Abstract
Background
The genetically encoded calcium (Ca2+) sensor GCaMP6 has been widely used for imaging Ca2+ transients in neuronal somata, dendrites, and synapses.
New method
Here we describe five new transgenic mouse lines expressing GCaMP6F (fast) or GCaMP6S (slow) in the central and peripheral nervous system under the control of the Thy1.2 promoter.
Results
These transgenic lines exhibit stable and layer-specific expression of GCaMP6 in multiple brain regions. They have several unique features compared to existing Thy1.2-GCaMP6 mice, including sparse expression of GCaMP6 in layer V pyramidal neurons of the cerebral cortex, motor neurons in the spinal cord, as well as sensory neurons in dorsal root ganglia (DRG). We further demonstrate that these mouse lines allow for robust detection of Ca2+ transients in neuronal somata and apical dendrites in the cerebral cortex of both anesthetized and awake behaving mice, as well as in DRG neurons.
Comparison with existing method(s)
These transgenic lines allows calcium imaging of dendrites and somas of pyramidal neurons in specific cortical layers that is difficult to achieve with existing methods.
Conclusions
These GCaMP6 transgenic lines thus provide useful tools for functional analysis of neuronal circuits in both central and peripheral nervous systems.
Keywords: calcium imaging, motor cortex, somatosensory cortex, dorsal root ganglia, in vivo, two-photon microscopy
1. Introduction
The measurement of calcium (Ca2+) transients in neurons has become increasingly important for detecting neuronal activity in living animals [1]. With the development of Genetically Encoded Calcium Indicators (GECIs), neuronal activity can be assessed over time by expressing GECIs in specific cell types or subcellular compartments [2–4]. Among all the GECIs, the GFP-based GCaMP has been the most widely used in biological systems [1, 5]. More recently, GCaMP has been improved via several rounds of mutagenesis to its current form, GCaMP6 [4, 6]. GCaMP6, similar to prior fluorescence resonance energy transfer based GECI, is capable of single action potential detection in vivo under certain conditions [7, 8]. There are three variants of GCaMP6 exhibiting either slow, medium, or fast fluorescence changes upon Ca2+ binding [4]. GCaMP6S is one of the most sensitive GECIs with slow kinetics, whereas GCaMP6F is one of the fastest GECIs with sensitivity comparable to synthetic Ca2+ indicator OGB1-AM [4].
Transgenic mice provide several advantages for driving GCaMP expression over the use of viruses [1, 4, 9–11]. The expression patterns of GCaMP6 are often highly reproducible across mice, and the expression levels are fairly stable, allowing individual cells to be imaged over long periods of time [4, 12, 13]. By using different promoters, transgenes can also be expressed in different cell types throughout the mouse nervous system [5, 14]. Furthermore, transgenic mice potentially enable GCaMP6 expression and subsequent imaging early in postnatal development.
Previous studies have reported Thy1.2-GCaMP6 transgenic mouse lines suitable for imaging the activity of neuronal somata in the cortex [12, 13]. Because in those lines GCaMP6 was expressed at high density and across several cortical layers, it was difficult, if not impossible, to image dendritic activity of cell populations from a defined cortical layer. Furthermore, there has been no report on lines of transgenic mice expressing GCaMP6 in the peripheral nervous system. Since AAV infection of peripheral neurons is technically challenging [15, 16], transgenic lines with GCaMP6 expression in sensory neurons would be particularly useful for studies of the activity of peripheral neurons during sensory processing.
In the present work, we characterized five transgenic mouse lines that express GCaMP6 under the control of the Thy1.2 promoter and in various neuronal populations. These transgenic lines allow Ca2+ imaging of pyramidal neuron somata in cortical layer II/III and layer V, as well as dendrites of layer V pyramidal neurons [17]. We also report for the first time that these lines are suitable for monitoring activity of sensory neurons in the dorsal root ganglia. We expect these new GCaMP6 transgenic mouse lines will greatly facilitate Ca2+ imaging of neurons in the living mice.
2. Experimental methods
2.1. Thy1.2-GCaMP6 transgenic mice generation and care
GCaMP6 fast and slow coding regions were PCR amplified from GCaMP6F and GCaMP6S containing plasmids (Addgene, Cambridge, MA), respectively, and a Kozak sequence was introduced. All constructs were verified by sequencing. GCaMP6 cDNAs were cloned into the SacII site of the mouse Thy1.2 promoter [18, 19]. The entire construct (the 6.5 kb of basic expression cassette and the 1.3 kb of transgene sequences) was purified after EcoRI and Pvul digestion and injected into fertilized B6SJLF1 mouse eggs at the Transgenic Mouse Lab facilities (Skirball Institute at NYU Langone Medical Center, NYULMC). Transgenic founders were backcrossed to C57BL/6J mice for the analysis of expression patterns. Primers for genotyping were: 5’- TCT GAG TGG CAA AGG ACC TTA GG −3’ (forward) 5’- TTA CGA CGT GAT GAG TCG ACC −3’ (reverse). Animals of both sexes were used for all the experiments. All mice were maintained at the NYULMC Skirball animal facility with controlled temperature and humidity conditions and had free access to food and water. All animal handling was in accordance with guidelines set forth by New York University Langone School of Medicine’s Institutional Animal Care and Use Committee (IACUC), approved protocol number: 160905. Thy1.2 -GCaMP6S Lines 1, 2, and 3, and GCaMP6F Lines 1 and 2 are available at The Jackson Laboratory (http://jaxmice.jax.org), with stock numbers 031892, 031893, 031894, 031898, and 031899, respectively. Following GCaMP expression or imaging experiments stated below, mice were euthanized according to NYULMC IACUC/American Veterinary Medical Association guidelines. In brief, mice were placed in the chamber before turning on CO2 gas. After mice became unconscious, flow rate was increased to obtain rapid euthanasia. Mice were left in the chamber until clinical death, waiting at least 60 seconds without seeing a breath before removing rodent from the chamber. To ensure death, mouse heartbeat was checked prior to cervical dislocation. If a mouse was not dead following CO2 exposure, it was placed back into the CO2 chamber until death can be verified, followed by cervical dislocation, and disposal.
2.2. Expression analysis
Mice (from postnatal days 14 and 30 to 35) were deeply anesthetized with ketamine (100 μg/g) and xylazine (10 μg/g) via an intraperitoneal injection and then perfused transcardially with room temperature PBS, followed by 4% paraformaldehyde (PFA) in PBS. Brain, spinal cord, dorsal root ganglia (DRG), sural and femoral nerves, and soleus and diaphragm muscles were harvested. Mouse brains and spinal cords were post-fixed in 4% PFA overnight at 4°C. Brains were then washed with PBS before being cut into 50 μm coronal and sagittal sections using a vibratome. Sections were permeabilized with 1% Triton X-100 in PBS for 3 h, washed with PBS three times for 5 min per wash, and incubated with blocking buffer (5% normal goat serum, 0.1% Triton X-100 in PBS) for 1 h at room temperature. Sections were then incubated with rabbit anti-GFP antibody (Molecular Probes, A11122; 1:300 dilution) overnight at room temperature to enhance GCaMP detection. Selected slices were also co-incubated with a mouse anti-NeuN antibody (Millipore, MAB377; 1:500). After incubation with primary antibodies, sections were washed with PBS three times for 20 min per wash, followed by incubation with Alexa 488 - conjugated goat anti-rabbit and Alexa 555 - conjugated goat anti-mouse secondary antibodies (Invitrogen) for 2 h at room temperature and then washed again with PBS. Sections were then transferred onto slides and mounted with 100% glycerol.
DRG specimens and spinal cords were cryoprotected in 30% sucrose and cryosections (20 μm thick) were obtained from the lumbar region. Muscles and nerves were stored in PBS at 4°C and stained with standard immunohistochemistry protocols [18]. The following antibodies were used: GFP (Molecular Probes, A11122; 1:300), SMI32 (Covance, SMI-32R; 1:1,000), and cy2- and cy3-conjugated secondary antibodies (Jackson ImmunoResearch; 1:250). Post-synaptic acetylcholine receptors were detected with tetramethylrhodamine-labeled α-bungarotoxin (BTX, Invitrogen; 1:500).
Images from coronal and sagittal brain slices were collected in a Zeiss 700 Confocal Microscope, using 10x and 20x lenses. Images from spinal cord sections and peripheral nervous system specimens were collected in a Leica TCS SP5 Confocal Microscope (Leica Microsystems), using 10x dry, 40x oil, and 63x oil immersion lenses, with NA 0.30, 1.25 and 1.4, respectively. Standard pinhole of 1 AU and optical intervals of 5, 1, and 0.5 μm (for 10x, 40x, and 63x lenses, respectively) were used.
2.3. Surgical preparation for in vivo two-photon imaging head-restrained mice
Dendritic imaging was carried out in awake, head restrained mice through a thinned-skull window [9, 20, 21]. Somatic imaging of layer II/III and layer V was performed through an open-skull, similar to methods previously described [22]. Briefly, mice were deeply anesthetized with an intraperitoneal injection of ketamine (100 μg/g) and xylazine (10 μg/g). The mouse head was shaved, and the skull surface was exposed with a midline scalp incision. The periosteum tissue over the skull surface was removed without damaging the temporal and occipital muscles. A head holder composed of two parallel micro-metal bars was attached to the animal’s skull to restrain its head and reduce motion-induced artifact during imaging. A small region (~0.2 mm in diameter) located over the primary motor cortex (defined by stereotactic coordinates: at bregma and 1.2 mm lateral from the midline) was marked and a layer of cyanoacrylate-based glue was applied to the top of the skull surface. The primary somatosensory cortex was imaged using the coordinates 0.5 mm posterior and 1.5 mm lateral to the bregma. Following marking of imaging location, a head holder was then mounted on top of the skull with dental acrylic cement (Lang Dental Manufacturing Co., IL, USA) such that the marked region in the skull was exposed between the two bars. Once the cement dried, the head holder was screwed to two metal cubes that were attached to a solid metal base, and a cranial window (either thin- or open-skull) was created over the previously marked region. The mice were then returned to their home cages to recover.
Imaging experiments started after awakening. Mice with head mounts were habituated 3 times (10 min each) in the imaging apparatus, which sits on top of a custom-built free-floating motorized linear treadmill (96 × 56 × 61 cm dimensions), to minimize potential stress effects of head restraining, motor training, and imaging. During motor training, the treadmill motor (Dayton, Model 2L010) was driven by a DC power supply (Extech). At the onset of a trial, the motor was turned on and the belt speed gradually increased from 0 cm s−1 to 8 cm s−1 within ~3 s. Each running trial lasted 30 s. After completion of each trial, the treadmill was turned off and the next trial started after 30 s rest period. We show the summation of running-induced activity by pooling 5 running trials together into 2.5 min running aggregate measures. In some experiments, mice were anesthetized following awake recordings with a single intraperitoneal ketamine and xylazine injection. Heart rate, respirations, and temperature were assessed following induction of anesthesia. Depth of anesthesia was checked prior to imaging by the lack of spontaneous limb movement and the response to hindlimb withdrawal after toe pinch.
2.4. Surgical preparation for imaging DRG in awake resting mice
Imaging DRG primary sensory neurons was performed as described previously [23]. In brief, Thy1.2-GCaMP6 mice were anesthetized with ketamine and xylazine at postnatal day 35. After a skin incision, a spinal holder was attached to the animal’s fourth lumbar vertebrae with cyanoacrylate-based glue and cement. The lumbar 4 DRG on the left side was exposed and a glass window was implanted on top. The mice were allowed 4 days of recovery before two-photon imaging. Selected regions of interest (ROI; 470 μm × 470 μm) were imaged before, during and after punctate mechanical stimulation (6 g von Frey filament) applied to the left hind paw to study sensory neuronal activities in DRG.
2.5. Imaging of neuronal somata and dendrites in awake, head-restrained mice
In vivo two-photon imaging was performed using an Olympus Fluoview 1000 two-photon system (tuned to 920 nm) equipped with a Ti:Sapphire laser (MaiTai DeepSee, Spectra Physics). The average laser power to image GCaMP6 signals in layers I, II/III and V was 20, 30–40, and 50–60 mW, respectively. We found that imaging GCaMP6 mice requires on average more laser power (2–4x) than AAV-GCaMP6 transduced mice (typically, 5–10 mW for layers I and II/III). All experiments were performed using a 25x objective (NA 1.1) immersed in ACSF solution, and 1.5x and 4x digital zoom (for soma and dendrites, respectively). All imaging was acquired at frame rates of 2 Hz (2-μs pixel dwell time). Typical imaging window for tuft dendrites was 160 μm × 80 μm. Image acquisition was performed using FV10-ASW v.2.0 software and analyzed post hoc using NIH ImageJ software.
2.6. Imaging data analysis
ROIs containing apical tuft dendrites, cortical neuronal somata, or DRG neuronal somata were selected for quantification. Ca2+ transients in dendrites and somata were measured according to recently published studies [9, 20]. During running trials, the lateral movement of the images was typically less than 1 μm. All imaging stacks were registered using ImageJ plugin StackReg. Active dendrites and pyramidal somata that could be identified in all imaging sessions were included in the data set. The dendritic segment or somatic fluorescence was measured by averaging all pixels within the ROI covering the dendrite or soma. The ΔF/F0 was calculated as ΔF/F0 = (F−F0)/F0 × 100, where F0 is the baseline fluorescence signal averaged over a 2-s period corresponding to the lowest fluorescence signal over the 2.5-min recording time. In order to compare neuronal activity among different cells and across transgenic mice, measurement of peak fluorescence signal, as well as an integrated measurement of a cell’s output activity over 2.5-min recording time were performed (termed “average calcium activity”). In all imaging sessions, resting awake images were collected prior to running trials. Stimuli-responsive neurons were defined as neurons presenting a change in fluorescence ΔF/F0 >20% or ΔF/F0 < −20%.
2.7. Data analysis
All imaging data were presented as means ± SEM. Parametric tests including unpaired t test and paired t tests were performed if distributions passed Kolmogorov-Smirnov tests for normality. Significant levels were set at P ≤ 0.05. All statistical analyses were performed using the GraphPad Prism.
3. Results
3.1. Patterns of GCaMP6 expression in the brains of Thy1.2-GCaMP6 transgenic mice
To generate GCaMP6 transgenic mice, we utilized the previously described GCaMP6 fast and slow cDNA constructs (referred as GCaMP6F and GCaMP6S, respectively) and the well-characterized Thy1.2 promoter, which drives transgene expression in subsets of neurons (Fig 1A) [5, 14]. We obtained several founder lines (five for GCaMP6F and seven for GCaMP6S) with different levels and patterns of transgene expression in the nervous system. The expression patterns of two Thy1.2-GCaMP6F lines (Line 1 and 2) and three Thy1.2-GCaMP6S lines (Line 1, 2 and 3) were further characterized.
Fig 1. GCaMP6 expression in the primary motor cortex of Thy1.2-GCaMP6 mouse lines.
A. Schematic representation of the expression cassette used for the generation of Thy1.2-GCaMP6 transgenic mice. GCaMP6 transgenes were cloned into the SacII site and the entire construct (6.5 kb basic mouse Thy1.2 expression cassette and 1.3 kb GCaMP6 transgene sequences), flanked by an EcoRI and a Pvul sites, was injected into mouse pronuclei. B. Motor cortical region containing layer II/III and deeper layers (V and VI) neurons. Note Line 1 for GCaMP6F and GCaMP6S have sparse expression in layer V. C. Higher magnification view of layers II/III and V in the primary motor cortex. Sections were co-stained with an antibody against the neuronal marker NeuN (red) to label all neurons. Note the differing density of GCaMP-positive neurons within the different layers and transgenic lines. L, layer. Scale bars, 100 μm (A), 20 μm (C).
All five lines of transgenic mice were born at the expected Mendelian rate, were healthy and fertile as non-transgenic mice, and presented the expected live span and no apparent histological or behavioral abnormalities. At the single cell level, all lines examined showed no obvious signs of cytotoxicity (GCaMP6 signal devoid of nucleus) based on live and fixed tissue imaging (S1 Fig).
Mice at postnatal day 30 (P30) from all five lines exhibited widespread GCaMP6 expression in multiple brain regions, including the olfactory bulb, cortex, hippocampus, thalamus, cerebellum and brain stem (Table 1, S2 and S3 Fig). Overall, high transgene expression was present in the amygdala (Table 1, S3 Fig) and moderate expression in the primary motor cortex (Figs 1B and 1C, Table 1) and somatosensory cortex (Table 1, S3 Fig). However, some notable differences in the expression patterns among these lines were also observed. For example, Thy1.2-GCaMP6F Line 2 presented the highest expression of GCaMP6 in thalamus and midbrain, as compared to other lines (Table 1). Thy1.2-GCaMP6F Line 1 and Thy1.2-GCaMP6S Line 1 have sparse expression in layer V of the primary motor cortex (M1) (50% and 26% of neurons, respectively), with no expression in layer II/III (Figs 1B and 1C). Whereas Thy1.2-GCaMP6S Line 2 and Line 3, and Thy1.2-GCaMP6F Line 2 have GCaMP6 expression in M1 layers II/III and V, as well as layer VI, they differed in the density of GCaMP6-positive neurons. Thus, Thy1.2-GCaMP6S Line 2 had the densest expression in layer V (51% of neurons) and layer II/III (47% neurons), as compared to Thy1.2-GCaMP6S Line 3 and Thy1.2-GCaMP6F Line 2 (41% and 42% of neurons in layer V, and 17% and 20% of neurons in layer II/III, respectively) (Figs 1B and 1C). Similar patterns of expression and densities were observed in the primary somatosensory cortex and visual cortex (Table 2).
Table 1. GCaMP6 expression patterns in the brain of Thy1.2-GCaMP6 mice.
GCaMP6 fast and slow lines are indicated on the left column. GCaMP6 expression was examined on postnatal day 30. M1, primary motor cortex; S1, primary somatosensory cortex; V1, primary visual cortex. Scale: − no signal, + weak signal, ++ moderate signal, +++ strong signal.
| Thy1.2 mouse line | Olfactory bulb | M1 | S1 | V1 | Piriform area | Hippocampus | Thalamus | Hypothalamus | Amygdala | Cerebellum | Midbrain | Pons | Medulla |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GCaMP6F Line 1 | + | ++ | ++ | + | + | ++ | − | − | +++ | − | + | + | + |
| GCaMP6F Line 2 | + | ++ | + + | + | + | + | +++ | + | +++ | ++ | +++ | ++ | ++ |
| GCaMP6S Line 1 | + | ++ | ++ | + | + | ++ | + | − | +++ | + | ++ | ++ | ++ |
| GCaMP6S Line 2 | + | ++ | ++ | + | + | ++ | + | + | +++ | ++ | ++ | ++ | + |
| GCaMP6S Line 3 | ++ | ++ | ++ | + | ++ | ++ | + | + | +++ | − | + | ++ | + |
(−) no signal
(+) weak signal
(++) moderate signal
(+++) strong signal
Table 2. GCaMP6 expression across layers in primary cortices.
Table summarizing GCaMP6 expression in the primary motor cortex (M1), somatosensory cortex (S1), and primary visual cortex (V1) of different transgenic mouse lines at postnatal day 30.
| Thy1.2 mouse line | Neocortex | Hippocampus | Basal Ganglia | Thalamus | Hypothalamus | Midbrain | Hindbrain |
|---|---|---|---|---|---|---|---|
| GCaMP6F Line 1 | ++ | ++ | + | − | − | ++ | ++ |
| GCaMP6F Line 2 | ++ | ++ | + | ++ | + | ++ | + |
| GCaMP6S Line 1 | ++ | ++ | + | ++ | + | ++ | ++ |
| GCaMP6S Line 2 | ++ | ++ | + | ++ | + | ++ | ++ |
| GCaMP6S Line 3 | ++ | ++ | + | ++ | + | + | + |
(−) no signal
(+) weak signal
(++) moderate signal
(+++) strong signal
In addition to P30 mice, we also examined the expression of GCaMP6 during early postnatal development, at P14 in all five transgenic lines. We observed widespread GCaMP6 expression in multiple brain regions, including the neocortex, hippocampus, basal ganglia, thalamus, hypothalamus, midbrain and hindbrain (Table 3 and S4 Fig). In general, expression patterns at P14 were similar to those observed at P30 for the same line, although the expression of GCaMP6 in most regions was more widespread at P30.
Table 3. GCaMP6 expression in different brain areas of postnatal day 14 transgenic mice.
GCaMP6 fast and slow lines are indicated on the left column. Scale: − no signal, + weak signal, ++ moderate signal.
| M1 Cortical layer | GCaMP6F Line 1 | GCaMP6F Line 2 | GCaMP6S Line 1 | GCaMP6S Line 2 | GCaMP6S Line 3 |
| L1 | |||||
| L2/3 | + | ++ | ++ | ||
| L4 | |||||
| L5 | +++ | ++ | +++ | +++ | ++ |
| L6 | + | + | ++ | + | ++ |
| S1 Cortical layer | GCaMP6F Line 1 | GCaMP6F Line 2 | GCaMP6S Line 1 | GCaMP6S Line 2 | GCaMP6S Line 3 |
| L1 | |||||
| L2/3 | + | + | ++ | ++ | |
| L4 | + | ||||
| L5 | ++ | +++ | ++ | +++ | ++ |
| L6 | + | +++ | ++ | + | ++ |
| V1 Cortical layer | GCaMP6F Line 1 | GCaMP6F Line 2 | GCaMP6S Line 1 | GCaMP6S Line 2 | GCaMP6S Line 3 |
| L1 | |||||
| L2/3 | + | + | + | + | |
| L4 | + | ||||
| L5 | + | + | + | +++ | ++ |
| L6 | + | +++ | ++ | ++ |
(−) no signal
(+) weak signal
(++) moderate signal
(+++) strong signal
Taken together, these results show that the expression level of GCaMP6 in these transgenic lines is moderate, without apparent neuronal toxicity, and therefore potentially suitable for Ca2+ imaging. Moreover, neuronal populations in multiple cortical and subcortical regions express GCaMP6 in both P14 and P30 transgenic mice.
3.2. GCaMP6 expression in spinal cord and peripheral axons of Thy1.2-GCaMP6 mouse lines
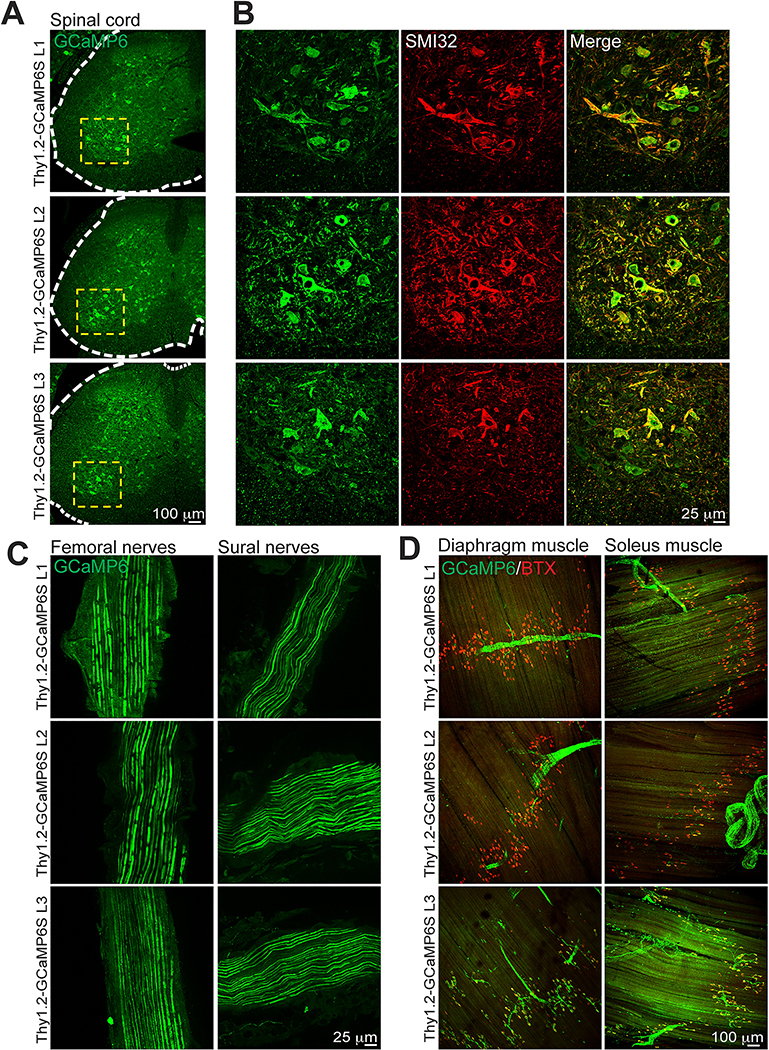
In addition to the expression patterns in the brain, we also evaluated GCaMP6 expression in spinal cord and peripheral nerves of all five transgenic lines. We chose to further evaluate GCaMP6S lines due to the higher expression level of GCaMP6 (Fig 2). GCaMP6 expression was detected in ventral and dorsal horns of the spinal cords from adult GCaMP6S mice (Figs 2A and 2B). Consistent with its expression in motor neurons, we found GCaMP6 expression in femoral nerves, which contain motor axons (Fig 2C), as well as at neuromuscular junctions of diaphragm and soleus muscles in all three GCaMP6S lines (Fig 2D). GCaMP6 expression was also detected in sural nerves containing sensory axons, where most fibers were positive across all three Thy1.2-GCaMP6S lines (Fig 2C, right panels).
Fig 2. Expression of GCaMP6 in the spinal cord and peripheral nerves of GCaMP6S lines.
A. Coronal spinal cord sections from Thy1.2-GCaMP6S Lines 1, 2, and 3. Note the sparse labeling of motor neurons in the ventral horns. B. Magnified inset region (yellow dashed boxes) from (A) showing GCaMP6 expressing neurons (green) co-labeled with the motor neuron neurofilament marker SMI32 (red). Merged images shown on the right. C. Representative confocal images of motor (femoral nerve) and sensory (sural nerve) axons from Thy1.2-GCaMP6S mouse lines. Note the comparable levels of GCaMP expression among the different lines. D. Representative confocal images of motor axon terminals labeled with GCaMP6 in two different muscles, diaphragm and soleus. The muscle side of the neuromuscular junction is labeled with bungarotoxin (BTX, red). Note the strong expression of Thy1.2-GCaMP6S Line 3 at the motor endplates. Scale bars, 100 μm (A and D), 25 μm (B and C).
3.3. GCaMP6 expression and imaging of neurons in dorsal root ganglia (DRG)
We examined DRG from spinal cord lumbar disc 4 region of four Thy1.2-GCaMP6 lines and observed more GCaMP6-labelled sensory neurons in Thy1.2-GCaMP6S Line 3 as compared to the other three lines (S Line 3: 1763 ± 39 cells; S Line 1: 673 ± 28 cells; S Line 2: 351 ± 33 cells; F Line 2: 367 ± 26 cells) (Fig 3A). To assess whether Ca2+ transients could be detected in sensory neurons in vivo, we performed two-photon Ca2+ imaging in the DRG of awake GCaMP6S Line 3 mice (Fig 3B). We observed low levels of spontaneous Ca2+ transients in sensory neurons during quiet wakefulness. Notably, a marked increase of neuronal activity was observed after punctate mechanical stimulation was applied to the animal’s ipsilateral hind paw using von Frey filaments (Figs 3B and 3C). Further analysis of imaged cells revealed that 28.2 ± 2.8% of DRG neurons (n = 343 neurons from 7 mice) responded to mechanical stimulation in awake mice (Fig 3D). Hind limb stimulation induced a substantial increase in Ca2+ transients in responsive neurons (P < 0.0001, paired t-test, t = 9.343, df = 97) (Fig 3E). These results demonstrate that the level of GCaMP6 expression in Thy1.2-GCaMP6 mice is suitable for detecting neuronal activity in the peripheral nervous system in vivo.
Fig 3. GCaMP6 expression and in vivo recordings in dorsal root ganglia (DRG) from Thy1.2-GCaMP6S mice.
A. Representative confocal images of primary sensory neurons from lumbar 4 DRGs from Thy1.2-GCaMP6S Lines 1, 2, 3 and Thy1.2-GCaMP6F Line 2. B. Representative frames obtained during in vivo two-photon imaging of DRG neurons in GCaMP6S Line 3 mouse, before (left panel) and during (right panel) hind limb stimulation using von Frey filaments (6 g of force). Yellow arrowheads point to six cells analyzed in (C). C. Representative fluorescence Ca2+ traces from six sensory neurons over time. Gray bar illustrates the point of hind limb stimulation. D. Percentages of responders (R) and non-responders (NR) of all sensory neurons observed upon von Frey stimulation (n = 343 neurons, seven mice). E. Average calcium signal over a period of 10 seconds before and after von Frey stimulation (n = 343 neurons, seven mice). ***P < 0.001. Scale bars, 100 μm (A), 20 μm (B).
3.4. In vivo imaging of somatic activity in the mouse motor cortex under anesthetized and awake behaving states
To further evaluate the suitability of these new GCaMP6 transgenic lines in detecting cortical neurons activity in vivo, we performed two-photon imaging in the motor cortex of P30–40 mice (Fig 4 and 5) [9]. GCaMP6 expression was perimembrane, devoid of the nuclei in layer V somas in Thy1.2-GCaMP6 (S1 Fig). We detected spontaneous Ca2+ transients in the somata of pyramidal neurons in layer V of the motor cortex (500–700 μm below the pial surface) under both ketamine-xylazine anesthesia and wakefulness (S5 Fig). The average Ca2+ activity over 2.5 min period of time in layer V somata under anesthesia was 15.4 ± 1.4 ΔF for Line 1 (n = 42 from 2 mice) and 28.1 ± 1.0 ΔF for Line 3 (n = 43 cells from 2 mice), while in the awake state it was 36.2 ± 1.8 ΔF for Line 1 and 42.2 ± 2.4 ΔF for Line 3. When comparing neuronal activity of layer V neurons between these two brain states for both GCaMP6 Line 1 and 3, we found significantly higher Ca2+ activity in awake mice than in anesthetized mice (P < 0.001, paired t-test). Similar Ca2+ responses were recorded in layer II/III and layer V neurons of the primary somatosensory cortex under wakefulness (S6 Fig) [17].
Fig 4. In vivo two-photon Ca2+ imaging in GCaMP6-expressing neocortical neurons.
A-D. Fluorescent traces of somatic Ca2+ transients in layer V (A–C) and layer II/III (D) neurons from the primary motor cortex of different lines of Thy1.2-GCaMP6 mice during quiet wakefulness and forward running (light green shaded area) on a linear treadmill. Scale for all traces is displayed in (D). Right panels of A-D show the transient frequency, Ca2+ transient amplitude, and average Ca2+ activity over 2.5 min recordings of somas between quiet wakefulness and forward running states. Grey circles (quiet wakefulness) and green circles (forward running) represent single cells for each transgenic line. Black bars depict the means of the population ± SEM. ***P < 0.001, paired t test. Representative traces and measures are from at least 3 animals per group.
Fig 5. In vivo Ca2+ imaging of apical dendrites in GCaMP6 mice.
A. Representative two-photon images of a dendrite located in layer I of primary motor cortex before and after a dendritic Ca2+ transient occurs. Multiple ROIs are indicated (yellow boxes). B. The ΔF/F0 of each individual ROI is shown during quiet wakefulness and forward running (light green shaded area). Graphs displayed to right of traces show the transient frequency, Ca2+ transient amplitude, and average Ca2+ activity between quiet wakefulness (grey circles) and forward running (green circles) in individual layer I dendrites of Thy1-GCaMP6S Line 1. C-F. Representative fluorescent images and traces of apical dendritic trunks (located 200–300 μm below the pial surface) from layer V neurons during wakefulness and forward running (light green shaded areas) states of Thy1.2-GCaMP6S Line 1 mice (C-D) and Thy1.2-GCaMP6F Line 1 (E-F). Yellow arrow heads denote activated trunks. Graphs displayed to right of traces show the transient frequency, Ca2+ transient amplitude, and average Ca2+ activity between wakefulness and forward running states in individual apical dendritic trunks of Thy1-GCaMP6 mice. Grey circles (quiet wakefulness) and green circles (forward running) represent single cells for each transgenic line. Black bars depict the means of the population ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, paired t test. Scale bar, 5 μm. Representative traces and measures are from at least 3 animals per group.
We also imaged the neuronal activity in awake behaving mice under quiet wakefulness and forward running on a free-floating linear treadmill using Thy1.2-GCaMP6F Line 1 and Thy1.2-GCaMP6S Line 1 and 3. In all transgenic lines tested, treadmill running induced significant increases in Ca2+ transient amplitude and average Ca2+ activity of layer V neurons, as compared to quiet wakefulness (Figs 4A–C; Thy1.2-GCaMP6F Line 1 (n = 23 from 3 mice) wakefulness: freq. 3.7 ± 0.8 transients/2.5 min, amplitude 78 ± 5.8 ΔF/F0, average Ca2+ activity 10 ± 0.7 ΔF; forward running: freq. 6.7 ± 0.8 transients/2.5 min, amplitude 137 ± 14 ΔF/F0, average Ca2+ activity 24 ± 1.9 ΔF; Thy1.2-GCaMP6S Line 1 (n = 21 from 3 mice) wakefulness: freq. 5.8 ± 0.8 transients/2.5 min, amplitude 110 ± 9.2 ΔF/F0, average Ca2+ activity 26 ± 1.6 ΔF; forward running: freq. 6.8 ± 0.6 transients/2.5 min, amplitude 161 ± 15 ΔF/F0, average Ca2+ activity 36 ± 2.4 ΔF; Thy1.2-GCaMP6S Line 3 (n = 30 from 3 mice) wakefulness: freq. 9.6 ± 0.5 transients/2.5 min, amplitude 95 ± 3.2 ΔF/F0, average Ca2+ activity 39 ± 2.2 ΔF; forward running: freq. 10 ± 0.6 transients/2.5 min, amplitude 152 ± 6.6 ΔF/F0, average Ca2+ activity 75 ± 5.1 ΔF). We were only able to detect an increased number of Ca2+ transients during forward running compared to wakefulness with Thy1.2-GCaMP6F Line 1 mice presumably due to the rapid decay of GCaMP signal to baseline. In addition, layer II/III neurons also presented a significant increase of Ca2+ transients in mice running on the treadmill (Fig 4D; Thy1.2-GCaMP6S Line 3 (n = 49 from 3 mice) wakefulness: freq. 9 ± 0.5 transients/2.5 min, amplitude 107 ± 5.9 ΔF/F0, average Ca2+ activity 32 ± 2.4 ΔF; forward running: freq. 8 ± 0.5 transients/2.5 min, amplitude 244 ± 17 ΔF/F0, average Ca2+ activity 96 ± 10 ΔF). These results indicate that Thy1.2-GCaMP6 transgenic mouse lines are useful for monitoring neuronal activity in both layers II/III and V neurons in the primary motor cortex and primary somatosensory cortex of living mice.
3.5. In vivo Ca2+ imaging of apical dendrites of layer V pyramidal neurons in the motor cortex
To evaluate whether these transgenic mice could also be used to detect dendritic Ca2+ transients of layer V pyramidal neurons, we performed transcranial two-photon imaging both in the superficial cortical layer (Fig 5A, layer I) and in deeper compartments of the apical dendritic tree (Fig 5C–F). The density of labeled dendrites in layer I of the motor cortex was comparable between Thy1.2-GCaMP6S Line 1 and Thy1.2-GCaMP6F Line 1 mice. Similar to layer V neuronal soma Ca2+ measurements, we recorded Ca2+ transients in the apical tuft dendrites both during quiet wakefulness and during bouts of treadmill forward running. Under quiet wakefulness, large elevations of Ca2+ were readily detected not only in dendritic shafts but also in dendritic spines (Fig 5A–B and S7 Fig). These dendritic Ca2+ signals occurred more frequently during forward running as compared to quiet wakefulness (light green shaded area in Fig 5B and S7 Fig).
We also examined Ca2+ transients in deeper compartments of the apical dendritic tree (trunk) of layer V pyramidal neurons. As we demonstrated for the thin branches in layer I, apical trunks (located 200–300 μm below the pial surface) were active during both quiet wakefulness and treadmill forward running (Fig 5C–F). A significant elevation of Ca2+ fluorescence was observed in apical trunks during forward running as compared to quiet wakefulness (Thy1.2-GCaMP6S Line 1 (n = 26 from 3 mice) wakefulness: freq. 1.8 ± 0.6 transients/2.5 min, amplitude 133 ± 17 ΔF/F0, average Ca2+ activity 29 ± 2.5 ΔF; forward running: freq. 3.2 ± 0.7 transients/2.5 min, amplitude 255 ± 26 ΔF/F0, average Ca2+ activity 58 ± 4.5ΔF; Thy1.2-GCaMP6F Line 1 (n = 30 from 3 mice) wakefulness: freq. 1.6 ± 0.2 transients/2.5 min, amplitude 98 ± 7.1 ΔF/F0, average Ca2+ activity 9.9 ± 0.4 ΔF; forward running: freq. 1.8 ± 0.3 transients/2.5 min, amplitude 155 ± 17 ΔF/F0, average Ca2+ activity 16 ± 1.4 ΔF). These results demonstrate that Thy1.2-GCaMP6S Line 1 and Thy1.2-GCaMP6F Line 1 are suitable for detecting dendritic Ca2+ transients in awake behaving animals.
4. Discussion
In this study, we generated and characterized five transgenic mouse lines that stably express Ca2+ sensor GCaMP6, with fast and slow kinetics, under the control of the Thy1.2 promoter. GCaMP6 expression was observed in multiple cortical and subcortical regions, spinal cord, and DRGs in each transgenic line from P14 to P40 (Tables 1–3, Figs 1–3). The patterns and levels of expression for all the GCaMP6 lines used were consistently preserved over several generations of mice (> 25 generations). Robust Ca2+ transients could be detected in primary motor and somatosensory cortices in somas of layers II/III and V neurons in living, head-restrained mice, under both anesthetized and awake behaving states (Fig 4, S5 Fig). Ca2+ transients were also readily detected in thin tuft dendrites and dendritic spines of layer V neurons in the motor cortex of awake behaving GCaMP6 mice (Fig 5, S7 Fig). Finally, in vivo imaging of Ca2+ transients in DRG allowed the analysis of sensory neuron responses to hind limb stimulation (Fig 3). Our findings indicate that these GCaMP6 lines provide excellent new tools for detecting patterns of neural activity at the level of individual neurons and dendrites in vivo in the central as well as the peripheral nervous systems.
GECIs, including GCaMP6, are becoming an important tool for in vivo detection of brain activities [4]. While AAV-mediated expression of GCaMP is widely used for Ca2+ imaging, the use of transgenic mice poses several advantages. In the case of our Thy1.2-GCaMP6S and Thy1.2-GCaMP6F lines, GCaMP6 is expressed homogeneously in the cytosol, with no signs of aggregation or nuclear expression (S1 Fig). Furthermore, the expression level of GCaMP6 in these lines is moderate and suitable for Ca2+ imaging without obvious neuronal toxicity. Comparing them with previously reported Thy1.2-GCaMP3 and Thy1.2-GCaMP6 mice [5, 13], our GCaMP6 transgenic lines provide a major advantage for subcellular in vivo imaging. First, two of our lines sparsely express GCaMP6 in layer V, with no expression in other layers, and thus allow the detection of dendritic Ca2+ transients specifically in layer V pyramidal neurons (Figs 1 and 5). Previously reported GCaMP mice exhibited dense expression of GCaMP6 in neurons across several cortical layers, making the imaging of dendritic activity from a defined cell population challenging.
Transgenic GCaMP6 mice could also serve as a useful tool in the detection of Ca2+ transients in the developing brain. We demonstrate that many of the GCaMP6 transgenic lines show strong expression of GCaMP6 in the neocortex, hippocampus, thalamus, and midbrain at postnatal day 14 (Table 2). Based on these expression patterns, we suspect that GCaMP signals could be detected in mice younger than P14 and therefore facilitate in vivo Ca2+ imaging in the developing brain.
In addition to two-photon Ca2+ imaging in the neocortex at the single cell level, we demonstrate GCaMP6 expression in the spinal cord, DRGs, and peripheral nerves (Figs 2 and 3). In the DRG, we identified two populations of sensory neurons: cells that were responsive to hindlimb stimulation and those that were not (Fig 3). Hind limb stimulation induced a significant increase in Ca2+ transients in responsive neurons. Although we did not further explore the characteristics of the unresponsive cells, they possibly belong to different sensory neuron subtypes. Future in vivo imaging of DRG, ventral horn of the spinal cord, and peripheral nerve activities will be useful for the study of diseases with a sensory neuropathy component or motor neuron diseases.
5. Conclusions
In conclusion, the transgenic mouse lines described in this study exhibit stable and layer-specific expression of GCaMP6 in multiple brain regions and allow robust detection of Ca2+ activities at the level of somata, dendrites and synapses in the living mouse brain. Therefore, these GCaMP6 mouse lines provide an important means for probing neuronal structure and function in vivo. Furthermore, by crossing GCaMP6 transgenic mice with mouse models of neurodegeneration, these mouse lines could greatly facilitate the studies of neuronal dysfunction during disease progression and help the development of new treatment strategies.
Supplementary Material
Highlights.
Development of transgenic mouse lines expressing GCaMP6F and GCaMP6S in subsets of neurons in the mouse nervous system under the control of the Thy1.2 promoter.
Characterization of GCaMP6 expression patterns in the central and peripheral nervous system.
Demonstration of the utility of two transgenic lines for calcium imaging in the central and peripheral nervous system.
Acknowledgements
This work was supported by National Institutes of Health grants R01 NS047325 and R01 NS087198 (to WB.G), R35 GM131765 (to G.Y.), the Friedreich’s Ataxia Research Alliance (FARA) grant (to J.M.), and Penn Anesthesia Dripps Research Scholarship (to J.C.).
Abbreviations
- Ca2+
calcium
- GECI
Genetically Encoded Calcium Indicator
- GCaMP
GFP-calmodulin protein
- GCaMP6F
GCaMP6 fast
- GCaMP6S
GCaMP6 slow
- DRG
dorsal root ganglia
Footnotes
Declarations of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature methods. 2009;6(12):875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, et al. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci. 2012;32(9):3131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shigetomi E, Bushong EA, Haustein MD, Tong X, Jackson-Weaver O, Kracun S, et al. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol. 2013;141(5):633–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, Cichon J, Wang W, Qiu L, Lee S-JR, Campbell NR, et al. Imaging Neural Activity Using Thy1-GCaMP Transgenic mice. Neuron. 2012;76(2):297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32(40):13819–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace DJ, Meyer zum Alten Borgloh S, Astori S, Yang Y, Bausen M, Kugler S, et al. Single-spike detection in vitro and in vivo with a genetic Ca2+ sensor. Nature methods. 2008;5(9):797–804. [DOI] [PubMed] [Google Scholar]
- 8.Lutcke H, Murayama M, Hahn T, Margolis DJ, Astori S, Zum Alten Borgloh SM, et al. Optical recording of neuronal activity with a genetically-encoded calcium indicator in anesthetized and freely moving mice. Frontiers in neural circuits. 2010;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cichon J, Gan W-B. Branch-specific dendritic Ca2+ spikes cause persistent synaptic plasticity. Nature. 2015;520(7546):180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huber D, Gutnisky DA, Peron S, O’Connor DH, Wiegert JS, Tian L, et al. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484(7395):473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Guo HF, Pologruto TA, Hannan F, Hakker I, Svoboda K, et al. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J Neurosci. 2004;24(29):6507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85(5):942–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dana H, Chen TW, Hu A, Shields BC, Guo C, Looger LL, et al. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS One. 2014;9(9):e108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41–51. [DOI] [PubMed] [Google Scholar]
- 15.Vulchanova L, Schuster DJ, Belur LR, Riedl MS, Podetz-Pedersen KM, Kitto KF, et al. Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Molecular pain. 2010;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C, et al. Coupled Activation of Primary Sensory Neurons Contributes to Chronic Pain. Neuron. 2016;91(5):1085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cichon J, Blanck TJJ, Gan WB, Yang G. Activation of cortical somatostatin interneurons prevents the development of neuropathic pain. Nat Neurosci. 2017;20(8):1122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caroni P Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods. 1997. Jan;71(1):3–9. [DOI] [PubMed] [Google Scholar]
- 19.Magrane J, Cortez C, Gan WB, Manfredi G. Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Human molecular genetics. 2014;23(6):1413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang G, Lai CSW, Cichon J, Ma L, Li W, Gan W-B. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344(6188):1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010;5(2):201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc. 2009;4(8):1128–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Zhang J, Sun L, Zhang Y, Gan WB, Tang P, Yang G (2019) Long-term imaging of dorsal root ganglia in awake behaving mice. Nat Commun 10:3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.