Abstract
Inflammatory myocardial diseases represent a diverse group of conditions in which abnormal inflammation within the myocardium is the primary driver of cardiac dysfunction. Broad causes of myocarditis include infection by cardiotropic viruses or other infectious agents, to systemic autoimmune disease, or to toxins. Myocarditis due to viral aetiologies is a relatively common cause of acute chest pain syndromes in younger and middle-aged patients and often has a benign prognosis, though this and other forms of myocarditis also cause serious sequelae, including heart failure, arrhythmia and death. Endomyocardial biopsy remains the gold standard tool for tissue diagnosis of myocarditis in living individuals, although new imaging technologies have a crucial and complementary role. This review outlines the current state-of-the-art and future experimental cardiovascular magnetic resonance (CMR) imaging approaches for the detection of inflammation and immune cell activity in the heart. Multiparametric CMR, particularly with novel quantitative T1- and T2-mapping, is a valuable and widely-available tool for the non-invasive assessment of inflammatory heart diseases. Novel CMR molecular contrast agents will enable a more targeted assessment of immune cell activity and may be useful in guiding the development of novel therapeutics for myocarditis.
Keywords: Myocarditis, cardiac magnetic resonance (CMR), inflammation
Immune cells and myocardial disease
The cells of the immune system have a myriad of roles in the prevention, development, maintenance and resolution of numerous cardiovascular diseases (1). These functions have historically been under-appreciated, in part due to the difficulty of detecting immune cells within the living human cardiovascular system. We now appreciate that all major immune cell classes are present in healthy mouse hearts (2) and that certain immune cell populations are essential for aspects of normal cardiac function including normal electrical activation (3). The immune system and the heart thus have a complex interaction in both steady state and perturbations of this steady state in disease (4). The consequences of immune responses in the heart can range from the essentially undetectable to myocyte loss and the development of fibrosis, heart failure, arrhythmia and death. Immune cells and their roles within the cardiovascular system are now a major focus of interest and are promising therapeutic targets (5,6).
Inflammatory cardiomyopathy
Although inflammatory activation is an essentially ubiquitous response to diverse forms of cardiac injury, the term myocarditis is generally reserved for situations in which inflammation is the primary driver of cardiac dysfunction. Three broad aetiologies of non-ischaemic myocarditis/inflammatory cardiomyopathy are recognised (7):
Infectious myocarditis may be caused by viral, bacterial, fungal, protozoal, parasitic, spirochaetal or Rickettsial infection. Viral infections may be the most common cause of myocarditis in humans, with adenovirus, parvovirus B19 and Coxsackie viruses most frequently implicated among numerous other organisms (8);
Immune-mediated myocarditis is associated with numerous systemic autoimmune or immune-mediated disorders, including systemic lupus erythematosus, rheumatoid arthritis, Churg-Strauss syndrome, inflammatory bowel disease, scleroderma, sarcoidosis, Kawasaki disease, granulomatosis with polyangiitis, and polymyositis, among others. Different forms of immune-mediated myocarditis are also seen following cardiac transplantation and in response to allergens;
Toxic myocarditis has numerous causes including drugs, heavy metals, hormones, envenomation, radiation and electrical shocks, among others.
Diagnosis of myocarditis using endomyocardial biopsy (EMB)
The gold standard method for assessing immune cell populations within the heart and obtaining a tissue diagnosis of myocarditis in living individuals is with EMB (9). The criteria for the diagnosis of myocarditis using histologic criteria for samples of myocardium obtained from biopsy differ from those of non-invasive imaging. The Dallas criteria are based on tissue histology and diagnose myocarditis according to the presence of an “inflammatory infiltrate of the myocardium with necrosis and/or degeneration of adjacent myocytes, not typical of ischaemic damage associated with coronary artery disease” (10). A more precise definition of this “inflammatory infiltrate” has been proposed by the ESC Working Group on Myocardial and Pericardial diseases (7) as “≥14 leucocytes/mm2 including up to 4 monocytes/mm2 with the presence of CD3 positive T-lymphocytes ≥7 cells/mm2”. EMB thus confirms the diagnosis of myocarditis and enables differentiation of certain key aetiologies of myocarditis (including giant cell, eosinophilic, sarcoidosis), which may have important treatment implications, as well as enabling detection of viruses using polymerase chain reaction (PCR) or other techniques (11). However, EMB is not entirely free from risk [reported rates vary by centre though one series reported a 2.7% complication rate during vascular access and 3.3% complication rate from the biopsy procedure (12)] and patients with a clinical diagnosis of myocarditis without high-risk features frequently do not require biopsy, given that the prognosis in those cases is generally benign and that biopsy results typically do not alter clinical management. A further potential limitation of EMB is of sampling error, which may not necessarily rule out the presence of myocarditis if negative. As a result, EMB is only indicated in selected clinical scenarios, such as rapidly progressive heart failure with haemodynamic compromise, suspected giant cell myocarditis, heart failure with suspected allergic reactions/eosinophilia and others (12). There exists an important and complementary role for imaging, which is non-invasive and could provide characterisation of the entire myocardium at serial timepoints to track progression or resolution of disease and the response to therapy (13).
Principles of using cardiovascular magnetic resonance (CMR) in the detection of myocardial inflammation
CMR, using new advances in tissue characterisation techniques, has important strengths in the assessment of myocardial inflammation. Alongside echocardiography and positron emission tomography (PET), CMR is now considered a key tool for the diagnosis and management of inflammatory heart diseases with a class I recommendation for the assessment of myocarditis and storage diseases in the current ESC guidelines for the diagnosis and treatment of acute and chronic heart failure (14). A further strength of CMR is the ability to reliably distinguish ischaemic from non-ischaemic myocardial injury in most cases, which may be particularly useful in acute clinical presentations and their in-hospital management. However, where the pre-test probability of acute coronary syndrome is not low, then alternative diagnostic strategies are usually undertaken prior to CMR.
Current non-invasive imaging techniques, including CMR, to assess for myocarditis do not directly resolve immune cells, but rather detect the macroscopic tissue responses that occur in response to inflammation and injury. These early tissue responses to myocarditis include myocardial oedema, vasodilatation, and myocyte necrosis. Late tissue consequences of myocarditis include myocardial fibrosis leading to impairment of systolic function. CMR, with its multiparametric capabilities for advanced myocardial tissue characterisation, is well placed to detect these responses.
The CMR imaging protocol for myocarditis and the Lake Louise Criteria
Given the importance of accurate diagnosis and standardisation, consensus criteria for the diagnosis of myocarditis using CMR have been defined according to the Lake Louise Criteria, first in 2009 (15) and recently updated in 2018, to reflect advances in imaging technology and new evidence (16).
The original Lake Louise Criteria (15) (in 2009) proposed a clinical CMR imaging protocol for evaluating the principle tissue targets in myocarditis, including: (I) myocardial oedema, using T2-based imaging; (II) hyperaemia and capillary leak, using early gadolinium enhancement (EGE) imaging; and (III) myocyte necrosis and fibrosis, using late gadolinium enhancement (LGE) imaging. Supportive criteria include associated impairment of global or regional systolic left ventricular (LV) function and pericardial effusion. The presence of “any 2 out of the 3” main criteria above would constitute a positive imaging diagnosis of myocarditis, with an overall diagnostic accuracy of 78%, with a sensitivity of 67% and a specificity of 91% (16).
In the subsequent decade, significant advancement in CMR technology enabled high resolution and clinically-applicable quantitative T1 and T2 mapping techniques, which are particularly sensitive to detecting significant increases in myocardial free water content, hence of utility in the detection of myocarditis. Accordingly, the Lake Louise Criteria were updated in 2018 to include parametric mapping.
According to the revised Lake Louise Criteria 2018 (Table 1), in the setting of clinically suspected acute myocarditis, CMR findings are consistent with myocardial inflammation if both T1- and T2- based criteria are present, as follows:
Table 1. Summary comparison of original and revised Lake Louise Criteria. The original criteria required the presence of 2 out of 3 of criteria based on T2 weighted imaging, early gadolinium enhancement or late gadolinium enhancement. The revised criteria require the presence of 2 out of 2 criteria from either T1-based or T2-based imaging.
| Original Lake Louise Criteria I (15) (2 out of 3 required) |
| T2-weighted imaging |
| Regional high T2 signal intensity or |
| Global T2 signal intensity ratio ≥2.0 on T2-weighted CMR imaging (myocardium versus skeletal muscle) |
| Early gadolinium enhancement |
| SI ratio myocardium/skeletal muscle (EGE ratio) of ≥4.0 on EGE images |
| Late gadolinium enhancement |
| Areas with high SI in a non-ischaemic distribution pattern on LGE imaging |
| Supporting criteria |
| Pericardial effusion |
| LV regional wall motion abnormality on cine imaging |
| Updated Lake Louise Criteria II (16) (2 out of 2 required) |
| T2-based imaging |
| Regional high T2 signal intensity or |
| Global T2 signal intensity ratio ≥2.0 on T2 weighted CMR imaging (myocardium versus skeletal muscle) or |
| Regional or global increase of myocardial T2 relaxation time |
| T1-based imaging |
| Regional or global increase of native myocardial T1 relaxation times or ECV or |
| Areas with high SI in a non-ischaemic distribution pattern on LGE imaging |
| Supporting criteria |
| Pericardial effusion or |
| High pericardial signal intensity on T1- or T2-mapping or LGE imaging, or |
| LV regional wall motion abnormality on cine imaging |
CMR, cardiovascular magnetic resonance; EGE, early gadolinium enhancement; LGE, late gadolinium enhancement; SI, signal intensity.
T2-based imaging: regional or global increase in myocardial T2 signal, either on T2-weighted imaging or T2-mapping;
T1-based imaging: regional or global increase in myocardial T1 signal, either on native myocardial T1-mapping, extracellular volume (ECV) quantification, or LGE imaging in a predominantly non-ischaemic pattern;
Supportive criteria include: (i) the presence of pericardial effusion in cine CMR images or high signal intensity (SI) of the pericardium in LGE images, T1-mapping or T2-mapping, and (ii) systolic LV wall motion abnormality in cine CMR images.
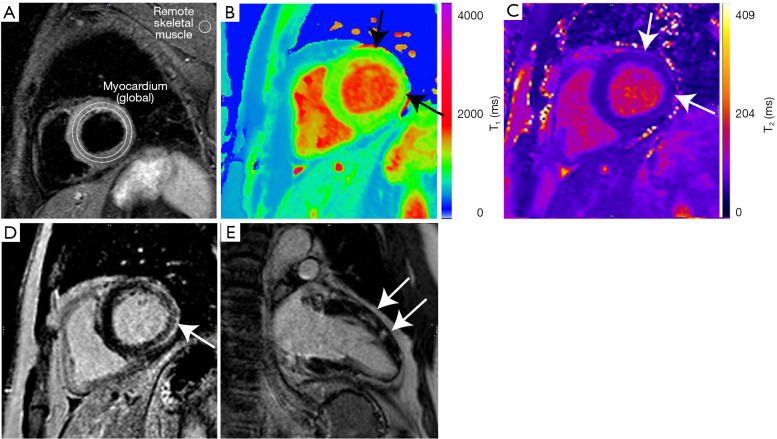
While having both a positive T2-based marker and a T1-based marker will increase specificity for diagnosing acute myocarditis, having only one (i.e., T2-based or T1-based) marker may still support a diagnosis of acute myocarditis in a relevant clinical scenario, but with less specificity. Reporting of the results for assessment of myocarditis should include the description of the presence or absence of the assessed criteria (17,18). These techniques are now widely available on many cardiac enabled clinical MRI systems. Example images from a patient with acute myocarditis are shown in Figure 1.
Figure 1.
Multiparametric CMR demonstrating Lake Louise Criteria for acute myocarditis. (A) T2 STIR imaging demonstrating high global myocardial signal intensity relative to remote skeletal muscle with a signal intensity ratio >2.0; (B) T1 mapping using ShMOLLI demonstrating significantly prolonged T1 times of >1,000 ms (local normal range 941±23 ms at 1.5T) in primarily the lateral and anterior walls; (C) T2 mapping demonstrating significantly prolonged T2 times of >55 ms (local normal range 44–52 ms at 1.5T) in the lateral and anterior walls. Quantification of T1 and T2 times is performed using specialist software rather than visual assessment; (D,E) late gadolinium enhancement imaging demonstrates patchy subepicardial and mid-wall enhancement in the anterior, lateral and inferior walls, consistent with myocarditis. CMR, cardiovascular magnetic resonance; STIR, short tau inversion recovery; ShMOLLI, shortened modified look-locker inversion recovery.
Anatomical and functional imaging
Cine imaging should cover the entire myocardium, using standard long axis views and a short axis stack covering both ventricles from base to apex. This provides a gold standard assessment of cardiac chamber volumes and systolic function while also allowing assessment for any associated pericardial effusion. The identification of associated regional wall motion abnormalities may support involvement from myocarditis. However, ventricular dysfunction and regional wall motion abnormalities detected in cine imaging are not specific to inflammatory aetiologies, and additional tissue characterisation sequences are therefore required.
Gadolinium enhanced imaging
The administration of gadolinium-based contrast agents (GBCAs) adds important information in the assessment of myocarditis. GBCAs include elemental gadolinium contained within large carrier molecules which prevent entry into myocardial intracellular space in normal conditions. GBCAs are paramagnetic and potently shortens T1 times; thus, relatively higher local concentrations of GBCA lead to a relative increase in myocardial SI on T1 weighted imaging, compared to nulled, normal myocardium.
EGE imaging
In myocarditis, the resulting hyperaemia and capillary leak can traditionally be assessed using EGE imaging by performing T1 weighted imaging early after the administration of GBCA. Semi-quantitative comparison is then made for the myocardial SI enhancement relative to a skeletal muscle reference region in the same image, before and after GBCA administration (16).
EGE imaging was in the original Lake Louise Criteria 2009 (15), though has limitations, including variability in SI that may be introduced by different MR systems and settings, and issues in image quality consistency. Recent data indicated that removing EGE from the original Lake Louise Criteria does not significantly reduce diagnostic accuracy for myocarditis, although the positive likelihood ratio may be slightly lowered (19). EGE imaging is therefore no longer required in the revised Lake Louise Criteria 2018, though may still be useful in experienced centres (16).
LGE imaging
The loss of myocytes in direct viral or immune-mediated myocyte injury opens an additional cellular compartment for GBCA accumulation. By imaging following a delay of around 10 minutes following injection of GBCA, the contrast agent will washout more quickly from non-injured myocardium, whilst persisting longer in areas with extracellular space expansion, including that due to myocyte loss and necrosis. This results in a relatively high concentration of GBCA, and hence a higher signal on T1 weighted imaging in areas of myocyte necrosis when compared to normal myocardium. LGE therefore allows localization and characterization of areas of myocyte loss as a result of the differential contrast enhancement. LGE images are acquired using inversion-recovery based T1-weighted imaging, typically using gradient-echo or FLASH read-out, with optimization of the sequence inversion time to correctly null signal from normal myocardium to highlight areas of abnormality.
Acquisition of a full short-axis stack and long-axis views for LGE is recommended and a finding of hyperintensity suggestive of focal injury should be confirmed in at least one additional orthogonal plane. LGE patterns in myocarditis are heterogenous, though the most common patterns include patchy, non-contiguous lesions, localised to the subepicardial or intramural layers of the lateral or septal walls (20). This typically allows for differentiation from ischaemic aetiologies where the enhancement is subendocardial (21). Less common LGE patterns, including subendocardial or even transmural LGE associated with inflammation-induced coronary vasospasm (22), can occur.
A limitation of LGE imaging is that they are not designed to image oedema or inflammation, and cannot differentiate active from chronic lesions; thus, LGE alone is limited in its ability to detect myocardial inflammation and guide the initiation of immunomodulatory therapies or the tracking of treatment response.
T2 weighted imaging
T2 weighted imaging is conventionally used to detect myocardial oedema, traditionally acquired using black-blood spin echo techniques with blood flow and fat suppression techniques, such as the short tau inversion recovery (STIR). Body coils, or surface coils with SI correction algorithms, are used to minimise hardware-derived variations in signal homogeneity. The increased water content of oedematous myocardium leads to prolonged T2 decay times, which can be detected as high signal on T2-weighted images. Conventional T2 weighted imaging can be assessed in a semi-quantitative fashioned by comparing the SI from myocardium to a reference region of interest (ROI) placed in skeletal muscle. Early clinical validation studies showed that a relative myocardium SI increase of >1.9 showed a sensitivity of 84% with 74% specificity for detection of significant myocarditis, with an overall accuracy of 79% (23). A regional increase in T2 signal, with SI more than 2 standard deviations above the mean value of a reference ROI placed in apparently normal myocardium, may also be considered a positive criterion (15,17). The semi-quantitative nature of these techniques has limitations, as the reference ROIs, whether they be within normal-appearing myocardium or in the skeletal muscle, may also be inflamed in a systemic inflammatory process, resulting in false negative results (16,24). Newer CMR techniques using directly quantitative, pixel-wise parametric T1- and T2-mapping circumvent this and other limitations of conventional CMR tissue characterisation techniques.
Parametric mapping techniques
Tissues in the body have characteristic T1 and T2 relaxation times (dependent on the method used to estimate them), and deviation from these relaxation times may indicate changes in the composition of the tissue that indicate disease or a change in physiology. Myocardial T1 and T2 relaxation times are markedly prolonged by an increased water content from oedema, making these techniques useful for the detection of acute myocardial injury and oedema in myocarditis.
Mapping techniques differ from conventional CMR techniques by enabling quantitative, pixel-by-pixel maps of the myocardium based on the T1 and T2 properties of the tissue. Mapping techniques can therefore overcome many of the technical limitations of semi-quantitative conventional techniques like T2-weighted and EGE imaging. Advantages include shorter breath-hold times and no requirement for a reference ROI for image processing to detect abnormal signal changes in the myocardium. Mapping techniques have been shown to be of particular utility in the diagnosis of myocarditis, are now increasingly used in clinical CMR protocols, and are expected to eventually replace some conventional CMR techniques.
Mapping techniques remain in active development, and standardised methods and protocols are still being established (25). For native T1 and ECV mapping, the most widely used approaches include inversion-recovery and saturation-recovery based sequences, or hybrid methods (26-29). For T2-mapping, the most common methods include gradient and spin echo using multi-echo readouts (30,31). Although superior to conventional techniques in this regard, measured T1 and T2 values in vivo using parametric mapping techniques are sensitive to the hardware and software used, and thus local validation is recommended at the present time in the absence of standardized, universal methods. As with comprehensive myocardial tissue characterisation using LGE, ideally, whole LV coverage should be obtained using long and short axis views in order to fully assess the regional and variable patterns of tissue injury in myocarditis, with regions of abnormal signal corroborated with views in other planes (32). Proper breath-holding, even if motion correction algorithms are inbuilt, and quality-control maps (33) increase the quality and reliability of pixel-wise mapping.
In the diagnosis of myocardial inflammation, T1 and T2 mapping have both demonstrated sensitivity to disease and are able to separate healthy control subjects and those with myocardial inflammation of various etiologies (16,34-36). Conditions and systemic diseases in which mapping have demonstrated clinical utility in the detection of myocardial involvement include among others:
The ability of CMR parametric mapping to provide novel therapy-responsive biomarkers in myocardial inflammation and to track disease course is promising (43), and is tested in ongoing and future studies.
Performance of CMR diagnostic criteria for detection of myocardial inflammation
The original Lake-Louise Criteria (2009) are well studied and provide good diagnostic accuracy in patients with suspected myocarditis. Two recent meta-analyses have evaluated the performance of the Lake Louise Criteria to identify acute myocarditis, with one reporting a pooled diagnostic accuracy of 83% (sensitivity, 80%; specificity, 87%) (44) and another reporting summary sensitivity of 78%, specificity of 88%, and area under the curve (AUC) of 83% (15,16,45). T1- and T2-mapping have demonstrated good performance for the detection of myocarditis in clinical studies (16,36,45). A recent meta-analysis of CMR mapping techniques in acute myocarditis showed that native T1 mapping (AUC 0.95) had superior diagnostic accuracy across all tissue characterisation techniques (45). The revised Lake Louise criteria 2018 show even greater diagnostic performance, with a validation cohort in 2019 demonstrating a significantly improved sensitivity of 87.5% and specificity of 96.2% for the diagnosis of acute myocarditis (46).
As T1 mapping and ECV are also sensitive to detection of water in more chronic settings, such as in areas of myocardial scarring, ischaemia or other causes of expanded extracellular space (47-49), there have been suggestions that T2 mapping may be more specific to acute myocardial inflammation (50). Whether parametric mapping techniques provide additional diagnostic accuracy that lead to improved clinical outcomes for patients with myocarditis or guide immunosuppressive therapy will be assessed by future larger and longer-term clinical studies.
Unmet needs and emerging magnetic resonance technologies for immune cell imaging
Whilst the powerful tissue characterisation techniques of proton T1, T2 and GBCA enhanced CMR provide important information for diagnosis and risk stratification in myocarditis and other inflammatory heart diseases, there remain several areas of unmet need from an imaging perspective.
First, whilst current MR techniques are highly sensitive to myocardial oedema and provide excellent whole-heart coverage, these MR signals lack sensitivity to detect or phenotype immune cells within the heart. This restricts the utility of CMR to understand biological mechanisms or identify the specific cause of an inflammatory heart disease. Although a versatile technique, MR has an intrinsically limited signal to noise ratio at biological temperatures despite high field strengths. Several magnetic resonance-based techniques which could provide a more direct measure of immune cell number and phenotype are under development. A more direct assessment of immune cell number and phenotype within the heart is most likely to be achieved by using novel molecular contrast agents which are sensitive immune cells. These include iron particle imaging, 19Flourine (19F) enhanced CMR, and hyperpolarized MRI.
Iron particle imaging
Particles of iron oxide provide tissue contrast due to a strong paramagnetic effect which leads to local shortening of T1, T2 and T2*. When ultra-small particles of iron oxide (USPIOs) are administered intravenously, the dimensions of these particles facilitate passive diffusion through capillary endothelia where they are taken up and engulfed by phagocytic cells, such as circulating and tissue resident macrophages. Regions of low signal on T1 and T2 weighted imaging, therefore, correspond to the presence of phagocytic immune cell populations. USPIOs are then cleared from the circulation and degraded by the reticuloendothelial system until they are incorporated into the body’s iron stores.
USPIOs have been used as contrast agents to detect phagocytic cell presence and infer inflammation in numerous diseases, including atherosclerotic plaques (51), abdominal aortic aneurysm (52,53), and neuroinflammation from multiple sclerosis (54), among many others. However, USPIOs have been less successful for the detection of cardiac inflammation. For example, in a study of 14 patients with acute myocarditis, USPIO administration did not cause detectable signal changes in regions demonstrating LGE and thus did not add incremental information and cannot rule out myocardial inflammation (55). This may reflect the fact that many forms of myocarditis are predominantly lymphocytic rather than macrophage-driven, at least at the timepoint studied. However, USPIOs did demonstrate inflammation in Takotsubo cardiomyopathy, highlighting the ability of novel contrast agents to inform disease mechanisms in situations where validated animal models of disease are lacking (56).
Larger microparticles particles of iron oxide (MPIOs) provide improved contrast-to-noise ratio compared to USPIOs, and, importantly, can be conjugated to antibodies, enabling in vivo targeting of specific molecular processes, including targeting to vascular cell adhesion molecule 1 (VCAM-1) on the endothelium of inflamed blood vessels (57). These agents have yet to be tested clinically for the evaluation of inflammatory myocardial diseases. Although there are some concerns about the biological persistence and degradability of the larger MPIO particles, this limitation has recently been overcome by binding smaller iron nanoparticles into larger particles using peptide linkers, which enable degradation in vivo and, hence, may be more suitable for clinical translation (58).
19F-enhanced CMR
Intravenously administered perfluorocarbons represent an alternative approach for imaging immune cells in the cardiovascular system with CMR. One important advantage over iron particles is that they provide positive, rather than negative, image contrast without disrupting the underlying proton image; furthermore, this contrast is directly proportional to the concentration of 19F nuclei within the tissue. 19F-based contrast agents, therefore, provide a quantitative assessment with high signal specificity, since 19F is essentially absent in living organisms. 19F nanoemulsions can be targeted to specific molecular targets (59) or used to exogenously label specific cell types (60,61) which can then be administered and tracked using 19F MRI. It remains a challenge to deliver a sufficient payload of 19F nuclei to provide adequate signal-to-noise ratio, and clinical translation of 19F agents for human use has been limited. Work towards the clinical translation of 19F-based imaging strategies continues.
Hyperpolarized magnetic resonance imaging
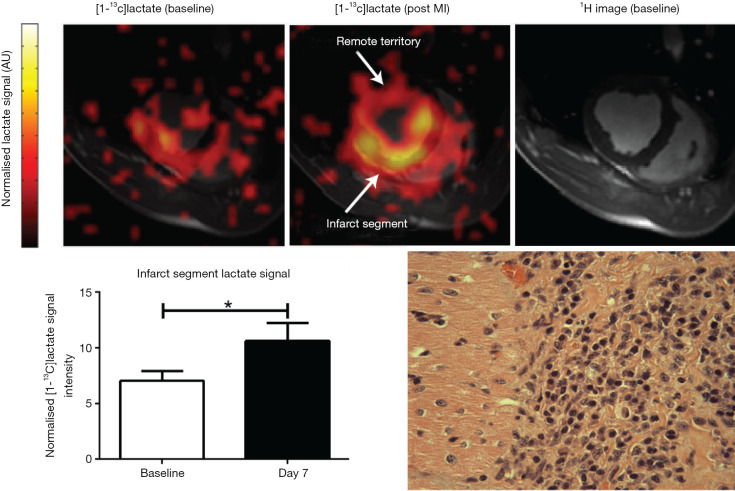
Hyperpolarized magnetic resonance is an emerging imaging technology in which the magnetic properties of external substances are manipulated to create molecular contrast agents with signal-to-noise ratio improvements of up to 4–5 orders of magnitude. Work using a key metabolic molecule, [1-13C]pyruvate highlights the potential to exploit the immunometabolic reprogramming of activated innate immune cells towards glycolysis to enable detection via a [13C]lactate signature (62). In rodent and large animal models of the immune response to myocardial infarction (MI), high lactate signature at day 3 and 7 corresponded to the macrophage driven local immune response with cell depletion experiments suggesting a high degree of specificity (Figure 2). Given that lymphocytes have similar immunometabolic properties to macrophages, hyperpolarized magnetic resonance using [1-13C]pyruvate may prove sensitive to lymphocyte driven myocardial inflammation unlike USPIOs. Further studies have established this concept in other models of inflammation including models of multiple sclerosis (63), lipopolysaccharide induced neuroinflammation (64), lung injury (65) and others. Early clinical cardiac studies of hyperpolarized [1-13C]pyruvate are underway in several centres worldwide highlighting the feasibility of this approach (66). The underlying biological process driving the MR contrast in this approach is similar to the mechanism underlying high uptake of 18FDG using PET, though hyperpolarized MR has the potential advantages of more rapid acquisition times, absence of ionising radiation and avoidance of the need for artificial suppression of cardiac carbohydrate metabolism.
Figure 2.
Experimental hyperpolarized magnetic imaging of post MI monocyte/macrophage response in a pig model of LAD occlusion. Activated innated immune cells give rise to high [1-13C]lactate signature at day 7, corresponding to immune cell infiltrate on tissue histology. Reproduced from Lewis et al. (62) under Creative Commons license. MI, myocardial infarction; LAD, left anterior descending.
A further hyperpolarized magnetic resonance molecule, hyperpolarized [1,4-13C2]fumarate provide a direct MR probe of active myocardial necrosis. The fumarate-to-malate hydration reaction is catalyzed by the intracellular enzyme fumarase as part of the tricarboxylic acid cycle. Myocyte necrosis exposes the [1,4-13C2] fumarate molecule to fumarase leading to production of [1,4-13C2]malate which does not occur when cell membranes are intact. In a rodent model of MI, malate was detectable from the hypokinetic infarcted region in the days following MI, though was not detected in sham operated rats (67). Although yet to be translated to clinical use, hyperpolarized [1,4-13C2]fumarate holds promise as a novel probe of active necrosis in myocarditis and other heart diseases, and could become an important tool in detecting disease activity and tracking response to therapy.
Conclusions
CMR is a valuable tool for the assessment of inflammatory heart diseases. Current techniques provide comprehensive visualisation and tissue characterisation of the left ventricle and provide complementary information to echocardiography and PET. Emerging MR imaging technologies based on novel molecular contrast agents based will enable a more immune cell specific assessment of inflammation and may be useful in targeting and tracking response to novel therapies.
Acknowledgments
Funding: AJ Lewis acknowledges funding from the British Heart Foundation and the Academy of Medical Sciences. VM Ferreira is supported by the British Heart Foundation, and acknowledges support from the British Heart Foundation Centre of Research Excellence in Oxford. MK Burrage is supported by a British Heart Foundation Clinical Research Training Fellowship (FS/19/65/34692). VM Ferreira and MK Burrage acknowledge support from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre at The Oxford University Hospitals NHS Foundation Trust, University of Oxford, UK.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Oliver Rider and Andrew J. Lewis) for the series “The use of advanced cardiac MRI in heart failure and cardiac hypertrophy” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The series “The Use of Advanced Cardiac MRI in Heart Failure and Cardiac Hypertrophy” was commissioned by the editorial office without any funding or sponsorship. AJML served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
References
- 1.Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol 2018;18:733-44. 10.1038/s41577-018-0065-8 [DOI] [PubMed] [Google Scholar]
- 2.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013;339:161-6. 10.1126/science.1230719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulsmans M, Clauss S, Xiao L, et al. Macrophages facilitate electrical conduction in the heart. Cell 2017;169:510-522.e20. 10.1016/j.cell.2017.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007;204:3037-47. 10.1084/jem.20070885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tardif JC, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med 2019. [Epub ahead of print]. 10.1056/NEJMoa1912388 [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119-31. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 7.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636-48. 10.1093/eurheartj/eht210 [DOI] [PubMed] [Google Scholar]
- 8.Dennert R, Crijns HJ, Heymans S. Acute viral myocarditis. Eur Heart J 2008;29:2073-82. 10.1093/eurheartj/ehn296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leone O, Veinot JP, Angelini A, et al. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol 2012;21:245-74. 10.1016/j.carpath.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 10.Aretz HT, Billingham ME, Edwards WD, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol 1987;1:3-14. [PubMed] [Google Scholar]
- 11.Bowles NE, Richardson PJ, Olsen EG, et al. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet 1986;1:1120-3. 10.1016/S0140-6736(86)91837-4 [DOI] [PubMed] [Google Scholar]
- 12.Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol 2007;50:1914-31. 10.1016/j.jacc.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz A, Ferreira V, Klingel K, et al. Role of cardiovascular magnetic resonance imaging (CMR) in the diagnosis of acute and chronic myocarditis. Heart Fail Rev 2013;18:747-60. 10.1007/s10741-012-9356-5 [DOI] [PubMed] [Google Scholar]
- 14.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 15.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol 2009;53:1475-87. 10.1016/j.jacc.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158-76. 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 17.Kramer CM, Barkhausen J, Flamm SD, et al. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson 2013;15:91. 10.1186/1532-429X-15-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. 10.1186/1532-429X-15-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu GC, Flewitt JA, Mikami Y, et al. Assessment of acute myocarditis by cardiovascular MR: diagnostic performance of shortened protocols. Int J Cardiovasc Imaging 2013;29:1077-83. 10.1007/s10554-013-0189-7 [DOI] [PubMed] [Google Scholar]
- 20.Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular Magnetic Resonance Assessment of Human Myocarditis: A Comparison to Histology and Molecular Pathology. Circulation 2004;109:1250-8. 10.1161/01.CIR.0000118493.13323.81 [DOI] [PubMed] [Google Scholar]
- 21.Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003;361:374-9. 10.1016/S0140-6736(03)12389-6 [DOI] [PubMed] [Google Scholar]
- 22.Mahrholdt H, Wagner A, Deluigi CC, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006;114:1581-90. 10.1161/CIRCULATIONAHA.105.606509 [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Aty H, Boye P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 2005;45:1815-22. 10.1016/j.jacc.2004.11.069 [DOI] [PubMed] [Google Scholar]
- 24.Ferreira VM, Piechnik SK, Dall'Armellina E, et al. T1 mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging 2013;6:1048-58. 10.1016/j.jcmg.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 25.Moon JC, Messroghli DR, Kellman P, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013;15:92. 10.1186/1532-429X-15-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messroghli DR, Greiser A, Frohlich M, et al. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging 2007;26:1081-6. 10.1002/jmri.21119 [DOI] [PubMed] [Google Scholar]
- 27.Piechnik SK, Ferreira VM, Dall'Armellina E, et al. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 2010;12:69. 10.1186/1532-429X-12-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow K, Flewitt JA, Green JD, et al. Saturation recovery single-shot acquisition (SASHA) for myocardial T(1) mapping. Magn Reson Med 2014;71:2082-95. 10.1002/mrm.24878 [DOI] [PubMed] [Google Scholar]
- 29.Roujol S, Weingartner S, Foppa M, et al. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology 2014;272:683-9. 10.1148/radiol.14140296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giri S, Chung YC, Merchant A, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 2009;11:56. 10.1186/1532-429X-11-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baeßler B, Schaarschmidt F, Stehning C, et al. A systematic evaluation of three different cardiac T2-mapping sequences at 1.5 and 3T in healthy volunteers. Eur J Radiol 2015;84:2161-70. 10.1016/j.ejrad.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 32.Ferreira VM, Piechnik SK, Dall'Armellina E, et al. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson 2014;16:36. 10.1186/1532-429X-16-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira VM, Piechnik SK, Dall'Armellina E, et al. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14:42. 10.1186/1532-429X-14-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puntmann VO, Isted A, Hinojar R, et al. T1 and T2 mapping in recognition of early cardiac involvement in systemic sarcoidosis. Radiology 2017;285:63-72. 10.1148/radiol.2017162732 [DOI] [PubMed] [Google Scholar]
- 35.Isted A, Grigoratos C, Bratis K, et al. Native T1 in deciphering the reversible myocardial inflammation in cardiac sarcoidosis with anti-inflammatory treatment. Int J Cardiol 2016;203:459-62. 10.1016/j.ijcard.2015.10.199 [DOI] [PubMed] [Google Scholar]
- 36.Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19:75. 10.1186/s12968-017-0389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ntusi NA, Piechnik SK, Francis JM, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: insights from CMR T1 mapping. JACC Cardiovasc Imaging 2015;8:526-36. 10.1016/j.jcmg.2014.12.025 [DOI] [PubMed] [Google Scholar]
- 38.Guo Q, Wu LM, Wang Z, et al. Early Detection of Silent Myocardial Impairment in Drug-Naive Patients With New-Onset Systemic Lupus Erythematosus: A Three-Center Prospective Study. Arthritis Rheumatol 2018;70:2014-24. 10.1002/art.40671 [DOI] [PubMed] [Google Scholar]
- 39.Mavrogeni S, Koutsogeorgopoulou L, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance detects silent heart disease missed by echocardiography in systemic lupus erythematosus. Lupus 2018;27:564-71. 10.1177/0961203317731533 [DOI] [PubMed] [Google Scholar]
- 40.Ntusi NA, Piechnik SK, Francis JM, et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis–a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson 2014;16:21. 10.1186/1532-429X-16-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greulich S, Mayr A, Kitterer D, et al. T1 and T2 mapping for evaluation of myocardial involvement in patients with ANCA-associated vasculitides. J Cardiovasc Magn Reson 2017;19:6. 10.1186/s12968-016-0315-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao XX, Tian Z, Lin L, et al. Successful Treatment of Type 1 Cryoglobulinemic Vasculitis With Cardiac Involvement. Can J Cardiol 2018;34:343.e1-343.e3. 10.1016/j.cjca.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 43.Ntusi NA, Francis JM, Sever E, et al. Anti-TNF modulation reduces myocardial inflammation and improves cardiovascular function in systemic rheumatic diseases. Int J Cardiol 2018;270:253-9. 10.1016/j.ijcard.2018.06.099 [DOI] [PubMed] [Google Scholar]
- 44.Lagan J, Schmitt M, Miller CA. Clinical applications of multi-parametric CMR in myocarditis and systemic inflammatory diseases. Int J Cardiovasc Imaging 2018;34:35-54. 10.1007/s10554-017-1063-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotanidis CP, Bazmpani MA, Haidich AB, et al. Diagnostic Accuracy of Cardiovascular Magnetic Resonance in Acute Myocarditis: A Systematic Review and Meta-Analysis. JACC Cardiovasc Imaging 2018;11:1583-90. 10.1016/j.jcmg.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 46.Luetkens JA, Faron A, Isaak A, et al. Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: results of a validation cohort. Radiology: Cardiothoracic Imaging 2019. doi:. 10.1148/ryct.2019190010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinojar R, Foote L, Arroyo Ucar E, et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging 2015;8:37-46. 10.1016/j.jcmg.2014.07.016 [DOI] [PubMed] [Google Scholar]
- 48.Lurz P, Luecke C, Eitel I, et al. Comprehensive Cardiac Magnetic Resonance Imaging in Patients With Suspected Myocarditis: The MyoRacer-Trial. J Am Coll Cardiol 2016;67:1800-11. 10.1016/j.jacc.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 49.Bohnen S, Radunski UK, Lund GK, et al. Performance of t1 and t2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging 2015. doi: . 10.1161/CIRCIMAGING.114.003073 [DOI] [PubMed] [Google Scholar]
- 50.Tahir E, Sinn M, Bohnen S, et al. Acute versus chronic myocardial infarction: diagnostic accuracy of quantitative native T1 and T2 mapping versus assessment of edema on standard T2-weighted cardiovascular MR images for differentiation. Radiology 2017;285:83-91. 10.1148/radiol.2017162338 [DOI] [PubMed] [Google Scholar]
- 51.Trivedi RA, Mallawarachi C, U-King-Im JM, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol 2006;26:1601-6. 10.1161/01.ATV.0000222920.59760.df [DOI] [PubMed] [Google Scholar]
- 52.MA3RS Study Investigators. Aortic Wall Inflammation Predicts Abdominal Aortic Aneurysm Expansion, Rupture, and Need for Surgical Repair. Circulation 2017;136:787-97. 10.1161/CIRCULATIONAHA.117.028433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richards JM, Semple SI, MacGillivray TJ, et al. Abdominal aortic aneurysm growth predicted by uptake of ultrasmall superparamagnetic particles of iron oxide: a pilot study. Circ Cardiovasc Imaging 2011;4:274-81. 10.1161/CIRCIMAGING.110.959866 [DOI] [PubMed] [Google Scholar]
- 54.Dousset V, Brochet B, Deloire MS, et al. MR imaging of relapsing multiple sclerosis patients using ultra-small-particle iron oxide and compared with gadolinium. AJNR Am J Neuroradiol 2006;27:1000-5. [PMC free article] [PubMed] [Google Scholar]
- 55.Stirrat CG, Alam SR, MacGillivray TJ, et al. Ferumoxytol-enhanced magnetic resonance imaging in acute myocarditis. Heart 2018;104:300-5. 10.1136/heartjnl-2017-311688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scally C, Abbas H, Ahearn T, et al. Myocardial and systemic inflammation in acute stress-induced (Takotsubo) cardiomyopathy. Circulation 2019;139:1581-92. 10.1161/CIRCULATIONAHA.118.037975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McAteer MA, Sibson NR, von Zur Muhlen C, et al. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat Med 2007;13:1253. 10.1038/nm1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perez-Balderas F, Van Kasteren SI, Aljabali AA, et al. Covalent assembly of nanoparticles as a peptidase-degradable platform for molecular MRI. Nat Commun 2017;8:14254. 10.1038/ncomms14254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Temme S, Grapentin C, Quast C, et al. Noninvasive imaging of early venous thrombosis by 19F magnetic resonance imaging with targeted perfluorocarbon nanoemulsions. Circulation 2015;131:1405-14. 10.1161/CIRCULATIONAHA.114.010962 [DOI] [PubMed] [Google Scholar]
- 60.Fink C, Gaudet JM, Fox MS, et al. 19 F-perfluorocarbon-labeled human peripheral blood mononuclear cells can be detected in vivo using clinical MRI parameters in a therapeutic cell setting. Sci Rep 2018;8:590. 10.1038/s41598-017-19031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouchlaka MN, Ludwig KD, Gordon JW, et al. 19F-MRI for monitoring human NK cells in vivo. Oncoimmunology 2016;5:e1143996. 10.1080/2162402X.2016.1143996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewis AJ, Miller JJ, Lau AZ, et al. Noninvasive immunometabolic cardiac inflammation imaging using hyperpolarized magnetic resonance. Circ Res 2018;122:1084-93. 10.1161/CIRCRESAHA.117.312535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guglielmetti C, Najac C, Didonna A, et al. Hyperpolarized 13C MR metabolic imaging can detect neuroinflammation in vivo in a multiple sclerosis murine model. Proc Natl Acad Sci U S A 2017;114:E6982-91. 10.1073/pnas.1613345114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Page LM, Guglielmetti C, Najac CF, et al. Hyperpolarized 13 C magnetic resonance spectroscopy detects toxin-induced neuroinflammation in mice. NMR Biomed 2019;32:e4164. 10.1002/nbm.4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pourfathi M, Xin Y, Kadlecek SJ, et al. In vivo imaging of the progression of acute lung injury using hyperpolarized [1‐13C] pyruvate. Magn Reson Med 2017;78:2106-15. 10.1002/mrm.26604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cunningham CH, Lau JY, Chen AP, et al. Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ Res 2016;119:1177-82. 10.1161/CIRCRESAHA.116.309769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller JJ, Lau AZ, Nielsen PM, et al. Hyperpolarized [1,4-13C2]Fumarate Enables Magnetic Resonance-Based Imaging of Myocardial Necrosis. JACC Cardiovasc Imaging 2018;11:1594-606. 10.1016/j.jcmg.2017.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]