To the Editor:
Tissue-resident memory T (TRM) cells persist in peripheral tissues such as skin and do not recirculate (Mueller and Mackay, 2016). TRM cells protect against infections and mediate anti-tumor responses, but have also been implicated in driving autoimmune and other immune-mediated diseases (Masopust and Soerens, 2019),(Park and Kupper, 2015). However, relatively little is known about their role in mediating delayed drug hypersensitivity reactions. Studies of cutaneous drug reactions such as fixed drug eruption that involve recurrent lesions at the same site after re-exposure to the offending agent, have indicated that localised skin TRM cells may be mediating inflammatory responses (Shiohara et al., 2002). However, it is not known if TRM cells play a causative role in other delayed (T cell-mediated) hypersensitivities, such as Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), severe maculopapular exanthema (MPE) and other T-cell mediated drug-induced cutaneous diseases. We sought to examine the role of TRM cells in a prospective cohort of patients with a history of antibiotic-induced DRESS or MPE.
Acute DRESS and MPE are both characterized by a widespread rash involving the skin surface indicating that drug-specific T cell-mediated responses are occurring in the skin. Indeed, we found that a T cell infiltrate of predominantly CD4+ T cells in skin sampled from a patient with acute DRESS (Figure S1a). Skin biopsies from a patients presenting with DRESS showed that CD4+ and CD8+ T cells were present in affected skin after disease onset (acute disease) and following resolution of skin pathology (post-disease), during which T cells persisted at relatively higher frequencies, with the majority of T cells expressing CD69 (Figure S1b, c).
This increase in T cells in the post-disease skin biopsy taken 44 days later suggested that the skin had not yet reached a quiescent state and that persistent smoldering inflammation in the absence of clinically overt disease may be occurring, or may reflect increased cell death in the lesional skin biopsy. To formally assess drug-reactive T cell responses in vivo, we applied the antibiotic implicated in the acute DRESS or MPE reaction intradermally in the recovery phase of the drug reaction and monitored skin for the development of a local response (Trubiano et al., 2019). As previously described (Trubiano et al., 2019), intradermal testing was performed in forearm skin, a site that was involved in the initial drug exanthema. Normal Saline was inoculated intradermally in an adjacent site as a negative control. A positive result was defined as induration of >5mm that persisted for > 72 hours in a patient with a negative Normal Saline control, as per previous definitions (Trubiano et al., 2018). Skin biopsies of the positive intradermal site reaction (‘lesion’) and non-affected control skin (‘control’) were obtained one day (acute IDT) and more than 8 weeks (post-IDT) after intradermal drug inoculation (Figure 1A). Post-IDT biopsies were taken from skin immediately adjacent to the index biopsy in the lesional and control areas. Five patients with a history of DRESS or severe MPE developed a positive intradermal site reaction one day after inoculation with causative drug (Table 1). We performed flow cytometry on lymphocytes extracted from skin biopsies to determine T cell content and expression of CD69 and the integrin CD103, markers that denote tissue-residency in skin (Mackay et al., 2013). Whereas CD69+CD103− T cells may represent cells in transit through the tissue (Mackay et al., 2015), CD8+CD69+CD103+ skin TRM cells are widely held to be a stable non-recirculating population (Mackay et al., 2013, Mackay et al., 2015, Watanabe et al., 2015), although some uncertainty about these distinctions remain (Klicznik et al., 2019). Shortly after drug inoculation, we found increased numbers of CD4+ T cells in the inoculated lesion skin site as compared to the control skin (median 4.8-fold increase in lesional skin) although in convalescence, this site-specific localisation of CD4+ T cells in lesional skin was reduced (2.8-fold increase in lesional skin) (Figure 1b, c, Figure S2a). In contrast, the number of CD8+ T cells did not vary substantially in lesional skin compared to the control skin site during acute and convalescent phases (Figure 1b, c, Figure S2a). Within the CD4+ T cell population, cells of a CD69+CD103− phenotype dominated lesional skin in the acute phase at a 5.1-fold increase over control skin, although this dominance reduced over time (Figure 1d, 1e, left panel; Figure S2b). Conversely, the ratio of CD4+CD69+CD103+ cells in lesion versus control skin was increased in the convalescence phase (median 2.9-fold increase) indicating conversion to the TRM cell phenotype over time (Figure 1d, e; Figure S2c). This observation was even more striking for CD8+ T cells expressing CD69 and CD103, which were found in lesional skin at a 5.4-fold increase over control skin in convalescence (Figure 1d, e, Figure S2c). Taken together, this data demonstrates that T cells infiltrate and persist as TRM cells following intradermal drug challenge, supporting the notion that skin TRM cells contribute to cutaneous drug hypersensitivity reactions.
Figure 1.
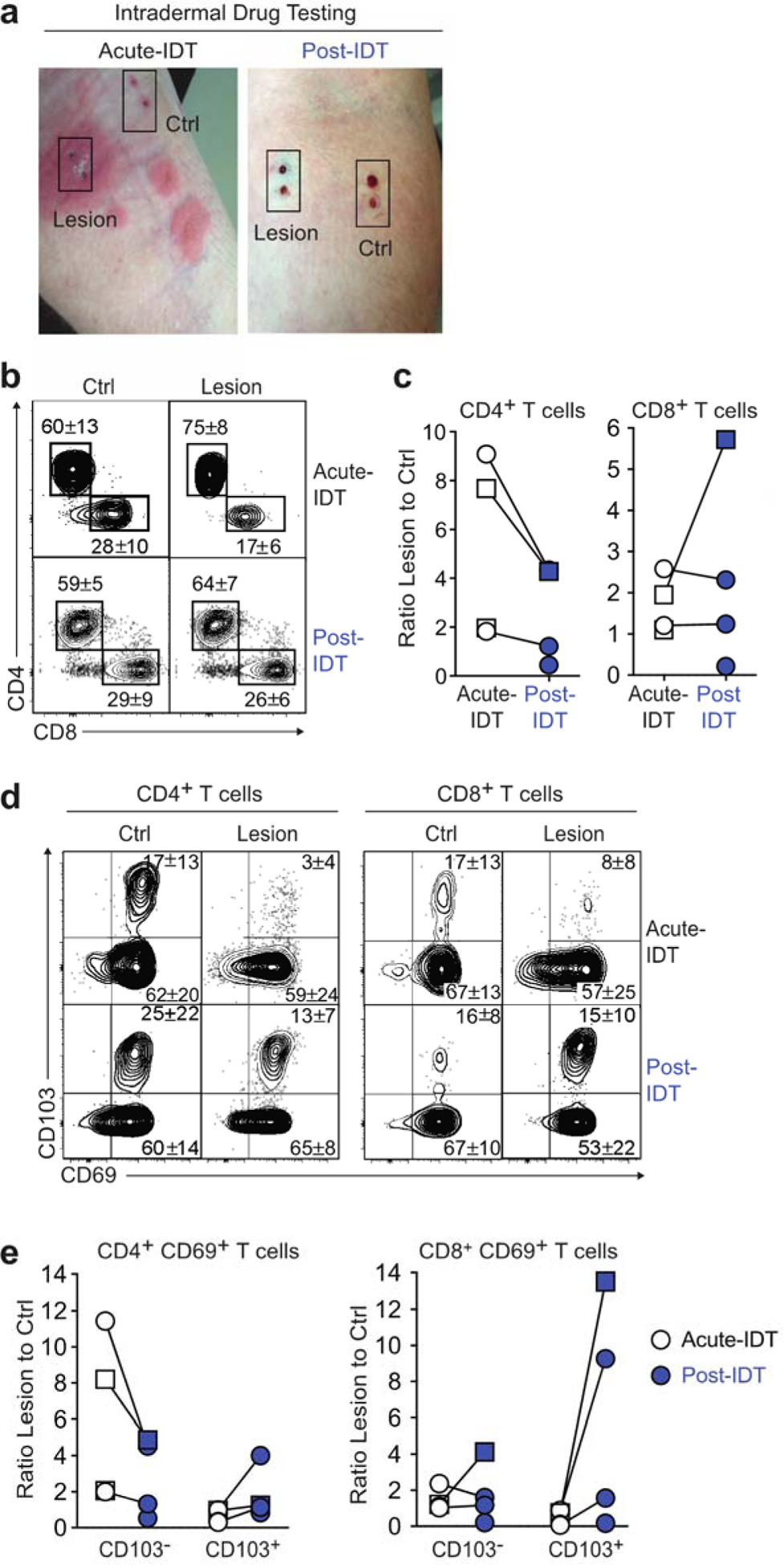
TRM cell persistence in skin following intradermal drug inoculation in patients with a history of DRESS or MPE. IDT with causative drugs was performed on five patients (3 DRESS [circles] and 2 MPE [squares]), and skin biopsies were taken from the reactive exanthema (Lesion) and control (Ctrl) skin 1 day (Acute-IDT) and > 8 weeks (Post-IDT, red) later. Both MPE patients had CLL. (a) Image shows Lesion and Ctrl skin biopsy sites during acute-IDT reaction and post-IDT from a representative patient (ID 1). The site of the control biopsy was distinct from the adjacent lesion. This patient was IDT positive to additional beta-lactam antibiotics (benzylpenicillin, ampicillin), which are routinely tested in the setting of a primary beta-lactam allergy (flucloxacillin). Patient consent for image publication was obtained. (b–e) Flow cytometry was performed on lymphocytes extracted from skin biopsies. Individual data points are shown (n = 5) and paired samples connected where available (n = 3). (b) Representative flow cytometry plots show CD4 and CD8 staining of CD3+CD45RO+ T cells. The mean frequency is shown (n = 4 Acute-IDT, n = 4 Post-IDT). (c) Ratio of the number of CD4+ (left) and CD8+ (right) T cells isolated from Lesion compared with Ctrl skin. (d) Representative flow cytometry plots show CD69 and CD103 expression by CD4+ (left) and CD8+ (right) CD3+CD45RO+ T cells. The mean frequency is shown (n = 5). (e) Ratio of the number of CD103− or CD103+ T cells isolated from Lesion compared with Ctrl skin of CD69+ CD4+ T cells (left) and CD69+ CD8+ T cells (right). CLL, chronic lymphocytic leukemia; Crtl, control; DRESS, drug reaction with eosinophilia and systemic symptoms; IDT, intradermal drug testing; Lesion, reactive exanthema; MPE, maculopapular exanthema; TRM, tissue-resident memory T cells.
Table 1 –
Clinical features of patients with positive intradermal drug testing
| ID | Age/Sex | ICHa | CCI | Pheno-type | RegiSCAR score | Internal organ involved | Primary implicated drugs | Days from phenotype onset to IDT | Positive IDT drugd | Days between Acute IDT and Post-IDT Bx | Time points analyzed by flow cytometry | ELISpot resultb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51F | - | 1 | DRESS | 4 | No | PTZ, FLU | 110 | FLUe | 105 | Acute, conv | Neg |
| 2 | 70M | CLL | 4 | MPE | 3 | No | ADF | 57 | CLAV | NA | Acute | Neg |
| 3 | 70F | CLL | 5 | MPE | 1 | No | ADF | 2118 | AMX | 63 | Acute, conv | Neg |
| 4 | 41F | - | 5 | DRESS | 7 | Yes (Liver, renal)c | PTZ, VAN | 1202 | PTZ | 56 | Conv | Pos -PTZ |
| 5 | 72F | - | 3 | DRESS | 5 | Yes (Liver, renal)c | CEF, VANC | 861 | CEF | 63 | Acute, conv | Pos- CEF |
Abbreviations– M, male; F, female; DRESS, drug reaction with eosinophilia and systemic symptoms; MPE, maculopapular exanthema; CLL, chronic lymphocytic leukemia; CCI, age-adjusted charlson comorbidity index; PTZ, piperacillin-tazobactam; FLU, flucloxacillin; ADF, amoxicillin clavulanate; CLAV, clavulanate; AMX, amoxicillin; VAN, vancomycin; CLI, clindamycin; CEF, ceftriaxone; NP, not performed; NA, not applicable; Neg, negative; Pos, positive; Conv, convalescent; Bx, biopsy; IDT, intradermal testing; NA, not applicable
ICH, immunocompromised host – HIV, cancer, haematological malignancy, transplant, autoimmune/connective tissue disorder, pred > 10mg daily for 1 month
IFN-γ release ELISpot performed to implicated drugs from PBMCs (see Supplementary Materials and Methods)
Liver injury defined as per drug induced livery injury definition (DILI) (Aithal et al., 2011) and renal injury as per a 50% increase in creatine from baseline or requirement of continuous renal replacement therapy.
Following intradermal drug inoculation, the acute exanthema develops rapidly. This response could be mediated by local or transiting skin cells that respond to cognate antigen, or bystander T cells that may have accumulated in the previously affected site. To assess whether circulating drug-reactive T cells were contributing to development of acute exanthema after intradermal testing, we measured circulating T cell responses to the implicated drug ex vivo using IFN-γ release ELISpot (Trubiano et al., 2018) at the time the intradermal testing (IDT) was administered or in convalescence. Only two patients had reactive IFN-γ release ELISpot tests, both of which had severe multiple internal organ involvement at presentation (Figure S3, Table 1). However, both uniquely had severe multiple internal organ involvement at presentation, suggesting that circulating T cells may be related to organ involvement rather than contributing to the lesional skin response.
Severe T cell mediated hypersensitivity reactions are associated with significant morbidity and mortality (Trubiano et al., 2016) yet the immunopathogenesis remains ill-defined and there is no consensus regarding optimal therapy. Previous case reports have noted an absence of circulating drug-reactive T cells in severe T cell mediated cutaneous reactions (Iriki et al., 2014) raising the possibility that skin-resident TRM cells are key mediators of such skin hypersensitivity reactions. Moreover, recent work has shown a reduction in TRM cell function after immune checkpoint blockade therapy may reduce the severity of the non-drug hypersensitivity disease allergic contact dermatitis (Gamradt et al., 2019). Here, our findings show that skin T cells persist and covert to the TRM cell phenotype following intradermal drug challenge, supporting the hypothesis that skin TRM cells may contribute to cutaneous drug hypersensitivity. This provides previously unreported insights into the pathogenesis of T cell-mediated cutaneous hypersensitivity reactions.
Supplementary Material
Acknowledgements:
We acknowledge Dr Sandy Darling from the Department of Pathology Austin Health for her contribution to reviewing the histopathology slides, and the Vanderbilt Translational Pathology Shared Resource supported by NCI/NIH Cancer Center Support Grant 5P30 CA68485-19.
Funding: This work was supported by the National Health and Medical Research Council (NHMRC) and the Austin Medical Research Foundation. J.A.T. is supported by a NHMRC postgraduate scholarship (GNT 1139902) and a postgraduate scholarship from The National Centre for Infections in Cancer, National Health and Medical Research Council, Centre for Research Excellence (App 1116876). C.L.G is supported by a NHMRC Early Career Fellowship (GNT 1160963). E.J.P. is supported in part by the National Institutes of Health (NIH) (1P50GM115305-01, R21AI139021, R34AI136815), NHMRC, and the Angela Anderson Foundation. K.C.K. is supported by the National Institutes of Health (NIH) (award nos. F30 AI131780, P50 GM115305 and T32 GM7347). S.L.P. is supported by a Cancer Council Victoria Postdoctoral Fellowship. L.K.M is a Senior Medical Research Fellow supported by the Sylvia and Charles Viertel Charitable Foundation.
Abbreviations:
- M
male
- F
female
- DRESS
drug reaction with eosinophilia and systemic symptoms
- MPE
maculopapular exanthema
- CLL
chronic lymphocytic leukemia
- CCI
age-adjusted charlson comorbidity index
- PTZ
piperacillin-tazobactam
- FLU
flucloxacillin
- ADF
amoxicillin clavulanate
- CLAV
clavulanate
- AMX
amoxicillin
- VAN
vancomycin
- CLI
clindamycin
- CEF
ceftriaxone
- NP
not performed
- NA
not applicable
- Neg
negative
- Pos
positive
- Conv
convalescent
- Bx
biopsy
- IFN-γ
interferon gamma
- ICH
immunocompromised host
- TRM
tissue-resident memory T cells
Footnotes
Data Availability Statement:
Datasets related to this article can be found at https://doi.org/10.26188/5d92f2dacbf6a, hosted at University of Melbourne Figshare.
Conflict of Interest Statement:
The authors state no conflict of interest
References:
- 1.Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 2011;89(6):806–15. [DOI] [PubMed] [Google Scholar]
- 2.Gamradt P, Laoubi L, Nosbaum A, Mutez V, Lenief V, Grande S, et al. Inhibitory checkpoint receptors control CD8(+) resident memory T cells to prevent skin allergy. J Allergy Clin Immunol 2019;143(6):2147–57 e9. [DOI] [PubMed] [Google Scholar]
- 3.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009;10(5):524–30. [DOI] [PubMed] [Google Scholar]
- 4.Iriki H, Adachi T, Mori M, Tanese K, Funakoshi T, Karigane D, et al. Toxic epidermal necrolysis in the absence of circulating T cells: a possible role for resident memory T cells. J Am Acad Dermatol 2014;71(5):e214–6. [DOI] [PubMed] [Google Scholar]
- 5.Klicznik MM, Morawski PA, Hollbacher B, Varkhande S, Motley S, Kuri-Cervantes L, et al. Human CD4+CD103+ cutaneous resident memory T cells are found in the circulation of healthy subjects. bioRxiv 2019;361758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 2013;14(12):1294–301. [DOI] [PubMed] [Google Scholar]
- 7.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, et al. T-box transcription factors combine with the cytokines TGF-beta and IL-15 to control tissue-resident memory T cell fate. Immunity 2015;43(6):1101–11. [DOI] [PubMed] [Google Scholar]
- 8.Masopust D, Soerens AG. Tissue-Resident T Cells and Other Resident Leukocytes. Annu Rev Immunol 2019;37:521–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 2016;16(2):79–89. [DOI] [PubMed] [Google Scholar]
- 10.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 2015;21(7):688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiohara T, Mizukawa Y, Teraki Y. Pathophysiology of fixed drug eruption: the role of skin-resident T cells. Curr Opin Allergy Clin Immunol 2002;2(4):317–23. [DOI] [PubMed] [Google Scholar]
- 12.Trubiano JA, Aung AK, Nguyen M, Fehily SR, Graudins L, Cleland H, et al. A Comparative Analysis Between Antibiotic- and Nonantibiotic-Associated Delayed Cutaneous Adverse Drug Reactions. J Allergy Clin Immunol Pract 2016;4(6):1187–93. [DOI] [PubMed] [Google Scholar]
- 13.Trubiano JA, Douglas AP, Goh M, Slavin MA, Phillips EJ. The safety of antibiotic skin testing in severe T-cell-mediated hypersensitivity of immunocompetent and immunocompromised hosts. J Allergy Clin Immunol Pract 2019;7(4):1341–3 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trubiano JA, Strautins K, Redwood AJ, Pavlos R, Konvinse KC, Aung AK, et al. The Combined Utility of Ex Vivo IFN-gamma Release Enzyme-Linked ImmunoSpot Assay and In Vivo Skin Testing in Patients with Antibiotic-Associated Severe Cutaneous Adverse Reactions. J Allergy Clin Immunol Pract 2018;6(4):1287–96 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med 2015;7(279):279ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


