Abstract
We examined activities of dense granule proteins (GRAs), which Toxoplasma gondii secretes within infected cells, to stimulate microglial IFN-γ production in vitro. We identified that the N-terminal region (amino acids 41–152) of GRA6 (GRA6Nt) stimulates IFN-γ production by both a microglia cell line and primary microglia purified from the brains of uninfected adult mice. In contrast, neither of GRA1, GRA2, GRA5Nt, nor the carboxyl-terminal (amino acids 174–224) of GRA6 stimulated microglial IFN-γ production. GRA6Nt appears to be a target molecule of the sentinel function of microglia to detect cerebral proliferation of T. gondii and activate their IFN-γ production for facilitating the protective immunity to control the pathogen.
Keywords: Toxoplasma, IFN-gamma, protective immunity, innate immunity, microglia, dense granule protein 6
1. Introduction
Toxoplasma gondii is an obligate intracellular parasite that can infect all mammals including humans. Tachyzoites proliferate in various organs during an acute acquired infection, which can cause serious diseases such as retinochoroiditis and congenital infection of the fetuses [1]. The immune responses mediated by IFN-γ restrict the tachyzoite growth, but the parasite forms tissue cysts and establishes a chronic infection, especially in the brain and skeletal muscle. Chronic T. gondii infection is one of the most common parasitic infections in humans worldwide [1]. This chronic infection can reactivate and cause serious encephalitis in immunocompromised individuals such as those with AIDS [1], indicating the requirement of the appropriate protective immunity to maintain the latency of the chronic infection.
We [2] and others [3] previously demonstrated that IFN-γ is required for maintaining the latency of chronic T. gondii infection. Both CD4+ and CD8+ T cells produce IFN-γ to prevent a reactivation of cerebral T. gondii infection [3–5]. In addition to T cells, the cells other than T or NK cells need to produce this cytokine for preventing the reactivation of infection [6]. Microglia and macrophages were identified to be major innate immune cells other than NK cells that produce IFN-γ during the reactivation of T. gondii infection [7, 8]. Our recent studies revealed that IFN-γ production by brain-resident cells (irradiation-resistant cell populations in the brain) including microglia is critical for facilitating both innate and T cell-mediated protective immunity to prevent reactivation of the infection [9]. In addition, an in vitro study demonstrated that a microglia cell line produces IFN-γ when stimulated with total lysate antigens of T. gondii tachyzoites [9]. These evidences together suggest that a detection of T. gondii molecules by microglia and an activation of their IFN-γ production is a key sentinel function in the brain to promptly activate the protective immunity to prevent reactivation of cerebral T. gondii infection.
Secretion of dense granule proteins (GRAs) of T. gondii within infected cells plays important roles in survival of tachyzoites within infected cells. It is possible that upon an occurrence of T. gondii proliferation in the brain, uninfected microglia detect GRAs released from infected cells to activate their IFN-γ production. In the present study, we examined whether recombinant proteins of either GRA1, 2, 5, or 6 activates microglial IFN-γ production using a microglia cell line (EOC20) and primary microglia purified from the brains of uninfected adult mice. We identified that the N-terminus region (amino acids 41–152) of GRA6 (GRA6Nt) activates IFN-γ production of both EOC20 cell line and the primary microglia. Thus, GRA6Nt appears to be an important target of the sentinel function of microglia to detect an occurrence of cerebral T. gondii tachyzoite growth and to activate their IFN-γ production to promptly activate the protective immune responses to prevent the tachyzoite growth in the brain.
2. Materials and methods
2.1. Mice
Female BALB/c mice of 11 weeks old (Jackson Laboratories, Bar Harbor, ME) were used for the study. All mice were maintained in micro-isolator cages with sterile bedding, chaw, and water. All animal experiments were conducted with the approval of the University of Kentucky Institutional Animal Care and Use Committee in accordance with the “Guide for the Care and Use of Laboratory Animals,” published by the National Research Council and endorsed by the NIH Office of Laboratory Animal Welfare.
2.2. Culture of EOC20 microglial cell line and stimulation with T. gondii GRA proteins
EOC20 mouse microglial cells (ATCC, Manassas, VA) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; ATCC) supplemented with 10% FBS (Gibco, Carlsbad, CA) and 20% LADMAC-conditioned medium as a source of colony stimulation factor-1. The LADMAC-conditioned medium was collected on the fifth day of culture of 2 × 105 LADMAC cells (ATCC)/ml in 10% FBS-Eagle’s Minimum Essential Medium (ATCC). For examining IFN-γ production by EOC20 microglia in response to recombinant GRA proteins (rGRAs), the cells (1 × 105 cells/well in 200 μl) in RPMI1640 medium (Gibco/Millipore Sigma, St. Louis, MO) containing 10% FBS (Hyclone/GE-Healthcare Life Science, Pittsburgh, PA) were placed in wells of a flat-bottom 96-well tissue culture plate (Costar, Corning, Lowell, MA). Two μl of rGRA1, rGRA2, rGRA5Nt, rGRA6Nt, or rGRA6Ct (100 μg/ml) in PBS were added to the cultures of the microglia cell line at 1 μg/ml of the final concentration and cultured for 72 hrs. As a control, the same volume of PBS was added to the cultures. Three to six wells (mostly 3 or 4 wells) were used for each experimental group. Production of the rGRA proteins was described elsewhere [10–13]. The rGRA1 and rGRA2 contains the amino acids 21–190 of GRA1 and among acids 21 to 185 of GRA2, respectively. The rGRA5Nt contains the amino acids 29–68. The rGRA6Nt and rGRA6Ct contain the amino acids 41–152 and 174–224 of GRA6, respectively. All of these rGRAs are excluded from their signal peptide. The rGRA2, rGRA6Nt, and rGRA6Ct were pUET fusion proteins. The rGRA1 and rGRA5Nt were GST fusion proteins. T. gondii has three predominant genotypes, types I, II and III [14], and the rGRA1 and rGRA5Nt were from the RH strain (type I). The rGRA2, rGRA6Nt, and rGRA6Ct were from the 76K strain (type II).
2.3. Purification of primary microglia from adult mouse brains and their stimulation with T. gondii rGRA proteins in vitro
Primary microglia were purified from the brains of normal adult BALB/c mice using a gentleMACS Octo Dissociator and MACS purification system (Miltenyi) using anti-CD11b antibody-coated magnetic beads by following manufacture’s manuals. Purity of CD11b+ cells in the purified microglia population was 90% by FACS analysis using FITC-labeled anti-CD11b antibody (clone M1/70) (BD Biosciences, San Jose, CA). The majority of the purified cells adhered to the plastic following an overnight incubation, and displayed morphological characteristics of microglia. The purified primary microglia (5 × 104 cells/well) were cultured in the 96-well tissue culture plate in 10% FBS-RPMI1640 culture medium in the presence of either rGRA6Nt (type I) or rGRA2 (type II) at 1 μg/ml of the final concentration for 72 hrs. In this experiment, in contrast to the experiment with EOC20 microglia cell line, rGRA6Nt of type I T. gondii was used due to an unavailability of a sufficient amount of this recombinant protein of the type II parasite. As a control, PBS was added to the culture. Four wells were used for each experimental group.
2.4. Quantification of IFN-γ and IL-12 levels in microglial culture supernatants
Amounts of IFN-γ in the culture supernatants of EOC20 cells were determined by an ELISA kit from R&D Systems (Minneapolis, MN) [9]. Amount of IFN-γ and IL-12 in the culture supernatants of primary microglia were measured by very sensitive Meso Scale Discovery electrochemiluminescence assay kits (Meso Scale Diagnostics, Rockville, MD) by following manufacture’s manuals. These kits provide the sensitivities to detect as low as 0.16 pg/ml of IFN-γ and 1.1 pg/ml of IL-12.
2.5. Statistical analyses
Differences between experimental groups were determined by One-way ANOVA with Dunnett’s multiple comparison post test vs. control group or Newman-Keuls multiple comparison post test.
3. Results and discussion
T. gondii tachyzoites infect and proliferate frequently within astrocytes and neurons in the brain [15]. Therefore, it is possible that microglia detect T. gondii molecules released from these infected cells to sense an occurrence of a growth of the parasite in the brain as the first line defense system and initiate their IFN-γ production. GRAs are a major group of proteins that T. gondii secretes within infected host cells [16]. To address a possibility that microglia activate their IFN-γ by detecting T. gondii GRAs, we first examined whether rGRAs stimulate this cytokine production in EOC20 murine microglia cell line. EOC20 microglia (1 × 105 cells) were placed in wells of a flat-bottom 96-well plate and cultured in the presence of rGRA1, rGRA2, GRA5Nt, GRA6Ct, or rGRA6Nt (1 μg/ml) for 72 hrs. As a control, PBS was added to the culture. Amounts of IFN-γ in the culture supernatants of EOC20 microglia stimulated with rGRA6Nt were significantly greater than those in the control culture (P<0.05, Fig. 1). In contrast, IFN-γ levels in the culture supernatants of the cells stimulated with either rGRA1, rGRA2, GRA5Nt, or GRA6Ct did not differ from those of the control culture with PBS (Fig. 1). All of the rGRA proteins used were produced in Escherichia coli [10]. Although lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria including E. coli, has previously been shown to stimulate microglial IFN-γ production [17, 18], EOC20 microglia cell line was originally established from the brain of C3H/HeJ mice, which lack Toll-like receptor 4, the receptor for LPS to activate innate immune cells. Therefore, the production of IFN-γ by EOC20 microglia cell line is not due to a possible contamination of rGRA6Nt with LPS. These results strongly suggest that the capability to activate microglial production of IFN-γ is not a common function of T. gondii GRA proteins, and that GRA6Nt is a unique target molecule among the GRAs for the sentinel function of microglia to activate their IFN-γ production.
Figure 1. Production of IFN-γ by EOC20 microglia cell line in response to rGRA6Nt of T. gondii.
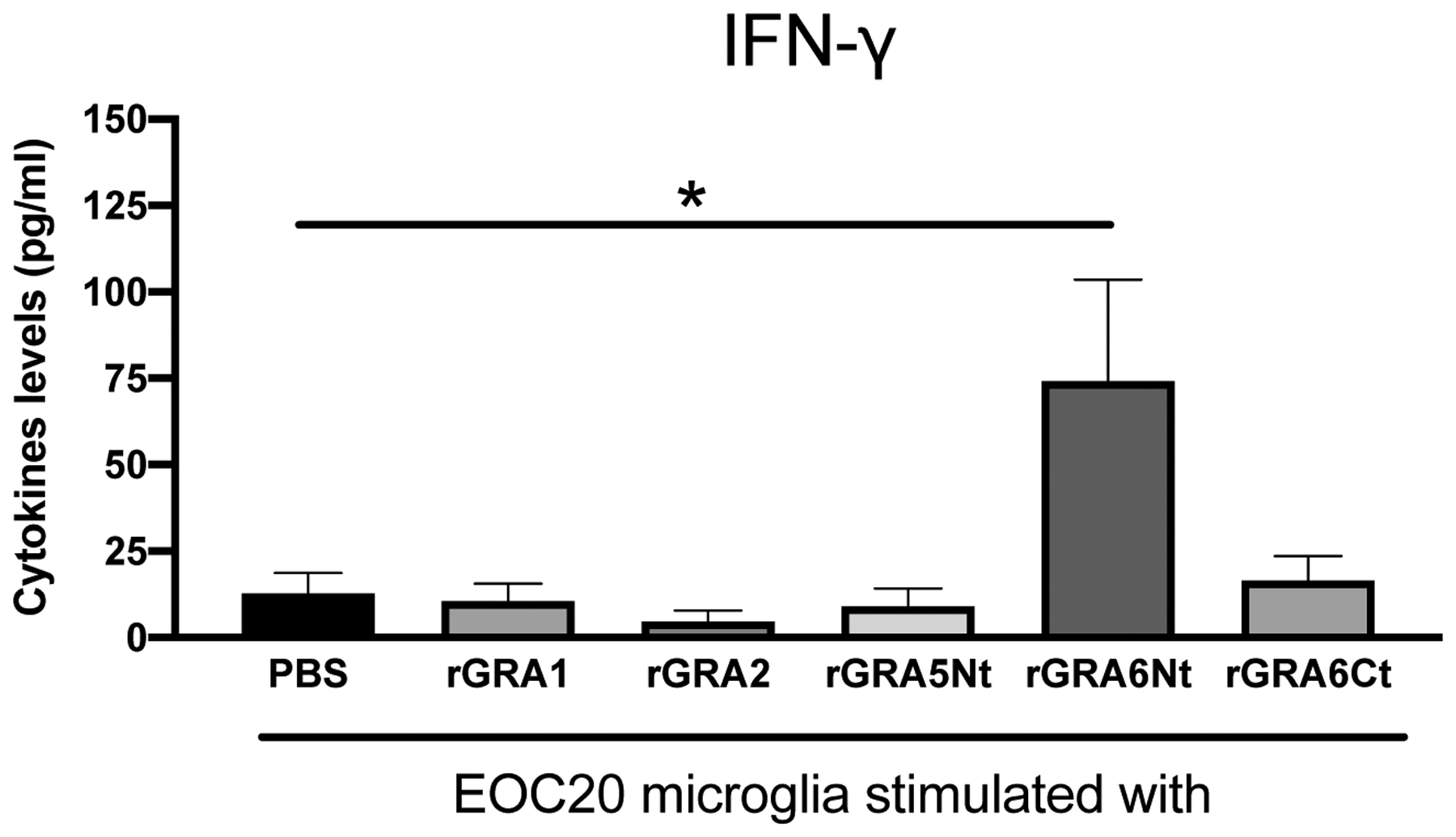
EOC20 cells were cultured in a 96 well culture plate (1 × 105 cells/well) in the presence of rGRA1, rGRA2, rGRA5Nt, rGRA6Ct, or rGRA6Nt (1 μg/ml at the final concentration) for 72 hrs. As a control, PBS was added to the culture. Amounts of IFN-γ in the culture supernatants were measured by ELISA. The rGRA2, rGRA6Nt, and rGRA6Ct were from the type II strain of T. gondii. The rGRA1 and rGRA5Nt were from the type I strain of the parasite. Three to six wells were used for each experimental group. The results from two independent experiments are combined. *P<0.05, **P<0.01.
To further confirm the activity of rGRA6Nt to stimulate microglial IFN-γ production, primary microglia were purified from the brains of normal adult BALB/c mice and cultured with rGRA6Nt or rGRA2 for 72 hrs. As a control, PBS was added to the primary microglia culture. Consistent with the results from EOC20 microglia cell line, IFN-γ levels of the culture supernatants of the primary microglia stimulated with rGRA6Nt were significantly greater than those of the control cultures with PBS (P<0.05, Fig. 2), and IFN-γ levels in the culture with rGRA2 did not differ from those of the control culture. In addition, the IFN-γ levels in the culture with rGRA6Nt were also significantly greater than those of the culture with rGRA2 (P<0.05, Fig. 2). In addition, in a separate experiment, the primary microglia from the brains of normal adult C57BL/6 mice were stimulated with rGRA6Nt or rGRA2. Consistent with BALB/c microglia, only rGRA6Nt stimulated IFN-γ production by the microglia from C57BL/6 mice at the levels around 1 pg/ml, which were similar to those observed with BALB/c microglia shown in Fig. 2 (data not shown).These results along with the results from EOC20 microglia cell line indicate that GRA6Nt is a unique target molecule among T. gondii GRA proteins to activate IFN-γ production by microglia. Therefore, GRA6Nt is a crucial target molecule of the sentinel function of microglia to detect an occurrence of cerebral proliferation of T. gondii and to activate the protective immunity to prevent the growth of the parasite.
Figure 2. Production of IFN-γ but not IL-12 by primary microglia cells in response to rGRA6Nt of T. gondii.
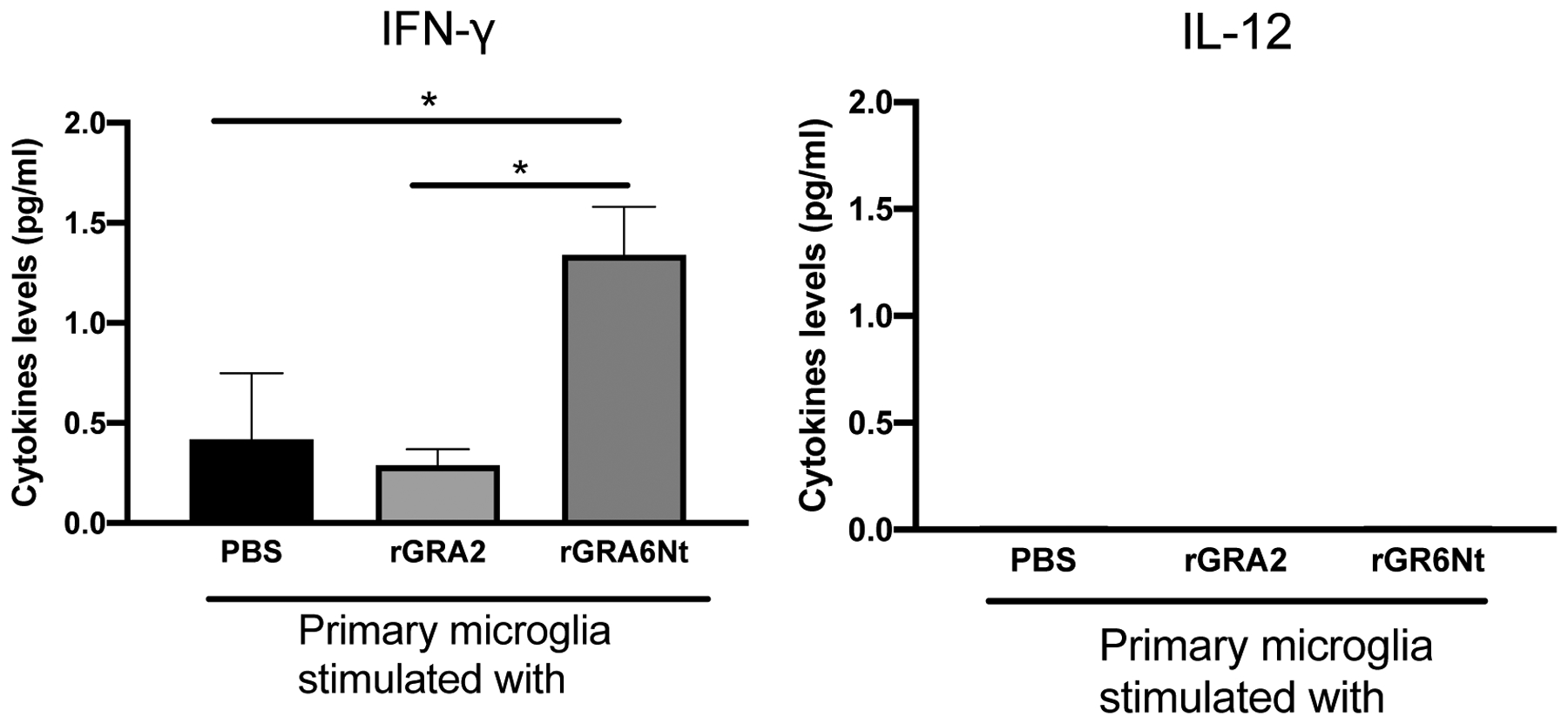
Primary microglia were purified from the brains of adult BALB/c mice and cultured in a 96 well culture plate (5 × 104 cells/well) in the presence of rGRA2, orrGRA6Nt (1 μg/ml at the final concentration) for 72 hrs. As a control, PBS was added to the culture. The rGRA2 and rGRA6Nt were from the type II and type I strains, respectively. Amounts of IFN-γ and IL-12 in the culture supernatants were measured by an electrochemiluminescence assay. Four wells were used for each experimental group. *P<0.05.
The results presented are from one experiment.
The levels of IFN-γ detected in the culture supernatants of primary microglia were much lower than those detected in the cultures of EOC20 microglia cell line. In the latter, higher than 10 pg/ml of this cytokine were detectable in the control cultures without any stimulants (Fig. 1). The low levels of IFN-γ production by the mouse primary microglia observed in the present study are consistent with those reported in a previous study using stimulation with IL-12 and/or IL-18 [19], in which IFN-γ protein was detectable by immunoblotting but not by a standard commercial ELISA kit. In the present study, we measured IFN-γ levels in the culture supernatants of the mouse primary microglia using a very sensitive advanced Meso Scale Discovery electrochemiluminescence assay kits that can detect the cytokine as low as 0.16 pg/ml. IFN-γ is a potent pro-inflammatory cytokine, and the brain is the organ where inflammatory responses are strictly restricted. Therefore, it is most likely that the results from the primary microglia represent more closely the physiological activity of microglia to produce IFN-γ in the brain than the results from the EOC20 microglia cell line. The production of the low levels of this cytokine by microglia could be ideal to induce anti-T. gondii protective immune responses only in the area where microglia become activated by detecting GRA6Nt released from infected cells. In this manner, the protective immune responses are limited only in the area just adjacent to the location of microbial proliferation, and an induction of unwanted inflammatory responses in the distant areas could be avoided.
Since IL-12 was previously shown to activate microglial IFN-γ production [19], we also examined whether rGRA6Nt stimulates IL-12 production by the primary microglia. In contrast to IFN-γ, IL-12 was undetectable in the culture supernatants from the most of the cultures stimulated with either rGRA6Nt or rGRA2 and those of the control culture with PBS (Fig. 2). IL-12 may not be a major mediator of inducing IFN-γ production of microglia activated by rGRA6Nt.
Previous in vitro studies demonstrated a production of IFN-γ by microglia following stimulation with LPS [17, 18], a component of gram-negative bacteria, as mentioned earlier. The present study now identified that GRA6Nt of T. gondii, a protozoan parasite, activates IFN-γ production of both EOC20 microglia cell line and primary microglia from adult mice. Thus, microglia possess the capability to detect molecules derived from both bacteria and protozoan parasite. To our knowledge, GRA6Nt is the first microbial protein molecule identified to activate microglial IFN-γ production.
The results from the present study using in vitro cultures of microglia are consistent with the observations in our previous ex vivo studies using mice. Flow cytometric analyses demonstrated an expression of IFN-γ by CD11b+CD45low microglia purified from the brains of mice with acute acquired infection with T. gondii [8] and those with a reactivation of chronic cerebral infection with this parasite [7, 8]. Furthermore, when these microglia were purified by sorting from the brains of mice with reactivation of the infection and applied to cultures, both CD11b+CD45low microglia and CD11b+CD45high blood-derived macrophages secreted IFN-γ into their culture supernatants without any additional stimulation in vitro [8].
Recently, our in vivo studies using bone marrow chimeric mice revealed that IFN-γ production by brain-resident cells (irradiation-resistant cells) including microglia is crucial for promptly activating both protective innate and T cell-mediated immunity following reactivation of cerebral T. gondii infection to control the infection [9]. The present studies now demonstrate that a stimulation of uninfected microglia with GRA6Nt is able to activate microglial IFN-γ production. Therefore, it is most likely that uninfected microglia sense an occurrence of cerebral T. gondii proliferation by detecting GRA6Nt released from infected brain cells, probably astrocytes and neurons, and activate their IFN-γ production as a critical sentinel system to promptly trigger both innate and T cell-mediated protective immune responses only in the areas of the parasite growth to control the infection.
We recently identified that GRA6Nt contains a key epitope(s) that activates CD8+ cytotoxic T cells capable of removing tissue cysts of the parasite from the brains of infected mice [11]. The present study uncovered a novel activity of GRA6Nt to activate microglial IFN-γ production for the protective immunity against T. gondii tachyzoites. These evidences together emphasize that GRA6Nt is a critical target molecule of the immune system in the brain to fight against both the acute stage form, tachyzoites, and chronic stage form, cysts, of T. gondii.
Acknowledgements
We appreciate Dr. Jenny Lutshumba for her assistance on preparing this manuscript. The studies are supported in part by NIH grants (AI078756, AI134323, AI136821, and AI095032) (Y.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare that there is no conflict of interest.
References
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004;363:1965–76. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki Y, Conley FK, Remington JS. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J. Immunol 1989;143:2045–50. [PubMed] [Google Scholar]
- 3.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol 1992;149:175–80. [PubMed] [Google Scholar]
- 4.Wang X, Kang H, Kikuchi T, Suzuki Y. Gamma interferon production, but not perforin-mediated cytolytic activity, of T cells is required for prevention of toxoplasmic encephalitis in BALB/c mice genetically resistant to the disease. Infect. Immun 2004;72:4432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Claflin J, Kang H, Suzuki Y. Importance of CD8+Vbeta8+ T Cells in IFN-gamma-mediated prevention of toxoplasmic encephalitis in genetically resistant BALB/c mice. J. Interferon Cytokine Res 2005;25:338–44. [DOI] [PubMed] [Google Scholar]
- 6.Kang H, Suzuki Y. Requirement of non-T cells that produce gamma interferon for prevention of reactivation of Toxoplasma gondii infection in the brain. Infect. Immun 2001;69:2920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki Y, Claflin J, Wang X, Lengi A, Kikuchi T. Microglia and macrophages as innate producers of interferon-gamma in the brain following infection with Toxoplasma gondii. Int. J. Parasitol 2005;35:83–90. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Suzuki Y. Microglia produce IFN-gamma independently from T cells during acute toxoplasmosis in the brain. J. Interferon Cytokine Res 2007;27:599–605.. [DOI] [PubMed] [Google Scholar]
- 9.Sa Q, Ochiai E, Tiwari A, Perkins S, Mullins J, Gehman M, et al. Cutting Edge: IFN-gamma produced by brain-resident cells is crucial to control cerebral infection with Toxoplasma gondii. J. Immunol 2015;195:796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golkar M, Shokrgozar MA, Rafati S, Musset K, Assmar M, Sadaie R, et al. Evaluation of protective effect of recombinant dense granule antigens GRA2 and GRA6 formulated in monophosphoryl lipid A (MPL) adjuvant against Toxoplasma chronic infection in mice. Vaccine 2007;25:4301–11. [DOI] [PubMed] [Google Scholar]
- 11.Sa Q, Ochiai E, Tiwari A, Mullins J, Shastri N, Mercier C, et al. Determination of a key antigen for immunological intervention to target the latent stage of Toxoplasma gondii. J. Immunol 2017;198:4425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrandiz J, Mercier C, Wallon M, Picot S, Cesbron-Delauw MF, Peyron F. Limited value of assays using detection of immunoglobulin G antibodies to the two recombinant dense granule antigens, GRA1 and GRA6 Nt of Toxoplasma gondii, for distinguishing between acute and chronic infections in pregnant women. Clin. Diagn. Lab. Immunol 2004;11:1016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bittame A, Effantin G, Petre G, Ruffiot P, Travier L, Schoehn G, et al. Toxoplasma gondii: biochemical and biophysical characterization of recombinant soluble dense granule proteins GRA2 and GRA6. Biochem. Biophys. Res. Commun 2015;459:107–12. [DOI] [PubMed] [Google Scholar]
- 14.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis 1995;172:1561–6. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson DJ, Hutchison WM. An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol. Res 1987;73:483–91. [DOI] [PubMed] [Google Scholar]
- 16.Mercier C, Cesbron-Delauw MF. Toxoplasma secretory granules: one population or more? Trends Parasitol. 2015;31:60–71. [DOI] [PubMed] [Google Scholar]
- 17.Dimayuga FO, Reed JL, Carnero GA, Wang C, Dimayuga ER, Dimayuga VM, et al. Estrogen and brain inflammation: effects on microglial expression of MHC, costimulatory molecules and cytokines. J. Neuroimmunol 2005;161:123–36. [DOI] [PubMed] [Google Scholar]
- 18.Makela J, Koivuniemi R, Korhonen L, Lindholm D. Interferon-gamma produced by microglia and the neuropeptide PACAP have opposite effects on the viability of neural progenitor cells. PLoS One 2010;5:e11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawanokuchi J, Mizuno T, Takeuchi H, Kato H, Wang J, Mitsuma N, et al. Production of interferon-gamma by microglia. Mult. Scler 2006;12:558–64. [DOI] [PubMed] [Google Scholar]


