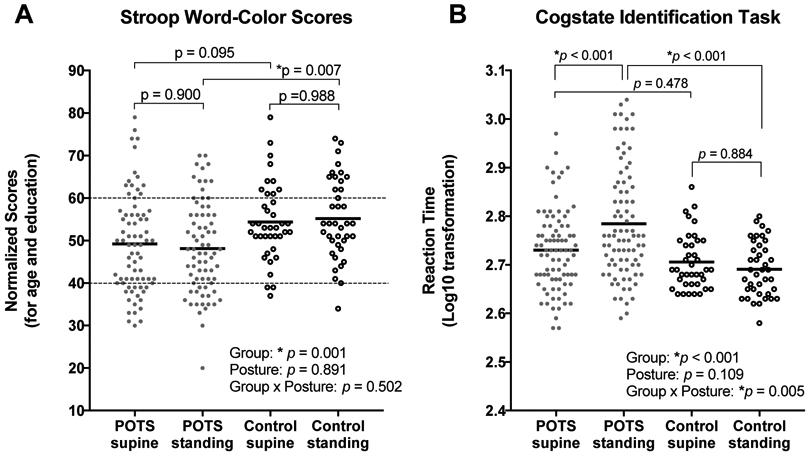
Stroop word-color test of executive function (A) and CogState Identification Task of visual attention (B) were worse in POTS participants compared to healthy controls in the standing posture. Values represent mean ± SD. Dotted lines in Panel A are ±1 SD of the mean for Stroop word-color test t-score of executive function representing within the average range, with normalized t-scores <40 representing clinically meaningful impairment.
CogState Identification scores of attention were also significantly different between POTS and healthy controls (Pgroup<0.001), with no main effect of posture (Pposture=0.109) and a group by posture interaction detected (PInt=0.005). Attention scores were higher (worse) in the standing versus supine posture in POTS (supine: 2.73±0.08, standing: 2.78±0.11, p<0.001) but not in controls (supine: 2.70±0.06, standing: 2.69±0.05, p=0.884, Figure 1B). Attention scores were worse in POTS compared to controls in the standing posture (p<0.001), but not in the supine posture (p=0.478). After adjustment for age using a general linear model, attention was still impaired in POTS participants during standing (p=0.001), but not in the supine posture (p=0.783).
We also assessed for potential clinical predictors to cognitive impairment during active standing in POTS participants. With standing executive function defined as the dependent variable, there were no significant main effects of age (p=0.545), systolic blood pressure (p=0.792), diastolic blood pressure (p=0.913), heart rate (p=0.602), or orthostatic changes in systolic blood pressure (p=0.741), diastolic blood pressure (p=0.429) or heart rate (p=0.387). With standing attention defined as the dependent variable, there were also no significant main effects of age (p=0.116), systolic blood pressure (p=0.410), diastolic blood pressure (p=0.081), heart rate (p=0.693), or orthostatic changes in systolic blood pressure (p=0.668), diastolic blood pressure (p=0.138) or heart rate (p=0.782). Importantly, for both executive function and attention, the participant group effect remained significant (p<0.01) after adjustment for these demographic and hemodynamic variables when compared both within POTS groups (normal versus impaired Stroop scores) and to healthy controls. Finally, we found a significant negative correlation between Stroop word-color and CogState identification scores in the standing position (r2=−0.191, p<0.001), with no correlation in the supine position (r2=−0.035, p=0.095). This suggests a relationship between mild impairment on the Stroop word-color test and slower reaction time on the CogState Identification task during standing in POTS.
DISCUSSION
Overall, we found that executive function and attention are impaired in POTS during active standing when compared to healthy controls of similar age, sex, and education level. These impairments in cognitive function were observed in POTS participants even on medications and with the majority demonstrating well-controlled orthostatic tachycardia. While most POTS participants had cognitive test scores within normal limits, approximately 25% exhibited clinically meaningful impairment in executive function. These findings suggest that active standing impairs cognitive domains of attention and executive function particularly within a subset of POTS participants and emphasizes the need for further research to better understand how orthostatic stress affects cognition in this patient population to help identify novel targeted treatment approaches.
Brain fog is described in POTS as “forgetful”, “cloudy”, and “difficulty focusing, thinking, and communicating” suggesting impairment in multiple cognitive domains including executive function, attention, and memory.(Ross et al., 2013) In the supine position, studies have shown no differences between POTS patients and healthy controls in memory (n-back, CogState One Card Learning, and WRAML2 memory tasks), and attention and psychomotor function (CogState Identification and Detection tasks, WRAML2 attention/concentration index).(Anderson et al., 2014; Ocon et al., 2012; Stewart et al., 2015; Stewart et al., 2012; Tsai Owens et al., 2019) In contrast, a study showed decreased attention (Ruff 2&7 Selective Attention speed), cognitive processing speed (Symbol Digits Modalities Test), and executive function (Stroop word-color and Trails B) in POTS when tested in the semi-recumbent posture to minimize orthostatic tachycardia (Arnold et al., 2015). This study noted selectivity in cognitive deficits in POTS with normal performance on measures of psychomotor speed, memory function, and verbal fluency. Finally, a handful of studies have tested cognition in POTS during passive upright posture induced by head-up tilt, and reported impairments in working memory (n-back) and attention and information processing (CogState Detection and Identification tasks).(Anderson et al., 2014; Ocon et al., 2012; Stewart et al., 2015; Stewart et al., 2012)
While previous studies measured cognition in response to head-up tilt, no studies have evaluated cognition during active standing. Active standing differs from passive upright posture induced by head-up tilt in that the body compresses and releases pressure on veins in the lower extremities to engage the skeletal muscle pump and to increase venous return to the heart.(Rowell, 1986) These reflex responses to counteract effects of initial and prolonged standing are significantly less present during passive tilt, resulting in reduced venous return over time. This results in marked differences in hemodynamic responses, with a previous study showing exaggerated orthostatic tachycardic responses in POTS patients during head-up tilt versus active standing.(Plash et al., 2013) In addition, active standing is more realistic to what people with POTS experience daily, and therefore induces less emotional stress than head-up tilt, which may affect cognition.
The current study shows that standing impairs attention in POTS, but not in healthy participants. These findings are consistent with a study showing no difference in CogState Identification scores between POTS participants and healthy participants while semi-recumbent, but worse scores in POTS participants following head-up tilt.(Anderson et al., 2014) We further provide new evidence that POTS participants exhibit worse executive function compared to controls during active standing, with a similar trend observed in the supine posture. This is consistent with a previous study showing impaired executive function in semi-recumbent POTS patients using the Stroop test.(Arnold et al., 2015) We acknowledge the tests used in this study do not assess all aspects of cognition, and future studies in POTS should not be limited to the tests used in the current study. Importantly, the impairment in executive function and attention in POTS participants remained significant after adjustment for age, suggesting these cognitive impairments were not driven by differences in performance between adolescent versus adult participants.
While cognitive test scores were generally within normal limits, they were on average significantly lower in POTS compared with healthy participants, with a higher proportion of POTS participants scoring in a range consistent with clinically meaningful impairment for executive function. This overlap in cognitive scores between POTS and healthy participants has been observed in previous studies (Arnold et al., 2015), and may reflect heterogeneity of this condition. There is also a clear disconnect between subjective cognitive complaints and objectively measured cognitive dysfunction in POTS. While most POTS patients report symptoms of “brain fog,” previous studies have only demonstrated objective cognitive impairment in 20-30% of patients.(Anderson et al., 2014; Arnold et al., 2015; Tsai Owens et al., 2019) Consistent with this, we found that only a subset of POTS participants had clinically meaningful impairment of executive function. This subjective-objective disconnect could reflect a mismatch of expectations (patients had higher pre-morbid intellectual functioning) as well as cognitive hypervigilance. We found, however, that the impairments in executive function are not solely driven by this subgroup of POTS participants.
Given the identification of a subgroup of POTS participants with clinically meaningful impairment of executive function, we assessed for potential clinical predictors to cognitive impairment during standing. Of interest, we did not find any relationship between executive function or attention scores and age or hemodynamic variables. POTS participants with clinically meaningful impairment in executive function, however, did have worse reaction times on the attention test, perhaps suggesting a more global cognitive impairment. Overall, this heterogeneity in response and subjective-objective mismatch could in part underlie the lack of difference in cognitive parameters observed in previous studies. In addition, future research may need to consider pre-selection of patients with objectively measured cognitive dysfunction, particularly for treatment trials in order to observe measurable improvement.
An advantage of the current study is that we included a large sample of POTS participants recruited from a patient conference, with participants remaining on any usual treatments to mimic real-world conditions. Previous studies testing cognition in POTS were performed in small groups of patients from tertiary care centers with variables such as medications, diet, and time of day controlled. We observed that, despite controlled orthostatic tachycardia in 90% of POTS participants, attention and executive function were still impaired during active standing. This finding is consistent with two previous studies in which cognitive impairment was present in POTS participants even when orthostatic tachycardia was minimized, such as in the semi-recumbent posture and at low angles of head-up tilt.(Arnold et al., 2015; Ocon et al., 2012) One study, however, did observe an association between orthostatic heart rate and the degree of cognitive impairment in POTS. (Anderson et al., 2014) As recently reviewed, other mechanisms that may contribute to cognitive dysfunction in POTS include central norepinephrine dysregulation, structural and functional brain abnormalities including changes in cerebral blood flow and neurovascular coupling, psychiatric symptoms, presence of chronic fatigue syndrome or other comorbidities, and sleep disturbances.(Raj et al., 2018)
There are several limitations to this study. First, we relied on self-reported diagnosis of POTS, and did not confirm with medical records. We could not confirm POTS diagnosis with orthostatic vital signs as participants remained on any usual treatments to manage their symptoms, and we only measured blood pressure and heart rate in each posture for 5 minutes prior to cognitive tests. Second, while most clinical research studies in POTS to date have relied on a convenience sample, this study is potentially influenced by sampling bias and therefore may not be representative of the generalized patient population. Finally, we did not control for potential effects of hemodynamic, psychiatric, or pain medications, all of which could impact cognitive functioning in POTS. While this study aimed to mimic real-word conditions where patients remain on usual medications, determining the impact of classes of medications on cognitive functioning is POTS is an important area for future research.
Overall, the current study adds to the limited literature on cognition in POTS by demonstrating that measures of attention and executive function are worse particularly in a subset of POTS participants when compared to healthy participants during active standing, despite standard treatment and management of orthostatic tachycardia. The mechanisms by which orthostatic stress affects cognition in POTS and treatment approaches to ameliorate this symptom remain to be determined.
ACKNOWLEDGEMENTS
Dysautonomia International funded this work. The authors are supported by NIH R00HL122507 (to A.C.A.) and by American Heart Association 18POST339660087 (to A.J.M.). The authors thank Dysautonomia International volunteers who helped with this study including Aly Aylward, Leslie Bassett, and Zachary Orban.
Footnotes
DISCLOSURES
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson JW, Lambert EA, Sari CI, Dawood T, Esler MD, Vaddadi G, Lambert GW 2014. Cognitive function, health-related quality of life, and symptoms of depression and anxiety sensitivity are impaired in patients with the postural orthostatic tachycardia syndrome (POTS). Front Physiol 5, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AC, Haman K, Garland EM, Raj V, Dupont WD, Biaggioni I, Robertson D, Raj SR 2015. Cognitive dysfunction in postural tachycardia syndrome. Clin Sci (Lond) 128, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AC, Ng J, Raj SR 2018. Postural tachycardia syndrome - Diagnosis, physiology, and prognosis. Auton Neurosci 215, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boris JR, Bernadzikowski T 2018. Demographics of a large paediatric Postural Orthostatic Tachycardia Syndrome Program. Cardiol Young 28, 668–674. [DOI] [PubMed] [Google Scholar]
- Jensen AR, Rohwer WD Jr. 1966. The Stroop color-word test: a review. Acta Psychol (Amst) 25, 36–93. [DOI] [PubMed] [Google Scholar]
- Karas B, Grubb BP, Boehm K, Kip K 2000. The postural orthostatic tachycardia syndrome: a potentially treatable cause of chronic fatigue, exercise intolerance, and cognitive impairment in adolescents. Pacing Clin Electrophysiol 23, 344–351. [DOI] [PubMed] [Google Scholar]
- Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, Pietrzak RH 2009. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol 24, 165–178. [DOI] [PubMed] [Google Scholar]
- McDonald C, Koshi S, Busner L, Kavi L, Newton JL 2014. Postural tachycardia syndrome is associated with significant symptoms and functional impairment predominantly affecting young women: a UK perspective. BMJ Open 4, e004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocon AJ, Messer ZR, Medow MS, Stewart JM 2012. Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clin Sci (Lond) 122, 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plash WB, Diedrich A, Biaggioni I, Garland EM, Paranjape SY, Black BK, Dupont WD, Raj SR 2013. Diagnosing postural tachycardia syndrome: comparison of tilt testing compared with standing haemodynamics. Clin Sci (Lond) 124, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V, Opie M, Arnold AC 2018. Cognitive and psychological issues in postural tachycardia syndrome. Auton Neurosci 215, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, Medow MS, Rowe PC, Stewart JM 2013. What is brain fog? An evaluation of the symptom in postural tachycardia syndrome. Clin Auton Res 23, 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB 1986. Orthostasis in Human Circulation: Regulation During Physical Stress (pp 150). Oxford University Press, New York, USA. [Google Scholar]
- Shaw BH, Stiles LE, Bourne K, Green EA, Shibao CA, Okamoto LE, Garland EM, Gamboa A, Diedrich A, Raj V, Sheldon RS, Biaggioni I, Robertson D, Raj SR 2019. The face of postural tachycardia syndrome - insights from a large cross-sectional online community-based survey. J Intern Med 286, 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon RS, Grubb BP 2nd, Olshansky B, Shen WK, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, Sutton R, Sandroni P, Friday KJ, Hachul DT, Cohen MI, Lau DH, Mayuga KA, Moak JP, Sandhu RK, Kanjwal K 2015. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 12, e41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Del Pozzi AT, Pandey A, Messer ZR, Terilli C, Medow MS 2015. Oscillatory cerebral blood flow is associated with impaired neurocognition and functional hyperemia in postural tachycardia syndrome during graded tilt. Hypertension 65, 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Medow MS, Messer ZR, Baugham IL, Terilli C, Ocon AJ 2012. Postural neurocognitive and neuronal activated cerebral blood flow deficits in young chronic fatigue syndrome patients with postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 302, H1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Owens M, Fischer P, Kirsch A, Harbeck-Weber C, Sim L, Zaccariello M, Homan K 2019. Neurocognitive Difficulties Among Youth with POTS within an Intensive Pain Rehabilitation Program. J Pediatr Psychol 44(5), 567–575. [DOI] [PubMed] [Google Scholar]


