Abstract
Introduction:
Offspring from a prenatal docosahexaenoic acid (DHA) supplementation trial, in which pregnant women were assigned to placebo or 600mg DHA/day, were followed to determine the effect of prenatal DHA supplementation on children’s behavior and brain function at 5.5 years (n=81 placebo, n=86 supplemented).
Methods:
Event-related potentials (ERP) were recorded during a visual task requiring a button press (Go) to frequent target stimuli and response inhibition to the rare stimuli (No-Go). Univariate ANOVAs were used to test differences between group and sex for each behavioral measure. A three-way mixed-design multivariate analysis of variance (MANOVA) was used to examine statistical ERP differences.
Results:
There was a significant sex × group interaction for hit rate and errors of omission; there was no difference between males and females in the placebo group, but DHA males were significantly better than DHA females For false alarms (failure to inhibit), males overall, and the placebo group made more false alarms while DHA females made significantly fewer than placebo females and DHA males. ERP P2 amplitude was larger in the DHA group. There was a significant ERP N2 amplitude condition effect observed in females and DHA group males, however, placebo group males did not generate this typical ERP condition difference.
Discussion:
Prenatal DHA supplementation improved inhibitory performance overall, especially for females in the DHA group, possibly accounting for their conservative behavior during Go trials. Development of brain regions responsible for visual processing may be sensitive to maternal DHA status, evidenced by greater P2 amplitude in children whose mothers received DHA. Males may benefit more from maternal DHA supplementation, indicated by the N2 condition effect seen only in males in the DHA group.
Keywords: Docosahexaenoic acid, long-chain polyunsaturated fatty acids, prenatal nutrition, event-related potentials
Introduction
The long-chain polyunsaturated fatty acids, docosahexaenoic acid (DHA) and arachidonic acid (ARA), are essential for fetal and infant neurodevelopment. DHA and its metabolites are involved in early developmental events such as neurogenesis, neurite outgrowth, and synaptogenesis [1]. In utero accretion of DHA in the fetal central nervous system takes place largely in the 3rd trimester, increases by nearly 30-fold in the first two years of life [2] and depends on maternal [3] and infant DHA intake [4].
A daily intake of 200 mg DHA has been suggested for pregnancy [5]. The Dietary Guidelines for Americans recommends 8 ounces of seafood per week, which would provide an estimated average of 200 mg DHA per day over the week with neurodevelopmental benefit outweighing the risk of mercury or other contamination [6]. However, a 2014 analysis of NHANES data revealed females of childbearing age in the United States consume only about 50 mg per day of DHA [7].
Even an intake of 200 mg/day may not be sufficient to gain the full benefit from DHA intake during pregnancy for infant neurodevelopment. Two previously reported randomized controlled trials (RCTs) found beneficial effects of DHA supplementation during pregnancy with provision of 500 and 600 mg DHA, respectively. In women randomized to a daily supplement of 500 mg DHA from 20 weeks of gestation to term, newborn cord blood DHA was associated with better neurological outcomes in their children at age 5.5 years [8]. Male neonates whose mothers received a supplement providing 600 mg of DHA during pregnancy had significantly larger total and regional brain volumes [9].
We studied the offspring of women assigned to either a placebo or 600 mg DHA/day beginning before 20 weeks gestation in the Kansas University DHA Outcomes Study (KUDOS; NCT00266825). The parent RCT was designed to test the effect of a daily prenatal dose of 600 mg of DHA on pregnancy and postnatal outcomes out to 18 months of age [10]. When children were 18 mo old, parents of 190 children gave permission for continued follow-up from 2 through 6 years of age ( NCT02487771) [11]. The primary aim of the follow-up study (2010-2014) was long-term evaluation of cognitive development. The results of that follow-up revealed positive effects on the quality of attention in infancy [12] and on spatial memory performance and an executive function task in early childhood [11]. However, no consistent pattern of long-term benefit was observed for IQ or other global measures of developmental status [11].
Here, we report the results of brain event-related potentials (ERP) recorded at 5.5 years of age. We hypothesized that prenatal DHA supplementation would result in differences in behavior and brain electrophysiology when children performed a Go/No-Go task; this task requires stimulus evaluation, conflict monitoring and response inhibition.
Methods
Subject characteristics
Pregnant women (n=350) were enrolled in the KUDOS parent RCT from March 2006 through September 2009, from local hospitals in the Kansas City metropolitan area. The Investigational Pharmacy at the University of Kansas Medical Center used a randomization schedule generated by the study biostatistician to provide placebo (3 capsules/day containing an equal mixture of soybean and corn oil) or DHA (3 capsules/day of algal oil providing a total of 600 mg of DHA). Capsules were mailed and bottles returned to the Pharmacy to count capsules remaining. All capsules were orange-flavored, supplied by DSM Nutritional Products (Columbia, MD). Women were enrolled between 12-20 weeks gestation (M = 14.5 weeks). Details of the parent trial study design, subject demographics, inclusion and exclusion criteria are provided in the published report on pregnancy outcomes [10].
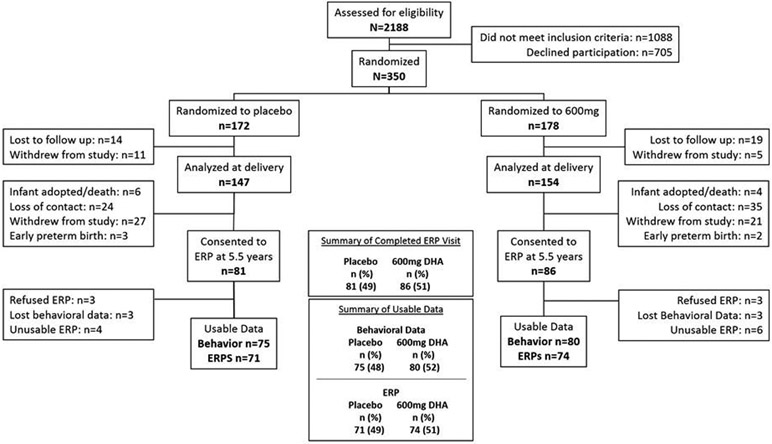
ERPs were a planned component of the follow-up for the preschool period; based upon observed attrition through the parent trial and further attrition projections, appropriate power was expected for the follow-up phase. ERPs were scheduled when children were 5.5 years of age. Details of subject enrollment, task completion and reasons for missing ERP data are seen in Figure 1. CONSORT flow chart. All study personnel were masked to capsule allocation until after results were entered for the last 6-year-old child. The research protocol and informed consent adhered to the Declaration of Helsinki. The parent RCT (HSC #10186) and follow-up study (HSC #11406) were approved by The University of Kansas Institutional Review Board.
Figure 1.
CONSORT flow diagram
Study Design
Go/No-Go task
The task used [13] was implemented using Neuroscan Stim2 software (Compumedics, Charlotte, NC). Children were told that they were going to play a “fishing game,” where the object was to “catch a fish” but “don’t catch a shark.” Prior to the test, children went through an introductory session where stationary cartoon images of fish and sharks were shown on a computer monitor. The experimenter pointed to examples of fish to familiarize the child with the images and showed the child how to press a button on a response pad as soon as they saw a fish (“Go” trial). When pictures of sharks appeared during the explanation of the task, children were reminded not to press the button (“No-Go” trial; inhibit the response). After explaining the task, we administered a practice session using the same stimulus timings and intervals as the test session to confirm that children understood the task and learned to respond quickly; the practice task was repeated a second time if catching a fish was missed due to slow response, or if sharks were caught. Experimenters would provide feedback as needed during this period. Regardless of performance on the practice task, all children advanced to the test session, and experimenter feedback ceased. The test session included 69 trials: 51 (74%) fish Go trials and 18 (26%) shark No-Go trials. The images were displayed on the monitor for up to 1500 milliseconds or until the child pressed the response button. After a button press, the stimulus disappeared. All button presses within the 1500 ms response window received feedback: a fish in a net with a bubble sound for correct Go trials or a shark in a ripped net with a ripping sound for incorrect No-Go trials. There was no feedback for incorrectly missed fish or correctly missed sharks.
Electrophysiologic recording
Brain activity was measured using electroencephalography (EEG) on a Neuroscan system (Synamps 2 and Neuroscan software version 4.3, Compumedics, Charlotte, NC). Silver/silver chloride electrodes were manually placed on the child’s scalp at 29 active recording sites following scalp preparation with NuPrep and secured with Elefix paste. Electrode sites used were according to the International 10-20 standardization. Active electrodes were Fp1, Fpz, Fp2, F7, F3, Fz, F4, F8, FC5, FC1, FC2, FC6, T3, Cz, C3, C4, T4, CP5, CP1, CP2, CP6, T5, P3, Pz, P4, T6, O1, Oz and O2. Scalp impedance was at or below 10 Kohm. Active electrodes were referenced to a left mastoid electrode, using a forehead ground. Infra- and supra-orbital electrodes placed above and below the center of the left eye recorded vertical eye movements while electrodes placed near the outer canthi of each eye recorded horizontal eye movements. Continuous EEG activity was recorded using a 1000 Hz sampling rate and filtered online from 0.05 to 70 Hz with a roll-off of 6 dB per octave. Behavioral button press data were recorded simultaneously with the EEG.
Data Analysis
Measures of Behavior
For Go stimuli, we obtained measures of mean reaction time (ms), percent correct responses (hit rate) and number of omissions (incorrect response). For the No-Go stimuli, we calculated the percent correct responses and number of times children failed to inhibit the response (false alarm). To determine the error rate for each child, the total errors were divided by the number of possible errors. Z-scores were calculated for reaction time, error rate, hit rate and false alarm rate. An efficiency score (speed-accuracy tradeoff) was generated by summing subject Z-scores for reaction time and error rate.
EEG and ERP Analysis
Details of the EEG analysis are explained in detail in a previous report [14]. Briefly, EEGs were filtered using a high pass filter of 0.1 Hz and a low pass filter of 40 Hz and re-referenced to an average reference [15]. After filtering, the EEG was decomposed using Independent Component Analysis (ICA), using the Infomax ICA algorithm from EEGLAB toolbox [16]. Independent components visually identified as artifacts (eye movement, blinking, electrocardiogram and electromyography) were removed. Individual trials were generated from the artifact-free, continuous EEG based on the event trigger; presentation of a fish or shark image. Epochs ranged from 100 ms pre- to 1500 ms post-stimulus with zero ms as the stimulus onset. Each epoch was examined for residual artifacts and rejected if the epoch exceeded a threshold of 250 microvolts. Trials with incorrect responses (missed fish, caught shark) were removed. Trials with correct responses were used to generate the average ERP to Go and No-Go stimuli for each child. Grand average ERPs for each group and sex were generated using the average of participants’ mean data.
Missing behavioral and ERP data are shown in the Consort Diagram (Figure 1). Of the 167 participants tested, 155 behavioral responses (69 male, 86 female) and 145 ERPs (65 male, 80 female) could be analyzed. Twelve participants provided no behavioral or ERP data; 6 refused ERP testing, and 6 records were lost due to equipment failure (n=1) or data storage error (n=5). Ten participants provided only behavioral data (no usable ERP) due to incorrect EEG montage (n=2), excessive EEG artifact from head or body movement (n=3), or data storage error (n=5).
ERP Components
Three ERP components (P2, N2 and P3) were identified from the grand average ERP data. The amplitude peak value and corresponding latency were calculated within temporal windows of interest. To avoid biasing component measurements [17], we used the same ERP component temporal windows and electrode clusters used in the prior study of children of the same age who were supplemented postnatally [14]. For component P2, we analyzed peak/latency between 150-300 ms, using 3 electrodes in the fronto-central region (FC1, FC2, and Cz). For component N2, we analyzed peak/latency between 300-500 ms using 8 frontal electrodes (Fp1, Fpz, Fp2, F7, F3, Fz, F4, and F8). For component P3, we analyzed peak/latency between 500-700 ms using 5 centro-parietal electrodes (P3, Pz, P4, CP1 and CP2). Grand average ERP waveforms for Go and No-Go conditions at Fpz, Fz, Cz, and Pz are shown in Figure 2.
Figure 2.
Grand average ERP waveforms of Go/No-Go condition. ERP components are marked at their representative electrodes (P2 at Cz, N2 at Fpz and Fz, P3 at Pz). Data are displayed from 0 to 1000 ms after the stimulus onset.
Statistical analysis
Univariate ANOVAs were used to test differences between group and sex for each behavioral measure. Arcsine or square root transformation were applied to data that were not normally distributed. A three-way mixed-design multivariate analysis of variance (MANOVA) was used to examine statistical ERP differences. The ERP amplitudes and latencies for each electrode served as an indicator of the overall cluster construct.
Group and Sex were included as between-subject factors and Condition (Go vs. No-Go) was included as a within-subject factor; the number of correct Go/No-Go trials were entered as a covariate. The MANOVA model was built and computed using SPSS software version 22 (IBM Corp., Chicago, IL). Wilks’ lamdba (Λ) was used as the test statistic. An alpha significance level of p ≤ 0.05 was adopted across all statistical comparisons.
Results
Behavioral Response
The mean behavioral variables for each group and sex are shown in Table 1. The statistical test results of ANOVA are shown in Table 2. There was a significant main effect of Sex for reaction time on Go trials, F(1, 151) = 4.38, p = .038, with faster reaction time in males (mean: 718.6 ms, SE = 15.7 ms) than in females (mean: 762.9 ms, SE = 14.1 ms).
Table 1.
Behavioral data for each group and sex
| Placebo | DHA | |||
|---|---|---|---|---|
| 37 male, 38 female | 32 male, 48 female | |||
|
|
|
|||
| Variable | Mean/SE | 95% CI | Mean/SE | 95% CI |
| Reaction time (ms) | ||||
| Male | 732.1/18.3 | 696-768 | 705.2/26.2 | 654-757 |
| Female | 761.8/20.8 | 721-803 | 763.9/19.1 | 726-801 |
| Hit Rate (arcsine transform) | ||||
| Male | 1.09/0.04 | 1.02-1.16 | 1.18/0.04 | 1.10-1.25 |
| Female | 1.15/0.04 | 1.08-1.22 | 1.06/0.03 | 1.00-1.13 |
| Omission error (Square root transform) | ||||
| Male | 2.39/0.18 | 2.03-2.75 | 1.98/0.20 | 1.59-2.36 |
| Female | 2.09/0.18 | 1.73-2.45 | 2.52/0.16 | 2.21-2.84 |
| False alarm (Square root transform) | ||||
| Male | 1.47/0.14 | 1.20-1.75 | 1.27/0.15 | 0.98-1.57 |
| Female | 1.19/0.14 | 0.92-1.46 | 0.84/0.12 | 0.60-1.08 |
| Efficiency Score | ||||
| Male | 0.04/0.11 | −0.23-0.31 | −0.19/0.15 | −0.48-0.10 |
| Female | 0.04/0.14 | −0.23-0.31 | 0.13/0.12 | −0.11-0.36 |
| Error Rate (arcsine transform) | ||||
| Male | 0.14/0.02 | 0.10-0.17 | 0.11/0.02 | 0.08-0.15 |
| Female | 0.12/0.03 | 0.08-0.15 | 0.13/0.02 | 0.10-0.16 |
Note: SE = standard error, CI: confidence interval. Terms used: Reaction time = correct response to Go stimuli (ms); Hit Rate = percentage of correct Go responses; Omission Error = missed Go (fish); False alarm = failure to inhibit No-Go (caught shark).
Table 2.
F-values of ANOVA comparing Group and Sex for behavioral result
| Variable | Group | Sex | Group × Sex |
|---|---|---|---|
| Reaction time | 0.35 | 4.38 * | 0.48 |
| Hit Rate | 0.004 | 0.50 | 5.50 * |
| Omission error | 0.002 | 0.46 | 5.60 * |
| False alarm | 4.05 * | 6.67 * | 0.30 |
| Efficiency score | 0.29 | 1.46 | 1.37 |
| Error rate | 0.08 | 0.002 | 1.44 |
Note.
: p< .05
Analyses of the hit rate (i.e., correct responses) and errors of omission (i.e., missed fish, incorrect response) on Go trials showed significant Sex × Group interactions, Hit Rate: F(1,151) = 5.50, p = .02; Errors of Omission; F(1,151) = 5.60, p = .019. For hit rate, males (mean: 1.09, SE =0.04) and females (mean: 1.15, SE = 0.04) were not statistically different in the placebo group, but males (mean: 1.18, SE = 0.04) were significantly (p = 0.043) better than females (mean: 1.06, SE = 0.03) in the DHA group. The same pattern was seen for errors of omission, such that males (mean: 2.39, SE = 0.18) and females (mean: 2.09, SE = 0.18) did not statistically differ in the placebo group, but males (DHA, mean: 1.98, SE = 0.20) were significantly (p = 0.044) better than females (mean: 2.52, SE = 0.16) in the DHA group. Thus, it would appear that the prenatal DHA intervention engendered a sex difference in accuracy on Go trials.
However, this puzzling pattern of results becomes somewhat more interpretable after analysis of false alarms on No-Go trials (i.e., when a child mistakenly “caught” a shark). This analysis yielded significant main effects for Sex, F(1,151) = 6.67, p = .011, as males (mean: 1.38, SE = 0.11) made more false alarms than females (mean: 1.00, SE = 0.09). The analysis also yielded a main effect for Group, F(1,151) = 4.05, p = .046, such that the placebo group (mean: 1.33, SE = 0.10) made more false alarms than the DHA group (mean: 1.01, SE = 0.09). We probed the data further, however, in an effort to elucidate the unexpected pattern seen on Go trials. Indeed, females in the DHA group had the lowest number of false alarms; significantly (p = 0.049) fewer than females in the placebo group and even fewer than males in the DHA group (p = 0.025). Thus, while the DHA intervention improved inhibitory performance on No-Go trials overall, it appears to have especially improved false alarm rates for females, perhaps as a result of making them more conservative, or likely to withhold a response. Note that this explanation would also account for DHA females missing more targets on Go trials.
There were no main effects or interactions for error rate or the efficiency score.
Event-Related Potentials
The mean amplitude and latency for each group, condition, and sex are shown in Table 3. The statistical test results of MANOVA for the 3 ERP components are shown in Table 4. Only significant results are discussed in the content below.
Table 3.
Mean amplitude and latency for ERP components of Go and No-Go conditions in male and female [Mean (SD)]
| Placebo | DHA | |||||||
|---|---|---|---|---|---|---|---|---|
| 36 male, 35 female | 29 male, 45 female | |||||||
|
|
|
|||||||
| Go | No-Go | Go | No-Go | |||||
|
|
|
|
|
|||||
| Variable | Male | Female | Male | Female | Male | Female | Male | Female |
| P2 | ||||||||
| Amplitude | 2.7(4.9) | 4.0(3.7) | 4.6(5.5) | 5.3(4.5) | 2.6(3.9) | 3.3(4.4) | 5.8(7.4) | 6.4(5.0) |
| Latency | 179.1(23.1) | 182.0(23.7) | 200.6(36.3) | 197.4(25.6) | 178.4(17.1) | 187.9(23.2) | 194.2(31.4) | 191.5(29.3) |
| N2 | ||||||||
| Amplitude | 11.0(5.0) | 11.2(3.7) | 13.3(4.9) | 15.4(5.3) | 11.0(4.6) | 12.1(5.1) | 16.5(8.2) | 15.0(5.1) |
| Latency | 396.1(37.5) | 381.5(36.2) | 424.0(30.3) | 408.5(36.1) | 393.7(28.6) | 387.0(38.2) | 413.5(30.6) | 414.8(37.2) |
| P3 | ||||||||
| Amplitude | 8.8(4.2) | 9.1(3.8) | 14.1(5.2) | 13.4(4.8) | 10.4(6.0) | 9.2(4.2) | 14.9(7.2) | 14.2(4.0) |
| Latency | 588.3(36.6) | 592.0(32.8) | 597.5(38.2) | 583.7(38.3) | 586.5(33.2) | 597.6(36.0) | 591.9(40.1) | 589.6(39.5) |
Note. The units of ERP components amplitude and latency are μV and ms respectively
Table 4.
F-values of MANOVA comparing Group, Condition and Sex for ERP components
| Variable | Group | Condition | Sex | Group × Condition | Condition × Sex | Group × Sex | Group × Condition × Sex |
|---|---|---|---|---|---|---|---|
| P2 | |||||||
| Amplitude | 2.80 * | 4.92 ** | 2.46 | 2.18 | 0.52 | 0.64 | 2.27 |
| Latency | 0.48 | 4.72 ** | 0.31 | 2.50 | 2.55 | 0.71 | 0.97 |
| N2 | |||||||
| Amplitude | 0.77 | 4.28 *** | 1.83 | 0.98 | 0.41 | 0.49 | 2.64 * |
| Latency | 0.98 | 3.13 ** | 1.62 | 0.54 | 1.30 | 0.80 | 0.86 |
| P3 | |||||||
| Amplitude | 0.66 | 6.25 *** | 2.06 | 0.58 | 0.57 | 1.14 | 0.62 |
| Latency | 0.34 | 1.54 | 0.37 | 0.79 | 1.44 | 1.60 | 1.70 |
Note.
: p< .05
:p< .01
:p< .001
P2 Component.
P2 Amplitude
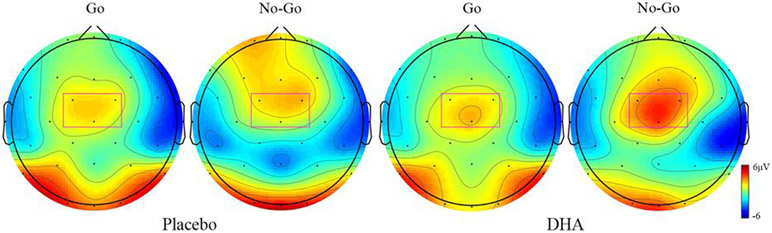
P2 amplitude showed a significant Group effect, F(3,137) = 2.80, p = .042, Wilks' Λ = .94, ηp2 = .06, with larger P2 amplitude in the DHA group (placebo: 4.14 μV, DHA: 4.60 μV). There was also an expected significant main effect of Condition, F(3,137) = 4.92, p = .003, Wilks' Λ = .90, ηp2 = .10, as the P2 amplitude was higher in the No-Go condition than in the Go condition (Go: 3.19 μV, No-Go: 5.56 μV). The P2 component topographies are shown in Figure 3.
Figure 3.
P2 ERP component in placebo and DHA groups. Topographies of Go and No-Go conditions (t = 180 ms). The electrodes used in P2 component analysis are highlighted by the rectangle. The placebo group responses are seen in the 2 left plots; the 2 right plots show the DHA group responses.
P2 Latency
There was a significant main effect of Condition on P2 latency, F(3, 137) = 4.72, p = .004, Wilks’ Λ = .906, ηp2 = .09. As expected, the No-Go condition had a longer latency than Go condition (Go: 182.4 ms, No-Go: 195.7 ms).
N2 Component
N2 Amplitude
For N2 amplitude there was a significant main effect for Condition, F(8, 132) = 4.28, p < .001, Wilks' Λ = .79, ηp2 = .21, since the No-Go condition had a greater amplitude than the Go condition (Go: 11.40 μV, No-Go: 14.96 μV). However, the Condition effect was qualified by a significant Group × Condition × Sex interaction, F(8, 132) = 2.64, p = .01, Wilks' Λ = .862, ηp2 = .14. To decompose this interaction, we conducted separate MANOVAs for each Sex and Group, with Condition (Go vs. No-Go) as a within-subject factor, controlling for the number of the correct Go/No-Go trials. The Condition effect (i.e., N2 amplitude higher on No-Go trials) was observed for all females (DHA, Go: 12.12 μV, No-Go: 15.00 μV, p = .008; placebo, Go: 11.22 μV, No-Go: 15.39 μV, p = .011), but for males, the significant Condition effect was observed only in the DHA group (Go: 11.03 μV, No-Go: 16.47 μV, p = .037). The Condition effect was not observed for males from mothers assigned to the placebo group (Go: 10.98 μV, No-Go: 13.28 μV, p = .707).
N2 Topography and Condition Difference
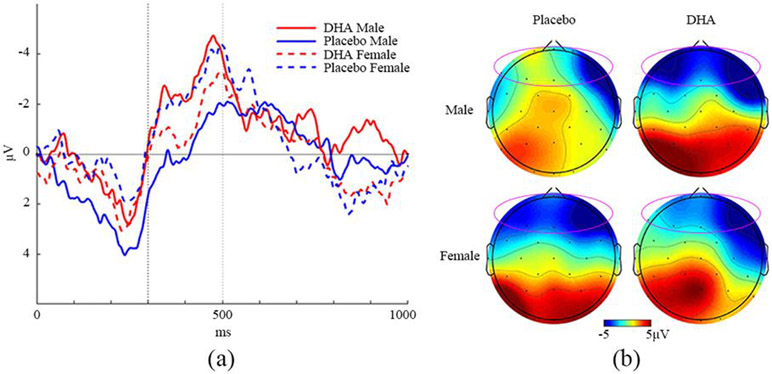
We examined the topography of the 3-way interaction of Group × Condition × Sex of N2 component amplitude, which is illustrated in Figure 4. First, a grand average ERP for the N2 component was generated using the 8 electrodes in the frontal cluster for each group, condition and sex. Then the condition difference ERP waveforms were generated by subtracting Go from No-Go. Males in the placebo group show the least N2 condition difference when compared to males in the DHA group and females in both groups (Figure 4a). This condition difference is also seen in the topographical maps in which the males in the DHA group and females in both groups present with greater increased negativity in the frontal region than males in placebo group (Figure 4b). This is in accordance with the failure of the Condition significance test in the male placebo group.
Figure 4.
N2 condition difference ERP waveforms and topographies. (a) Grand average of difference ERP waveforms (No-Go minus Go condition). The N2 component analysis temporal window is between 300-500ms (indicated by vertical dash lines). (b) Topography difference between No-Go and Go condition (t = 485 ms). Electrodes used in N2 component are circled.
N2 Latency
As expected. a significant main effect of Condition existed for N2 component latency, F(8, 132) = 3.13, p = .003, Wilks' Λ = .84, ηp2 = .16, as the No-Go condition had a longer latency than Go condition (Go: 389.3 ms, No-Go: 415.3 ms). There were no significant effects involving Group.
P3 Component
P3 Amplitude
A significant main effect of Condition emerged for P3 amplitude, F(5, 135) = 6.25, p < .001, Wilks' Λ = .812, ηp2 = .19, with amplitudes significantly higher on No-Go trials than on Go trials (Go: 9.29 μV, No-Go: 14.12 μV). Again, there were no significant effects involving Group.
Discussion
Summary of Main Findings
Our main objective in the ERP assessment of this long-term follow-up was to determine if prenatal DHA supplementation would result in long-term differences in children’s behavior and brain function at 5.5 years using a Go/No-Go task. Although previous long-term follow-up with this sample did not reveal pervasive benefit on global neurodevelopmental outcomes, there were some indications of advantages in specific cognitive functions [11]; these indications were further corroborated in the results from the current data.
There were significant differences in behavior related to prenatal supplementation. All children whose mothers were randomized to DHA supplementation during pregnancy made fewer false alarms than children in the placebo group, confirming our hypothesis that prenatal DHA would result in a greater ability to inhibit a response. As noted above, while the prenatal intervention improved inhibitory function overall, this effect may have been exaggerated in females to the point that it affected females’ response bias for responding during the Go-task. That is, prenatal DHA may have made females more inhibited to the point where, to avoid the mistake of catching a shark (false alarm), they avoided pressing the button to all stimuli, sacrificing fish. If females in the DHA group were merely inattentive, they would have likely made errors to both Go and No-Go stimuli. There are reported sex differences in fatty acid synthesis and randomized controlled trials of long-chain polyunsaturated fatty acid supplementation, however, the effects are mixed; see Decsi et al, for a review [18]. Nonetheless, the accumulating evidence that fatty acid supplementation may affect males and females differently underscores the important of including analyses by sex in randomized controlled trials.
In this trial, prenatal DHA supplementation also resulted in differences in brain function, as indicated by ERP components. Children in the supplemented group had larger P2 amplitude. The P2 component is considered a representation of endogenous attention orienting, facilitating the process of identifying, comparing, and analyzing target stimuli [19]. Thus, greater P2 amplitude should indicate improved performance on visual tasks. This is supported in part by the lower false alarm rate in the DHA group as the task requires children to attend to, compare, and analyze the stimuli before inhibiting their response. This is not the first evidence of increased performance linked to visual processing in this cohort. Over the first year of life, we reported a beneficial effect of prenatal DHA supplementation on the development of the quality of visual attention [12, 20]. Prenatal supplementation also conferred a positive effect on spatial memory tested at 24 and 30 months using the delayed response task [11].
Typically, N2 amplitude shows a condition effect where amplitude is greater (more negative) to the rare No-Go condition than the frequent Go condition [21]. Males in the placebo group failed to show an N2 condition effect, and these males also had the highest false alarm rate. The N2 component is thought to reflect conflict monitoring and inhibitory control, therefore, the finding of a typical task condition effect for N2 amplitude in males whose mothers were supplemented with DHA could be interpreted as development of more mature inhibitory control [22].
We employed identical ERP acquisition and analysis methodology used in an earlier RCT ( NCT00753818) [23] where newborn infants were randomized to formulas with varying levels of DHA plus ARA, or formula with no added long-chain polyunsaturated fatty acids and followed to 9 years of age. At 5.5 years, 60 children in the formula study provided adequate data for ERP analysis [14]. There are similarities in ERP results between the current prenatal and former postnatal supplementation cohorts that may elucidate the effect of early life long-chain polyunsaturated fatty acid supplementation on later life brain function. For example, children who received DHA-ARA supplemented formula also had greater P2 amplitude [14] and improved visual attention over the first year of life [20].There was also a significant Group × Condition interaction for N2 amplitude, where unsupplemented children failed to show the typical N2 condition effect. In the current study, only males in the placebo group failed to show the N2 amplitude condition effect. In the previous postnatal supplementation study, we consistently found better performance in the DHA-ARA groups for tasks requiring rule learning and inhibition, measured between 3 to 5 years [24]. For the current cohort, the cognitive group differences were more modest, showing a significant acceleration of performance on rule learning and flexibility at 36 months and a trend for children in the supplemented group to show better inhibitory control [11]. Both studies provide evidence that DHA and ARA are implicated in developmental programming of visual attention and inhibitory mechanisms that affect brain function and behavior in later life. While we don’t know the precise neurobiological mechanism behind these effects, we do know that effects of prenatal DHA supplementation can be detected in the fetus as early as the third trimester as evidenced by more rapid neural integration of the autonomic nervous system [25, 26].
Infants born to women who consumed a DHA-enriched cereal bar (300 mg/day) during the last two trimesters of pregnancy were reported to have a more mature sleep/wake state [27]. Supplementing pregnant women with 600 mg/day DHA during the last two trimesters of pregnancy reduced early preterm birth [10], improved standardized measures of fetal heart rate variability and resulted in higher autonomic and motor scores in neonates [25]. Later, we were able to identify selective increases in factors consistent with a physiological increase of vagal input and integration of sympatho-vagal activity in the supplemented fetuses [26]. Therefore, DHA may program greater regulatory physiology of the autonomic nervous system. The autonomic nervous system in turn, plays an important role in higher order cognitive functions, in particular, those related to attention and arousal [28].
Neuroimaging studies may elucidate structural and functional differences associated with DHA and other long-chain polyunsaturated fatty acid intake. In adults, higher dietary intake had a positive association with gray matter volume in the anterior cingulate cortex (ACC), amygdala and hippocampus; regions supporting emotional arousal and regulation [29]. Although limited in number, trials using imaging modalities in children have also linked DHA to the ACC and cortical attention networks, suggesting that insufficient dietary DHA can limit brain maturation. Darcey and colleagues found adolescent males were more vulnerable to low n-3 levels (DHA-EPA) showing reduced gray matter volume in the dorsal region of the cingulate and lower caregiver-rated impulse control; results not found in female subjects [30].
When children from the postnatal DHA-ARA formula study described above were 9 years old, we used multi-modal imaging to investigate the long-term effects of early life supplementation on brain structure, function and metabolism [31]. We reported greater white matter volume in the ACC and parietal regions, and greater brain activation during a functional MRI task in children who received supplemented formula the first year of life. Human milk consumption during infancy has been linked to greater white matter development in 8 year old males, but not females [32]. In 2017, Almeida and colleagues reported reduced event-related functional connectivity between the ACC and several brain regions in males with low DHA biostatus [33]. Additionally, differences in brain metabolic function have also been reported in studies of early life long-chain polyunsaturated fatty acid supplementation [31] and current DHA biostatus [34].
In summary, we posit that the earliest evidence of the effects of DHA supplementation can be measured in utero, where maternal supplementation results in more mature neurophysiologic regulatory systems indicated by measureable differences in fetal autonomic control and newborn behavior [25, 26], differences that could potentially contribute to the reduction of early preterm birth [10]. In this cohort, we observed greater visual attention in the first year of life [12], a positive effect on attention and spatial memory at 24 and 36 months of age [11] and, at 5.5. years, greater inhibition and differences in ERP components indicating greater visual processing, attention and inhibitory processes, especially for males.
Strengths and Limitations
The current results are strengthened by a large sample size and steps to avoid biasing ERP component measurement procedures by using previously established temporal windows and electrode clusters before analyzing the results [17].There are some limitations to the current study. The ERP design was simplified for children, making it more likely that they could complete the task. While the use of cartoon images kept children engaged, it does not allow for a more targeted assessment of spatial and temporal components typically used to test selective visual attention, a consideration for future investigation. Likewise, considering the limits of child cooperation, we chose to use an electrode montage with fewer electrodes. A more complex, cued ERP design, using high density EEG or magnetoencephalography in older children, could improve our understanding of the role of DHA in long-term programming of brain regions involved in attention, inhibition and impulse control.
We can only speculate on brain mechanisms related to the findings reported here and draw from previous imaging studies from our group and others. Future studies using a combination of neurophysiologic measures and multi-modal imaging would bring us closer to understanding the role of DHA in the developing brain.
Conclusion
Our findings suggest that prenatal DHA affects brain and behavioral responses during the performance of an inhibitory task during the preschool period. Overall inhibitory performance (i.e., errors on No-Go trials) was improved for children from DHA-supplemented mothers. Secondary analyses also suggest that prenatal DHA may have made females more conservative in their responses to both Go and No-Go stimuli. The ERP component attributed to visual processing (P2) may be sensitive to maternal DHA status during pregnancy for both sexes indicated by greater amplitude observed in the supplemented group. Unlike males in the DHA group, males in the placebo group failed to show the N2 condition effect, suggesting that males may benefit more from maternal DHA supplementation.
Acknowledgements
Supported by a grant from the National Institutes of Health (R01 HD047315). The authors thank Lori Blank and Sara Macone for their expertise and assistance with data acquisition. The authors thank the families for their time and commitment to the long-term follow up of their children.
Funding
This study was supported by the Eunice Shriver National Institute of Child Health and Development under Grant R01 HD047315.
List of abbreviations:
- DHA
docosahexaenoic acid
- EEG
electroencephalography
- ARA
arachidonic acid
- RCT
randomized controlled trial
- KUDOS
Kansas University DHA Outcomes Study
- ERP
event-related potential
- ICA
Independent Components Analysis
- MANOVA
Multivariate Analysis of Variance
- fMRI
Functional Magnetic Resonance Imaging
- ACC
Anterior Cingulate Cortex
Footnotes
Trial Registration: ClinicalTrials.gov NCT00266825 (https://clinicaltrials.gov/ct2/show/NCT00266825) and ClinicalTrials.gov NCT0248771 (https://clinicaltrials.gov/ct2/show/NCT02487771)
Disclosure of interest
Gustafson, Liao, Mathis, Shaddy, Kerling, and Christfano declare no conflicts of interest with the study. Carlson and Colombo designed the parent and follow up studies that were funded by NICHD from 2006 to 2018. Both Carlson and Colombo have consulted and received honoraria from formula and ingredient companies that have a commercial interest in DHA.
References
- [1].Kim HY, Spector AA. N-Docosahexaenoylethanolamine: A neurotrophic and neuroprotective metabolite of docosahexaenoic acid. Mol Aspects Med 64:34–44, 2018. [DOI] [PubMed] [Google Scholar]
- [2].Lauritzen L, Carlson SE. Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern Child Nutr 7 Suppl 2:41–58, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Haggarty P Fatty acid supply to the human fetus. Annu Rev Nutr 30:237–255, 2010. [DOI] [PubMed] [Google Scholar]
- [4].Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr 60(2):189–194, 1994. [DOI] [PubMed] [Google Scholar]
- [5].Koletzko B, Lien E, Agostoni C, Bohles H, Campoy C, Cetin I, Decsi T, Dudenhausen JW, Dupont C, Forsyth S, Hoesli I, Holzgreve W, Lapillonne A, Putet G, Secher NJ, Symonds M, Szajewska H, Willatts P, Uauy R, World Association of Perinatal Medicine Dietary Guidelines Working G. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med 36(1):5–14, 2008. [DOI] [PubMed] [Google Scholar]
- [6].Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, Clinton S, Hu F, Nelson M, Neuhouser ML, Perez-Escamilla R, Siega-Riz AM, Story M, Lichtenstein AH. The 2015 Dietary Guidelines Advisory Committee Scientific Report: Development and Major Conclusions. Adv Nutr 7(3):438–444, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Papanikolaou Y, Brooks J, Reider C, Fulgoni VL 3rd. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003-2008. Nutr J 13:31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Escolano-Margarit MV, Ramos R, Beyer J, Csabi G, Parrilla-Roure M, Cruz F, Perez-Garcia M, Hadders-Algra M, Gil A, Decsi T, Koletzko BV, Campoy C. Prenatal DHA status and neurological outcome in children at age 5.5 years are positively associated. J Nutr 141(6):1216–1223, 2011. [DOI] [PubMed] [Google Scholar]
- [9].Ogundipe E, Tusor N, Wang Y, Johnson MR, Edwards AD, Crawford MA. Randomized controlled trial of brain specific fatty acid supplementation in pregnant women increases brain volumes on MRI scans of their newborn infants. Prostaglandins Leukot Essent Fatty Acids 138:6–13, 2018. [DOI] [PubMed] [Google Scholar]
- [10].Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, Georgieff MK, Markley LA, Kerling EH, Shaddy DJ. DHA supplementation and pregnancy outcomes. Am J Clin Nutr 97(4):808–815, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Colombo J, Shaddy DJ, Gustafson K, Gajewski BJ, Thodosoff JM, Kerling E, Carlson SE. The Kansas University DHA Outcomes Study (KUDOS) clinical trial: long-term behavioral follow-up of the effects of prenatal DHA supplementation. Am J Clin Nutr 109(5):1380–1392, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Colombo J, Gustafson KM, Gajewski BJ, Shaddy DJ, Kerling EH, Thodosoff JM, Doty T, Brez CC, Carlson SE. Prenatal DHA supplementation and infant attention. Pediatr Res 80(5):656–662, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wiebe SA, Sheffield TD, Andrews Espy K. Separating the fish from the sharks: a longitudinal study of preschool response inhibition. Child Dev 83(4):1245–1261, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liao K, McCandliss BD, Carlson SE, Colombo J, Shaddy DJ, Kerling EH, Lepping RJ, Sittiprapaporn W, Cheatham CL, Gustafson KM. Event-related potential differences in children supplemented with long-chain polyunsaturated fatty acids during infancy. Dev Sci 20(5), 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Micah MM, De Lucia M, Brunet D, Michel CM: Principles of Topographic Analyses for Electrical Neuroimaging. In Handy TC (ed): "Brain Signal Analysis: Advances in Neuroelectric and Neuromagnetic Methods." MIT Press, pp 21–54, 2009. [Google Scholar]
- [16].Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134(1):9–21, 2004. [DOI] [PubMed] [Google Scholar]
- [17].Luck SJ, Gaspelin N. How to get statistically significant effects in any ERP experiment (and why you shouldn't). Psychophysiology 54(1):146–157, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am J Clin Nutr 94(6 Suppl):1914S–1919S, 2011. [DOI] [PubMed] [Google Scholar]
- [19].Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci U S A 95(3):781–787, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Colombo J, Carlson SE, Cheatham CL, Fitzgerald-Gustafson KM, Kepler A, Doty T. Long-chain polyunsaturated fatty acid supplementation in infancy reduces heart rate and positively affects distribution of attention. Pediatr Res 70(4):406–410, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hoyniak C Changes in the NoGo N2 Event-Related Potential Component Across Childhood: A Systematic Review and Meta-Analysis. Dev Neuropsychol 42(1):1–24, 2017. [DOI] [PubMed] [Google Scholar]
- [22].Kenemans JL, Bekker EM, Lijffijt M, Overtoom CC, Jonkman LM, Verbaten MN. Attention deficit and impulsivity: selecting, shifting, and stopping. Int J Psychophysiol 58(1):59–70, 2005. [DOI] [PubMed] [Google Scholar]
- [23].Birch EE, Carlson SE, Hoffman DR, Fitzgerald-Gustafson KM, Fu VL, Drover JR, Castaneda YS, Minns L, Wheaton DK, Mundy D, Marunycz J, Diersen-Schade DA. The DIAMOND (DHA Intake And Measurement Of Neural Development) Study: a double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am J Clin Nutr 91(4):848–859, 2010. [DOI] [PubMed] [Google Scholar]
- [24].Colombo J, Carlson SE, Cheatham CL, Shaddy DJ, Kerling EH, Thodosoff JM, Gustafson KM, Brez C. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am J Clin Nutr 98(2):403–412, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gustafson KM, Carlson SE, Colombo J, Yeh HW, Shaddy DJ, Li S, Kerling EH. Effects of docosahexaenoic acid supplementation during pregnancy on fetal heart rate and variability: a randomized clinical trial. Prostaglandins Leukot Essent Fatty Acids 88(5):331–338, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hoyer D, Schmidt A, Schneider U, Gustafson K. Fetal developmental deviations reflected in a functional autonomic brain age score. In. Fetal developmental deviations reflected in a functional autonomic brain age score. Computing in Cardiology; 23-26 Sept 2018; Maastricht, Netherlands: IEEE; 2018. [Google Scholar]
- [27].Judge MP, Cong X, Harel O, Courville AB, Lammi-Keefe CJ. Maternal consumption of a DHA-containing functional food benefits infant sleep patterning: an early neurodevelopmental measure. Early Hum Dev 88(7):531–537, 2012. [DOI] [PubMed] [Google Scholar]
- [28].Gustafson KM, Colombo J, Carlson SE. Docosahexaenoic acid and cognitive function: Is the link mediated by the autonomic nervous system? Prostaglandins Leukot Essent Fatty Acids 79(3-5):135–140, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Conklin SM, Gianaros PJ, Brown SM, Yao JK, Hariri AR, Manuck SB, Muldoon MF. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett 421(3):209–212, 2007. [DOI] [PubMed] [Google Scholar]
- [30].Darcey VL, McQuaid GA, Fishbein DH, VanMeter JW. Relationship between whole blood omega-3 fatty acid levels and dorsal cingulate gray matter volume: Sex differences and implications for impulse control. Nutr Neurosci:1–11, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lepping RJ, Honea RA, Martin LE, Liao K, Choi IY, Lee P, Papa VB, Brooks WM, Shaddy DJ, Carlson SE, Colombo J, Gustafson KM. Long-chain polyunsaturated fatty acid supplementation in the first year of life affects brain function, structure, and metabolism at age nine years. Dev Psychobiol 61(1):5–16, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ou X, Andres A, Cleves MA, Pivik RT, Snow JH, Ding Z, Badger TM. Sex-specific association between infant diet and white matter integrity in 8-y-old children. Pediatr Res 76(6):535–543, 2014. [DOI] [PubMed] [Google Scholar]
- [33].Almeida DM, Jandacek RJ, Weber WA, McNamara RK. Docosahexaenoic acid biostatus is associated with event-related functional connectivity in cortical attention networks of typically developing children. Nutr Neurosci 20(4):246–254, 2017. [DOI] [PubMed] [Google Scholar]
- [34].McNamara RK, Jandacek R, Tso P, Weber W, Chu WJ, Strakowski SM, Adler CM, Delbello MP. Low docosahexaenoic acid status is associated with reduced indices in cortical integrity in the anterior cingulate of healthy male children: a 1H MRS Study. Nutr Neurosci 16(4):183–190, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]